Abstract
Background
Women have increased lifetime stroke risk and more disabling strokes compared with men. Insights into the association between menopause and stroke could lead to new prevention strategies for women. The objective of this study was to examine the association of age at natural menopause with ischemic stroke risk in the Framingham Heart Study.
Methods
Participants included women who survived stroke-free until age 60, experienced natural menopause, did not use estrogen prior to menopause, and who had complete data (n=1,430). Participants were followed until first ischemic stroke, death, or end of follow-up (2006). Age at natural menopause was self-reported. Cox proportional hazards models were used to examine the association between age at natural menopause (<42, 42-54, ≥55) and ischemic stroke risk adjusted for age, systolic blood pressure, atrial fibrillation, diabetes, current smoking, cardiovascular disease and estrogen use.
Results
There were 234 ischemic strokes identified. Average age at menopause was 49 years (sd=4). Women with menopause at ages 42-54 (HR=0.50; 95% CI:0.29-0.89) and at ages ≥55 (HR=0.31; 95% CI:0.13-0.76) had lower stroke risk compared with those with menopause <42 years adjusted for covariates. Women with menopause before age 42 had twice the stroke risk compared to all other women (HR=2.03; 95% CI: 1.16-3.56).
Conclusion
In this prospective study, age at natural menopause before age 42 was associated with increased ischemic stroke risk. Future stroke studies with measures of endogenous hormones are needed to inform the underlying mechanisms so that novel prevention strategies for mid-life women can be considered.
Keywords: Stroke, cerebrovascular disease, women, menopause, bone mineral density
Average life expectancy for women in the US is 80 years, five years longer than that of men.1 While men have an increased stroke risk, more women than men will experience a stroke during their lifetime because of their increased life span.2 Studies consistently show that women are more functionally impaired following stroke and are less likely to receive tPA compared with men.3 Given the increased stroke burden and barriers to acute stroke therapy in women, it is critical to understand risk factors unique to women so that new strategies for stroke prevention can be considered.
Results from a meta-analysis demonstrated that menopause before age 50 was associated with a 25% increased risk of cardiovascular disease.4 Three of the 12 studies in the meta-analysis included stroke,5-7 with only one focused on incident stroke versus stroke mortality.7 This investigation from the Nurse's Health Study failed to find an association between age at natural menopause and stroke risk; however, a protective effect of older age at menopause and ischemic stroke risk was suggested.8 With the exception of this study, prospective data on the association of age at natural menopause and stroke risk among US women are lacking.
Beyond age at natural menopause, duration of ovarian activity may be a marker of stroke risk. A recent case-control study found that a longer lifetime estrogen exposure, defined as the difference between age at menopause and age at menarche, was associated with decreased stroke risk.8 An alternative measure of cumulative endogenous estrogen exposure is bone mineral density (BMD). BMD is associated with age at menarche,9, 10 age at menopause,11, 12 and endogenous estrogen levels among peri- and post-menopausal women.13, 14 Data from one prospective US study of elderly women demonstrated a strong association between low BMD and risk of incident stroke,15 while data from NHANES I failed to find an association.16 Further investigation of the relationship between BMD and stroke incidence is warranted.
The primary objective of this study was to prospectively examine the association of age at natural menopause with risk of ischemic stroke in the Framingham Heart Study (FHS). A secondary objective was to examine the association of BMD and risk of ischemic stroke. Analyses were limited to ischemic stroke given the purported role of estrogen deficiency in promoting atherosclerosis.
