Abstract
Brain neuropathy target esterase (NTE), associated with organophosphorus (OP)-induced delayed neuropathy, has the same OP inhibitor sensitivity and specificity profiles assayed in the classical way (paraoxon-resistant, mipafox-sensitive hydrolysis of phenyl valerate) or with lysophosphatidylcholine (LysoPC) as the substrate. Extending our earlier observation with mice, we now examine human erythrocyte, lymphocyte and brain LysoPC hydrolases as possible sensitive targets for OP delayed neurotoxicants and insecticides. Inhibitor profiling of human erythrocytes and lymphocytes gave the surprising result of essentially the same pattern as with brain. Human erythrocyte LysoPC hydrolases are highly sensitive to OP delayed neurotoxicants, with in vitro IC50 values of 0.13–85 nM for longer alkyl analogs, and poorly sensitive to the current OP insecticides. In agricultural workers, erythrocyte LysoPC hydrolyzing activities are similar for newborn children and their mothers and do not vary with paraoxonase status but have high intersample variation that limits their use as a biomarker. Mouse erythrocyte LysoPC hydrolase activity is also of low sensitivity in vitro and in vivo to the OP insecticides whereas the delayed neurotoxicant ethyl n-octylphosphonyl fluoride inhibits activity in vivo at 1–3 mg/kg. Overall, inhibition of blood LysoPC hydrolases is as good as inhibition of brain NTE as a predictor of OP inducers of delayed neuropathy. NTE and lysophospholipases (LysoPLAs) both hydrolyze LysoPC, yet they are in distinct enzyme families with no sequence homology and very different catalytic sites. The relative contributions of NTE and LysoPLAs to LysoPC hydrolysis and clearance from erythrocytes, lymphocytes and brain remain to be defined.
Keywords: erythrocytes, lysophosphatidylcholine, lysophosphatidylcholine hydrolase, neuropathy target esterase, organophosphorus
Introduction
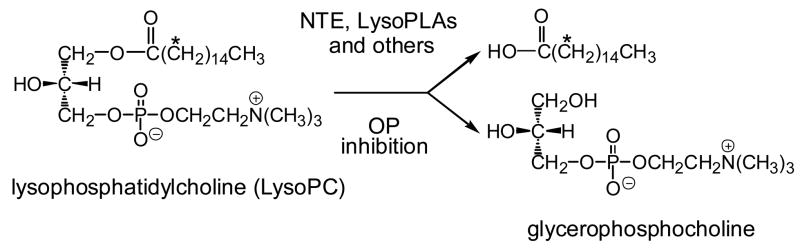
Organophosphorus (OP)- induced delayed neuropathy (OPIDN), with >70,000 human cases in the last 75 years, is associated with inhibition of neuropathy target esterase (NTE) and aging of the phosphorylated enzyme (Johnson and Glynn, 2001; Ehrich and Jortner, 2001). NTE is normally assayed with brain membranes as the paraoxon-resistant and mipafox-sensitive portion of phenyl valerate (PV) hydrolysis (Johnson and Glynn, 2001). PV is an abiotic substrate modeled on a potent OP NTE inhibitor rather than an endogenous compound (Casida and Quistad, 2004). NTE also hydrolyzes lysophosphatidylcholine (LysoPC) (van Tienhoven et al., 2002; Quistad et al., 2003), yet belongs to a different enzyme family with a catalytic site unique from the well-characterized lysophospholipase (LysoPLA) I (Wang and Dennis, 1999). Mouse brain homogenate assayed as either PV or LysoPC hydrolysis has similar reduced activity in nte +/− versus wild type mice and the same OP sensitivity and specificity profiles (Quistad and Casida, 2004). LysoPC hydrolytic activity is therefore a more mechanism-based approach to OPIDN assayed directly or as the paraoxon-resistant and mipafox-sensitive portion (Quistad et al., 2003) (Fig. 1). Lymphocyte NTE inhibition assayed with PV has been proposed to monitor exposure to insecticides and OPIDN agents (Lotti et al., 1986), but this approach has limited value due to poor enzyme stability and reproducibility (Lotti, 1992; Mutch et al., 1992). Erythrocyte and lymphocyte LysoPC hydrolases are possible alternative biomarkers for OP delayed neurotoxicant exposure.
Fig. 1.
OP-sensitive LysoPC hydrolases assayed with [14C-palmitoyl]LysoPC
The monitoring of human exposure to OP insecticides relies on inhibition of erythrocyte acetylcholinesterase (AChE) or plasma butyrylcholinesterase (BChE) activity (Ballantyne and Salem, 2006) or analysis of blood and urine for the OPs or their metabolites. OP pesticides are of special concern for pregnant women, infants and children (National Research Council, 1993; Neri et al., 2006). Plasma levels of chlorpyrifos among women in New York City are associated with low birth weight and length and adverse effects on neurodevelopment (Perera et al., 2003; Rauh et al., 2006). Maternal urinary dialkyl phosphate metabolite levels in an agricultural population are correlated with shortened gestational length, a higher number of abnormal reflexes in neonates, and poorer neurodevelopment in young children (Eskenazi et al., 2004, 2007; Young et al., 2005). Paraoxonase 1 (PON1) is an OP-hydrolyzing enzyme with high activity for detoxifying chlorpyrifos oxon (CPO) among many OPs (Geldmacher-von Mallinckrodt and Diepgen, 1988; Furlong et al., 1988). PON1 levels in children are often lowest at birth and can take up to 24 months or longer to reach adult levels (Augustinsson and Barr, 1963; Cole et al., 2003). The PON192 genotype may be a factor in OP detoxification and therefore sensitivity (Furlong et al., 2006; Holland et al., 2006).
