Abstract
The effects of topiramate were evaluated in two groups of rats trained to discriminate administration of either 0.4 mg/kg nicotine or 10 mg/kg cocaine from administration of saline under a fixed-ratio 10 schedule of food delivery. Topiramate (1−60 mg/kg, i.p.) did not produce any nicotine- or cocaine-like discriminative effects by itself and did not produce any shift in the dose-response curves for nicotine or cocaine discrimination. Thus, the ability to discriminate nicotine's or cocaine's effects does not appear to be altered by topiramate administration. Also, topiramate, given either alone or in combination with nicotine or cocaine, did not depress rates of responding. These experiments indicate that topiramate does not enhance or reduce the ability of rats to discriminate the effects of nicotine or cocaine.
Keywords: Subjective effects, Drug discrimination, Reward, Nicotine, Rats, Cocaine, Topiramate
1. Introduction
Topiramate is a novel anticonvulsant that is approved by the Food and Drug Administration for the treatment of childhood epilepsy. Topiramate displays a complex pharmacological profile: effects ranging from agonistic properties at GABAA receptors, antagonistic properties at glutamate receptors, inhibition at L-type calcium channels and voltage-dependent sodium channels and activation of potassium conductance (see Johnson, 2004; Shank et al., 2000) and references herein) have been described. Some of these pharmacological targets are critically involved in drug dependence processes (Cornish and Kalivas, 2000; Kenny and Markou, 2004; Markou, 2007; Paterson et al., 2004) and topiramate has been recently studied for the treatment of various types of drug dependence. The initial studies were conducted in alcohol- (Johnson, 2004; Johnson et al., 2003; Johnson et al., 2004; Ma et al., 2006; Rubio et al., 2004; Swift, 2003) and opiate-dependent subjects (Zullino et al., 2002). More recently, topiramate has been suggested as a potential treatment for cocaine (Cubells, 2006; Kampman et al., 2004; Vocci and Elkashef, 2005) or nicotine (Schiffer et al., 2001) dependence. This is particularly important, since there is no current pharmacological treatment available for cocaine dependence and tobacco dependence is the chief preventable cause of death in developed countries (Fiore, 2000).
The efficacy of topiramate for cocaine dependence has been tested in a 13-week, double-blind, placebo-controlled pilot trial involving 40 human subjects (Kampman et al., 2004). Topiramate-treated subjects were more likely to be abstinent from cocaine compared to placebo-treated subjects (Kampman et al., 2004), suggesting that topiramate may be effective for the treatment of cocaine dependence. In another clinical trial examining the effects of topiramate in alcohol dependent subjects, those assigned to topiramate were more likely to quit smoking than those assigned to placebo (19.4 vs 6.9% at week 12) (Johnson et al., 2005). These preliminary findings concerning the potential use of topiramate for the treatment of cocaine and nicotine dependence are encouraging.
Although addictive drugs produce their effects through actions at various receptors in the brain, it is thought that their common effects on the activity of dopaminergic brain reward pathways are primarily responsible for their addictive properties (Koob, 1992; Wise, 2004). It has been suggested that the reduction of activity of mesocorticolimbic dopamine function may account for the effects of topiramate on dependence processes (Johnson, 2004; Schiffer et al., 2001). Surprisingly, in human studies, most of the subjective responses induced by methamphetamine in humans were not affected by topiramate administration (Johnson et al., 2006). Although topiramate administration did not affect craving or reinforcement, it accentuated the appreciation of methamphetamine-induced stimulation and euphoria significantly (Johnson et al., 2006). Topiramate also showed a non significant trend towards mild reductions in positive mood and reinforcement. Therefore, these results suggest that topiramate does not attenuate the positive subjective effects of methamphetamine in humans in the conditions tested (Johnson et al., 2006). Moreover, when topiramate was evaluated in combination with intravenous nicotine in overnight abstinent smokers, it appeared to enhance the ratings of subjective effects of nicotine (Sofuoglu et al., 2006).
The subjective effects of drugs are most frequently assessed in humans through the use of subject self-rating scales or self-reports, but may be evaluated in experimental animals using two-lever drug-discrimination procedures (Le Foll and Goldberg, 2005). For this purpose, rats can be trained under a schedule of food delivery to respond on one lever during sessions following an injection of a particular dose of drug and on another lever during sessions following an injection of saline. This procedure allows assessment of ‘drug-like’ subjective responses by determining whether different drugs or different doses of the training drug result in the animal preferentially pressing the drug-associated lever. This procedure also allows assessment of the effects of drug treatments on the animal's ability to discriminate the effects of administered drug and on non-specific effects of the administered drug on food-maintained behavior. Here, we used the two-lever drug discrimination choice procedure as an animal model for assessing topiramate's ability to alter the psychomotor and subjective effects of nicotine and cocaine.
