Abstract
Sustained pretreatment with Angiotensin II AT1 receptor antagonists prevents the sympathoadrenal and hormonal responses to 24 hour isolation stress. To elucidate the mechanism of the anti-stress effects of AT1 receptor antagonism, we examined the effect of subcutaneous infusion of candesartan, a non-competitive AT1 receptor antagonist, 0.5 mg/kg/day for 14 days, to Wistar rats on the hypothalamic pituitary adrenal (HPA) axis after 24 hour isolation stress. In the morning of day 15, we measured AT1 receptors corticotropin releasing factor (CRF) mRNA and immunoreactive CRF in the paraventricular nucleus (PVN), the pituitary adrenocorticotropin hormone (ACTH) and adrenal corticosterone content, and the urinary corticosterone excretion. In rats not treated with candesartan, 24 hour isolation stress increased pituitary ACTH, adrenal corticosterone content and AT1 receptor binding in the PVN but decreased CRF mRNA and CRF content in the PVN. This indicates enhanced CRF utilization not compensated by CRF gene transcription and effective glucocorticoid feedback inhibition in spite of the increase in AT1 receptor expression. The effects of stress on HPA axis activation and CRF mRNA and content in the PVN were prevented by candesartan pretreatment, suggesting that activation of AT1 receptors is required for the HPA axis response to isolation. Our results support the hypothesis that the activity of PVN AT1 receptors is part of the mechanism necessary for development of a full stress-induced HPA axis activation. Inhibition of central AT1 receptors limits the CRF response to stress and should be considered as a therapeutic tool to preserve homeostasis under chronic stress conditions.
Keywords: Renin-Angiotensin System, Hypothalamic-Pituitary-Adrenal axis, Paraventricular nucleus, Brain Angiotensin II receptors, Stress
1. INTRODUCTION
Responses to stress are initiated through stimulation of sensory pathways, not necessarily including conscious experiences, leading to a series of adaptive central mechanisms culminating in rapid activation of the hypothalamic-pituitary-adrenal (HPA) axis and enhanced sympathoadrenal response (Goldstein and McEwen, 2002). The hallmark of the HPA axis response is a rapid increase in the release of hypothalamic corticotropin releasing factor (CRF) into the portal circulation and enhanced CRF transcription in the hypothalamic paraventricular nucleus (PVN), with the consequent increases in anterior pituitary ACTH and adrenocortical glucocorticoid secretion. In addition to their actions on systemic adaptation to stress, glucocorticoids exert a negative feedback control on CRF expression and release, limiting the HPA response in time and extent (Itoi et al., 2004). Such a complex and finely tuned response involves multiple neural pathways and loci of action.
Recent interest on angiotensin II (Ang II) as an important stress hormone is based on observations indicating that Ang II contributes to regulating the sympathoadrenal and hormonal responses to stress through stimulation of peripheral and brain AT1 receptors. Acute stress increases the formation of renin, the rate-limiting enzyme in Ang II formation, leading to increased Ang II generation (Xang et al., 1993). The increased circulating Ang II contribute to the enhanced anterior pituitary ACTH secretion during stress (Saavedra, 1992), mediate increases in aldosterone secretion from the adrenal zona glomerulosa, and catecholamine release from the adrenal medulla (Aguilera, 1993; Keller-Wood et al., 1983; Livett et al., 1990), through stimulation of AT1 receptors in these target tissues In addition, Ang II activates central stress pathways by interacting with AT1 receptors located in brain areas outside the blood brain barrier, the circumventricular organs, such as the subfornical organ (SFO) (Saavedra, 1992), as well as in brain nuclei inside the blood brain barrier such as the PVN, where AT1 receptors are localized in parvocellular CRF neurons (Aguilera et al., 1995a). The number of AT1 receptors in these areas increases during stress (Aguilera et al., 1995a, b; Sumitomo et al., 1991; Castrén and Saavedra, 1988). There is evidence suggesting that AT1 receptors play a role in increasing CRF formation (Aguilera et al., 1995b; Sumitomo et al., 1991) and release into the hypothalamic portal system, contributing to the stimulation of pituitary ACTH (Aguilera et al., 1983) and adrenal corticoid production and release. Ang II also stimulates vasopressin (AVP) formation in the PVN and its release from the median eminence to participate with CRF in enhancing ACTH production (Volpi et al., 2004; Antoni 1993). Sustained pretreatment with a peripherally and centrally acting AT1 receptor antagonist, candesartan, prevented the HPA axis and sympathoadrenal activation and the cortical CRF and benzodiazepine responses to isolation stress (Armando et al., 2001; Saavedra, 2005; Saavedra et al., 2006). These results highlighted the important role of AT1 receptors during stress.
The precise role of Ang II and AT1 receptors in the regulation of CRF formation and release during stress is not yet completely understood. To further clarify these mechanisms, we studied the CRF mRNA levels and CRF content after 24 hours of isolation in rats pretreated with the AT1 antagonist candesartan. This drug is an insurmountable antagonist which readily crosses the blood-brain barrier (Nishimura et al., 2000) and inhibits both peripheral and central AT1 receptors (Nishimura et al., 2000). The present results confirm our earlier findings of prevention of the HPA axis activation during isolation stress by pretreatment with candesartan (Armando et al., 2001) and provide additional information on the effects of AT1 receptor blockade on the CRF mRNA expression and content in the PVN during isolation.
2. RESULTS
In group-housed rats, on the third day (day 14 of the treatment) the blood pressures for vehicle-treated animals were 125 ± 5 mmHg, and for the candesartan-treated animals 110 ± 3 mmHg (P<0.05).