This study considers the LysoPC hydrolases as sensitive targets for OP delayed neurotoxicants, insecticides and other inhibitors. The first goal is to characterize the LysoPC hydrolases of human erythrocytes, lymphocytes and brain and profile their inhibitor sensitivities. The second is to determine the potency of OP neurotoxicants and insecticides for inhibition of human erythrocyte LysoPC hydrolases in vitro and mouse erythrocyte activity in vivo. Finally, factors affecting human erythrocyte LysoPC hydrolases are evaluated including PON1 genotype using both newborn children and mothers. We conclude that although blood LysoPC hydrolases are poorly sensitive to current OP insecticides, they are as good as brain NTE as a predictor of OP inducers of delayed neuropathy.
Materials and methods
Chemicals
The OPs and other inhibitors were selected for major differences in structure and potency thereby serving as a suitable set to examine the inhibitor specificity and binding pocket conformation of the serine hydrolases (Casida and Quistad, 2004). Alternatively, they were widely-used insecticides in Monterey County, California (California DPR, 2002), the study area described below for human environmental exposure. Insecticides are referred to by their common names (Tomlin, 2003) or are identified in the abbreviations and table footnotes. ChemService (West Chester, PA) was the source of the seven widely-used OP insecticides (acephate, chlorpyrifos, diazinon, dimethoate, malathion, methamidophos and oxydemeton-methyl) and activated metabolites of some of these compounds (CPO, diazoxon, malaoxon, and o-methoate). The other candidate inhibitors were obtained from commercial sources or synthesized in the Environmental Chemistry and Toxicology Laboratory.
Tissues
Whole human blood from seven subjects and lymphocytes (isolated by aphaeresis using a leukopac filter) from three subjects were obtained from AllCells LLC (Emeryville, CA). Mouse blood from Swiss-Webster males was obtained by cardiac puncture and collected in heparin. Erythrocytes were separated from whole blood by centrifugation and washed with three volumes of isotonic saline. To determine the contribution of blood fractions to LysoPC hydrolase activity, human whole blood was layered with Ficoll and centrifuged to obtain plasma, lymphocyte and erythrocyte portions. To minimize loss of LysoPC hydrolase activity, erythrocytes and lymphocytes were aliquoted and kept at −80 °C until use. Storage of erythrocytes for 3 months at −80 °C or at liquid nitrogen temperature preserved 75% of the original LysoPC hydrolase activity, compared to 40% at −20 °C. The activity loss was >50% within 48 h at 4 °C. An internal standard erythrocyte sample was used to correct for variability in assays on separate days (4.1 ± 1.1 pmol/min/mg protein, n= 28). Human brain was obtained from a medical examiner’s office (Cole et al., 1984) and samples from the frontal cortex were used for assays.
Human erythrocytes were obtained from 25 randomly-selected pregnant women (at approximately 26 weeks gestation) and their newborns (cord blood) who participated in the “Center for the Health Assessment of Mothers and Children of Salinas” (CHAMACOS) project in Monterey County, California, a longitudinal birth cohort study to evaluate the effects of pesticides and other environmental exposures on the health of children and pregnant women (Eskenazi et al., 1999). After the samples were divided into newborn and mother groups and according to PON1192 genotype (Q/Q slow metabolizers; Q/R; R/R fast metabolizers) (Holland et al., 2006), 100 μl of erythrocytes from each subject was pooled. Written informed consent was obtained from all women in the study and protocols were approved by the Institutional Review Board at the University of California, Berkeley.
Mouse studies
Mice (three per compound) were treated ip with the seven widely-used insecticides at the highest non-lethal dose or with ethyl n-octylphosphonyl fluoride (EOPF) at 1 and 3 mg/kg and sacrificed 4 h later. EOPF is the most commonly used OP delayed neurotoxicant in recent studies (Wu and Casida, 1996). Erythrocytes were obtained as above and brains were removed and frozen at −80 °C until analyzed.
Enzyme activity assays
LysoPC hydrolases were assayed with [14C-palmitoyl]LysoPC (Amersham, 56 mCi/mmol, 99% radiochemical purity) by a specific partitioning method (Fig. 1). Brain was homogenized and lymphocytes were sonicated in assay buffer (1 mM CaCl2, 0.01% Triton-X100, 100 mM Tris-HCl, pH 8.0, 25 °C). Erythrocytes were sonicated without prior dilution. In one study the brain homogenate and sonicated erythrocytes were centrifuged at 100,000 × g for 1 h at 4 °C to obtain membrane and cytosol fractions. Protein was quantitated (Bradford, 1976) and an amount within the linear range for protein level- percent hydrolysis was used. The tissue preparation in assay buffer (490 μl) was treated with the test OP in dimethyl sulfoxide (DMSO) (5 μl) and after 15 min at room temperature with [14C]LysoPC (70,000 dpm) in DMSO (5 μl). The reaction in a 7.4-ml vial was then incubated at 37 °C for 15 min. The [14C]palmitic acid hydrolysis product was separated from unhydrolyzed [14C]LysoPC by a modified Dole extraction method (Zhang et al., 1991) with hexane substituted for heptane. A solution (2.5 ml) of isopropanol: hexane: 1N sulfuric acid (20:5:1) was added to stop the reaction. After introduction of ~100 mg silica gel, the vial was vortexed for 10 sec. Hexane (1.5 ml) and water (1.5 ml) were added followed by vortexing for 15 sec. After 2–5 min for phase separation, most of the top organic layer containing the [14C]palmitic acid was removed (1.0 ml) and the 14C content was determined in a scintillation counter. A control sample was assayed with isopropyl n-dodecylfluorophosphonate (IDFP) (100 μM, 15 min preincubation) to completely block the LysoPC hydrolase activity and this value, averaging 7% of the total, was subtracted to obtain the specific enzymatic hydrolysis. All assays were done in triplicates. LysoPC hydrolase activity is reported as pmol [14C]LysoPC hydrolysis per min per mg protein.