2. Materials and Methods
Subjects
Male Sprague-Dawley rats (n = 12; 330 − 370g) were obtained from Charles River (Wilmington, MA) and housed in a temperature- and humidity-controlled room. Animals used in this study were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and all experiments were conducted in accordance with the guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse (NIDA), National Institutes of Health and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003). Experiments were conducted during the light phase of a 12-h/12-h light/dark cycle (lights on at 0700 hours). Rats were housed individually and water was available ad libitum. A diet restriction was maintained throughout the study (3 NIH07 biscuits/day). Rats weighed 350 g prior to food restriction and the feeding was adjusted to reduce body weight by no more than 10% per week until reaching the target training weight, which was not less than 85% of its highest recorded body weight prior to food restriction.
Drugs
Nicotine [(−)-nicotine hydrogen tartrate] and topiramate [2,3:4,5-Bis-O-(1-methylethylidene)-36-D-fructo-pyranose sulfamate] were purchased from Sigma Chemical Company (St Louis, Mo., USA). The pH of the nicotine solution was adjusted to 7.0 with dilute NaOH. (−)-Cocaine HCl was obtained from NIDA, NIH (Rockville, MD). Nicotine was administered subcutaneously (s.c.) and cocaine was administered intraperitoneally (i.p.) in a volume of 1.0 ml/kg. All drugs were diluted in saline. All doses of nicotine and topiramate are expressed as mg free base per kg body weight. Doses of cocaine refer to the weight of the salt.
Nicotine discrimination
Apparatus
Twelve standard operant-conditioning chambers (Coulbourn Instruments, Lehigh Valley, PA) were used. Each chamber contained a white house light and two levers, separated by a recessed tray into which a pellet dispenser could deliver 45 mg food pellets (F0021, Bioserv, Frenchtown, NJ). Each press of a lever with a force of 0.4 N or more through 1 mm was recorded as a response and was accompanied by an audible click. The operant-conditioning chambers were controlled by microcomputers using the MED Associates MED-PC software package (MED Associates Inc., East Fairfield, VT).
Procedure
Training of the rats have been previously reported (Le Foll and Goldberg, 2004). Rats were trained, as described previously, under a discrete-trial schedule of food-pellet delivery to respond on one lever after an injection of a training dose of 0.4 mg/kg nicotine or 10 mg/kg cocaine and on the other lever after an injection of 1 ml/kg of saline vehicle (n = 6 for each experiment). For the nicotine discrimination studies, injections of nicotine or saline were given subcutaneously 10 min before the start of the session, whereas for the cocaine discrimination studies the injections were given intraperitoneally 15 min before the start of the session. At the start of the session, a white house light was turned on and in its presence the rats were required to make ten consecutive responses (fixed-ratio 10 schedule of food delivery) on the lever appropriate to the pre-session treatment. The completion of ten consecutive responses on the appropriate lever produced delivery of a 45 mg food pellet and initiated a 45-s time-out during which lever-press responses had no programmed consequences and the chamber was dark. Responses on the incorrect lever had no programmed consequences other than to reset the fixed-ratio requirement on the correct lever. After each time-out, the white house light was again turned on and the next trial began. Each session ended after completion of 20 fixed-ratio trials or after 30 min elapsed, whichever occurred first. Discrimination-training sessions were conducted 5 days per week under a double alternation schedule (i.e. DDSSDDSS etc., D = drug; S = saline). Training continued until there were eight consecutive sessions during which rats completed at least 90% of their responses during the session on the appropriate lever and no more than four responses occurred on the inappropriate lever during the first trial. Test sessions with other doses of nicotine, or cocaine and with topiramate were then initiated.
Test sessions
A range of doses of topiramate was substituted for the training dose of nicotine or cocaine. Topiramate was also administered together with various doses of nicotine or cocaine to assess possible alterations of the dose-response curves for nicotine or cocaine discrimination. Topiramate was administered intraperitoneally 60 minutes before the session. Test sessions were identical to training sessions, with the exception that both levers were active and ten consecutive responses on either one of the two levers resulted in delivery of a food pellet. Switching responding from one lever to the other lever reset the ratio requirement. In a test phase, a single alternation schedule was introduced and test sessions were usually conducted on Tuesdays and Fridays. Thus, a 2-week sequence starting on Monday was: DTSDTSTDST (T = test). In this way, test sessions occurred with equal probability after saline and drug sessions. Test sessions were conducted only if the criterion of 90% accuracy and not more than 4 incorrect responses during the first trial was maintained in the two preceding training sessions.
