Abstract
Vascular smooth muscle (VSM) growth is integral in the pathophysiology of blood vessel diseases, and identifying approaches that have capacity to regulate VSM growth is critically essential. Cyclic nucleotide signaling has been generally considered protective in cardiac and vascular tissues and has been the target of numerous basic science and clinical studies. In this project, the influence of BAY 41-2272 (BAY), a recently described soluble guanylate cyclase (sGC) stimulator and inducer of cyclic guanosine monophosphate (cGMP) synthesis, on VSM cell growth was analyzed. In rat A7R5 VSM cells BAY significantly reduced proliferation in dose- and time-dependent fashion. BAY activated cGMP and cyclic adenosine monophosphate (cAMP) signaling evidenced through elevated cGMP and cAMP content, increased expression of cyclic nucleotide-dependent protein kinases, and differential vasodilator-stimulated phosphoprotein (VASP) phosphorylation. BAY significantly elevated cyclin E expression, decreased expression of the regulatory cyclin-dependent kinases (Cdk)-2 and -6, increased expression of cell cycle inhibitory p21WAF1/Cip1 and p27Kip1, and reduced expression of phosphorylated focal adhesion kinase (FAK). These comprehensive findings provide first evidence for the anti-growth, cell cycle-regulatory properties of the neoteric agent BAY 41-2272 in VSM and lend support for its continued study in the clinical and basic cardiovascular sciences.
Keywords: BAY 41-2272, cyclic GMP, cyclins, protein kinases, vascular smooth muscle
Introduction
Abnormal growth of vascular smooth muscle (VSM) provides an etiologic underpinning of many forms of cardiovascular disease (CVD) and has been historically targeted for therapeutic intervention. Our laboratories [1,2] and others [3,4,5] have identified cell cycle-related proteins including protein kinases, G1 cyclins, cyclin-dependent kinases (Cdks), and Cdk inhibitors (CKIs) as integral mediators of VSM growth under in vitro and in vivo settings. Cyclin/CDK complexes play pivotal roles in regulating G0/G1-to-S phase progression while CKIs inhibit cyclin/CDK activity and lead to static G1 arrest.
Cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) are critically important second messengers with wide-ranging roles in VSM. Our previous findings provide strong evidence that cGMP-generating heme oxygenase (HO)/carbon monoxide (CO) signaling has capacity to reduce abnormal VSM growth through anti-proliferative and pro-apoptotic mechanisms [1,6,7,8,9]. Of particular interest we found that exogenous CO is capable of reducing neointimal development through reduction in cyclins A and E and transforming growth factor (TGF)-β1 signaling but without marked effects on cyclin D1 or p21WAF1/Cip1 and p27Kip1 [1].
A family of synthetic soluble guanylate cyclase (sGC) agonists has been identified and characterized over the past 15 years that is rapidly gaining interest in terms of scientific utility [6,9,10]. The prototype, a benzyl indazole termed YC-1, has been shown to confer protection to cardiovascular and hematological tissues [11,12,13,14]. BAY 41-2272 (BAY), a recently described pyrazolopyridine derivative and “second-generation” YC-1 mimetic, is an attractive agonist for NO- and/or CO-mediated signaling [15,16,17]. BAY possesses anti-hypertensive [18] and anti-platelet [19] properties and reduces load during experimental heart failure [20], yet the influence of BAY on VSM growth has not been documented to date. Therefore, the overall goal of this project is to characterize the influence of BAY on VSM cell growth.
Methods
This investigation abided by guidelines of the Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (U.S. National Institutes of Health, Publication No. 85-23, revised 1996).
Vascular smooth muscle cell culture
Rat thoracic aorta A7R5 VSM cells (ATCC, Manassas, VA) were cultured in Dulbecco's Modified Eagles Medium (DMEM) supplemented with fetal bovine serum (FBS, 0.2-10%), 2 mM L-glutamine, 500 U penicillin, and 100 mg/L streptomycin at 37 °C in 95% air/5% CO2. Cells were serially split and used through passage 6.
Cell proliferation assays
Three approaches were used to measure cell growth: hemacytometry, automated cell counting (Vi-Cell, Beckman Coulter), and fluorescent nucleic acid staining (CyQUANT Cell Proliferation Assay; Molecular Devices, Sunnyvale, CA) [21]. In general, cells were plated in multi-well plates (25,000 cells/well in 24-well plates) in 10% FBS until adherent, quiesced in low serum (0.2% FBS) conditions overnight, and then growth-stimulated with 10% serum in DMEM and varying concentrations of BAY with/without the following agents: the heme substrate and HO inducer hemin (20 μM) [22], the HO inhibitor tin protoporphyrin-IX (SnPP-IX; 20 μM) [23], the NO donor sodium nitroprusside (SNP; 1 mM) [24,25], or the NO synthase inhibitor L-NAME (20-2000 μM). At specific times cells were trypsinized (0.5%, 3-4 minutes, 37 °C) and whole cell suspensions were prepared for use in specific assays. For the fluorescent assays, standard curves were prepared for extrapolation of random units into cell numbers.
Trypan blue exclusion staining
To estimate viability, trypan blue exclusion staining was performed in parallel with each proliferation assay. Cells were trypsinized (0.5%, 3-4 min, 37 °C) and suspensions were incubated with 0.4% trypan blue (1:1, 2 min, MediaTech CellGro). Numbers of stained cells and total (stained and unstained) cells were counted and viability determined as the percentage of unstained cells.
ELISA for cGMP and cAMP
Cyclic GMP and cAMP levels were measured using CatchPoint Assays and a Spectra Max Gemini EX microplate reader (Molecular Devices). Briefly, VSM cells were pre-treated with 0.75mM IBMX (Calbiochem) in dimethyl sulfoxide (DMSO) for 10 min. BAY/DMSO treatments were added for specific times (≤ 60 min), after which reactions were stopped with lysis buffer. Protein concentrations were determined with a BCA protein assay kit (Pierce). Results were calculated with SoftmaxPro software (Molecular Devices).