In the PVN, we found AT1 binding located close to the 3rd ventricle and in the parvocellular region (Figure 1). CRF mRNA was expressed in the parvocellular PVN, and could be superimposed on the parvocellular AT1 binding (Figure 1).
Figure 1.
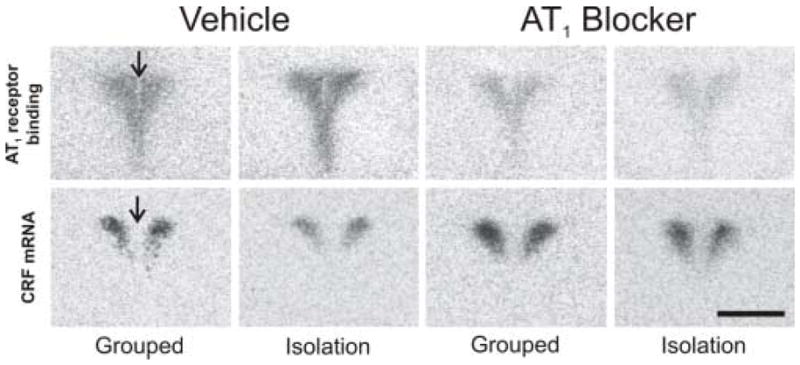
Representative autoradiograms of AT1 receptor binding (upper row) and CRF mRNA (lower row) in the paraventricular nucleus of grouped or isolated animals treated with vehicle or the AT1 blocker. AT1 receptor binding is localized to the parvocellular and periventricular regions of the PVN. CRF mRNA is expressed in the parvocellular PVN and can be superimposed to the parvocellular AT1 receptor binding. Arrows point to the 3rd ventricle. Scale bar = 1 mm.
Isolation stress significantly increased the number of AT1 receptors in the PVN (Figures 1 and 2). AT1 receptor blockade decreased AT1 binding in the PVN of control grouped animals by about 75%, and a similar decrease in binding occurred when the animals were submitted to isolation stress (Figures 1 and 2). This indicates that receptor blockade was equally effective in control and stressed rats.
Figure 2.
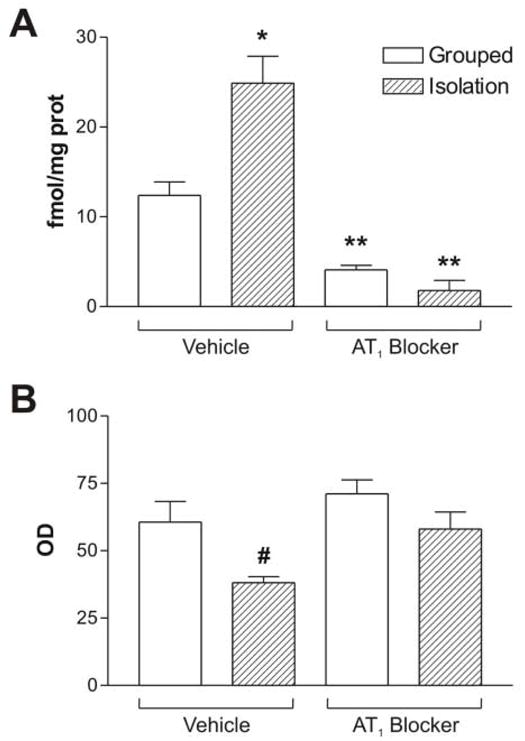
Quantitative autoradiography of AT1 receptor binding (A) and expression of CRF mRNA (B) in the paraventricular nucleus of grouped or isolated animals treated with vehicle or the AT1 blocker. Bars represent means ± S.E.M. of groups of six rats measured individually. * P<0.05 vs. grouped treated with vehicle; ** P<0.01 vs. grouped or isolated rats treated with vehicle; # p<0.05 vs. all others.
Isolation stress significantly decreased both CRF mRNA (Figures 1 and 2) and CRF content (Figure 3) in the PVN. Pretreatment with the AT1 receptor antagonist did not affect CRF mRNA or content in grouped-housed animals, but completely prevented the decrease of both CRF mRNA and content in stressed rats (Figures 1, 2 and 3).
Figure 3.
Content of CRF in the paraventricular nucleus of grouped or isolated animals treated with vehicle or the AT1 blocker. Results are expressed as the mean ± S.E.M. of groups of six rats measured individually. * P<0.05 vs. all others.
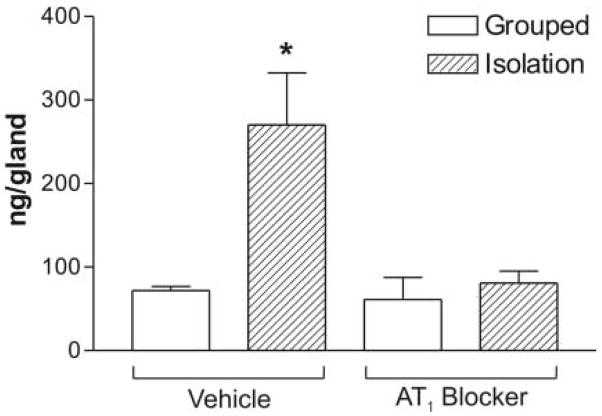
Isolation stress increased ACTH content in the pituitary gland by two-fold (Figure 4). Pretreatment with the AT1 receptor antagonist had no effect on pituitary ACTH content in grouped-housed animals but completely eliminated the isolation-induced increase in pituitary ACTH content (Figure 4).