METHODS
FHS is an ongoing prospective cohort study of 5,209 participants (2,873 women, ages 28-62 at the time of enrollment) that began in 1948 in the town of Framingham, Massachusetts. Participants undergo biennial examinations including medical histories, physical examinations, laboratory tests for vascular risk factors, and, at some examinations, brain imaging studies. Details of the study methods have been published.17, 18
This study investigated the association of age at natural menopause and incident ischemic stroke after age 60. Among the 2,873 women in the original cohort, there were 2,461 who attended an examination within 3 years of age 60; this examination was designated the participant's baseline. Participants were excluded if they had no information on age at menopause (n=7), surgical menopause or menopause of unknown cause (n=702), prevalent ischemic stroke at entry (n=18), no follow-up after entry (n=11), estrogen use before menopause (n=26), or missing risk factor data (n=267). The remaining 1,430 participants comprised the study sample for the analysis of the association of age at natural menopause with risk of ischemic stroke (primary objective).
Of the 2,873 women in the original cohort, 866 were alive and attended examination 20 (1986-1990), when BMD was measured; this examination was designated the participant's baseline for the analysis of BMD and risk of ischemic stroke (secondary objective). Participants were excluded from this analysis if they had prevalent ischemic stroke at entry (n=43) or no follow-up after entry (n=7). Of the remaining 816, BMD was measured in 654 women. These women comprised the study sample for the secondary objective. All participants provided written informed consent and the study was approved by the Boston Medical Center Institutional Review Board.
Baseline Covariates
Baseline covariates were assessed at age 60 (± 3 years) for the primary objective and at the time of BMD measurement for the secondary objective. The following covariates were considered: systolic blood pressure (SBP), diabetes, atrial fibrillation, cardiovascular disease, current smoking status, body mass index (BMI), and estrogen use. SBP was recorded as the average of two physician recorded measurements. Diabetes was defined as a random blood glucose >200mg/dl, previous diagnosis or treatment with diabetes medication (insulin or oral hypoglycemia agent). Prior cardiovascular disease included coronary heart disease, congestive heart failure and intermittent claudication. Atrial fibrillation was obtained from a standard 12 lead electrocardiogram completed at or before the baseline examination. Estrogen use was defined as someone taking estrogen at their baseline assessment. Women taking estrogen prior to menopause were excluded from the analysis so this covariate measured estrogen started after menopause. Analyses were limited to those with complete covariate data.
Stroke Ascertainment
The primary outcome was incident ischemic stroke. Stroke was defined clinically as a focal neurological deficit of sudden or rapid onset that persisted for more than 24 hours. Continuous surveillance for cerebrovascular events included daily hospital monitoring, tracking of medical encounters, and examination of those with possible stroke symptoms identified at routine biennial examinations. Events were adjudicated by at least two neurologists, and with verification of stroke by imaging when available. Stroke occurrence and characteristics, including subtypes, were determined at the end of the acute stroke phase according to uniform criteria and a standardized protocol.18, 19
Age at Natural Menopause
At each biennial examination, women were queried as to whether periods had stopped for one year or more, the age at which periods ceased, the cause of stopped periods (natural, surgical, other), whether a hysterectomy was performed, and number of ovaries removed. Natural menopause occurred if a woman had ceased menstruating naturally for at least one year. Age at natural menopause was retrospectively assigned as the self-reported age at last menstrual period.
Bone Mineral Density
BMDs of the femur (neck and trochanter) and distal third of the radius were measured in members of the cohort who came for their 20th biennial examination in 1986-1990.
Measurements were done using dual-photon absorptiometry for the hip (DP3; Lunar Corp, Madison, WI) and single-photon absorptiometry for the distal third of the radius (LUNAR SP2; Lunar Corp).
Statistical Analysis
Baseline characteristics were calculated using frequencies and percents or means and standard deviations (sd). Cox proportional hazards models were used to examine the association between age at natural menopause and risk of ischemic stroke. Individuals were censored at death, hemorrhagic stroke, last examination or contact date, or end of follow-up (December 2006). Survival age was used as the outcome in all models, with entry age used as the left truncation limit. Given an observed non-linear relationship, age at natural menopause was modeled categorically (<42 (referent), 42-54, ≥55). Models were run age-adjusted and adjusted for age plus baseline covariates (SBP, atrial fibrillation, diabetes, current smoking, cardiovascular disease, estrogen use). All covariates were modeled dichotomously with the exception of SBP and age which were modeled continuously. A Wald chi-square test was used to test the overall association between age at natural menopause and risk of stroke in the adjusted model. Models were also run limited to never smokers and never estrogen users given the potential confounding effects of these covariates.