Western blot
Cells were lysed in buffer (50mM Tris, pH 6.8; 1% SDS; 0.1% Triton; protease inhibitor cocktail (Roche); phosphate inhibitor cocktail (Santa Cruz)) and sonicated on ice followed by centrifugation (14000g, 20 min). Protein concentration of whole cell lysates was determined by the BCA assay (Pierce). Proteins were separated on SDS-PAGE gels and transferred to Immobilon-P membranes (Millipore) using Mini-Protean Electrophoresis and Mini Trans-Blot Cells (Bio-Rad). Blots were blocked with 5% non-fat milk in TTBS (25mM Tris/Tris-HCl; 0.0027M KCl; 0.13M NaCl; 0.1% Tween 20) or 5% BSA in TTBS for 1 hour and incubated with primary antibodies directed against sGCα1 and sGCα2 (1:500; Santa Cruz), protein kinase G (PKG) and protein kinase A (PKA) (1:1000; Abcam), phospho-PKASer338 (1:1000; Biosource), VASP, phospho-VASPSer157, and phospho-VASPSer239 (1:1000; Cell Signaling), cyclins A, D1, and E and p27Kip1 and p21WAF1/Cip1 (1:200; Santa Cruz), Cdk-2 and Cdk-6 (1:1000; Millipore), FAK and phospho-FAKTyr397 (1:500), apurinic/apyrimidinic endonuclease/redox factor 1 (APE/Ref1; 1:500), α-tubulin (1:1000; Sigma), and GAPDH (1:400; Santa Cruz) overnight at 4 °C. Blots were washed with TTBS, incubated with peroxidase-linked secondary antibodies (1:5000) for 1 hour, visualized using Pierce ECL Western blotting kit, documented using an Alpha Imager 2200, and analyzed with ImageJ 1.40g software (NIH).
Statistical analyses
Data were stored and analyzed on personal computers using Excel 2007 (Microsoft) and Sigma Plot 10.0 and Sigma Stat 3.1 (SPSS, Inc.). One-way analysis of variance (ANOVA) and post-hoc Holm-Sidak multiple comparison tests were used to detect changes between individual cohort groups [26]. Data are expressed as mean ± SEM, and a P < 0.05 was considered statistically significant.
Results
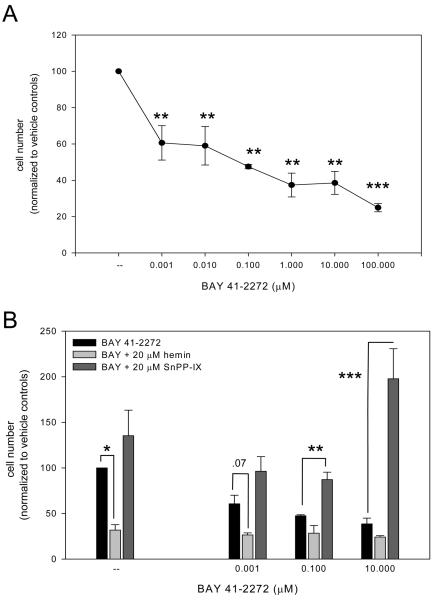
The three approaches used to measure cell growth yielded consistent and reproducible results within each cohort and across multiple experiments (see Table). In summary, treatment of VSM cells with BAY (0.001-100 μM) in 10% FBS for 48 or 72 hours resulted in significant (p < .001) dose-dependent reduction in cell numbers (results from fluorescent nucleic acid staining 72 h post-treatment are shown in Fig. 1A). Not surprisingly, detectable differences in proliferation from BAY treatment were not detected after 24 hours (an estimated cell cycling time for VSM cells is ∼22 hours). In effort to examine upstream involvement by the HO/CO pathway, concomitant treatment of cells with BAY and the HO inducer hemin (20 μM) caused significantly lower cell numbers (p = .026) at each BAY concentration than that observed with BAY alone (Fig. 1B). In corresponding fashion, HO inhibition by 20 μM SnPP-IX reversed BAY-induced growth inhibition and led to significantly increased cell numbers (p = .022) compared to sole BAY treatment (Fig. 1B). Likewise, simultaneous exposure of cells with BAY and the NO donor SNP (1.0 mM) exhibited a trend (not statistically significant) for reduced cell numbers at each BAY concentration compared to BAY alone; however, concomitant BAY and the NO synthase inhibitor L-NAME (20-2000 μM) failed to noticeably alter cell growth compared to exclusive BAY treatment (data not shown). Combined BAY with hemin and SNP did not noticeably alter cell growth compared to the BAY and hemin or BAY and SNP cohorts, respectively. Trypan blue exclusion staining run in parallel with each assay within each cohort did not reveal statistically significant reduction in viability in any treatment group (baseline: 87.5 ± 12.4%; 0.001 μM BAY: 78.2 ± 16.5%; 100 μM BAY: 66.8 ± 22.3% (p = .17)).
Table. Comparison of Experimental Approaches to Estimate Cell Numbers.