Data analysis
Two independent measures of behavior were collected in the discrimination study: a measure of discrimination performance expressed as the percentage of nicotine- or cocaine-appropriate responses and a measure of motor performance expressed as response rate. The percentage of nicotine- or cocaine-appropriate responses during each session (training or test) reflected the percentage of the number of responses emitted on the nicotine- or cocaine-appropriate lever relative to the total number of responses emitted on both levers during a session. Response rate (responses/s) during each session was calculated by dividing the total number of responses emitted on both levers during a session by the total session length. Response rate and percentage of nicotine- or cocaine-appropriate responses were individually calculated for each rat and then expressed as group means (±S.E.M.). For the analysis of the discrimination performance of the rats, nicotine- or cocaine-associated lever selection data were excluded from analysis if the response rate of the rat was below 0.5 responses/s (results from 2 testing sessions were excluded from the nicotine-discrimination study but no results were excluded from the cocaine-discrimination study). No generalization to the nicotine-training stimulus was defined as 20% or lower responding on the nicotine- or cocaine-appropriate lever. The doses of nicotine or cocaine predicted to produce 50% of responses on the nicotine- or cocaine-appropriate lever were calculated by linear regression analyses of the log dose-effect function in generalization tests when different doses of nicotine or cocaine were tested in combination with topiramate (using Bioassay software from Med Associates Inc v6.2). These doses were expressed as ED50 values (in mg/kg) with 95% confidence intervals (CI). Analysis of variance (ANOVA) was used to analyze experimental data using Statistica v6 software. Statistical analyses were performed on raw (rate of responding) or transformed (percentage of nicotine-appropriate lever selection) data. Data were considered statistically significant at P<0.05. Two ED50 values were considered statistically different if their 95% confidence limits did not overlap.
3. Results
Establishment of the Drug -Discrimination Baseline
All of the rats reached the final level of accuracy (eight consecutive sessions with at least 90% of the responses on the correct lever and no more than four incorrect responses during the first trial), as previously reported with this procedure (Justinova et al., 2003; Le Foll and Goldberg, 2005; Le Foll et al., 2005). Once the training criterion was reached, discrimination performance during training sessions was maintained with a high degree of accuracy (95−100% responding on the appropriate lever) with both nicotine and cocaine. When the dose of nicotine or cocaine was varied, there was a dose-dependent increase in drug-lever selection with maximal selection at the training dose of nicotine or cocaine (see Fig. 1B and Fig. 2B).
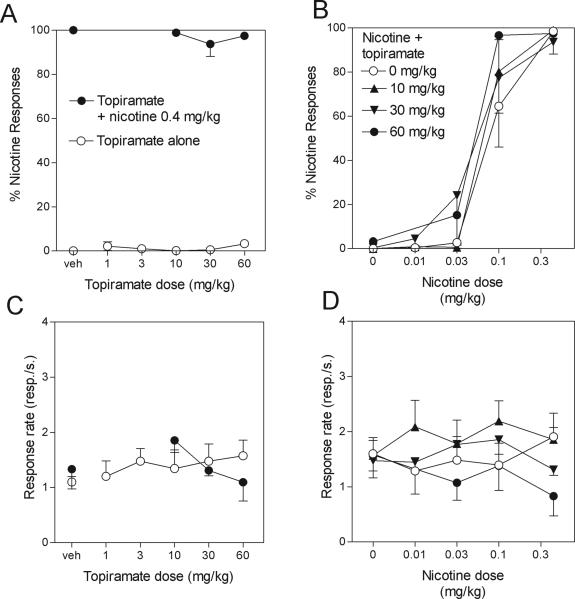
Fig. 1.
Lack of effect of topiramate on nicotine discrimination. Data are means ± S.E.M of the percentage of responses on the lever associated with nicotine administration (upper panels) or rates of responding (lower panels) during the session. The discrimination performance of the rats is indicated in the upper panels Left panels: Topiramate administered alone (A, open symbols) did not produce nicotine-like discriminative effects. Topiramate administered in combination with the training dose of 0.4 mg/kg nicotine (A, filled symbols) did not alter the discrimination performance (A, open symbols). Topiramate alone (C, open symbols) or in combination with nicotine (C, filled symbols) did not affect rates of responding. Right panels: doses of 10, 30 and 60 mg/kg topiramate did not shift the dose-response curve for nicotine discrimination (B) and did not significantly decreased rates of responding of rats when given alone or in combination with nicotine (D). ED50 values with 95% CIs for these dose-response curves are given in Table 1.