Cox proportional hazards models were used to examine the association between BMD and risk of ischemic stroke, with individuals censored as described above. Survival age was again used as the outcome in all models, with age at the 20th examination used as the left truncation limit. Models were run separately for each BMD site. BMD was modeled categorically based on quintiles of the distribution of BMD at each site with the middle quintile as the referent. BMD quintiles were determined within age groups (67-69, 70-74, 75-79, ≥80). Models were run age-adjusted and adjusted for the covariates described above with additional adjustment for BMI. Using the adjusted models, Wald chi-square tests were used to test the overall associations of BMD at each site and risk of stroke. Models were also run limited to those not taking antihypertensives given the potential confounding effects of this covariate.
Results
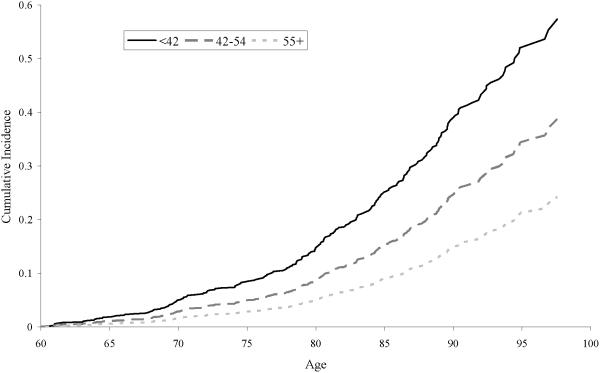
For the primary objective, there were 1,430 women with complete data. Baseline covariate data for these women is included in Table 1. Average age at menopause was 49 years (sd=4). Women were followed for an average of 22 years (sd=9). There were 234 incident ischemic strokes occurring at an average age of 80 years (sd=9). Figure 1 displays cumulative incidence of ischemic stroke by age and age at natural menopause, and Table 2 displays the model results. In the age-adjusted model, women with menopause at ages 42-54 (HR=0.57; 95% CI:0.33-1.01) and at ages ≥55 (HR=0.33; 95% CI:0.14-0.79) had lower stroke risk compared with those with menopause <42 years. Women with menopause before age 42 had twice the risk of ischemic stroke compared to all other women (HR=2.03; 95% CI:1.16-3.56). These associations were relatively unchanged with adjustment for baseline covariates. In the adjusted model, there was a significant overall association between age at natural menopause and ischemic stroke risk (p=0.02). Limiting to never smokers or to never HRT users, results were similar (Table 2).
Table 1.
Baseline characteristics (percent or mean ± sd) at age 60 among women in the Framingham Heart Study (n=1,430).
| Baseline covariate | |
|---|---|
| Age (years) | 60.0 ± 0.8 |
| Systolic blood pressure | |
| (mm/Hg) | 141 ± 24 |
| History of diabetes | 4% |
| History of CVD | 7% |
| History of AF | 1% |
| Current smoking | 32% |
| Hormone replacement | |
| therapy | 19% |
| BMI (kg/m2) | 27 ± 5 |
CVD = cardiovascular disease, AF = atrial fibrillation, BMI = body mass index
Figure 1.
Cumulative incidence of ischemic stroke by age and age at natural menopause among women in the Framingham Heart Study (n=1,430).
Table 2.
