Summary
Objective
The purpose of this study was to define the cardiovascular abnormalities present in young and adult cats affected with the lysosomal storage diseases mucopolysaccharidosis (MPS) I and MPS VI.
Method
Eighteen cats affected with MPS I and fifteen cats affected with MPS VI were evaluated by physical examination, electrocardiography and echocardiography. Electrocardiograms were performed on all MPS I and all but 7 of the MPS VI cats. Ten unaffected cats underwent complete examinations for comparison purposes.
Results
No cardiovascular physical examination abnormalities were noted. ECG intervals were normal in affected cats; however, changes consistent with aberrant conduction were noted more frequently than in unaffected cats. Significant echocardiographic abnormalities included valve thickening and regurgitation (aortic and mitral) and aortic root dilation, particularly in the older cats.
Conclusion
As affected animals increased in age, more cardiac abnormalities were found with increasing severity. MPS I and MPS VI cats have similar cardiovascular findings to those seen in children and MPS VII dogs.
Keywords: mucopolysacharidosis, valve disease, animal models
Introduction
Degenerative valve disease is the most common pathologic valve disease in the USA and Europe and valves on the left side of the heart are most often involved (3). Animal models of mitral regurgitation (MR) are useful to study disease pathogenesis and develop therapies. In particular, large animal models such as dogs and cats are optimal because their body size more closely resembles humans.
The mucopolysaccharidoses (MPS) are a family of lysosomal storage diseases resulting from defective catabolism of glycosaminoglycans (GAGs) by 1 of 11 enzymes. In children affected with MPS, the most common cardiovascular lesion is thickening of the mitral valve secondary to GAG accumulation in the valve leaflets resulting in fibrosis and nodular deformation (14). Lesions are progressive with risk of death as a result of congestive heart failure (4). The overall incidence is 1 in every 27,000 live births (12).
Cardiovascular abnormalities seen by echocardiography have been reported previously in a colony of MPS VII (β–glucuronidase-deficient) dogs including: mitral valve thickening and regurgitation, increased aortic dimensions, and thickening and regurgitation of the aortic valves (12,14,15), and similar findings were noted in cats affected with MPS I and VI on post mortem examination (9,10), but ante mortem findings were not described. The purpose of this study was to compare electrocardiographic and echocardiographic data from young and old MPS I and MPS VI cats to those obtained from unaffected cats.
Methods
Colonies of cats affected with MPS I and VI were established at the University of Pennsylvania, School of Veterinary Medicine. Animals were raised under National Institutes of Health and US Department of Agriculture guidelines for the care and use of animals in research. Eighteen cats affected with MPS I (Hurler, Scheie, Hurler/Scheie syndrome, α–L–iduronidase deficiency) and fifteen with MPS VI (Maroteaux-Lamy syndrome, 4-sulfatase deficiency) were evaluated by physical examination, electrocardiography (9 of the MPS VI cats had ECG) using a Hewlett-Packard Page Writer (Sacramento, CA), and echocardiography using a Philips 7500 Sonos ultrasound machine (Wilmington, MA) and a 12 MHz transducer. Six lead electrocardiograms were obtained and the PR, QRS, and QT intervals and mean electrical axis were measured. Rhythm assessment was also performed. Ten unaffected cats underwent complete cardiac evaluation for comparison purposes.