Figure 4.
Content of ACTH in the pituitary gland of grouped or isolated animals treated with vehicle or the AT1 blocker. Results are expressed as the mean ± S.E.M. of groups of six rats measured individually. * P<0.05 vs. all others.
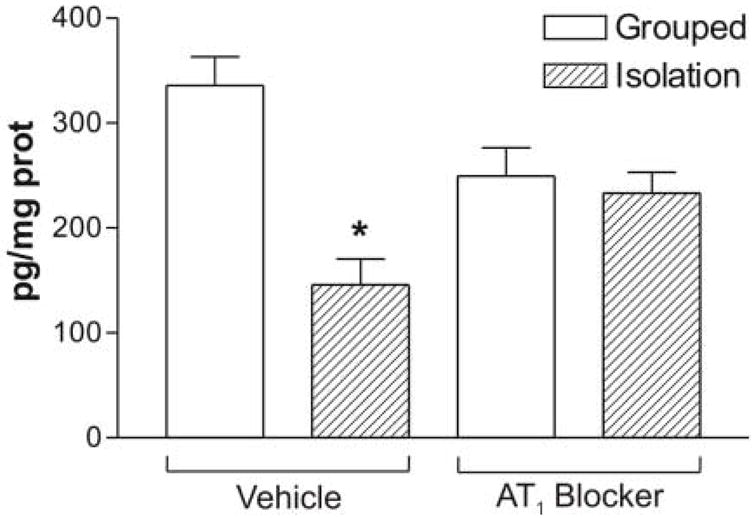
The adrenal corticosterone content was significantly higher after isolation (Figure 5). Treatment with the AT1 receptor antagonist significantly decreased the adrenal corticosterone content in grouped-housed rats and prevented the increase in adrenal corticosterone produced by isolation (Figure 5). Urinary corticosterone was only determined in isolated animals.
Figure 5.
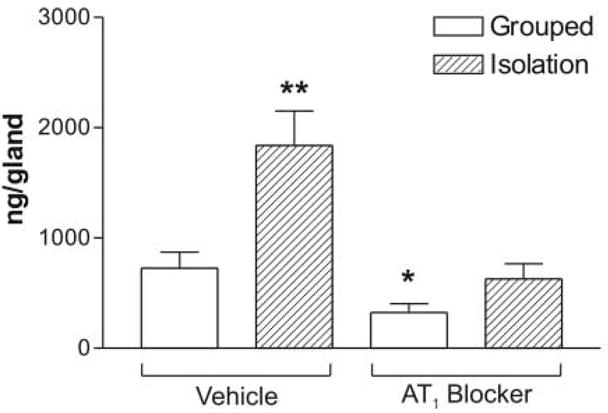
Content of corticosterone in the adrenal gland of grouped or isolated animals treated with vehicle or the AT1 blocker. Results are expressed as the mean ± S.E.M. of groups of six rats measured individually. * P<0.05 vs. all others ** P<0.01 vs. grouped treated with vehicle.
Urinary corticosterone was significantly lower in animals pretreated with the AT1 receptor antagonist than in rats treated with vehicle (Figure 6).
Figure 6.
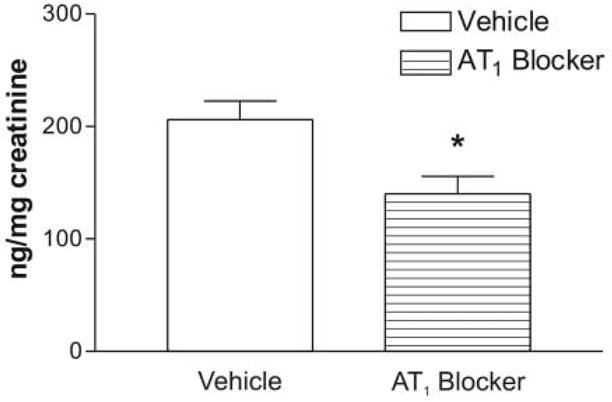
Urinary excretion of corticosterone in isolated rats treated with vehicle or the AT1 blocker. Results are expressed as the mean ± S.E.M. of groups of six rats measured individually. * P<0.05.
3. DISCUSSION
The present study confirms our previous observations of prevention of the isolation-induced HPA axis activation by pretreatment with the AT1 receptor antagonist candesartan (Armando et al., 2001). In addition, we provide novel information demonstrating that AT1 receptor blockade prevents the isolation-induced changes in CRF mRNA and protein expression in the PVN.
Housing rats individually in unfamiliar metabolic cages is a clinically relevant model of emotional stress (Misslin et al., 1982). Isolation for 24 hours produces a typical HPA axis stimulation (Armando et al., 2001). In agreement with our previous report (Armando et al., 2001), we have confirmed that two-week pretreatment of rats with the peripheral and central Ang II AT1 antagonist candesartan, a compound that readily crosses the blood brain barrier, (Nishimura et al., 2000) completely prevented the HPA axis stimulation as a consequence of isolation. After 24 hours of isolation, we found increased AT1 receptor expression in the PVN, similar to the increase in AT1 receptors that occurs during repeated immobilization stress (Castrén and Saavedra, 1988; Leong et al., 2002; Aguilera et al., 1995a). Consistent with previous observations (Armando et al., 2001), sustained pretreatment with the AT1 receptor antagonist decreased AT1 receptor binding in the PVN in both control and stressed rats. This finding indicates that candesartan was effective in blocking AT1 receptors in the PVN of both control and stressed rats.