Associations of age at natural menopause and risk of incident ischemic stroke among women in the Framingham Heart Study (n= 1,430).
| Age at Natural Menopause | Number of Participants | Number of Ischemic Strokes | Age-adjusted | Multivariable-adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| <42 | 56 | 13 | 1.00 | 1.00 | ||||
| 42-54 | 1,299 | 213 | 0.57 | 0.33-1.01 | 0.05 | 0.50 | 0.29-0.89 | 0.02 |
| ≥55 | 75 | 8 | 0.33 | 0.14-0.79 | 0.01 | 0.31 | 0.13-0.76 | 0.01 |
| Never Smokers at Age 60 | ||||||||
| <42 | 30 | 9 | 1.00 | 1.00 | ||||
| 42-54 | 605 | 96 | 0.38 | 0.19-0.76 | 0.01 | 0.39 | 0.20-0.78 | 0.01 |
| ≥55 | 40 | 6 | 0.34 | 0.12-0.96 | 0.04 | 0.40 | 0.14-1.12 | 0.08 |
| Never Estrogen Users at Age 60 | ||||||||
| <42 | 51 | 12 | 1.00 | 1.00 | ||||
| 42-54 | 1158 | 193 | 0.54 | 0.30-0.97 | 0.04 | 0.48 | 0.27-0.87 | 0.02 |
| ≥55 | 63 | 7 | 0.31 | 0.12-0.79 | 0.01 | 0.30 | 0.12-0.77 | 0.01 |
HR = hazard ratio, CI = confidence interval
Adjusted for age, systolic blood pressure, atrial fibrillation, diabetes, current smoking, cardiovascular disease, and estrogen use.
Six hundred fifty-four women had at least one BMD measurement with an average age at measurement of 76 years (sd=5). Women were followed an average of 12 years (sd=5). In this subset, there were 92 ischemic strokes. Table 3 displays the model results. Cut-points for defining quintiles of BMD are provided in Table 4 (online). In adjusted models, there were borderline significant associations with BMD at the trochanter (p=0.07) and radius (p=0.07) and ischemic stroke risk. For BMD measured at the trochanter, a U-shaped pattern in risk was observed with women in the lowest (HR=2.36, 95% CI:1.15-4.83) and highest (HR=1.94, 95% CI:0.94-3.95) quintiles of BMD having elevated stroke risk compared with women in the middle quintile. A similar pattern was observed at the radius (Q1-HR=2.92, 95% CI:1.55-6.34; Q5-HR=2.34, 95% CI:1.12-5.30). Limiting the analysis to those not using antihypertensives, results were similar (data not shown).
Table 3.
Associations of bone mineral density measured at three sites and risk of incident ischemic stroke among women in the Framingham Heart Study (n=654).
| Quintile of BMD | Number of Participants | Number of Ischemic Strokes | Age-adjusted | Multivariable-adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Femoral neck | ||||||||
| Q1 | 122 | 18 | 1.37 | 0.69-2.73 | 0.36 | 1.33 | 0.66-2.68 | 0.42 |
| Q2 | 124 | 18 | 1.25 | 0.63-2.38 | 0.53 | 1.30 | 0.65-2.59 | 0.45 |
| Q3 | 126 | 15 | 1.00 | 1.00 | ||||
| Q4 | 124 | 17 | 1.18 | 0.59-2.36 | 0.65 | 0.92 | 0.45-1.88 | 0.81 |
| Q5 | 124 | 24 | 1.72 | 0.90-3.29 | 0.10 | 1.41 | 0.72-2.76 | 0.32 |
| Trochanter | ||||||||
| Q1 | 125 | 25 | 2.17 | 1.07-4.38 | 0.03 | 2.36 | 1.15-4.83 | 0.02 |
| Q2 | 127 | 19 | 1.13 | 0.51-2.52 | 0.76 | 1.07 | 0.48-2.41 | 0.87 |
| Q3 | 128 | 9 | 1.00 | 1.00 | ||||
| Q4 | 131 | 18 | 1.90 | 0.93-3.86 | 0.08 | 1.77 | 0.86-3.64 | 0.12 |
| Q5 | 126 | 24 | 2.38 | 1.19-4.77 | 0.01 | 1.94 | 0.94-3.95 | 0.07 |
| Radius | ||||||||
| Q1 | 122 | 22 | 3.18 | 1.48-6.82 | 0.00 | 2.92 | 1.55-6.34 | 0.01 |
| Q2 | 123 | 12 | 2.10 | 0.95-4.65 | 0.07 | 1.94 | 0.87-4.32 | 0.11 |
| Q3 | 125 | 12 | 1.00 | 1.00 | ||||
| Q4 | 123 | 21 | 2.07 | 0.93-4.60 | 0.08 | 1.68 | 0.74-3.80 | 0.21 |
| Q5 | 123 | 24 | 2.92 | 1.36-6.28 | 0.01 | 2.43 | 1.12-5.30 | 0.03 |
Q = quintile, HR = hazard ratio, CI = confidence interval, BMD = bone mineral density
Adjusted for age, systolic blood pressure, atrial fibrillation, diabetes, current smoking, cardiovascular disease, estrogen use, and body mass index.