Mouse brain NTE activity assays were done as described above for LysoPC hydrolases, with one additional step. Mipafox (50 μM final concentration) was added in 50 mM tris-citrate buffer (pH 6.0) followed by a 20-min incubation at 25 °C before [14C]LysoPC was added. In contrast to assays in which NTE is measured using PV as the substrate, there is no inhibition by paraoxon when LysoPC is the substrate. Thus, in this study mipafox was added but not paraoxon to determine NTE activity.
IC50 values and statistical analysis
OP sensitivity is expressed as the IC50 value (concentration which inhibits 50% of enzymatic activity) with the standard deviation and number of replicates. The IC50s were used to compare different compounds and enzyme sources to determine the specificity of the binding pocket rather than as indicators of toxicity. Correlation plots were made by linear regression using SigmaPlot 8.0. Activity values (means ± SD) also allowed comparison of the differential activity between selected groups (newborns vs mothers and PON1 genotype pools) using the students t-test to evaluate significant differences. In dose-response considerations, the alpha value for significance was adjusted for Bonferroni inequality to account for multiple hypothesis testing.
Results
Activity and distribution
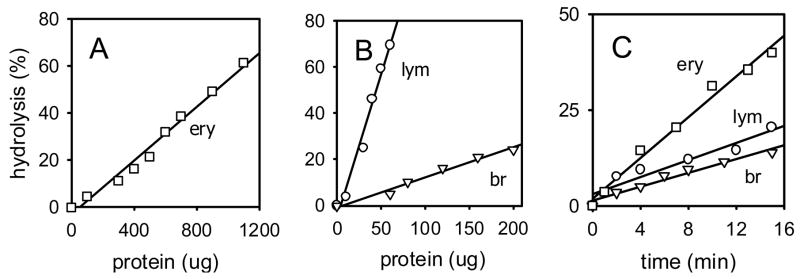
The LysoPC hydrolase activities (pmol/min/mg protein, mean ± SD) observed for human erythrocytes averaged 21 ± 25, range 4–75 (n= 7 subjects), for lymphocytes 135 ± 28, range 110–165 (n= 3 subjects) and for brain 56 ± 8 (single subject, variability of 23 analyses), in each case with good linearity for protein level or incubation time versus substrate hydrolysis (Fig. 2). Mouse erythrocyte LysoPC hydrolytic activity was considerably lower (0.51 ± 0.05 pmol/min/mg, n= 5 subjects) than that of human erythrocytes. The LysoPC hydrolase activity of human erythrocytes was predominantly (98 ± 2%) in the cytosol, of human brain was mostly in the membrane fraction (96 ± 7%), and of human blood was almost entirely (>99%) in the erythrocyte fraction, with the lymphocyte and plasma portions contributing < 1% of the total activity.
Fig. 2.
[14C]LysoPC hydrolytic activities of human erythrocytes (ery), lymphocytes (lym) and brain (br). A and B. Dependence on protein level in assays for 15 min. C. Dependence on time with protein levels (μg) of 700 for erythrocytes, 25 for lymphocytes and 80 for brain.
OP sensitivity and specificity profiles
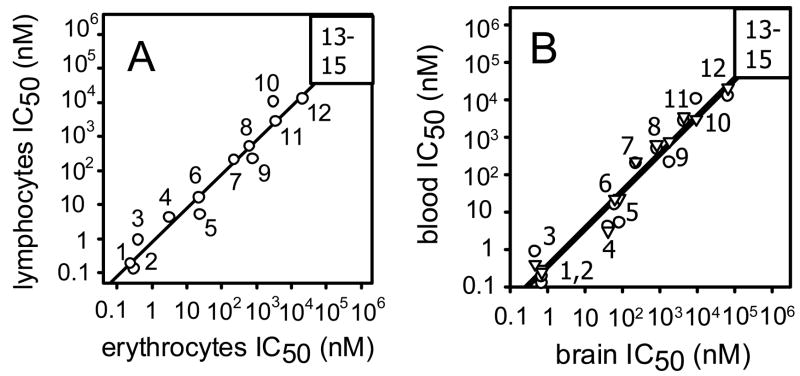
Erythrocyte, lymphocyte and brain LysoPC hydrolases are ultra sensitive to S-OBDPO, IDFP and EOPF (IC50s 0.13– 0.93 nM), highly sensitive to DP-dichlorvos, DP-CPO and R-OBDPO (IC50s 3.2 – 85 nM), moderately sensitive to CPO, TBDPO, DSF, DFP and OSF (IC50s 210 – 10,500 nM), and somewhat less sensitive to phenyl benzylcarbamate (Table 1). The other candidate inhibitors (compounds 13–15) are generally less potent. Using data for the 12 compounds with discrete IC50 numbers for all enzyme sources, the r2 values (0.93–0.97) indicate a conserved inhibitor specificity or binding site conformation (Fig. 3). This is considered to be preliminary evidence that the erythrocyte, lymphocyte and brain enzyme(s) are very similar to each other.