Complete echocardiograms were obtained (including 2-D, M-mode, and color flow Doppler of all valves) from all but one MPS 1 cat. For the echocardiographic assessment, 22/27 cats were sedated with butorphanol (0.3-0.5 mg/kg) intramuscularly. Two also received diazepam (0.15-0.2 mg/kg) intramuscularly. Sedation was not necessary in any of the unaffected cats. Left ventricular measurements including interventricular diastolic septal thickness, left ventricular diastolic free wall thickness, and diastolic and systolic left ventricular chamber diameter were obtained. The aortic diameter was measured in the short axis at the level of the sinus of Valsalva and in the long axis at the sinotubular junction. Left atrial diameter was measured using a diastolic short axis frame at the level of the aortic valve. All measurements were obtained using the leading edge to leading edge technique as recommended by the American Society of Echocardiography (16). A fractional shortening was calculated to assess systolic function using the equation: (left ventricular diastolic diameter minus left ventricular systolic diameter)/ (left ventricular diastolic diameter). All valves were interrogated with color flow Doppler and pulsed-wave Doppler. A subjective score was assigned for thickness of the mitral and aortic valves, aortic root diameter (assessed at the level of the sinotubular junction from a long-axis plane), and valvular regurgitation (aortic and mitral). The subjective score ranged from 0 to 4 with 0 = normal/none; 4 = severe. Physical examination, ECG and echocardiography were performed in twelve unaffected cats for comparison. To compare disease (MPS VI, MPS I, unaffected cats) and age groups (≤12 months, >12 months) with regard to electrocardiography parameters, a 2-way analysis of variance performed. To adjust for multiple pairwise comparisons the Tukey-Kramer method was used. All analyses were performed using SAS statistical software (Version 9.1, SAS Institute, Cary NC).
Results
Cardiovascular physical examination
No cardiovascular abnormalities were detected on physical examination of normal, affected MPS I, or affected MPS VI cats. However, all but 1 MPS I cat was purring loudly during examination, which would have made auscultation of a cardiac murmur unlikely. In these cats, even the normal heart sounds (S1 and S2) were not detectable over purring sounds. However, femoral pulse quality, mucous membrane color, and capillary refill time were normal in all cats.
Electrocardiography
When less than 12 months of age, MPS I cats had a shorter PR interval (0.07 sec ±0.007) than MPS VI (0.09 sec ±0.001; p=0.01) and unaffected (0.08 sec ±0.005; p=0.01) cats, but there was no difference between MPS VI and normal cats, nor was there a difference in older cats. No other differences in ECG intervals were significant. Three of the MPS I cats and six of the MPS VI cats had a sinus arrhythmia, however a regular sinus rhythm was present in all twelve unaffected cats (p=.0004). The mean electrical axis was deviated anteriorly in three of the 12 normal cats, four of the 18 MPS I cats, and four of the nine MPS VI cats, which was not significantly different. Three of the MPS I cats had QRS notching, but none of the MPS VI or normal cats did. Electrocardiographic interval measurements are presented in Table 1.
Table 1.
Electrocardiographic interval measurements in MPS I and MPS VI cats. All of the measurements were within normal limits for this species.
| Age (mo) | N | HR (bpm) | PR (sec) | QRS (sec) | QT (sec) | |
|---|---|---|---|---|---|---|
| Unaffected | ≤ 12 | 6 | 171 ±26 | 0.08 ± 0.005** | 0.05 ± 0.005 | 0.18 ± 0.024 |
| > 12 | 6 | 198 ± 25 | 0.07 ± 0.007 | 0.04 ± 0.005 | 0.17 ± 0.017 | |
| MPS I | ≤ 12 | 8 | 211 ± 18 | 0.07 ± 0.007* | 0.03 ± 0.008 | 0.15 ± 0.015 |
| > 12 | 10 | 214 ± 36 | 0.07 ± 0.008 | 0.04 ± 0.012 | 0.16 ± 0.027 | |
| MPS VI | ≤ 12 | 4 | 195 ± 25 | 0.09 ± 0.011** | 0.04 ± 0.010 | 0.18 ± 0.016 |
| > 12 | 5 | 198 ± 33 | 0.08 ± 0.013 | 0.04 ± 0.006 | 0.15 ± 0.062 | |
(p<0.05 MPS I* vs MPS VI**; p<0.05 MPS I* vs. normal**)
ECG measurements [mean and (standard deviation)]
Normal feline ECG ranges: HR- 160-240 bpm; PR-0.05-0.09 sec; QRS- maximum of 0.04 sec; QT- 0.12-0.18 sec.