Table 4.
(online only). Quintiles of bone mineral density by site and age group among women in the Framingham Heart Study. Values represent the upper end of the quintile with greater values falling to the next quintile.
| Age Group | ||||
|---|---|---|---|---|
| 67-69 | 70-74 | 75-79 | 80+ | |
| Femoral Neck | ||||
| N | 56 | 239 | 189 | 136 |
| 0.498- | 0.182- | 0.475- | 0.442- | |
| Range | 1.062 | 1.164 | 1.027 | 0.984 |
| Q1 | 0.669 | 0.640 | 0.622 | 0.590 |
| Q2 | 0.737 | 0.698 | 0.671 | 0.648 |
| Q3 | 0.813 | 0.745 | 0.730 | 0.704 |
| Q4 | 0.856 | 0.813 | 0.783 | 0.774 |
| Q5 | 1.062 | 1.164 | 1.027 | 0.984 |
| Trochanter | ||||
| N | 55 | 239 | 186 | 136 |
| 0.423- | 0.281- | 0.352- | 0.335- | |
| Range | 1.110 | 0.964 | 1.200 | 0.886 |
| Q1 | 0.554 | 0.531 | 0.521 | 0.498 |
| Q2 | 0.620 | 0.583 | 0.589 | 0.545 |
| Q3 | 0.674 | 0.640 | 0.646 | 0.623 |
| Q4 | 0.748 | 0.717 | 0.702 | 0.699 |
| Q5 | 1.110 | 0.964 | 1.200 | 0.886 |
| Radius | ||||
| N | 56 | 253 | 189 | 139 |
| 0.392- | 0.217- | 0.297- | 0.241- | |
| Range | 0.755 | 0.821 | 0.743 | 0.649 |
| Q1 | 0.459 | 0.439 | 0.423 | 0.377 |
| Q2 | 0.520 | 0.500 | 0.475 | 0.443 |
| Q3 | 0.559 | 0.547 | 0.522 | 0.494 |
| Q4 | 0.621 | 0.598 | 0.568 | 0.544 |
| Q5 | 0.755 | 0.821 | 0.743 | 0.649 |
Discussion
In this prospective study, we observed a significant association between age at natural menopause and ischemic stroke risk in a cohort of women followed from age 60. This association was non-linear and reflected an increased risk of ischemic stroke in those with natural menopause before 42. Menopause at ≤40 years is termed premature ovarian failure (POF). The etiology of POF is unknown, although POF is thought to arise from different processes than those leading to natural menopause around age 50. Prevalence of POF is 1-2% among women, with an additional 3-10% of women experiencing “early” menopause defined as natural menopause before age 45.20, 21 Although women with menopause before 42 years represent a small subgroup of the total population, data from this study suggest that 4-5% of strokes in all women can be attributed to this risk factor. Reasons for increased ischemic stroke risk among women with POF or early menopause are not clear but early loss of ovarian function coupled with a prolonged low estrogen state is a plausible hypothesis.