Table 1.
Inhibitor sensitivity and specificity profiles in vitro for human erythrocyte, lymphocyte and brain LysoPC hydrolases
| IC50 ± SD (nM) (n = 3) |
|||
|---|---|---|---|
| Compounds a | erythrocytes | lymphocytes | brain |
| 1 S-OBDPO | 0.25 ± 0.07 | 0.19 ± 0.00 | 0.71 ± 0.09 |
| 2 IDFP | 0.31 ± 0.03 | 0.13 ± 0.00 | 0.72 ± 0.18 |
| 3 EOPF | 0.39 ± 0.06 | 0.93 ± 0.08 | 0.48 ± 0.03 |
| 4 DP-dichlorvos | 3.2 ± 0.4 | 4.2 ± 0.8 | 41 ± 5.3 |
| 5 DP-CPO | 24 ± 5 | 5.2 ± 0.8 | 85 ± 22 |
| 6 R-OBDPO | 23 ± 6 | 16 ± 3 | 62 ± 9 |
| 7 CPO | 230 ± 53 | 210 ± 30 | 230 ± 9 |
| 8 TBDPO | 640 ± 92 | 510 ± 180 | 813 ± 70 |
| 9 DSF | 800 ± 170 | 220 ± 20 | 1800 ± 80 |
| 10 DFP | 3100 ± 460 | 10,500 ± 3000 | 9600 ± 2800 |
| 11 OSF | 3600 ± 530 | 2800 ± 500 | 4300 ± 600 |
| 12 phenyl benzylcarbamate | 21,000 ± 5200 | 13,000 ± 600 | 66,000 ± 17,000 |
| 13 mipafox | < 50,000 (65 ± 5) b | < 50,000 (62 ± 2) b | > 50,000 (40 ± 5) b |
| 14 paraoxon | > 40,000 (32 ± 3) b | < 40,000 (77 ± 1) b | > 40,000 (22 ± 10) b |
| 15 phenylmethanesulfonyl fluoride | > 100,000 (17 ± 13) b | > 100,000 (26 ± 2) b | > 100,000 (23 ± 2) b |
Three types of compounds are represented: 1–8, 10, 13 and 14 are OPs; 12 is a carbamate; 9, 11 and 15 are sulfonyl fluorides. See the abbreviations for compound identifications.
Inhibition (%) at indicated concentration.
Fig. 3.
Correlation of OP sensitivity and specificity profiles for human erythrocyte, lymphocyte and brain LysoPC hydrolases. A. Erythrocytes compared with lymphocytes. B. Brain compared with erythrocytes (▷) and lymphocytes (○). The data plotted are from Table 1. Correlation coefficients for compounds 1–12 with discrete IC50 values: A r2= 0.96; B erythrocytes r2= 0.97 and lymphocytes r2= 0.93.
Delayed neurotoxicant inhibitors
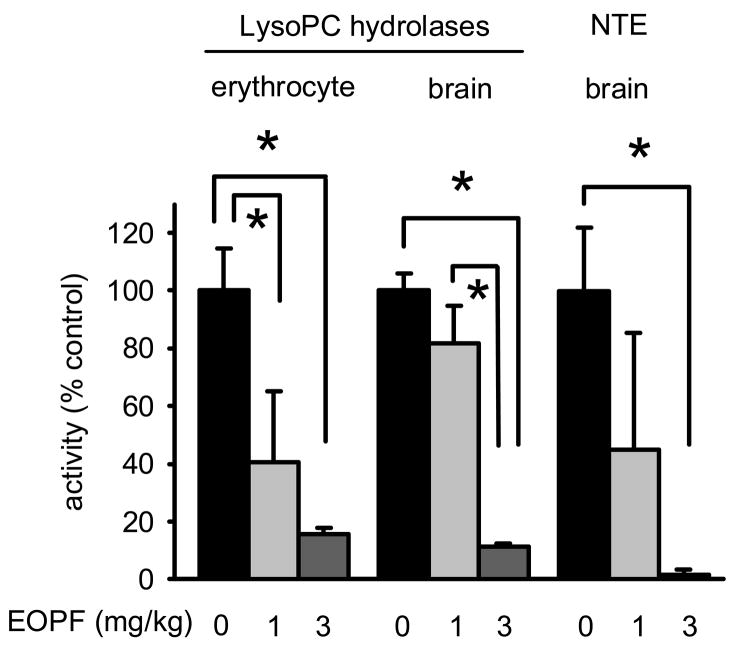
EOPF was used to determine the relative in vivo sensitivity of erythrocyte and brain LysoPC hydrolase and brain NTE activities at 4 h after ip treatment. EOPF at 1 and 3 mg/kg caused dose-dependent reduction in erythrocyte LysoPC hydrolase activity closely paralleling the brain LysoPC hydrolase and NTE diminished activities (Fig. 4). At 1 mg/kg, erythrocyte LysoPC hydrolase activity was inhibited 59% while brain LysoPC hydrolase and NTE activities were inhibited 18 and 55%, respectively. At 3 mg/kg, erythrocyte inhibition was 84%, while brain LysoPC hydrolase and NTE activities were inhibited 88 and 99%, respectively. Mice 4 h post- treatment with EOPF at 1 mg/kg appeared normal without significant hyperactivity measured as vertical rearing and distance traveled (data not shown).