The table contains summary (normalized to DMSO vehicle controls) data for BAY 41-2272-treated VSM cell numbers after 48 or 72 hours' incubation derived from three different experimental approaches: fluorescent nucleic acid staining, automated cell counting, and hemacytometry. At these time points, BAY significantly reduces VSM cell numbers in dose-dependent fashion
| 48 hours | 72 hours | |||||
|---|---|---|---|---|---|---|
| BAY 41-2272 (mM) | BAY 41-2272 (mM) | |||||
| 0.001 | 0.1 | 10.0 | 0.001 | 0.1 | 10.0 | |
| Fluorescent nucleic acid staining |
93.5 ± 15.5 | 92.6 ± 12.7 | 72.4 ± 9.1 | 60.6 ± 9.5 | 47.5 ± 1.2 | 38.5 ±6.3 |
| Automated cell counting |
88.2 ± 15.4 | 77.2 ± 21.9 | 59.1 ± 11.1 | 54.9 ± 12.3 | 41.6 ± 6.3 | 35.9 ± 12.2 |
| Hemacytometry | 79.4 ± 5.6 | 69.9 ± 11.2 | 55.0 ± 8.5 | 61.5 ± 8.8 | 46.2 ± 8.9 | 28.6 ± 4.0 |
Figure 1.
A: Incubation of rat A7R5 VSM cells with BAY 41-2272 (0.001-100 μM) in 10% FBS significantly (p < .001) reduces cell growth in dose-dependent fashion after 72 hours as measured with fluorescent nucleic acid staining. Comparable growth-inhibitory effects of BAY were observed using hemacytometry and automated cell counting (data not shown). Results are mean ± SEM, and n = 3 experiments/group. *Statistically significant with p < .05, ***statistically significant with p < .001 versus normalized DMSO vehicle controls. B: Concomitant treatment of cells with BAY and the HO inducer hemin (20 μM) significantly reduced cell numbers (p = .026) at each BAY concentration while HO inhibition by tin protoporphyrin-IX (SnPP-IX; 20 μM) significantly increased cell numbers (p = .022) compared to exclusive BAY treatments. Post-hoc results: *statistically significant with p < .05, **statistically significant with p < .01, ***statistically significant with p < .001 versus BAY 41-2272 treatment only. Trypan blue exclusion staining did not reveal significant reduction in cell viability in any treatment group (data not shown). Results are mean ± SEM, and n = 3 experiments/group.
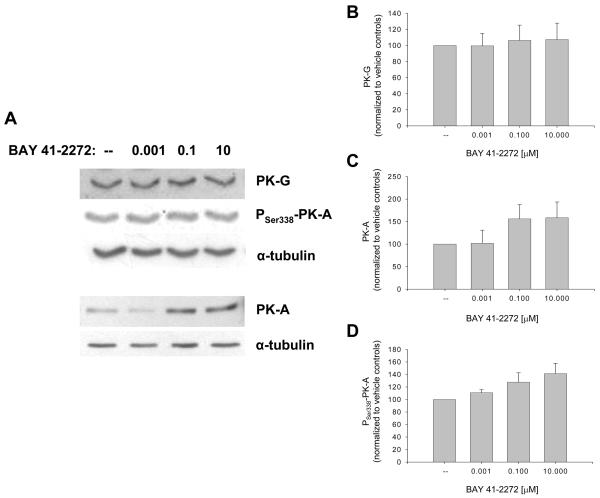
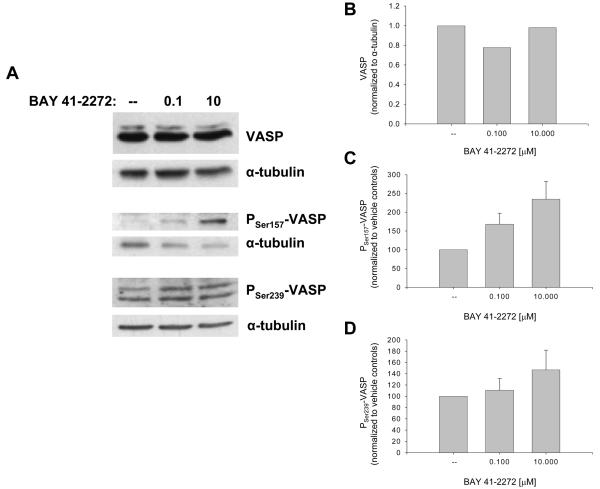
Ensuing studies analyzed the sGC/cGMP/cAMP-specific actions of BAY in VSM cells. BAY 41-2272 approximately doubled the content of cGMP (23,421 vs. 10,357 nM/mg protein) and cAMP (10,500 vs. 5607 nM/mg protein) compared to vehicle-treated cells between 10 and 30 min incubation without significant effects on protein expression of sGCα1 or sGCα2 over 4 hours (data not shown). Western analyses of cyclic nucleotide-dependent protein kinases showed that BAY (0.001-10 μM) had minimal effect on total PK-G but increased expression of PK-A (60%) and phosphorylated (at Serine 338) PK-A (40%; p = .08) after 24 hours (Fig. 2). Using differential VASP phosphorylation as a biochemical marker for activated kinase signaling in VSM [27,28], with Ser239 phosphorylation primarily indicative of a PK-G event and Ser157 phosphorylation predominantly PK-A-mediated (although Ser157 can also be phosphorylated by PK-G) [29,30,31,32], BAY did not alter total VASP content but increased VASPSer239 (∼40%) and more than doubled VASPSer157 after 24 hours (Fig. 3).
Figure 2.
Effects of BAY 41-2272 on cyclic nucleotide-regulated protein kinases in VSM. A: Western blot showing expression of PK-G, PK-A, and phosphorylated PK-A (at Serine 338) following 24 hours of BAY treatment. B-D: Densitometry (normalized to α-tubulin) reveals that BAY has minimal effect on PK-G but increases PK-A (∼60%) and phosphorylated PK-ASer338 (∼40%; p = 0.8). Results are mean ± SEM, and n = 5-6 experiments/group.
Figure 3.
Effects of BAY 41-2272 on VSM VASP after 24 hours. A: Western blots for total VASP and phosphorylated VASP (at Ser157 and Ser 239). B-D: Densitometry (normalized to α-tubulin) shows that BAY has no discernable effect on total VASP but markedly increases VASPSer157 (∼140%; p = .17) and VASPSer239 (∼40%) in dose-dependent fashion. Results are mean ± SEM, and n = 1 experiment for VASP and n = 6 experiments/group for phosphorylated VASP.