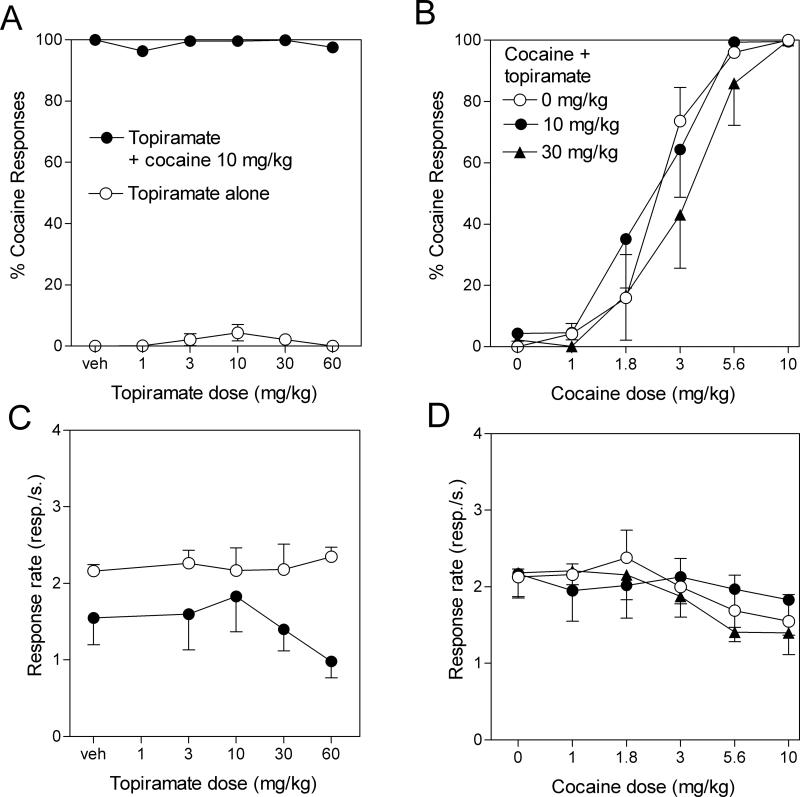
Fig. 2.
Lack of effect of topiramate on cocaine discrimination. Data are means ± S.E.M of the percentage of responses on the lever associated with cocaine administration (upper panels) or rates of responding (lower panels) during the session. The discrimination performance of the rats is indicated in the upper panels Left panels: Topiramate administered alone (A, open symbols) did not produce cocaine-like discriminative effects. Topiramate administered in combination with the training dose of 10 mg/kg cocaine (A, filled symbols) did not alter the discrimination performance (A, open symbols). Topiramate alone (C, open symbols) or in combination with cocaine (C, filled symbols) did not affect rates of responding. Right panels: 10 or 30 mg/kg topiramate did not shift the dose-response curve for cocaine discrimination (B) and did not significantly decreased rates of responding of rats when given alone or in combination with cocaine (D). ED50 values with 95% CIs for these dose-response curves are given in Table 1.
Effects of topiramate on rats trained to discriminate nicotine
a) Generalization Tests (Fig.1, left panels)
The open symbols in the left panels of Figure 1 show the percentage of responses made on the nicotine lever (Fig. 1A) and overall rates of responding (Fig. 1C) during sessions when different doses of topiramate were tested for their ability to substitute for the training dose 0.4 mg/kg of nicotine. Topiramate failed to generalize to the nicotine training stimulus over a large range of doses (less than 5% of responses emitted on the nicotine-associated lever with doses of topiramate ranging from 1 to 60 mg/kg, F5,24 = 1.7, P = 0.18). One-way ANOVA also indicated an absence of effect of topiramate treatment alone on rates of responding (F5,24 = 0.4, P = 0.8).
b) Effect of topiramate on discrimination of the training dose of nicotine (Fig.1, left panels)
The filled symbols in the left panels of Figure 1 show the percentage of responses made on the nicotine-associated lever (Fig. 1A) and overall rates of responding (Fig. 1C) during sessions when different doses of topiramate were tested for their ability to alter the discriminative-stimulus effects of the 0.4 mg/kg training dose of nicotine. Analysis of the discrimination performance of the rats indicates that topiramate did not alter the discriminative-stimulus effects of the 0.4 mg/kg training dose of nicotine, even at the highest (i.e. 60 mg/kg) topiramate dose tested (F3,18 = 0.7, P = 0.5; Fig. 1A). Topiramate given in combination with nicotine slightly depressed rates of responding at high doses, but this effect was not significant (F3,18 = 1.49, P = 0.25; Fig. 1C).