Candesartan administration prevents the enhanced peripheral sympathetic activation (Armando et al., 2001) and tyrosine hydroxylase increase in the locus coeruleus (Saavedra et al., 2006) that are characteristic of isolation stress. The treatment also blocked the HPA axis stimulation during isolation, confirming previous observations (Armando et al., 2001). We interpreted these findings as the result of a combined blockade of AT1 receptors in the PVN, the median eminence, the anterior pituitary gland, and the adrenal gland (Armando et al., 2001). The association between AT1 receptor levels in the PVN and HPA axis activation suggests that AT1 receptors are required for parvocellular neuron activation and supports the hypothesis that Ang II acts a major stress hormone.
In the present study we focused on the effect of isolation and pretreatment with candesartan on the CRF expression in the PVN. We found that CRF mRNA expression was restricted to the parvocellular region of the PVN, the same area expressing AT1 receptor binding. This is in agreement with our observations demonstrating, using double-labeling hybridization, that in the PVN, the AT1 receptor mRNA is located in cells stained for CRF mRNA (Aguilera et al., 1995b).
Following 24 hours isolation stress, the CRF mRNA and CRF content in the PVN were significantly decreased. The decrease in CRF mRNA expression during isolation suggests an increased mRNA utilization without sufficient transcriptional activation to restore mRNA levels, and/or decreased CRF transcription as a consequence of glucocorticoid feedback. Under these conditions, continuous stimulation of CRF release probably through stimulation of the abundant AT1 receptors present in the median eminence (Tsutsumi and Saavedra, 1991a) will reduce the CRF content in the PVN.
The stress-induced decreased CRF mRNA which may be caused by direct or indirect glucocorticoid feedback to the PVN decreases CRF formation and release (Itoi et al., 1998). This is important to preserve homeostasis, limiting the duration and magnitude of the HPA axis response during stress (Aguilera, 1994; McEwen, 1998; Itoi et al., 1998; Ma and Aguilera, 1999a, b; Ginsberg et al., 2003). Activation of intracellular feedback mechanisms, such as cAMP-mediated repression of CRF transcription, may contribute to limiting the CRF response (Shepard et al., 2005).
Our observations of reduced PVN CRF content is at variance with the increase or lack of change in CRF content in the PVN observed during chronic stress paradigms such as repeated hypertonic saline injection (Kiss and Aguilera, 2000), foot shock (Imaki et al., 1996), immobilization (Mamalaki et al., 1991) or restraint (Kiss et al, 1966; Ma et al, 1999). Consistent with the present findings with isolation stress, other chronic stress conditions, including osmotic stress (Jessop et al., 1990) and chronic immune challenge (Harbuz and Lightman, 1992) are associated with decreased CRF expression. While many stressors increase CRF gene transcription (Jezova et al., 1998; Watts and Watts, 2002) cellular dehydration decreases CRF mRNA in the PVN and restrains CRF gene activation during hypovolemia (Watts and Watts, 2002). Reports of variations in CRF transcription and utilization are dependent on the type and duration of the stress, and are subject to multiple regulatory mechanisms. This suggests that the mechanisms activating secretion and gene transcription are distinct to a certain degree.
As shown by the present and a previous study (Armando et al., 2001), isolation enhanced AT1 receptor expression in the PVN, an effect seen also with other kinds of stress (Leong et al., 2002, Aguilera et al, 1995a). Glucocorticoids stimulate AT1 receptor transcription by acting on a glucocorticoid response element in the AT1 receptor promoter (Guo et al., 1995) and normal glucocorticoid formation and release is necessary for physiological expression of PVN AT1 receptors (Castrén and Saavedra, 1989). Increased AT1 receptor expression in the PVN after stress may be the result of direct effects of glucocorticoids stimulating AT1 receptor transcription (Castrén and Saavedra, 1998; 1989; Guo et al., 1995), enhanced stress-related sympathetic drive (Armando et al., 2001), or factors still to be determined.
Although activation of the HPA axis is usually associated with increases in AT1 receptors in the PVN, our results demonstrate that increased expression of PVN AT1 receptors is not sufficient to maintain enhanced CRF transcription and expression, because CRF mRNA expression and content were decreased in the PVN after 24 hours of isolation. This suggests that after one day of isolation, CRF expression in the PVN has been reduced because of glucocorticoid feedback, increased utilization, or additional regulatory factors in spite of enhanced AT1 receptor expression.
We next determined the effect of sustained pretreatment with the AT1 receptor antagonist candesartan on CRF mRNA and content in the PVN. The pretreatment completely blocked the HPA axis stimulation during isolation as reported earlier (Armando et al., 2001) and prevented the isolation-induced decrease in CRF mRNA and CRF levels in the PVN. The decrease in CRF mRNA and CRF expression following 24 hours of isolation is probably due to a combination of increased stress-induced CRF release and mRNA utilization and glucocorticoid feedback, as discussed above. It is likely that blockade of AT1 receptors by candesartan prevents the activation of the CRF neurons during isolation stress, Since the HPA axis activation does not occur, it also abolishes the glucocorticoid feedback to the PVN. Sustained pretreatment with AT1 receptor antagonists prevents the alterations in CRF mRNA and content in the PVN during isolation. The activity of the central RAS, and that of the Ang II AT1 receptors in the PVN act as alternative mechanism involved in stress-induced HPA axis activation, and they are an important factor in the mediation of the full HPA axis response during stress.