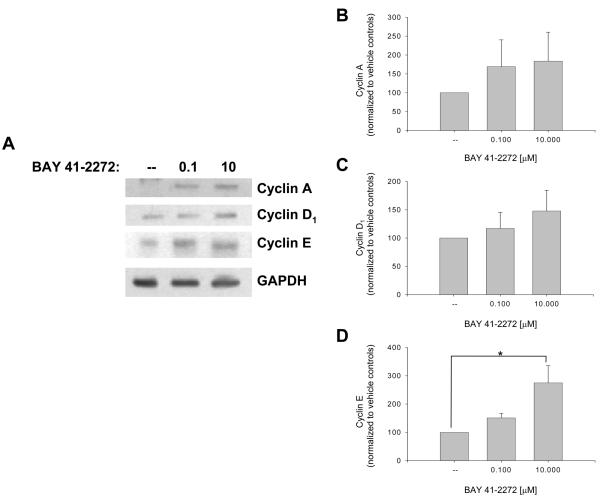
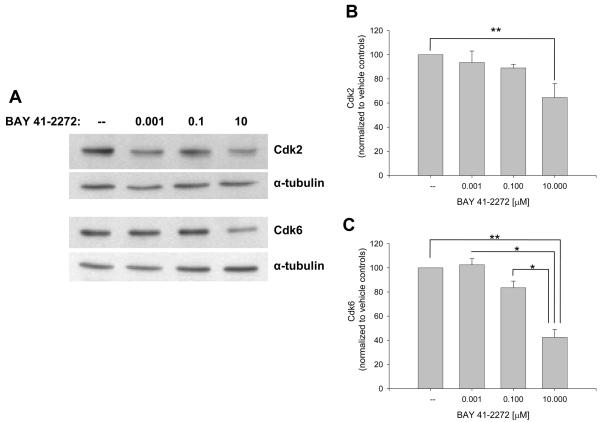
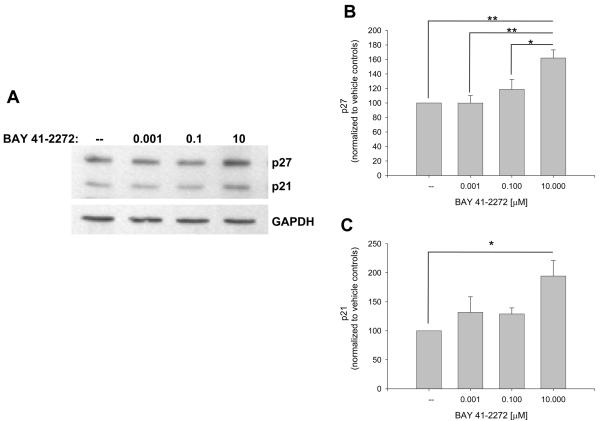
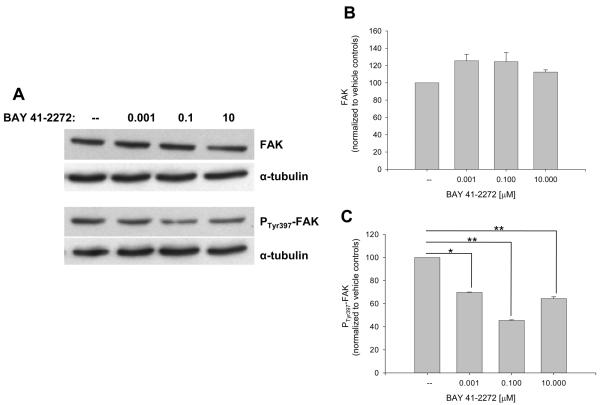
The influence of BAY on recognized VSM cell cycle-regulatory factors was then investigated. Figure 4 shows that BAY increases expression of cyclins A (∼80%) and D1 (∼50%) and significantly cyclin E (p < .05) after 48 hours. At this time point BAY significantly reduced expression of Cdk-2 (p < .05) and Cdk-6 (p < .001; Fig. 5) and significantly increased protein expression of the cell cycle-inhibitory p27Kip1 (p < .001) and p21WAF1/Cip1 (p < .05; Fig. 6). In these experiments, verification of the nuclear fraction was verified in each sample by stripping and re-probing for nuclear-specific APE/Ref1, a key protein in the base excision repair pathway (data not shown). Lastly, BAY did not alter total FAK but significantly reduced expression of phosphorylated (at Tyrosine 397) FAK (Fig. 7).
Figure 4.
Effects of BAY 41-2272 on nuclear cyclin expression in VSM after 48 hours incubation. A: Western blot showing protein expression for the G1 cyclins A, D1, and E. B-D: Densitometry (normalized to nuclear GAPDH) reveals that BAY (0.001-10 μM) elevates expression of cyclins A (∼80%) and D1 (∼50%) and significantly increases cyclin E (∼200%; p = .015). Concomitant staining for APE/Ref1 verified nuclear localization of all samples (data not shown). Results are mean ± SEM, and n = 5 experiments/group. Post-hoc results: *statistically significant versus DMSO controls with p < .05.
Figure 5.
Effects of BAY 41-2272 on VSM nuclear Cdk expression after 48 hours. A: Western blot showing Cdk-2 and Cdk-6 expression. B-C: Densitometry (normalized to α-tubulin) shows that BAY significantly and dose-dependently reduces expression of Cdk-2 (∼35%; p = 0.031) and Cdk-6 (∼60%; p < .001). Post-hoc results: *statistically significant with p < .05; **statistically significant with p < .01. Concomitant staining for APE/Ref1 verified nuclear localization of all samples (data not shown). Results are mean ± SEM, and n = 4 experiments/group.