Effects of topiramate on discrimination of various doses of nicotine (right panels Fig. 1)
The right panels of Figure 1 show the effects of 10, 30 and 60 mg/kg topiramate on the dose-response curve for nicotine discrimination (Fig. 1B) and on overall rates of responding (Fig. 1D). A two-way ANOVA analysis of results of discrimination performance indicated a significant effect of nicotine dose (F3,80 = 109, P < 0.0001), no significant effect of topiramate pretreatment (F2,80 = 0.8, P = 0.45), and no significant interaction between topiramate pretreatment and nicotine dose (F11,80 = 0.6, P = 0.8). A two-way ANOVA analysis of results of rates of responding indicated no significant effect of nicotine dose (F3,83 = 0.34, P = 0.8), no significant effect of topiramate pretreatment (F2,83 = 1.2, P = 0.32), and no significant interaction between topiramate pretreatment and nicotine dose (F11,83 = 0.7, P = 0.7). ED50 values for drug-lever selection with 95% CIs are shown in Table 1. The ED50 values overlap confirming that topiramate produced no significant shift of the dose-response curves for nicotine discrimination.
Table 1.
ED50 values (95% CIs) for percentage of drug-lever selection when nicotine or cocaine were administered alone and with various doses of topiramate
| ED50 (95% CI) as mg/kg | |
|---|---|
| Nicotine alone | 0.04 (0.02 − 0.06) |
| Nicotine + 10 mg/kg topiramate | 0.05 (0.02 − 0.08) a |
| Nicotine + 30 mg/kg topiramate | 0.06 (0.01 − 0.1) a |
| Nicotine + 60 mg/kg topiramate | 0.06 (0.04 − 0.08) a |
| Cocaine alone | 0.06 (0.04 − 0.08) |
| Cocaine + 10 mg/kg topiramate | 0.05 (0.03 − 0.07) a |
| Cocaine + 30 mg/kg topiramate | 0.05 (0.02 − 0.07) a |
Overlapping 95% CI compared with the dose-response curves of nicotine or cocaine alone.
Effects of topiramate on rats trained to discriminate cocaine
a) Generalization Tests (Fig. 2, left panels)
The open symbols in the left panels of Figure 2 show the percentage of responses made on the cocaine lever (Fig. 2A) and overall rates of responding (Fig. 2C) obtained during sessions when different doses of topiramate were tested for their ability to substitute for the 10 mg/kg training dose of cocaine. Topiramate failed to generalize to the cocaine training stimulus over a large range of doses (less than 5% of responses emitted on the cocaine-associated lever with doses of topiramate ranging from 3 to 60 mg/kg, F5,30 = 1.45, P = 0.23). One-way ANOVA also indicated an absence of effect of topiramate treatment alone on rates of responding (F5,30 = 0.48, P = 0.8).
b) Effect of topiramate on discrimination of the training dose of cocaine (Fig. 2, left panels)
The filled symbols in left panels of Figure 2 show the percentage of responses made on the cocaine-associated lever (Fig. 2A) and overall rates of responding (Fig. 2C) during sessions when different doses of topiramate were tested for their ability to alter the discriminative-stimulus effects of the 10 mg/kg training dose of cocaine. Analysis of the discrimination performance of the rats indicates that topiramate did not alter the discriminative-stimulus effects of the 10 mg/kg training dose of cocaine, even at the highest topiramate dose tested (F5,30 = 1.04, P = 0.4; Fig. 2A). Topiramate given in combination with 10 mg/kg cocaine did not affect rates of responding compared to cocaine alone, even at high doses (F5,30 = 0.7, P = 0.6; Fig. 2C).
Effects of topiramate on discrimination of various doses of cocaine (right panels Fig. 2)
The right panels of Figure 2 show the effects of 10 and 30 mg/kg topiramate on the dose-response curve for cocaine discrimination (Fig. 2B) and on overall rates of responding (Fig. 2D). A two-way ANOVA analysis of results of discrimination performance indicated a significant effect of cocaine dose (F5,90 = 64.5, P < 0.0001), no significant effect of topiramate pretreatment (F2,90 = 0.95, P = 0.39), and no significant interaction between topiramate pretreatment and cocaine dose (F10,90 = 0.63, P = 0.78). A two-way ANOVA analysis of results of rates of responding indicated a significant effect of cocaine dose (F5,90 = 2.8, P = 0.02), no significant effect of topiramate pretreatment (F2,90 = 0.27, P = 0.76), and no significant interaction between topiramate pretreatment and cocaine dose (F10,90 = 0.3, P = 0.98). However, post-hoc analysis did not reveal significant effect of cocaine administration compared to saline-receiving rats. ED50 values for drug-lever selection with 95% CIs are shown in Table 1. The ED50 values overlap confirming that topiramate produced no significant shift of the dose-response curves for cocaine discrimination. The 60 mg/kg dose of topiramate was not tested against the whole cocaine dose-response curve due to the absence of significant effects of this dose on discrimination performance with the training dose of cocaine and due to the fact that this range of doses is certainly not relevant for human studies evaluating topiramate as a drug candidate for treatment of drug dependence (see discussion below).