The present results strongly suggest that activation of central AT1 receptors is necessary for the HPA axis stimulation during isolation stress. Although participation of AT1 receptors located in circumventricular organs and other areas of the brain with connections to the PVN cannot be excluded, the marked upregulation of AT1 receptors in the PVN suggests that the modulatory action of Ang II on HPA axis activity is mediated by AT1 receptors in the CRF neurons (Aguilera et al., 1995a; Armando et al., 2001).
We have analyzed, in the present study, only the effects of AT1 blockade in the PVN, and the resulting modulation of CRF. Administration of candesartan, however, decreases AT1 binding throughout the brain, including the amygdala and hippocampus (Nishimura et. al., 2000). It is likely that AT1 receptor inhibition in these areas participates in the central regulation of the glucocorticoid feedback and HPA axis activation during isolation. Candesartan administration is anxiolytic in rodents, and prevents the isolation-induced alterations in benzodiazepine and CRF1 receptor binding in the cerebral cortex (Saavedra et al., 2006). A decreased cognitive and emotional reaction to isolation may therefore decrease the activation of all downstream mechanisms, including that of the HPA axis. We conclude that the effects of AT1 receptor blockade during isolation are the result of inhibition of the hormonal and sympathetic response, and are exerted at multiple levels including cortical, subcortical and hypothalamic mechanisms.
In the normotensive rats studied here, the decrease in blood pressure produced by candesartan was small, and the blood pressure remained within normotensive limits. Only RAS inhibition with candesartan or ramipril significantly reduced the ACTH response to CRF in hypertensive rats, while a similar blood pressure reduction with a calcium channel blocker was not effective (Raasch et al., 2006). This indicates that the anti-stress effects of candesartan described here are not likely to be the consequence of alterations in blood pressure.
Our results may have important implications. AT1 receptor antagonists could be considered as a novel treatment of stress and anxiety related disorders (Saavedra et al., 2005). Sustained pretreatment with candesartan prevents gastric ulcerations produced by cold-restraint stress (Bregonzio et al., 2003) and reduces anxiety in rats (Saavedra et al., 2006). Increased brain AT1 receptor activity, such as that present in genetic hypertension (Saavedra et al, 1986), may mediate the pathological, long term stimulation of the HPA axis during stress characteristic of genetically hypertensive rats (Horie et al., 1991). Excessive central AT1 receptor activation may decrease the efficiency of glucocorticoid feedback and may be a factor involved in the maintenance of sustained HPA axis activity in the presence of stress-induced hypercorticism (Itoi et al., 1998; Ma et al., 2001; Ginsberg et al., 2003). Whether such a mechanism plays a role in the development of stress-induced disorders, and whether centrally-acting AT1 receptor antagonists should be considered as therapeutic anti-stress compounds remain open questions.
4. EXPERIMENTAL PROCEDURE
Animals and preparation of tissues
Wistar Hanover male rats purchased from Taconic Farms (Germantown, NY), were kept at 22°C in groups of four animals per cage under a 12:12-h dark-light cycle with lights on at 07.00 h and were given free access to normal rat diet and tap water. The NIMH Animal Care and Use Committee (Bethesda, MD, USA) approved all procedures.
Ten week old rats weighing 250–300 g were anesthetized with 30 mg/kg pentobarbital, and Alzet osmotic minipumps (Alza Scientific Products, Palo Alto, CA) were implanted subcutaneously. Twenty animals received minipumps containing candesartan (CV 11974, AstraZeneca, Mölndal, Sweden) dissolved in 1 mol/l sodium carbonate and further diluted in saline at a final pH of 7.5 to 8.0, to be delivered at the rate of 0.5 mg/kg/day; another 20 animals received osmotic minipumps filled with vehicle. After minipump implantation the rats were kept in their cages in groups of 4 under standard conditions for 13 days. On day 13, half of the animals were randomly selected and individually housed in standard, plastic metabolic cages (Nalgene, Rochester NY). Regular rat food and water were provided ad libitum throughout the experiment. Urine from isolated animals was collected during the 24 hours of isolation and stored at −80°C until used. At the end of the experiment, on day 14, the animals were sacrificed by decapitation and the brain, pituitary gland, and adrenal glands were removed. These organs were frozen in isopentane at −70°C on dry ice and stored at −80°C until used.
Blood pressures were measured by the tail cuff technique in different groups of 6 animals each, during three consecutive days, four consecutive times each day, starting 12 days after minipumps implantation with candesartan or vehicle as described above, in conscious animals.
Hormone and CRF determinations
For determination of ACTH, pituitary glands were homogenized in 0.1 N acetic acid. For determination of corticosterone, adrenal glands were homogenized in 0.3 N perchloric acid. The clear supernatants obtained after centrifugation were stored frozen until assayed. Urine, pituitary and adrenal hormones were determined by RIA using commercially available kits (ICN Biomedicals Irvine, CA) after appropriate dilutions. Urinary corticosterone was determined only in isolated animals.
For determination of CRF in the PVN, serial brain frontal sections, 300 μm-thick, were cut in a cryostat at −10°C. The PVN was micropunched as described previously (Saavedra et al., 1974) with special stainless steel needles, under stereomicroscopic control. Bilateral tissue samples from each rat were pooled in 200 μl of cold 0.1N acetic acid and homogenized by ultrasonic disruption. Protein concentrations were determined on 10 μl aliquots of the homogenate by the Bradford procedure (Bio Rad, Hercules, CA). The rest of each homogenate was centrifuged and the clear supernatant was stored at −80°C until assayed. The amount of CRF in tissue extracts was determined by RIA as described earlier (Vale et al., 1983) using a CRF antibody provided by W. Vale (Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, La Jolla, California, USA).