The menopausal transition represents a change in endogenous hormones including decreasing estradiol levels several years before menopause and relative estrogen deficiency within 2-3 years of the final menstrual period.22 Estrogen deficiency is thought to promote cardiovascular disease,23 perhaps through functional or structural changes in the arteries,24 and as such early onset of estrogen loss in women with POF may contribute to increased stroke risk. However, the role of estrogen deficiency has become controversial in light of the higher stroke risk associated with hormone replacement therapy in clinical trials.25-27 Recent analyses of WHI data suggest that the timing of HRT initiation may modify the association of HRT and cardiovascular risk, with the effects of HRT being favorable in women initiating therapy in close proximity to menopause. Interestingly, this pattern does not hold for stroke, further complicating an understanding of the hormone-stroke association.28, 29
No published study has assessed the association between endogenous estrogens and stroke risk. Studies of other non-stroke cardiovascular disease endpoints in postmenopausal women have found no association between endogenous estrogen and peripheral artery disease,30 intima media thickness,31 and cardiovascular disease.32, 33 In contrast, proxy measures of endogenous estrogen exposure, including measures of lifetime ovarian activity and BMD, have been associated with stroke risk in some15, 34 but not all studies.16 Unlike previous studies, which suggested a linear association of decreasing BMD and increasing stroke risk, we observed a U-shaped pattern. Women in the lowest quintiles of BMD (trochanter and radius) had elevated risk. This finding supports the estrogen deficiency-stroke hypothesis, although other explanations are possible. Bone metabolism and atherosclerosis share factors including osteopontin and osteocalcin, as well as other potential pathogenic contributors such as oxidized lipids and hypertension.35, 36 This link is supported by an association between low BMD and carotid plaques.37 The finding of elevated stroke risk with the highest quintile of BMD is unexpected and could be real but could also be the result of misspecification of our model, residual confounding, or selection bias given the age at which BMD was measured in this study.
More research is needed to understand the impact of endogenous estrogen on stroke risk. However, given the harmful association of HRT with stroke in recent trials and negative findings of studies of endogenous estrogen and non-stroke cardiovascular disease endpoints, alternate hormonal pathways, including changes in androgens and sex-hormone binding globulin (SHBG) with menopause, should be explored. Lower levels of SHBG and higher levels of free androgen index (FAI) have been associated with cardiovascular disease,32 but again, data on stroke are lacking. Low SHBG and high FAI were also related to an adverse cardiovascular risk factor profile, including higher insulin, glucose, lipids, and hemostatic and inflammatory markers, in a study of peri-menopausal women.38 Estradiol was also associated with an adverse risk factor profile but to a lesser degree. These findings suggest that the association of age at menopause and stroke risk may be mediated through changes in risk factors which occur with menopause, although associations remained after adjustment for risk factors in this study. Alternatively, an adverse cardiovascular risk factor profile in premenopausal women may be associated with earlier menopause.39
Some limitations warrant discussion. The population was limited to Caucasian women who were recruited in 1948 and therefore results may not be generalizable to different populations or to more recent birth cohorts. Although age at natural menopause in the Framingham population is similar to estimates in more recent cohorts,40, 41 there have been temporal trends in increasing age at menopause.42 Oral contraceptive use and use of hormone replacement therapy were uncommon in this cohort due to the study time period limiting generalizability to more recent birth cohorts with a greater prevalence of these medications. Similarly, secular trends in stroke risk factors or their treatment may limit generalizability. Women with stroke before age 60 were excluded. Although ischemic stroke was rare before 60, if early menopause is associated with stroke at younger ages, the association of age at menopause and stroke may differ from that presented. Similarly, the secondary analysis followed women prospectively from BMD measurement, which occurred on average at 76 years. Women who experienced stroke or who died prior to BMD measurement were not included. This may have introduced bias and suggests that our results should be confirmed in different populations and across a broader range of ages. While we adjusted for confounders, with a focus on factors known to influence stroke in this population, there may be other unaccounted for confounders. For example, we did not include metabolic syndrome, measures of central adiposity or parity, which may be confounders, as they were not available for this population for the time frame under study. Sample sizes and numbers of events were small in some analyses, which may have limited power. This study relied on self-reported menopausal status which may be subject to recall bias, although the prospective biennial exams minimize this possibility.