4. Discussion
In the present study, topiramate, administered alone, did not produce either nicotine-like or cocaine-like discriminative effects in rats (Fig. 1A and 2A). Over a large range of doses, topiramate also produced no significant change in the discriminative-stimulus effects of the 0.4 mg/kg training dose of nicotine or the 10 mg/kg training dose of cocaine. Finally, when topiramate was administered in combination with different doses of nicotine or cocaine, it did not produce any significant shift of the dose-response curves for nicotine or cocaine discrimination (Table 1 and Fig. 1C and 2C).
This is the first report of the effects of topiramate on nicotine or cocaine discrimination in rats. Clearly, topiramate, over a large range of doses, did not produce any nicotine-like effects in rats trained to discriminate nicotine from saline and did not alter the discriminative stimulus effects of nicotine (Fig.1A). The discriminative-stimulus effects of nicotine are mainly mediated by neuronal nicotinic acetylcholine receptors (Kumar et al., 1987; Pratt et al., 1983; Shoaib et al., 2002; Stolerman et al., 1997; Stolerman et al., 1984), but dopaminergic receptors may be also involved (Desai et al., 2003; Le Foll et al., 2005). The absence of effects of topiramate on nicotine discrimination is in agreement with the absence of any reported pharmacological effects of topiramate on nicotinic systems (Shank et al., 2000). However, there was a slight shift to the left of the nicotine discrimination curve, suggesting a modest potentiation of the discriminative stimulus effects of some doses of nicotine in some rats, but this effect was not significant by post-hoc analysis. Such a shift could reflect a dopaminergic component of the nicotine discriminative-stimulus effect, however, this dopaminergic component is only partial and has been difficult to demonstrate (Corrigall and Coen, 1994; Le Foll et al., 2005) and this appears unlikely since topiramate inhibits nicotine-induced dopamine release (Schiffer et al., 2001).
Some complex effects of topiramate on dopaminergic systems have been described (Eltayb et al., 2005; Schiffer et al., 2001) and it has been suggested that the effects of topiramate on dopamine transmission may mediate its effects on drug dependence (Johnson, 2004). Since the discriminative stimulus effects of cocaine are mediated by mesocorticolimbic dopamine systems (Callahan et al., 1997), we decided to explore the effects of topiramate in rats trained to discriminate cocaine from saline. As with nicotine, topiramate did not produce any cocaine-like discriminative effects and did not significantly alter the discriminative effects of cocaine (Fig. 2 and Table 1). This result is in agreement with the absence of effect of topiramate on dopamine release described in previous studies (Eltayb et al., 2005; Schiffer et al., 2001). We cannot directly compare these results in animals to human results, since no direct evaluation of the effects of topiramate on subjective effects of cocaine has yet been reported. However, since the discriminative effects of cocaine and methamphetamine present many similarities (Justinova et al., 2003), it is of interest to analyze the results obtained with topiramate on various methamphetamine-responses in human subjects (Johnson et al., 2006). This study evaluated several outcomes measures, including a multiple-choice questionnaire (measuring preference for drug over a monetary reward), an end of day questionnaire, a visual analogue scale of methamphetamine effects, a global rating of stimulation, the addiction research center inventory and the profile of mood states questionnaire (Johnson et al., 2006). There was no interaction between topiramate and methamphetamine on these out-comes measures, except on the peak ‘euphoria’ and the peak ‘stimulate’ components of the visual analogue scale for methamphetamine effects. Interestingly, there was no significant effect of topiramate on the peak ‘crave’ component of the visual analogue scale for methamphetamine effects. These results indicate that most of the subjective responses induced by methamphetamine in humans, including the ability to discriminate the effects of methamphetamine administration, are not altered by topiramate and this is in agreement with the absence of topiramate effects on nicotine and cocaine discrimination in the present study.