Fig. 4.
Effect of EOPF on mouse erythrocyte and brain LysoPC hydrolase activities and brain NTE activity assayed with [14C]LysoPC 4 h after ip treatment. The mipafox-sensitive brain NTE activity represents 12 ± 4% (n= 4) of the total LysoPC hydrolase activity. * p < 0.017, adjusted for Bonferroni inequality.
OP insecticide inhibitors
Seven of the OPs (or their bioactivated metabolites) were compared for inhibition of LysoPC hydrolases in vitro and in vivo. The most potent inhibitor was CPO with IC50 values of 210–900 nM while diazoxon and oxydemeton-methyl were much less potent (Table 2). Interestingly, the mouse enzyme preparations appeared to be less sensitive than the human enzymes to CPO, diazoxon and oxydemeton-methyl (IC50s 900 vs 230 nM, >100,000 vs 42,000 nM and >100,000 vs 68,000 nM respectively). The remaining insecticides and metabolites had IC50 values over 100 μM. A similar sensitivity comparison was made for in vivo inhibition of mouse erythrocyte LysoPC hydrolases at 4 h post- treatment (Table 3). At the highest non-lethal dose, acephate and diazinon showed the greatest inhibition (24–28%), while dimethoate and chlorpyrifos were less potent with 14–16% inhibition. The remaining compounds did not inhibit LysoPC hydrolases at the test doses.
Table 2.
OP insecticide or activated metabolite potencies for in vitro inhibition of human and mouse erythrocyte and lymphocyte LysoPC hydrolases
| IC50 ± SD (nM) (n =3) a |
|||
|---|---|---|---|
| Human |
Mouse |
||
| Compound | lymphocyte | erythrocyte | erythrocyte |
| CPO | 210 ± 30 | 230 ± 53 | 900 ± 330 |
| diazoxon | 67,000 ± 2300 | 42,000 ± 3000 | >100,000 (37 ± 3) b |
| oxydemeton-methyl | >100 000 (5 ± 4) b | 68,000 ± 6000 | >100 000 (15 ± 27) b |
OPs with IC50s > 100,000 nM for all three assay systems were acephate, malaoxon, methamidophos and o-methoate.
Inhibition (%) at indicate concentration.
Table 3.
OP insecticide in vivo inhibition of mouse erythrocyte LysoPC hydrolases and AChE at 4 h post- treatment
| Inhibition (%) | |||
|---|---|---|---|
| Compound | Dose (mg/kg) | LysoPC hydrolases | AChE a |
| acephate | 30 | 28 ± 7 | 59 ± 12 |
| diazinon | 30 | 24 ± 27 | 85 ± 4 |
| dimethoate | 100 | 16 ± 7 | 74 ± 16 |
| chlorpyrifos | 30 | 14 ± 15 | 93 ± 7 |
| malathion | 30 | 0 ± 0 | 2 ± 2 |
| methamidophos | 3 | 1 ± 2 | 64 ± 12 |
| oxydemeton-methyl | 10 | 0 ± 0 | 72 ± 2 |
Activity and OP sensitivity of erythrocytes from agricultural workers
Some enzymes are not fully functional at birth, so possible differences were considered in erythrocyte LysoPC hydrolases between children and adults. Newborn children and their mothers were examined in two independent experiments using pooled erythrocyte samples to minimize intersample differences. The first experiment (n= 16) indicated that newborns had 60% of the activity of their mothers, while a second larger experiment (n= 31) showed similar LysoPC hydrolase activities (7.9 ± 1.0 and 7.3 ± 1.7 pmol/min/mg for newborns and mothers, respectively; p > 0.5). PON1 activity is indirectly a potential source of variability since by possibly detoxifying OPs it may reduce their effectiveness as inhibitors of LysoPC hydrolases in erythrocytes. If true, we expected that the PON1192 QQ subjects, especially newborns, would show lower levels of LysoPC hydrolase activity compared with the normal PON1192 RR due to the greater availability of OPs in their blood. However, although the data are not shown, the PON1192 QQ and PON1192 RR groups showed no significant difference in erythrocyte LysoPC hydrolase activities in both mothers and newborns. The erythrocytes from pooled samples of newborns and their mothers were compared for sensitivity to five OPs of varying potency (Table 4). There was no significant difference in the IC50 values for IDFP, CPO, OOS, diazoxon or oxydemeton-methyl (p values between 0.09 and 0.87). Finally, we investigated the possibility that PON1192 genotype could influence the availability of OPs to inhibit other secondary targets. To test this hypothesis, the LysoPC hydrolase activity of erythrocyte pools created according to the PON1 genotype were tested for sensitivity to 200 nM CPO but no difference was found in three experiments (percent inhibition ± SD), i.e. 53 ± 11 and 59 ± 7 for PON192 QQ and RR newborn pools, respectively, and 52 ± 4 and 62 ± 8 for PON192 QQ and RR mothers.