Figure 6.
Effects of BAY 41-2272 on the nuclear cyclin-dependent kinase inhibitors (CKIs) p27Kip1 and p21WAF1/Cip1 after 48 hours incubation. A: Western blot showing p27 and p21 induction by BAY. B-C: Densitometry (normalized to nuclear GAPDH) shows that BAY significantly increases p27 expression (> 60%; p < .001) and approximately doubles p21 expression (p = .02). Post-hoc results: *statistically significant with p < .05; **statistically significant with p < .01. Concomitant staining for APE/Ref1 verified nuclear localization of all samples (data not shown). Results are mean ± SEM, and n = 5 experiments/group.
Figure 7.
Effects of BAY 41-2272 on VSM FAK after 72 hours. A: Western blot for FAK and phosphorylated FAK (at Tyrosine 397). B-C: Densitometry (normalized to α-tubulin) shows that BAY has minimal effects on total FAK but significantly (p < .001) reduces phosphorylated FAK. Post-hoc results: *statistically significant with p < .05 versus DMSO controls; *statistically significant with p < .01 versus DMSO controls. Results are mean ± SEM, and n = 2 for FAK group and n = 4 for phosphorylated FAK group.
Discussion
This study provides the first scientific evidence for the anti-growth properties of the recently characterized sGC stimulator BAY 41-2272 in VSM and offers insights into its cell cycle-regulatory mechanisms of action. In rat A7R5 VSM cells BAY significantly inhibited serum-induced growth which was directly influenced by upstream HO and NO signaling. BAY stimulated VSM cGMP and cAMP signaling, including downstream kinases and VASP, markedly reduced cell cycle-regulatory Cdks and FAK, and increased cell cycle inhibitory CKIs p27 and p21 therefore leading to static G1 arrest. These novel results provide compelling support for the biologic relevance of BAY as an anti-mitogenic agent in VSM, offer promise for its therapeutic utility under conditions of cardiovascular adversity, and provide sound foundation for continued investigation of this attractive compound.
In this study BAY dose-dependently inhibited serum-induced VSM cell growth. Notably, this is the first report documenting growth-inhibitory effects of BAY in VSM. These findings corroborate earlier results by our laboratory [11,12,13] and others [33] demonstrating anti-mitogenic effects of the BAY archetype YC-1 in VSM and provides further support for the therapeutic utility of this promising class of sGC stimulators. Reliance of BAY on upstream HO/CO signaling was also observed, indicating that BAY, a cyclase agonist, operates more effectively in the presence of the activating gaseous ligand CO. This is therapeutically significant, as CO as well as NO signaling is induced during injury-mediated vascular growth [11]. Another notable aspect of these data is that, in the absence of BAY, induced HO signaling caused approximately a 70% reduction in growth while HO inhibition resulted in a ∼40% increase in growth, demonstrating the importance of HO/CO signaling (in the absence of BAY-mediated sGC stimulation) on cellular growth and confirming some of our earlier findings. Adding to its therapeutic attraction, cytotoxicity analyses failed to reveal discernable reduction in viability from BAY in any treatment group, suggesting that BAY operates via cytostatic rather than cytotoxic action.
These unique data demonstrating anti-mitogenic effects of sGC stimulation by BAY in VSM are complimented by similar studies in diverse tissues. Soluble GC stimulation by BAY has been shown to reduce proliferation of rat mesangial cells through cyclic GMP-dependent [34] and cyclic AMP-dependent [35] processes, lending credibility for the clinical utility of BAY against renal proliferative disorders. Paradoxically, in vascular endothelial cells sGC activation by BAY [36] or cyclic GMP/PK-A induction by phosphodiesterase (PDE) inhibition [37] leads to enhanced growth and promotion of angiogenesis, and these are theorized to occur through both cyclic GMP-dependent and cyclic GMP-independent pathways [38]. This is of particular significance as choice therapies against vascular proliferative diseases should incorporate both mitigation of VSM proliferation and enhancement of reparative vascular endothelial growth.
Acute exposure of VSM cells to BAY stimulated cyclic nucleotide signaling, observed through increases in cGMP and cAMP, elevated cGMP/cAMP-directed kinases, and activation of the kinase target VASP, thus confirming bioactivity of BAY in cultured VSM. Not surprisingly, as a sGC stimulator BAY directly induced the cGMP system, in accordance with our earlier findings using the parent compound YC-1 in cultured VSM cells [12]. These values, nonetheless, are markedly lower than those reported in isolated ovine pulmonary [39] and mouse femoral [40] arteries and rat brain stem vasculature [41]. Indeed, preliminary data in rat primary arteries (data not shown) reveal much higher cGMP levels after 30 min incubation with BAY, suggesting that cGMP stimulation by BAY may be reduced in cell lines versus primary preparations and/or may be largely contextual and based on experimental conditions. Caution is thus warranted when extrapolating data derived from in vitro studies to the in vivo environment. Specifically in terms of cyclic nucleotide biology, a potential limitation of this extrapolation would be an under-estimation of their physiological significance in the intact body. This caveat also argues for continued studies aimed at analyzing these phenomena in primary tissues.