Quantitative autoradiography of Angiotensin II AT1 receptors
Sixteen-μm-thick Brain coronal sections containing the PVN were cut in a cryostat at −20°C, thaw-mounted on poly-1-lysine-coated slides (Labscientific Inc., Livingston, NJ), dried overnight in a desiccator at 4°C, and stored at −80°C until used. Sections were labeled in vitro with 0.5 nM of [125I]Sarcosine1-Ang II ([125I]Sar1-Ang II, Peninsula Laboratories, Belmont, CA; iodinated by the Peptide Radioiodination Service Center, School of Pharmacy, University of Mississippi, to a specific activity of 2176 Ci/mmol). Sections were preincubated for 15 min at 22°C in 10 mM Na phosphate buffer, pH 7.4, containing 120 mM NaCl, 5 mM Na2EDTA, 0.005% bacitracin (Sigma Chemical, St Louis, MO), and 0.2% proteinase-free bovine serum albumin (Sigma Chemical), followed by incubation for 120 min in fresh buffer containing 0.5 nM of [125I]Sar1-Ang II. We determined total binding by incubating the sections as described above (Tsutsumi and Saavedra, 1991b). Nonspecific binding was determined in consecutive sections incubated as above in the presence of 1 μM unlabeled Ang II (Peninsula), and was defined as the binding remaining in the presence of excess unlabeled agonist. Since the PVN expresses only Ang II AT1 receptors (Tsutsumi and Saavedra, 1991b), to determine selective binding to the Ang II AT1 we incubated consecutive sections with 0.5 nM of [125I]Sar1-Ang II in the presence of the selective AT1 receptor antagonist losartan (10 μM; DuPont-Merck, Wilmington, DE, USA) to give maximum specific displacement. The number of AT1 receptors was defined as the binding displaced by the AT1 receptor antagonist (Tsutsumi and Saavedra, 1991b).
After incubation, slides were rinsed four consecutive times, for 1 min each, in fresh ice-cold 50 mM Tris-(hydroxymethyl)aminomethane) HCl buffer, pH 7.6, followed by a dip in ice-cold distilled water, and the sections were dried under air. Sections were exposed to Kodak Biomax MR film (Eastman Kodak Company, Rochester, NY) together with 14C-labeled microscales (American Radiolabeled Chemicals, St Louis, MO). Films were developed in ice-cold GBX developer (Eastman Kodak) for 4 min, fixed in Kodak GBX fixer for 4 min at 22°C, and rinsed in water for 15 min. Optical densities of autoradiograms generated by incubation with the 125I-labeled ligands were quantified by computerized densitometry using the Image 1.6 Program (National Institute of Mental Health, Bethesda, MD) after calibration with 14C-labeled standards as described (Miller and Zahniser, 1987). The films were exposed for different times to obtain film images within the linear portion of the standard curve and transformed to corresponding values of fmol per mg protein (Nazarali et al, 1989; Miller and Zahniser, 1987). Because we used single ligand concentrations below saturation, there is no information as to whether the changes described represent alterations in receptor number or receptor affinity. Each animal was quantified independently. The PVN was identified according to Paxinos and Watson (1986) by staining of consecutive sections with toluidine blue.
Determination of CRF mRNA by in situ hybridization
The rat CRF probe (kindly provided by Dr. Scott Young, NIH, Bethesda, MD) was an EcoRI cDNA fragment corresponding to 770 bp of exon 2 of the CRF gene subcloned into pBluescript SK (Stratagene, La Jolla, CA). High specific activity antisense probe was synthesized using the Riboprobe® System—T3 (Promega Co, Madison, WI) containing 100 μCi each of 35S UTP and 35S CTP (Amersham, Arlington Heights, IL, USA), 480 μM each of unlabelled ATP and GTP, 24 μM each of unlabelled UTP and CTP, 10 mM dithiothreitol, 1x Transcription buffer, 15 U RNAsin, 1 μg linearized template, and 15 U of T3 RNA polymerase. After 60 min incubation at 42 °C, the cDNA template was digested with DNAase I for 10 min at 37 °C. Radiolabeled RNA product was purified using quick-spin columns (Boehringer Mannheim, Indianapolis, IN, USA), precipitated with ethanol and resuspended in 100 μl of 10 mM Tris-HCl, pH 7.5 containing 20 mM dithiothreitol.
Prehybridization and hybridization procedures were performed as follows. Briefly, prior to hybridization, sections were thawed at room temperature and fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 5 min, and acetylated with 0.25% acetic anhydride in 0.1 M triethalolamine/0.9% NaCl, pH 8, for 10 min at room temperature. Sections were then dehydrated in 70%, 80%, 95%, and 100% ethanol, treated with chloroform to remove lipids, and hybridized overnight at 55 °C with 2 × 106 cpm. of 35S-labelled probe in 100 μl of hybridization buffer (50% formamide, 200 mM NaCl, 2.5 mM EDTA, 10% dextran sulphate, 250 μg/ml yeast tRNA, 50 mM dithiothreitol, and 1x Denhardt’s solution). After incubation, sections were subjected to consecutive washes in 4x standard saline citrate (SSC) for 15 min at room temperature and 50% formamide/250 mM NaCl for 10 min at 60 °C. After treatment with RNAase-A (20 μg/ml) for 30 min at 37 °C, sections were washed with 2x SSC, 1x SSC and 0.5x SSC for 5 min at room temperature, followed by washes with 0.1x SSC containing dithiothreitol for 15 min at 50 °C and with 0.1x SSC for 5 min at room temperature.