Table 4.
OP sensitivities of erythrocyte LysoPC hydrolase of newborn children and their mothers
| IC50 ± SD (nM) (n =3) |
||
|---|---|---|
| Compounds a | children | mothers |
| IDFP | 0.055 ± 0.006 | 0.048 ± 0.002 |
| CPO | 190 ± 30 | 170 ± 10 |
| OOS | 15,700 ± 2300 | 16,200 ± 3500 |
| diazoxon | 29,700 ± 6000 | 40,300 ± 5500 |
| oxydemeton-methyl | > 100,000 (18 ± 4) b | > 100,000 (1) b |
See the abbreviations for compound identifications.
Inhibition (%) at indicated concentration.
Discussion
OP sensitivity and specificity profiles
The LysoPC hydrolases of human erythrocytes, lymphocytes and brain are observed here to be surprisingly similar in OP sensitivity and specificity. All three activities are extremely sensitive to OPs with long alkyl chains (IC50 values <1 nM for S-OBDPO, IDFP and EOPF) but less sensitive to widely-used OP insecticides.
Delayed neurotoxicant inhibitors
Two of the three most potent inhibitors of LysoPC hydrolases (S-OBDPO and EOPF, Table 1) are delayed neurotoxicants in mice and hens (Wu and Casida, 1996). Human erythrocyte LysoPC hydrolase activity in this study is > 300-fold more sensitive than mouse brain AChE to two OP NTE inhibitors (IDFP and EOPF) (Casida and Quistad, 2004). Thus, inhibition of blood LysoPC hydrolases is a candidate marker of OP-delayed neurotoxicants. However, high intersample variation of human erythrocyte LysoPC hydrolase activities would necessitate a pre-exposure baseline for each individual. The lipid profile of erythrocytes is influenced by dietary intake (Popp-Snijders et al., 1986), and the impact of diet on erythrocyte LysoPC hydrolases is unexplored.
OP insecticide inhibitors
The traditional enzymes for OP exposure monitoring are erythrocyte AChE and to some extent plasma BChE. However, each serine hydrolase has a unique inhibitor profile and is worthy of consideration for monitoring exposure to complex mixtures of pesticides and neurotoxicants (Quistad et al., 2006). For example, erythrocyte acylpeptide hydrolase is a sensitive marker for exposure to some OP pesticides and metabolites (Richards et al, 2000) and relative to erythrocyte LysoPC hydrolases is 11-fold more sensitive to CPO (with an IC50 of 21 nM), 45-fold to diazoxon and >16-fold to acephate (Quistad et al., 2005). The most potent insecticide metabolite inhibitor of human blood LysoPC hydrolases is CPO, with an IC50 of 210 nM for lymphocytes and 230 nM for erythrocytes. The other insecticides or metabolites tested were less potent or completely inactive at 100 μM. OP compounds administered to mice were generally weak in vivo inhibitors of erythrocyte LysoPC hydrolases, i.e. at the highest non-lethal doses, acephate and diazinon inhibited about one quarter of the total activity and the other compounds showed less inhibition. These in vivo data suggest that even at large doses of OPs, erythrocyte LysoPC hydrolases are not likely to be appreciably inhibited. Erythrocyte AChE was a more sensitive indicator of OP insecticide exposure (59- 93% inhibition with the exclusion of malathion, Table 3) (Quistad et al., 2005). Most of the acute toxicity is due to inhibition of AChE, while LysoPC hydrolases do not appear to be involved. Thus, erythrocyte LysoPC hydrolases are very sensitive biomarkers for OP delayed neurotoxicants but AChE is better for the commonly-used OP insecticides.
LysoPLAs and NTE regulate LysoPC levels
LysoPC-hydrolyzing enzymes, particularly the LysoPLAs, are the major route by which LysoPC levels are controlled (Zhang et al., 1991; Ross and Kish, 1994; Wang and Dennis, 1999). LysoPLA activity is reported in brain (Ross and Kish, 1994; Quistad et al., 2003) and erythrocytes (Mulder et al., 1965), and the current study describes LysoPC hydrolase activity in lymphocytes as well. NTE is a contributor to brain LysoPC hydrolyzing activity (Quistad and Casida, 2004). NTE is found in lymphocytes (Lotti et al., 1986), but the blood enzymes responsible for LysoPC hydrolytic activity are not identified. Although LysoPLAs and NTE hydrolyze the same LysoPC substrate, they are not homologous enzymes. The catalytic site of LysoPLA I is a typical α/β Ser-Asp-His triad (Wang et al., 1997, Devedjiev et al., 2000), while that of the modeled NTE patatin domain consists of a Ser-Asp dyad (Wijeyesakere et al., 2007), also present in the active sites of the patatin protein and phospholipase A2(Dessen et al., 1999; Rydel at al., 2003). LysoPLAs and other phospholipases regulate phospholipid levels to maintain membrane homeostasis, flexibility and permeability (Selle at el., 1993; Wang and Dennis, 1999), and NTE apparently performs the same function relative to LysoPC. This is critical to cell maintenance, as excess LysoPC leads to shape change and lysis in erythrocytes (Shohet and Beutler, 1983) and is associated with several diseases such as atherosclerosis, inflammation and hyperlipidemia (Wang and Dennis, 1999). Both NTE and LysoPLAs are involved in LysoPC hydrolysis and their inhibition may contribute to the delayed effects of some OP toxicants.