Additionally, a remarkable aspect of these data is that BAY has clear capacity to activate the parallel cAMP cascade. Doubling of cAMP by BAY has been reported in a human leukemia cell line [42], yet other studies have failed to show discernable effects of BAY on cAMP in rat brain stem [41] or rabbit isolated perfused atria [43]. Findings from our latest article suggest that YC-1 operates through sGC and cyclic GMP to activate the cyclic AMP pathway in order to induce upstream NO signaling [44]. BAY 41-2272 may operate via a similar mechanism although this mandates further study. Cyclic GMP has capacity to directly stimulate cyclic AMP/PK-A [45] and inhibit cyclic AMP-specific phosphodiesterase [46], and it was recently proposed in a study examining vascular relaxation in murine PK-G-deficient tissues that cGMP operates primarily through PK-A in VSM [28]. Moreover, YC-1 can operate through cAMP-mediated signals [47]. Nonetheless, this is the first report documenting ability of BAY to increase cAMP and to activate cAMP signaling in VSM. In fact, these combined results (larger increases in PK-A and PSer338-PK-A compared to PK-G, markedly greater increases in PSer157-VASP compared to PSer239-VASP) strongly suggest that BAY preferentially activates cAMP signaling secondary to sGC stimulation, yet further studies are needed, perhaps in primary preparations, to verify these observations.
In mammalian VSM cell cycle division, progression through G1 and entry into the S phase is rate-limiting and is regulated by formation and activation of cyclin/Cdk complexes, and endogenous CKIs inactivate these complexes and leads to G1 arrest [3,48]. Early G1 is largely governed by cyclin D/Cdk-2, -4, or -6, and at this stage p21 and/or p27 can bind and exert cell cycle inhibition. Cyclin E/Cdk2 accumulates during late G1 and triggers passage into S phase, during which cyclin A/Cdk2 levels increase and provoke G2 transition. Exciting results from this study show that, although BAY increases cyclin expression and in particular cyclin E, BAY significantly reduces catalytic Cdk expression and markedly elevates the Cdk-inhibitory CKIs p27 and p21. Direct correlation between over-expression of p27/p21 and growth inhibition in VSM substantiates earlier findings in pig VSM [3] and suggests that the mechanism of BAY-mediated growth reduction in rat VSM involves p21/p27-mediated cyclin/Cdk inhibition. Similar results that cAMP and not cGMP is associated with p27 upregulation during VSM growth inhibition have been reported in human VSM cells [49]. Additionally, we show that BAY reduces phosphorylated FAK at Tyrosine 397, its major auto-phosphorylation site and the binding site for the SH2 domain of Src tyrosine kinase in an essential step for FAK activation. FAK-mediated actin dynamics are critically involved in the ability of cells to proliferate, and reduction in FAK activation is considered growth-inhibitory. Furthermore, these findings support results from a recent study that suggest cAMP inhibits VSM cell proliferation through the p27-related kinase Skp2 along with reduction in PTyr397-FAK [50].
Of note, these studies were performed using commercial A7R5 VSM cells. In general, cell lines may not accurately reproduce the in vivo milieu that exists in primary preparations or whole animals, and phenotypic alterations have been suggested for commercial or serially-passaged cell lines. Thus, verification of these findings in primary VSM cells or in whole animal models is warranted for rigorous interpretation. Nonetheless, A7R5 VSM cells represent a valuable model with which to initially examine the VSM response to experimental intervention.
Conclusion
In summary, this is the first report documenting anti-growth properties of the sGC stimulator BAY 41-2272 in VSM and offers biologically relevant mechanisms for this promising therapeutic agent. BAY inhibited growth of rat VSM cells in a manner influenced by upstream HO and NO signals. Mechanistically, results suggest that BAY operates primarily through cAMP signaling leading to cytostatis via reduced Cdks and FAK and enhanced CKI involvement. These original findings provide compelling initial evidence for therapeutic significance of BAY as growth-inhibitory agent in VSM cells and offer promise for continued study of its pharmacological efficacy in cardiovascular disorders.
Acknowledgements
Authors would like to acknowledge Dr. W. Durante, University of Missouri, and Drs. E. Jackson, Jr., and S. Mukhopadhyay, North Carolina Central University, for valuable discussions regarding this project. The authors would like to acknowledge the kind donation of FAK antibodies from Dr. Mike Schaller, University of North Carolina at Chapel Hill, and BAY 41-2272 from Drs. J.P. Stasch and A. Knorr, BAYER HealthCare AG, Germany.
Sources of Support/Declaration of Funding Sources: This work was funded by a grant from the American Heart Association and by NIH NHLBI grants HL59868 and HL81720.
Footnotes
Potential Reviewers:
Chris Wingard, Ph.D., East Carolina University
William Durante, Ph.D., University of Missouri
Nair Sreejayan, Ph.D., University of Wyoming
Conflict of Interest Disclosure: The authors do not have any conflicts of interest to declare.