For analysis of CRF mRNA in the PVN, the sections from all experimental groups were placed in the same X-ray cassette, and exposed to Kodak BioMax MR film (Kodak, Rochester, NY, USA) along with [14C] micro-scales (American Radiolabeled Chemicals, St. Louis, MO Amersham). Films were developed as described above. The intensities of hybridization signals were quantified in a computerized image-analysis system as optical densities (Wisden and Morris, 1994) using the public domain NIH Image 1.61 program (developed at the National Institutes of Health and available at http://rsb.info.nih.gov/nih-image), after calibration with the [14C] micro-scales. Two consecutive sections per rat were averaged and used to calculate group means. Each animal was quantified independently. Results are presented as means ± SEM. Tissue background was not different from the film background and no hybridization signal over background was observed using sense RNA probes (results not shown).
Statistics
Data are means ± S.E.M. Two way ANOVA followed by post-hoc analysis using the Newman-Keuls multiple comparison test was used to assess the significance of differences among groups. P<0.05 was considered statistically significant.
Acknowledgments
This study was supported by the Division of Intramural Research Programs, National Institute of Mental Health, Department of Health and Human Services, USA. Candesartan (CV11974) was kindly provided by AstraZeneca, Mölndal, Sweden. We thank Julius Benicky for his help in the preparation of the Figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera G. Factors controlling steroid biosynthesis in the zona glomerulosa of the adrenal. J Steroid Biochem Mol Biol. 1993;45:147–151. doi: 10.1016/0960-0760(93)90134-i. [DOI] [PubMed] [Google Scholar]
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Harwood JP, Wilson JX, Morell J, Brown JH, Catt KJ. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J Biol Chem. 1983;258:8039–8045. [PubMed] [Google Scholar]
- Aguilera G, Kiss A, Luo X. Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol. 1995a;7:775–783. doi: 10.1111/j.1365-2826.1995.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology. 1995b;61:437–444. doi: 10.1159/000126866. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral Administration of an Angiotensin II AT1 Receptor Antagonist Decreases the Hypothalamic-Pituitary-Adrenal Response to Isolation Stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G414–423. doi: 10.1152/ajpgi.00058.2003. [DOI] [PubMed] [Google Scholar]
- Castrén E, Saavedra JM. Repeated stress increases the density of angiotensin II binding sites in the rat paraventricular nucleus and subfornical organ. Endocrinology. 1988;122:370–372. doi: 10.1210/endo-122-1-370. [DOI] [PubMed] [Google Scholar]
- Castrén E, Saavedra JM. Angiotensin II receptors in paraventricular nucleus, subfornical organ, and pituitary gland of hypophysectomized, adrenalectomized, and vasopressin-deficient rats. Proc Natl Acad Sci USA. 1989;86:725–729. doi: 10.1073/pnas.86.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15:1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, McEwen B. Allostasis, homeostasis, and the nature of stress. Stress. 2002;5:55–58. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- Guo DF, Uno S, Ishihata A, Nakamura N, Inagami T. Identification of a cis-acting glucocorticoid responsive element in the rat angiotensin II type 1A promoter. Circ Res. 1995;77:249–257. doi: 10.1161/01.res.77.2.249. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Stress and hypothalamo- pituitary-adrenal axis: Acute, chronic and immunological activation. J Endocrinol. 1992;134:327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- Horie R, Yamori Y, Nara Y, Sawamura M, Mizushima S. Aggravating effects of isolated caging on the development of hypertension and its complications in stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar-Kyoto rats (WKY) Clin Exp Hyper-Theory Pract A. 1991;13:859–864. doi: 10.3109/10641969109042090. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosc. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol 2004. 2004;16:348–355. doi: 10.1111/j.0953-8194.2004.01172.x. [DOI] [PubMed] [Google Scholar]
- Itoi K, Seasholtz AF, Watson SJ. Cellular and extracellular regulatory mechanisms of hypothalamic corticotropin-releasing hormone neurons. Endocr J. 1998;45:13–33. doi: 10.1507/endocrj.45.13. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Chowdrey HS, Lightman SL. Inhibition of rat corticotropin-releasing factor and adrenocorticotropin secretion by an osmotic stimulus. Brain Res. 1990;523:1–4. doi: 10.1016/0006-8993(90)91628-t. [DOI] [PubMed] [Google Scholar]
- Jezova D, Ochedalski T, Kiss A, Aguilera G. Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J Neuroendocrinol. 1998;10:67–72. doi: 10.1046/j.1365-2826.1998.00182.x. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, Kimura B, Shinsako J, Phillips MI. Interaction between CRF and angiotensin II in control of ACTH and adrenal steroids. Am J Physiol. 1983;250:R396–R402. doi: 10.1152/ajpregu.1986.250.3.R396. [DOI] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Role of alpha-1-adrenergic receptors in the regulation of corticotropin-releasing hormone mRNA in the paraventricular nucleus of the hypothalamus during stress. Cell Mol Neurobiol. 2000;20:683–94. doi: 10.1023/A:1007098724683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Palkovits M, Aguilera G. Neural regulation of corticotropin releasing hormone (CRH) and CRH receptor mRNA in the hypothalamic paraventricular nucleus in the rat. J Neuroendocrinol. 1996;8:103–112. doi: 10.1111/j.1365-2826.1996.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Leong DS, Terrón JA, Falcón-Neri A, Armando I, Ito T, Jöhren O, Tonelli LH, Hoe KL, Saavedra JM. Restraint Stress Modulates Brain, Pituitary and Adrenal Expression of Angiotensin II AT1A, AT1B and AT2 Receptors. Neuroendocrinology. 2002;75:227–240. doi: 10.1159/000054714. [DOI] [PubMed] [Google Scholar]