Summary
Given the increased stroke burden in women, it is critical to understand risk factors unique to women so that new strategies for prevention can be considered. Results from the current study demonstrated an elevated risk of ischemic stroke in women with early menopause and possible POF and in women with low BMD. These findings raise the hypothesis that estrogen deficiency may play a role in ischemic stroke but current evidence regarding this hypothesis is inconsistent. Alternate hypotheses, including the role of androgens and/or a common cause of BMD and stroke, are also possible. Future studies, with measures of endogenous hormones, are needed to unravel the relationship between hormonal changes that occur with menopause, either premature or at the usual onset, and ischemic stroke.
Acknowledgements/Funding
Dr. Lisabeth is funded by National Institute for Neurologic Disorders and Stroke K23 NS050161.
This work was supported by the Framingham Heart Study's National Heart, Lung and Blood Institute's contract (N01-HC-25195) and grants from the National Institute of Neurological Disorders and Stroke (5R01-NS 17950) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging (R01 AR/AG 41398).
Footnotes
Conflicts of Interest: None
References
- 1.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: Final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 2.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: Estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 3.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 5.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 6.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339–345. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 8.de Lecinana MA, Egido JA, Fernandez C, Martinez-Vila E, Santos S, Morales A, Martinez E, Pareja A, Alvarez-Sabin J, Casado I. Risk of ischemic stroke and lifetime estrogen exposure. Neurology. 2007;68:33–38. doi: 10.1212/01.wnl.0000250238.69938.f5. [DOI] [PubMed] [Google Scholar]
- 9.Varenna M, Binelli L, Zucchi F, Ghiringhelli D, Gallazzi M, Sinigaglia L. Prevalence of osteoporosis by educational level in a cohort of postmenopausal women. Osteoporos Int. 1999;9:236–241. doi: 10.1007/s001980050143. [DOI] [PubMed] [Google Scholar]
- 10.Ito M, Yamada M, Hayashi K, Ohki M, Uetani M, Nakamura T. Relation of early menarche to high bone mineral density. Calcif Tissue Int. 1995;57:11–14. doi: 10.1007/BF00298989. [DOI] [PubMed] [Google Scholar]
- 11.Nilas L, Christiansen C. Bone mass and its relationship to age and the menopause. J Clin Endocrinol Metab. 1987;65:697–702. doi: 10.1210/jcem-65-4-697. [DOI] [PubMed] [Google Scholar]
- 12.Parazzini F, Bidoli E, Franceschi S, Schinella D, Tesio F, La Vecchia C, Zecchin R. Menopause, menstrual and reproductive history, and bone density in northern Italy. J Epidemiol Community Health. 1996;50:519–523. doi: 10.1136/jech.50.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97:14–21. doi: 10.1172/JCI118382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 15.Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The Study of Osteoporotic Fractures. Stroke. 1993;24:940–946. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- 16.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and stroke. Stroke. 2003;34:e20–22. doi: 10.1161/01.STR.0000065826.23815.A5. [DOI] [PubMed] [Google Scholar]
- 17.Dawber TR, Meadors GF, Moore FE., Jr. Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 19.Wolf PA, D'Agostino RB, O'Neal MA, Sytkowski P, Kase CS, Belanger AJ, Kannel WB. Secular trends in stroke incidence and mortality. The Framingham Study. Stroke. 1992;23:1551–1555. doi: 10.1161/01.str.23.11.1551. [DOI] [PubMed] [Google Scholar]
- 20.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18:199–206. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- 21.Torgerson DJ, Thomas RE, Reid DM. Mothers and daughters menopausal ages: Is there a link? Eur J Obstet Gynecol Reprod Biol. 1997;74:63–66. doi: 10.1016/s0301-2115(97)00085-7. [DOI] [PubMed] [Google Scholar]