References
- 1.Tulis DA, Keswani AN, Peyton KJ, Wang H, Schafer AI, Durante W. Local administration of carbon monoxide inhibits neointima formation in balloon injured rat carotid arteries. Cell Mol Biol. 2005;51:441–446. [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Thomas DP, Zhang X, Culver B, Alexander BM, Murdoch WJ, Rao MNA, Tulis DA, Ren J, Sreejayan N. Curcumin inhibits PDGF-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:85–90. doi: 10.1161/01.ATV.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- 3.Tanner FC, Boehm M, Akyurek LM, San H, Yang Z-Y, Tashiro J, Nabel GJ, Nabel EG. Differential effects of the cyclin-dependent kinase inhibitors p27Kip1, p21Cip1, and p16Ink4 on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 4.Lee B, Kim CH, Moon SK. Honokiol causes the p21Waf1-mediated G(1)-phase arrest of the cell cycle through inducing p38 mitogen activated protein kinase in vascular smooth muscle cells. FEBS Lett. 2006;580:5177–5184. doi: 10.1016/j.febslet.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 5.Jin YR, Han XH, Zhang YH, Lee JJ, Lim Y, Kim TJ, Yoo HS, Yun YP. Hesperetin, a bioflavanoid, inhibits rat aortic vascular smooth muscle cells proliferation by arresting cell cycle. J Cell Biochem. 2007;104:1–14. doi: 10.1002/jcb.21592. [DOI] [PubMed] [Google Scholar]
- 6.Jackson E, Mukhopadhyay S, Tulis DA. Pharmacologic modulators of soluble guanylate cyclase/cyclic guanosine monophosphate in the vascular system - from benchtop to bedside. Curr Vasc Pharmacol. 2007;5:1–14. [PubMed] [Google Scholar]
- 7.Tulis DA, Durante W, Liu X-M, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 8.Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis. 2001;155:113–122. doi: 10.1016/s0021-9150(00)00552-9. [DOI] [PubMed] [Google Scholar]
- 9.Tulis DA. Novel therapies for cyclic GMP control of vascular smooth muscle growth. Am J Ther. 2008 doi: 10.1097/MJT.0b013e318140052f. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulis DA, Durante W, Peyton KJ, Chapman GB, Evans AJ, Schafer AI. YC-1, a benzyl indazole derivative, stimulates vascular cGMP and inhibits neointima formation. Biochem Biophys Res Comm. 2000;279:646–652. doi: 10.1006/bbrc.2000.3942. [DOI] [PubMed] [Google Scholar]
- 12.Tulis DA, Bohl Masters KS, Lipke EA, Schiesser RL, Evans AJ, Peyton KJ, Durante W, West JL, Schafer AI. YC-1-mediated vascular protection through inhibition of smooth muscle cell proliferation and platelet function. Biochem Biophys Res Commun. 2002;291:1014–1021. doi: 10.1006/bbrc.2002.6552. [DOI] [PubMed] [Google Scholar]
- 13.Tulis DA. Salutary properties of YC-1 in the cardiovascular and hematological systems. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:343–359. doi: 10.2174/1568016043356200. [DOI] [PubMed] [Google Scholar]
- 14.Liu YN, Pan SL, Peng CY, Guh JH, Huang DM, Chang YL, Lin CH, Pai HC, Kuo SC, Lee FY, Teng CM. YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole] inhibits neointima formation in balloon-injured rat carotid through suppression of expressions and activities of matrix metalloproteinases 2 and 9. J Pharmacol Exp Ther. 2006;316:35–41. doi: 10.1124/jpet.105.090563. [DOI] [PubMed] [Google Scholar]
- 15.Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feure A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schröder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- 16.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schröder H, Stahl E, Steinke W, Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol. 2002;136:773–83. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerrigter G, Burnett JC. Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41-2272 in cardiovascular disease. Cardiovasc Drug Rev. 2007;25:30–45. doi: 10.1111/j.1527-3466.2007.00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Zanfolin M, Faro R, Araujo EG, Guaraldo AM, Antunes E, De Nucci G. Protective effects of BAY 41-2272 (sGC stimulator) on hypertension, heart, and cardiomyocyte hypertrophy induced by chronic L-NAME treatment in rats. J Cardiovasc Pharmacol. 2006;47:391–395. [PubMed] [Google Scholar]
- 19.Hobbs AJ, Moncada S. Antiplatelet properties of a novel, non-NO-based soluble guanylate cyclase activator, BAY 41-2272. Vascul Pharmacol. 2003;40:149–154. doi: 10.1016/s1537-1891(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 20.Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Tsuruda T, Harty GJ, Lapp H, Stasch JP, Burnett JC., Jr. Cardiorenal and humoral properties of a novel direct soluble guanylate cyclase stimulator BAY 41-2272 in experimental congestive heart failure. Circulation. 2003;107:686–689. doi: 10.1161/01.cir.0000055737.15443.f8. [DOI] [PubMed] [Google Scholar]
- 21.Jones LJ, Gray M, Yue ST, Haugland RP, Singer VL. Sensitive determination of cell number using the CyQUANT cell proliferation assay. J Immunol Methods. 2001;254:85–98. doi: 10.1016/s0022-1759(01)00404-5. [DOI] [PubMed] [Google Scholar]
- 22.Christodoulides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation. 1995;91:2306–2309. doi: 10.1161/01.cir.91.9.2306. [DOI] [PubMed] [Google Scholar]
- 23.Durante W, Peyton KJ, Schafer AI. Platelet-derived growth factor stimulates heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2666–2672. doi: 10.1161/01.atv.19.11.2666. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu S, Nara Y, Yamori Y, Keiser HR. Differences in response between SHRSP and WKY vascular smooth muscle cells to inhibition of cell proliferation by sodium nitroprusside. Clin Exp Hypertens A. 1991;13:787–795. doi: 10.3109/10641969109042081. [DOI] [PubMed] [Google Scholar]
- 25.Liu X-K, Abernethy DR, Andrawis NS. Nitric oxide inhibits Oct-1 DNA binding activity in cultured vascular smooth muscle cells. Life Sci. 1998;62:739–749. doi: 10.1016/s0024-3205(97)01172-7. [DOI] [PubMed] [Google Scholar]
- 26.Glantz SA. Primer of Biostastics. 6th Ed. McGraw Hill Medical Publishing Division; 2005. [Google Scholar]