Acknowledgments
This work was supported by Grants 2 P01 ES09605 from the National Institute of Environmental Health Sciences (NIEHS) and US EPA (to B.E. and N.H.) and by Grant R01 ES08762 (to J.E.C.) from the NIEHS, NIH, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. We thank Gary Quistad, Daniel Nomura and Kevin Ford of the Environmental Chemistry and Toxicology Laboratory for advice and assistance. We acknowledge the help of Kim Harley and Asa Bradman on the CHAMACOS project and the participation of the CHAMACOS families and staff. We are also grateful to Karen Huen and Nishat Shaikh of the Children’s Environmental Health Laboratory for their help with statistical analysis and biological samples. The authors declare that there are no conflicts of interest.
ABBREVIATIONS
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- CPO
chlorpyrifos oxon
- DFP
diisopropyl fluorophosphate
- DMSO
dimethyl sulfoxide
- DP-CPO
di-n-pentyl analog of CPO
- DP-dichlorvos
di-n-pentyl analog of dichlorvos
- DSF
n-dodecanesulfonyl fluoride
- EOPF
ethyl n-octylphosphonyl fluoride
- IC50
concentration inhibiting 50% of enzyme activity
- IDFP
isopropyl n-dodecylfluorophosphonate
- LysoPC
lysophosphatidylcholine
- LysoPLA
lysophospholipase
- NTE
neuropathy target esterase
- OOS
trimethyl phosphorothiolate
- OP
organophosphorus
- OPIDN
organophosphorus-induced delayed neuropathy
- OSF
n-octanesulfonyl fluoride
- PON1
paraoxonase 1
- PV
phenyl valerate
- R- and S-OBDPO
R- and S-enantiomers of n-octyl benzodioxaphosphorin oxide
- TBDPO
o-tolyl benzodioxaphosphorin oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augustinsson KB, Barr M. Age variation in plasma arylesterase activity in children. Clin Chim Acta. 1963;8:568–573. doi: 10.1016/0009-8981(63)90106-2. [DOI] [PubMed] [Google Scholar]
- Ballantyne B, Salem H. Occupational toxicology and occupational hygiene aspects of organophosphate and carbamate anticholinesterases with particular reference to pesticides. In: Gupta RC, editor. Toxicology of Organophosphate & Carbamate Compounds. Elsevier; San Diego: 2006. pp. 567–595. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;7:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- California Department of Pesticide Regulation. [Accessed July 2004];California Pesticide Information Portal, Pesticide Use Reporting. 2002 http://calpip.cdpr.ca.gov/cfdocs/calpip/prod/main.cfm.
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, Tward A, Lusis AJ, Jack RM, Costa LG, Furlong CE. Expression of human paraoxonase (PON1) during development. Pharmacogen. 2003;13:357–364. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- Cole LM, Lawrence LJ, Casida JE. Similar properties of [35S]t-butylbicyclophosphorothionate receptor and coupled components of the GABA receptor-ionophore complex in brains of human, cow, rat, chicken and fish. Life Sci. 1984;35:1755–1762. doi: 10.1016/0024-3205(84)90272-8. [DOI] [PubMed] [Google Scholar]
- Dessen A, Tang J, Schmidt H, Stahl M, Clark JD, Seehra J, Somers WS. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97:349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- Devedjiev Y, Dauter Z, Kuznetsov SR, Jones TLZ, Derewenda ZS. Crystal structure of the human acyl protein thioesterase I from a single X-ray data set to 1.5 Å. Structure. 2000;8:1137–1146. doi: 10.1016/s0969-2126(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Ehrich M, Jortner BS. Organophosphorus-induced delayed neuropathy. In: Krieger RI, editor. Handbook of Pesticide Toxicology. Vol. 2. Academic Press; San Diego: 2001. pp. 987–1012. [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Env Health Perspect. 1999;107:409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Env Health Perspect. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Env Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong CE, Richter RJ, Seidel SL, Motulsky AG. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am J Hum Genet. 1988;42:230–238. [PMC free article] [PubMed] [Google Scholar]
- Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet Genomics. 2006;16:183–190. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- Geldmacher-von Mallinckrodt M, Diepgen TL. The human serum paraoxonase- polymorphism and specificity. Toxicol Environ Chem. 1988;18:79–196. [Google Scholar]
- Holland N, Furlong C, Bastaki M, Richter R, Bradman A, Huen K, Beckman K, Eskenazi B. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Env Health Perspect. 2006;114:985–991. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Glynn P. Neuropathy target esterase. In: Krieger RI, editor. Handbook of Pesticide Toxicology. Vol. 2. Academic Press; San Diego: 2001. pp. 953–965. [Google Scholar]
- Lotti M. The pathogenesis of organophosphate polyneuropathy. Crit Rev Toxicol. 1992;21:465–487. doi: 10.3109/10408449209089884. [DOI] [PubMed] [Google Scholar]