Echocardiography
Echocardiographic measurements obtained that were within normal limits for the species (data not shown) (2) included the left ventricular systolic and diastolic chamber diameters, diastolic wall thickness, and left atrial diameter. Significant findings included aortic enlargement, mitral and aortic valve thickening. Aortic diameters were measured in the short axis at the level of the sinus of Valsalva (Figure 1A) and in the long axis at the sinotubular junction (Figure 1B). No difference was found between young and old unaffected cats at any time point (Figures 2 and 3). However, when older than 12 months of age, the aorta measured significantly larger in MPS VI cats (1.2 cm) than in unaffected cats (0.81 cm) at the sinusof Valsalva level (p =.003). At the sinutubular junction older MPS I (1.0 cm) and MPS VI (0.96 cm) affected cats were significantly dilated compared to unaffected cats (0.72 cm; p=.0009 for NR vs. MPS I; p=.02 for NR vs. MPSVI) (see Figure 2).
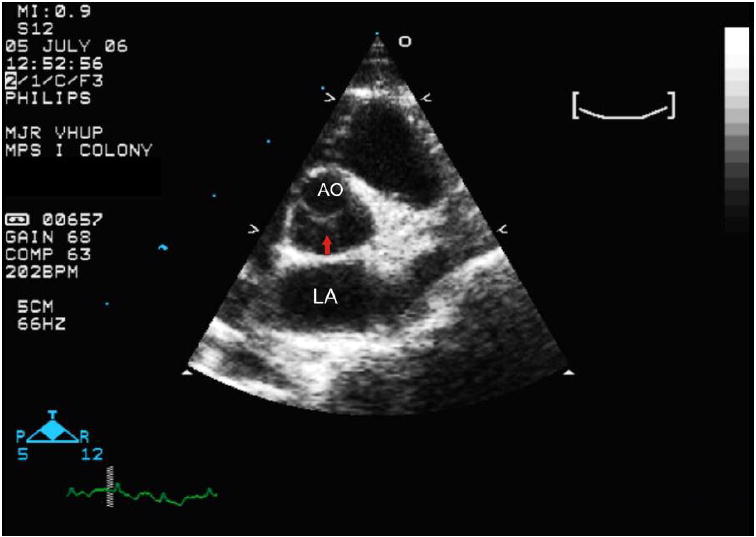
Figure 1.
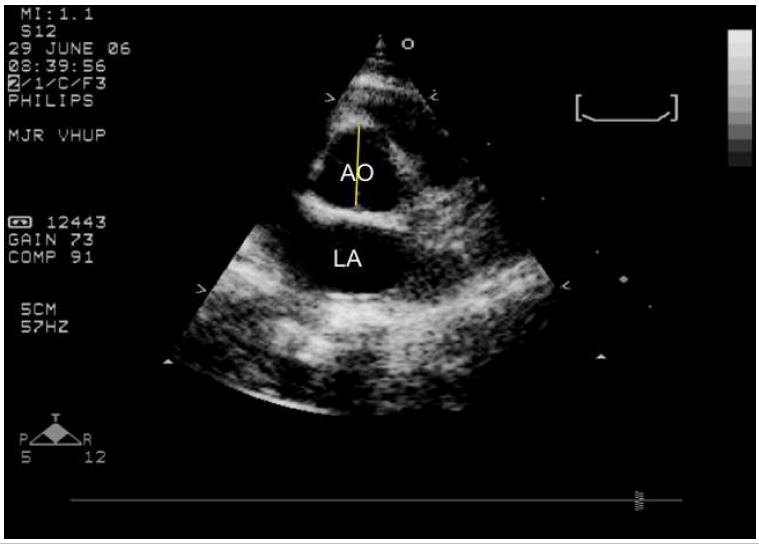
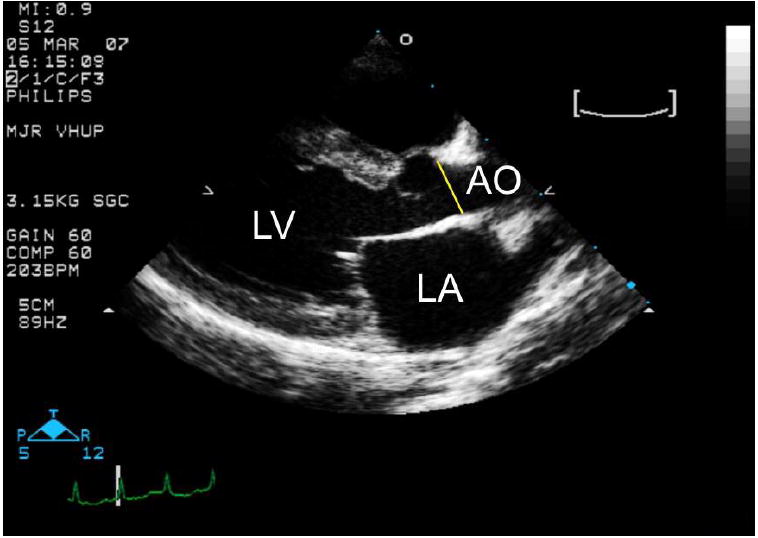
- A short axis right parasternal echocardiogram from an unaffected cat showing where the aortic diameter is measured at the level of the aortic valve (yellow line). AO= aorta; LA= left atrium.
- A long axis right parasternal echocardiogram from an unaffected cat showing the sinotubular junction, the aortic region most frequently dilated in cats with MPS I and VI. AO= aorta; LA= left atrium.
Figure 2.
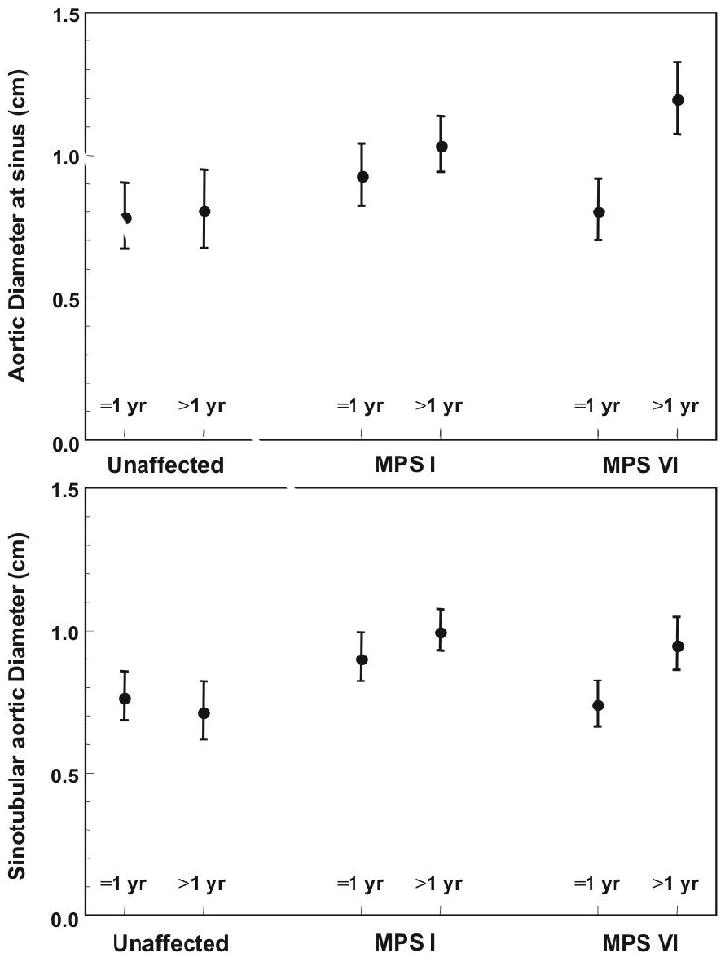
- Aortic dilation was present in MPS VI cats at 1 year of age compared to unaffected cats of the same age when measured at the sinus of Valsalva (p=.003)
- Aortic dilation was present in MPS I (p=.0009) and MPS VI (p=.02) cats at 1 year of age compared to unaffected cats of the same age when measured at the sinotubular junction.