- 22.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–4030. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 23.van der Graaf Y, de Kleijn MJ, van der Schouw YT. Menopause and cardiovascular disease. J Psychosom Obstet Gynaecol. 1997;18:113–120. doi: 10.3109/01674829709085577. [DOI] [PubMed] [Google Scholar]
- 24.Wildman RP, Colvin AB, Powell LH, Matthews KA, Everson-Rose SA, Hollenberg S, Johnston JM, Sutton-Tyrrell K. Associations of endogenous sex hormones with the vasculature in menopausal women: The Study of Women's Health Across the Nation (SWAN) Menopause. 2008;15:414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative Randomized Controlled Trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 26.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study Follow-up (HERS II) Jama. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative Randomized Controlled Trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 28.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 29.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women's Health Initiative: A randomized trial. Jama. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 30.Price JF, Lee AJ, Fowkes FG. Steroid sex hormones and peripheral arterial disease in the Edinburgh Artery Study. Steroids. 1997;62:789–794. doi: 10.1016/s0039-128x(97)00103-7. [DOI] [PubMed] [Google Scholar]
- 31.Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: A case-control study of the Atherosclerosis Risk in Communities cohort. Am J Epidemiol. 2002;155:437–445. doi: 10.1093/aje/155.5.437. [DOI] [PubMed] [Google Scholar]
- 32.Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 33.Barrett-Connor E, Goodman-Gruen D. Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. Bmj. 1995;311:1193–1196. doi: 10.1136/bmj.311.7014.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgensen L, Engstad T, Jacobsen BK. Bone mineral density in acute stroke patients: Low bone mineral density may predict first stroke in women. Stroke. 2001;32:47–51. doi: 10.1161/01.str.32.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Demer LL. Vascular calcification and osteoporosis: Inflammatory responses to oxidized lipids. Int J Epidemiol. 2002;31:737–741. doi: 10.1093/ije/31.4.737. [DOI] [PubMed] [Google Scholar]
- 36.Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: A prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354:971–975. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen L, Joakimsen O, Rosvold Berntsen GK, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: A population-based study. Am J Epidemiol. 2004;160:549–556. doi: 10.1093/aje/kwh252. [DOI] [PubMed] [Google Scholar]
- 38.Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torrens JI. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 39.Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PH, Wilson PW, Pearson PL, Grobbee DE. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1983. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 40.Hefler LA, Grimm C, Heinze G, Schneeberger C, Mueller MW, Muendlein A, Huber JC, Leodolter S, Tempfer CB. Estrogen-metabolizing gene polymorphisms and age at natural menopause in caucasian women. Hum Reprod. 2005;20:1422–1427. doi: 10.1093/humrep/deh848. [DOI] [PubMed] [Google Scholar]
- 41.Kok HS, Onland-Moret NC, van Asselt KM, van Gils CH, van der Schouw YT, Grobbee DE, Peeters PH. No association of estrogen receptor alpha and cytochrome p450c17alpha polymorphisms with age at menopause in a Dutch cohort. Hum Reprod. 2005;20:536–542. doi: 10.1093/humrep/deh600. [DOI] [PubMed] [Google Scholar]
- 42.Nichols HB, Trentham-Dietz A, Hampton JM, Titus-Ernstoff L, Egan KM, Willett WC, Newcomb PA. From menarche to menopause: Trends among US women born from 1912 to 1969. Am J Epidemiol. 2006;164:1003–1011. doi: 10.1093/aje/kwj282. [DOI] [PubMed] [Google Scholar]