- Livett BG, Marley PD, Wan DC, Zhou XF. Peptide regulation of adrenal medullary function. J Neurol Transm. 1990;29 (Suppl):77–89. doi: 10.1007/978-3-7091-9050-0_9. [DOI] [PubMed] [Google Scholar]
- Ma XM, Aguilera G. Transcriptional responses of the vasopressin and corticotropin-releasing hormone genes to acute and repeated intraperitoneal hypertonic saline injection in rats. Brain Res Mol Brain Res. 1999a;68:129–140. doi: 10.1016/s0169-328x(99)00080-7. [DOI] [PubMed] [Google Scholar]
- Ma XM, Aguilera G. Differential regulation of corticotropin releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology. 1999b;140:5642–5650. doi: 10.1210/endo.140.12.7214. [DOI] [PubMed] [Google Scholar]
- Ma XM, Camacho C, Aguilera G. Regulation of corticotropin releasing hormone (CRH) transcription and CRH mRNA stability by glucocorticoids. Cell Mol Neurobiol. 2001;21:465– 475. doi: 10.1023/A:1013863205647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Mamalaki E, Kvetnansky R, Brady LS, Gold PW. Changes in mRNA levels of POMC, CRH and steroid hormone receptors in rats exposed to acute and repeated immobilization stress. In: Kvetnansky R, McCarthy R, Axelrod J, editors. Stress: Neuroendocrine and Molecular Approaches. Gordon and Breach Science Publishers; 1991. p. 247. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miller JA, Zahniser NR. The use of 14 C-labelled tissue paste standards for the calibration of 125I-labelled ligands in quantitative autoradiography. Neurosci Lett. 1987;81:345–350. doi: 10.1016/0304-3940(87)90408-3. [DOI] [PubMed] [Google Scholar]
- Misslin R, Herzog F, Koch B, Ropartz P. Effects of isolation, handling and novelty on the pituitary-adrenal response in the mouse. Psychoneuroendocrinology. 1982;7:217–22. doi: 10.1016/0306-4530(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Nazarali AJ, Gukind JS, Saavedra JM. Calibration of [125I]-polymer standards with [125I]-brain paste standards for use in quantitative receptor autoradiography. J Neurosci Methods. 1989;30:247–253. doi: 10.1016/0165-0270(89)90135-0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ito T, Hoe KL, Saavedra JM. Chronic peripheral administration of the angiotensin II AT1 receptor antagonist Candesartan blocks brain AT1 receptors. Brain Res. 2000;871:29–38. doi: 10.1016/s0006-8993(00)02377-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. pp. 1–262. [Google Scholar]
- Raasch W, Wittmershaus C, Dendorfer A, Voges I, Pahlke F, Dodt C, Dominiak P, Jöhren O. Angiotensin II inhibition reduces stress sensitivity of hypothalamo-pituitary-adrenal axis in Spontaneously Hypertensive Ras. Endocrinol. 2006;147:3539–3546. doi: 10.1210/en.2006-0198. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain Angiotensin II: New Developments, Unanswered Questions and Therapeutic Opportunities. Cell Mol Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sanchez-Lemus E. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharm. 2006;31:1123–1134. doi: 10.1038/sj.npp.1300921. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Correa FM, Plunkett LM, Israel A, Kurihara M, Shigematsu K. Binding of angiotensin and atrial natriuretic peptide in brain of hypertensive rats. Nature. 1986;320:758–760. doi: 10.1038/320758a0. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Palkovits M, Brownstein MJ, Axelrod J. Serotonin distribution in the nuclei of the rat hypothalamus and preoptic region. Brain Res. 1974;77:157–165. doi: 10.1016/0006-8993(74)90812-9. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of Glucocorticoids and cAMP-Mediated Repression in Limiting Corticotropin-Releasing Hormone Transcription during Stress. J Neurosc. 2005;25:4073–4081. doi: 10.1523/JNEUROSCI.0122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo T, Suda T, Nakano Y, Tozawa F, Yamada M, Demura H. Angiotensin II increases the corticotropin-releasing factor messenger ribonucleic acid level in the rat hypothalamus. Endocrinology. 1991;128:2248–2252. doi: 10.1210/endo-128-5-2248. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K, Saavedra JM. Angiotensin-II receptor subtypes in median eminence and basal forebrain areas involved in regulation of pituitary function. Endocrinology. 1991a;129:3001–3008. doi: 10.1210/endo-129-6-3001. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptors subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991b;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- Vale W, Vaughan J, Yamamoto G, Bruhn T, Douglas C, Dalton D, Rivier C, Rivier J. Methods in Enzymology. Vol. 103. Academic Press, Inc.; 1983. Assay of Corticotropin Releasing Factor; pp. 565–577. [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7:75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. J Neurosci. 2002;22:6282–6289. doi: 10.1523/JNEUROSCI.22-14-06282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. In situ hybridization protocols for the brain. San Diego: Academic Press; 1994. pp. 9–34. [Google Scholar]
- Xang G, Xi ZX, Wan Y, Wang H, Bi G. Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals. 1993;2:166–172. doi: 10.1159/000109488. [DOI] [PubMed] [Google Scholar]