- 27.Chen L, Daum G, Chitaley K, Coats SA, Bowen-Pope DF, Eigenthaler M, Thumati NR, Walter U, Clowes AW. Vasodilator-stimulated phosphoprotein regulates proliferation and growth inhibition by nitric oxide in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:1403–1408. doi: 10.1161/01.ATV.0000134705.39654.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worner R, Lukowski R, Hofmann F, Wegener JW. cGMP signals mainly through cAMP kinase in permeabilized murine aorta. Am J Physiol Heart Circ Physiol. 2007;292:H237–H244. doi: 10.1152/ajpheart.00079.2006. [DOI] [PubMed] [Google Scholar]
- 29.Halbrugge M, Friedrich C, Eigenthaler M, Schanzenbacher P, Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990;265:3088–3093. [PubMed] [Google Scholar]
- 30.Nolte C, Eigenthaler M, Schanzenbacher P, Walter U. Endothelial cell-dependent phosphorylation of a platelet protein mediated by cAMP- and cGMP-elevating agents. J Biol Chem. 1991;266:14808–14812. [PubMed] [Google Scholar]
- 31.Walter U, Eigenthaler M, Geiger J, Reinhard M. Role of cyclic nucleotide-dependent protein kinases and their common substrate VASP in the regulation of human platelets. Adv Exp Med Biol. 1993;344:237–249. doi: 10.1007/978-1-4615-2994-1_19. [DOI] [PubMed] [Google Scholar]
- 32.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins : regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 33.Wu C-H, Chang W-C, Chang G-Y, Kuo S-C, Teng C-M. The inhibitory mechanism of YC-1, a benzyl indazole, on smooth muscle cell proliferation: an in vitro and in vivo study. J Pharmacol Sci. 2004;94:252–260. doi: 10.1254/jphs.94.252. [DOI] [PubMed] [Google Scholar]
- 34.Hohenstein B, Daniel C, Wagner A, Stasch JP, Hugo C. Stimulation of soluble guanylyl cyclase inhibits mesangial cell proliferation and matrix accumulation in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2005;288:F685–F693. doi: 10.1152/ajprenal.00280.2004. [DOI] [PubMed] [Google Scholar]
- 35.Yao J, Zhu Y, Sun W, Sawada N, Hiramatsu N, Takeda M, Kitamura M. Irsogladine maleate potentiates the effects of nitric oxide on activation of cAMP signalling pathways and suppression of mesangial cell mitogenesis. Br J Pharmacol. 2007;151:457–466. doi: 10.1038/sj.bjp.0707255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pyriochou A, Beis D, Koika V, Potytarchou C, Papadimitriou E, Zhou Z, Papapetropoulos A. Soluble guanylyl cyclase activation promotes angiogenesis. J Pharm Exp Ther. 2006;319:663–671. doi: 10.1124/jpet.106.108878. [DOI] [PubMed] [Google Scholar]
- 37.Pyriochou A, Zhou Z, Koika V, Petrou C, Cordopatis P, Sessa WC, Papapetropoulos A. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J Cell Physiol. 2007;211:197–204. doi: 10.1002/jcp.20929. [DOI] [PubMed] [Google Scholar]
- 38.Pyriochou A, Vassilakopoulos T, Zhou Z, Papapetropoulos A. cGMP-dependent and -independent angiogenesis-related properties of nitric oxide. Life Sci. 2007;81:1549–1554. doi: 10.1016/j.lfs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Bawankule DU, Sathishkumar K, Sardar KK, Chanda D, Krishna AV, Prakash VR, Mishra SK. BAY 41-2272 [5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridine-4-ylamine]-induced dilation in ovine pulmonary artery: role of sodium pump. J Pharmacol Exp Ther. 2005;314:207–213. doi: 10.1124/jpet.105.083824. [DOI] [PubMed] [Google Scholar]
- 40.Nimmegeers S, Sips P, Buys E, Brouckert P, Van de Voorde J. Functional role of the soluble guanylyl cyclase α1 subunit in vascular smooth muscle relaxation. Cardiovasc Res. 2007;76:149–159. doi: 10.1016/j.cardiores.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira CE, Priviero FBM, Todd JT, Jr., Webb RC. Vasorelaxing effect of BAY 41-2272 in rat basilar artery: involvement of cGMP-dependent and independent mechnanisms. Hypertension. 2006;47:596–602. doi: 10.1161/01.HYP.0000199914.36936.1b. [DOI] [PubMed] [Google Scholar]
- 42.de Oliviera-Junior EB, Thomazzi SM, Rehder J, Antunes E, Condino-Neto A. Effects of BAY 41-2272, an activator of nitric oxide-independent site of soluble guanylate cyclase, on human NADPH oxidase system from THP-1 cells. Eur J Pharmacol. 2007;567:43–49. doi: 10.1016/j.ejphar.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Wen JF, Cui X, Jin JY, Kim SM, Kim SZ, Kim SH, Lee HS, Cho KW. High and low gain switches for regulation of cAMP efflux concentration: distinct roles for particular GC- and soluble GC-cGMP-PDE3 signaling in rabbit atria. Circ Res. 2004;94:936–943. doi: 10.1161/01.RES.0000123826.70125.4D. [DOI] [PubMed] [Google Scholar]
- 44.Liu XM, Peyton KJ, Mendelev NN, Wang H, Tulis DA, Durante W. YC-1 stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells. Mol. Pharmacol. 2009;75:1–10. doi: 10.1124/mol.108.048314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am. J. Physiol. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 46.Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 47.Hwang TL, Jung HW, Kao SH, Teng CM, Wu CC, Cheng SJ. Soluble guanylyl cyclase activator YC-1 inhibits human neutrophil functions through a cGMP-independent but cAMP-dependent pathway. Mol. Pharmacol. 2003;64:1419–1427. doi: 10.1124/mol.64.6.1419. [DOI] [PubMed] [Google Scholar]
- 48.Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, Badimon JJ. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- 49.Fukumoto S, Koyama H, Hosoi M, Yamakawa K, Tanaka S, Morii H, Nishizawa Y. Distinct role of cAMP and cGMP in the cell cycle control of vascular smooth muscle cells: cGMP delays cell cycle transition through suppression of cyclin D1 and cyclin-dependent kinase 4 activation. Circ Res. 1999;85:985–991. doi: 10.1161/01.res.85.11.985. [DOI] [PubMed] [Google Scholar]
- 50.Yih-Jer W, Bond M, Sala-Newby GB, Newby AC. Altered S-phase kinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circ Res. 2006;98:1141–1150. doi: 10.1161/01.RES.0000219905.16312.28. [DOI] [PubMed] [Google Scholar]