- Lotti M, Moretto A, Zoppellari R, Dainese R, Rizzuto N, Barusco G. Inhibition of lymphocytic neuropathy target esterase predicts the development of organophosphate-induced delayed polyneuropathy. Arch Toxicol. 1986;59:176–179. doi: 10.1007/BF00316329. [DOI] [PubMed] [Google Scholar]
- Mulder E, van den Berg JWO, van Deenen LLM. Metabolism of red-cell lipids. II Conversions of lysophosphoglycerides. Biochim Biophys Acta. 1965;106:118–127. doi: 10.1016/0005-2760(65)90100-1. [DOI] [PubMed] [Google Scholar]
- Mutch E, Blain PG, Williams FM. Interindividual variations in enzymes controlling organophosphate toxicity in man. Hum Exp Toxicol. 1992;11:109–116. doi: 10.1177/096032719201100209. [DOI] [PubMed] [Google Scholar]
- National Research Council. Pesticides in the diets of infants and children. Washington: National Academy Press; 1993. [PubMed] [Google Scholar]
- Neri M, Bonassi S, Knudsen LE, Sram RJ, Holland N, Ugolini D, Merlo DF. Children’s exposure to environmental pollutants and biomarkers of genetic damage. I Overview and critical issues. Mutat Res. 2006;612:1–13. doi: 10.1016/j.mrrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, Bernet T, Garfinkel R, Tu YH, Diaz D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Env Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp-Snijders C, Schouten JA, van Blitterswijk WJ, van der Veen EA. Changes in membrane lipid composition of human erythrocytes after dietary supplementation of (n-3) polyunsaturated fatty acids. Maintenance of membrane fluidity. Biochim Biophys Acta. 1986;854:31–37. doi: 10.1016/0005-2736(86)90061-1. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Casida JE. Lysophospholipase inhibition by organophosphorus toxicants. Toxicol Appl Pharmacol. 2004;196:319–326. doi: 10.1016/j.taap.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Barlow C, Winrow CJ, Sparks SE, Casida JE. Evidence that mouse brain neuropathy target esterase is a lysophospholipase. Proc Natl Acad Sci USA. 2003;100:7983–7987. doi: 10.1073/pnas.1232473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Casida JE. Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants. Toxicol Sci. 2005;86:291–299. doi: 10.1093/toxsci/kfi195. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Liang SN, Fisher KJ, Nomura DK, Casida JE. Each lipase has a unique sensitivity profile for organophosphorus inhibitors. Toxicol Sci. 2006;91:166–172. doi: 10.1093/toxsci/kfj124. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards PG, Johnson MK, Ray DE. Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol Pharmacol. 2000;58:577–583. doi: 10.1124/mol.58.3.577. [DOI] [PubMed] [Google Scholar]
- Ross BM, Kish SJ. Characterization of lysophospholipid metabolizing enzymes in human brain. J Neurochem. 1994;63:1839–1848. doi: 10.1046/j.1471-4159.1994.63051839.x. [DOI] [PubMed] [Google Scholar]
- Rydel TJ, Williams JM, Krieger E, Moshiri F, Stallings WC, Brown SM, Pershing JC, Purcell JP, Alibhai MF. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a ser-asp catalytic dyad. Biochemistry. 2003;42:6696–6708. doi: 10.1021/bi027156r. [DOI] [PubMed] [Google Scholar]
- Selle H, Chapman BE, Kuchel PW. Glycerophosphocholine release in human erythrocytes. 1H spin-echo and 31P-NMR evidence for lysophospholipase. Eur J Biochem. 1993;212:411–416. doi: 10.1111/j.1432-1033.1993.tb17676.x. [DOI] [PubMed] [Google Scholar]
- Shohet SB, Beutler E. The red cell membrane. In: Williams WJ, Beutler E, Erslev AJ, Lichtman MA, editors. Hematology. 3. McGraw-Hill; New York: 1983. pp. 345–353. [Google Scholar]
- Tomlin CDS, editor. British Crop Protection Council. 13. Alton; Hampshire, UK: 2003. The Pesticide Manual. [Google Scholar]
- van Tienhoven M, Atkins J, Li Y, Glynn P. Human neuropathy target esterase catalyzes hydrolysis of membrane lipids. J Biol Chem. 2002;23:20942–20948. doi: 10.1074/jbc.M200330200. [DOI] [PubMed] [Google Scholar]
- Wang A, Dennis EA. Mammalian lysophospholipases. Biochim Biophys Acta. 1999;1439:1–16. doi: 10.1016/s1388-1981(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Wang A, Loo R, Chen Z, Dennis EA. Regiospecificity and catalytic triad of lysophospholipase I. J Biol Chem. 1997;272:22030–22036. doi: 10.1074/jbc.272.35.22030. [DOI] [PubMed] [Google Scholar]
- Wijeyesakere SJ, Richardson RJ, Stuckey JA. Modeling the tertiary structure of the patatin domain of neuropathy target esterase. [(accessed Jan 15, 2007)];Protein J. 2007 doi: 10.1007/s10930-006-9058-8. [Online early access] Published online: Jan 10, 2007. http://dx.doi.org/ [DOI] [PMC free article] [PubMed]
- Wu SY, Casida JE. Subacute neurotoxicity induced in mice by potent organophosphorus neuropathy target esterase inhibitors. Toxicol Appl Pharmacol. 1996;139:195–202. doi: 10.1006/taap.1996.0158. [DOI] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pederson L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199– 209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Deems RA, Dennis EA. Lysophospholipases I and II from P388D1 macrophage-like cell line. Meth Enzym. 1991;197:456–468. doi: 10.1016/0076-6879(91)97171-t. [DOI] [PubMed] [Google Scholar]