Since topiramate did not alter rates of responding in the rats, it is important to assess whether topiramate was used at pharmacologically active doses in this study. This was likely the case, since the ED50 values of topiramate for blocking different types of seizure in rodent models of epilepsy have been consistently found a dose of around 7 mg/kg, indicating that we used pharmacologically active doses in the present study (Shank et al., 2000). In rats and mice, anticonvulsant activity is evident within 30 min of administration, peaks 1 to 6 h after dosing, and gradually declines thereafter (Shank et al., 1994). Therefore, our pretreatment time of 60 min was in an appropriate time-range for detecting any effects. It is unlikely that using longer pretreatment time would have resulted in greater effects, since plasma and brain concentrations of topiramate would have been lower (Shank et al., 2000) and a recent study failed to detect motor impairment with high doses of topiramate using various pretreatment times (Alaverdashvili et al., 2005). It is also well known that there is a wide separation between effective anticonvulsant doses compared to doses causing motor impairment (Shank et al., 2000). Importantly, topiramate has been evaluated in clinical trials for drug dependence at doses ranging from 200 to 300 mg daily (Johnson et al., 2005; Johnson et al., 2003; Kampman et al., 2004). It is likely that the blood concentration of topiramate was in the range of 20 μM in those studies, since in a study of 11 patients with epilepsy, the peak and average concentrations in blood plasma were ∼25 and 18 μM after repeated dosing with 200 mg twice daily (Sachdeo et al., 1996). In rats, the plasma concentration of topiramate are ∼30 μM one hour after administration of 10 mg/kg topiramate (Shank et al., 2000). Therefore, it is likely that plasma levels in our experiments with 10 mg/kg topiramate were comparable to those in clinical trials evaluating topiramate for drug dependence.’ .
Interestingly, although considerable overlap exists, there is no direct relationship between the ability of a test drug to produce reinstatement of extinguished drug-seeking behavior and its ability to elicit drug-like, discriminative-stimulus effects (Spealman et al., 1999). This dissociation has been illustrated recently with several distinct pharmacological ligands. For example, ligands blocking dopamine D3 receptors are able to block the influence of nicotine-associated cues on various types of behavior (Le Foll et al., 2003; Le Foll and Goldberg, 2005; Le Foll et al., 2003; Le Foll et al., 2005) without influencing the discriminative-stimulus effects of nicotine (Le Foll et al., 2005). Similarly, ligands blocking cannabinoid CB1 receptors are also able to block the influence of these nicotine-associated cues (Cohen et al., 2005; Cohen et al., 2002; Le Foll and Goldberg, 2004, 2005) without altering the discriminative-stimulus effects of nicotine (Cohen et al., 2002; Le Foll and Goldberg, 2004)) (see (Le Foll and Goldberg, 2005; Le Foll et al., 2005; Le Foll et al., 2000; Newman et al., 2005; Sokoloff et al., 2006) for reviews on the role of dopamine D3 and cannabinoid CB1 receptors in drug addiction). Therefore, the inability of topiramate to block or enhance the discriminative-stimulus effects of nicotine or cocaine is not in contradiction with its apparent ability to enhance relapse and drug-seeking behavior in animals and humans.
In conclusion, this study is one of the few exploring the effects of topiramate on operant responding. The present findings provide evidence that topiramate does not possess any nicotine-like or cocaine-like discriminative effects of its own and does not significantly reduce or enhance the subjective effects of nicotine or cocaine assessed with drug-discrimination procedures in rats. It should be noted however, that rats can be trained to discriminate other doses of nicotine or cocaine than used in the present study and that we cannot rule out potential effects of topiramate under other training conditions (Stolerman et al., 1984; Terry et al., 1994). However, the dissociation that exists between the ability of drugs to interfere with motivational properties of drugs of abuse, without altering their subjective effects, suggests that studies of topiramate's effects on relapse and drug-taking and drug-seeking behavior should continue in animals and humans to further explore its potential as a treatment for cocaine and nicotine dependence.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH, DHHS. We thank Eric Thorndike for the programming. BLF is supported by a New Investigator Award from CIHR-TUSP program.
References
- Alaverdashvili M, Kubova H, Mares P. Motor performance and behavior of immature rats are not compromised by a high dose of topiramate. Epilepsy Behav. 2005;7:222–230. doi: 10.1016/j.yebeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Callahan PM, de la Garza R, Cunningham KA. Mediation of the discriminative stimulus properties of cocaine by mesolimbic dopamine systems. Pharmacol. Biochem. Behav. 1997;57:601–607. doi: 10.1016/s0091-3057(96)00434-0. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-Associated Cues Maintain Nicotine-Seeking Behavior in Rats Several Weeks after Nicotine Withdrawal: Reversal by the Cannabinoid (CB(1)) Receptor Antagonist, Rimonabant (SR141716). Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Dopamine mechanisms play at best a small role in the nicotine discriminative stimulus. Pharmacol Biochem Behav. 1994;48:817–820. doi: 10.1016/0091-3057(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Cubells JF. Topiramate for cocaine dependence. Curr Psychiatry Rep. 2006;8:130–131. doi: 10.1007/s11920-006-0011-5. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 2003;167:335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Eltayb A, Wadenberg ML, Schilstrom B, Svensson TH. Topiramate augments the antipsychotic-like effect and cortical dopamine output of raclopride. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:195–202. doi: 10.1007/s00210-005-0014-5. [DOI] [PubMed] [Google Scholar]
- Fiore MC. US public health service clinical practice guideline: treating tobacco use and dependence. Respir Care. 2000;45:1200–1262. [PubMed] [Google Scholar]
- Johnson BA. Topiramate-induced neuromodulation of cortico-mesolimbic dopamine function: a new vista for the treatment of comorbid alcohol and nicotine dependence? Addict Behav. 2004;29:1465–1479. doi: 10.1016/j.addbeh.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Uses of topiramate in the treatment of alcohol dependence. Expert Rev Neurother. 2004;4:751–758. doi: 10.1586/14737175.4.5.751. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med. 2005;165:1600–1605. doi: 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, et al. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol. 2006:1–14. doi: 10.1017/S1461145705006401. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Swift RM, Ait-Daoud N, DiClemente CC, Javors MA, Malcolm RJ., Jr. Development of novel pharmacotherapies for the treatment of alcohol dependence: focus on antiepileptics. Alcohol Clin Exp Res. 2004;28:295–301. doi: 10.1097/01.alc.0000113409.47937.6c. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, Antoniou K, Solinas M, Pappas LA, et al. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dopamine, addiction and reward. Sem. Neurosci. 1992;4:139–148. [Google Scholar]
- Kumar R, Reavill C, Stolerman IP. Nicotine cue in rats: effects of central administration of ganglion-blocking drugs. Br J Pharmacol. 1987;90:239–246. doi: 10.1111/j.1476-5381.1987.tb16845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioural sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotineconditioned place preferences. Neuroreport. 2004;15:2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J. Pharmacol. Exp. Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol Sci. 2005;26:287–293. doi: 10.1016/j.tips.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Ethanol does not affect discriminative-stimulus effects of nicotine in rats. Eur J Pharmacol. 2005;519:96–102. doi: 10.1016/j.ejphar.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. Dopamine D3 receptor and drug dependence: effect on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Schwartz J-C, Sokoloff P. Dopamine D3 receptor agents as potential new medications for drug addiction. Eur Psychiatry. 2000;15:140–146. doi: 10.1016/s0924-9338(00)00219-4. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Schwartz J-C, Sokoloff P. Disruption of nicotine conditioning by dopamine D3 receptor ligands. Molecular Psychiatry. 2003;8:225–230. doi: 10.1038/sj.mp.4001202. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561–1568. doi: 10.1111/j.1360-0443.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- Markou A. Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol Psychiatry. 2007;61:17–22. doi: 10.1016/j.biopsych.2006.03.053. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academy Press; Washington, DC, USA: 2003. [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 Receptor Partial Agonists and Antagonists as Potential Drug Abuse Therapeutic Agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABA(B) receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C. Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology (Berl) 1983;81:54–60. doi: 10.1007/BF00439274. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Jimenez-Arriero MA, Palomo T, Manzanares J, Ferre F. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37:37–40. doi: 10.1055/s-2004-815473. [DOI] [PubMed] [Google Scholar]
- Sachdeo RC, Sachdeo SK, Walker SA, Kramer LD, Nayak RK, Doose DR. Steady-state pharmacokinetics of topiramate and carbamazepine in patients with epilepsy during monotherapy and concomitant therapy. Epilepsia. 1996;37:774–780. doi: 10.1111/j.1528-1157.1996.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Gerasimov MR, Marsteller DA, Geiger J, Barnett C, Alexoff DL, et al. Topiramate selectively attenuates nicotine-induced increases in monoamine release. Synapse. 2001;42:196–198. doi: 10.1002/syn.10000. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl 1):S3–9. [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Vaught JL, Davis CB, Schupsky JJ, Raffa RB, et al. Topiramate: preclinical evaluation of structurally novel anticonvulsant. Epilepsia. 1994;35:450–460. doi: 10.1111/j.1528-1157.1994.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Mouratidis M, Kosten T. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2006;184:645–651. doi: 10.1007/s00213-005-0296-9. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. Current Drug Targets - CNS & Neurological Disorders. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Swift RM. Topiramate for the treatment of alcohol dependence: initiating abstinence. Lancet. 2003;361:1666–1667. doi: 10.1016/S0140-6736(03)13378-8. [DOI] [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Zullino DF, Cottier AC, Besson J. Topiramate in opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1221–1223. doi: 10.1016/s0278-5846(02)00251-8. [DOI] [PubMed] [Google Scholar]