Figure 3.
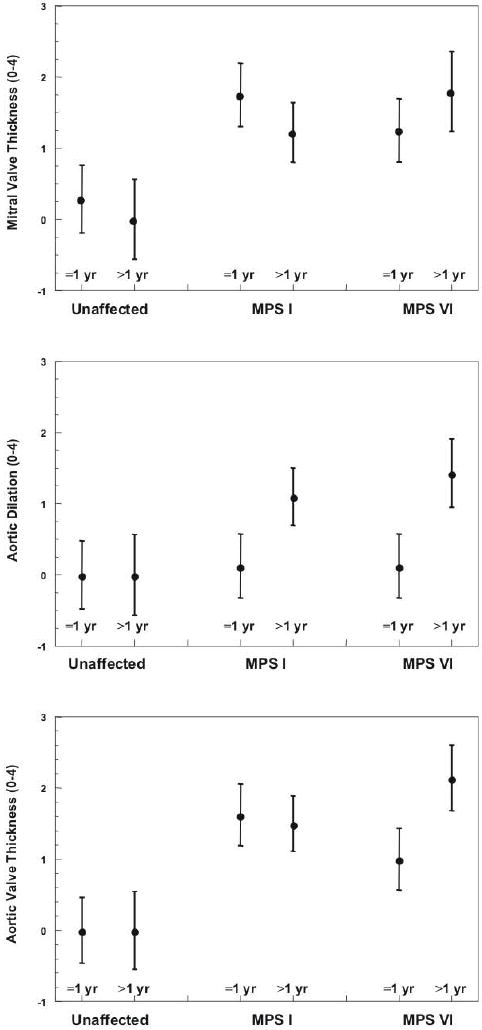
- Significant mitral valve thickening was present in young (p=.001) and old (p=.02) MPS I cats compared to unaffected cats of the same age. Significant valve thickening was present in older MPS VI cats compared to older unaffected cats (p=.001).
- Significant aortic valve thickening was present in young (p=.0002) and old (p=.001) MPS I cats and young (p=.04) and old (p<.0001) MPS VI cats compared to unaffected cats of the same age. Thickening worsened with age in the MPS VI cats (p=.01).
- Significant aortic dilation was present in older MPS I (p=.04) and MPS VI (p=.0007) cats compared to unaffected cats. Dilation was significantly worse in older affected cats of both groups (MPS I p=.03; MPS VI p=.005).
Subjective echocardiographic assessments were scored from 0 (normal or none) to 4 (severe). Significant mitral valve thickening was noted in young MPS I (1.75) versus unaffected cats (0.29; p=.001), and in both affected groups when older than 1 year of age (unaffected cats: 0; MPS I: 1.5; MPS VI: 1.3; p=.02 for NR vs. MPS I; p=.001 for NR vs. MPS VI). Significant aortic valve thickening was present in both groups of young, affected cats (unaffected cats: 0; MPS I: 1.56; MPS VI: 1.0; p=.0002 for NR vs. MPS I; p=.04 for NR vs. MPS VI). Older affected cats also had significant thickening (unaffected cats: 0; MPS I: 1.5; MPS VI: 2.1; p=.001 for NR vs. MPS I; p<.0001 for NR vs. MPS VI) and thickening worsened with age in the MPS VI cats (p=.01). Subjective aortic dilation scores in all groups of young cats were similar (unaffected: 0; MPS I: 0.13; MPS VI: 0.13, however aortic dilation was significantly larger in the older affected groups compared to the unaffected cats (unaffected: 0; MPS I: 1.1 [p=.04]; MPS VI: 1.43 [p=.007]. Moreover, dilation worsened with age in MPS I (p=.03) and MPS VI (p=.005) (see Figure 3). An aortic aneurysm was noted in the ascending aorta of one MPS 1 cat evaluated at age 2.5 years (Figure 4). No significant difference between groups was found for mitral or aortic regurgitation (data not shown).
Figure 4.
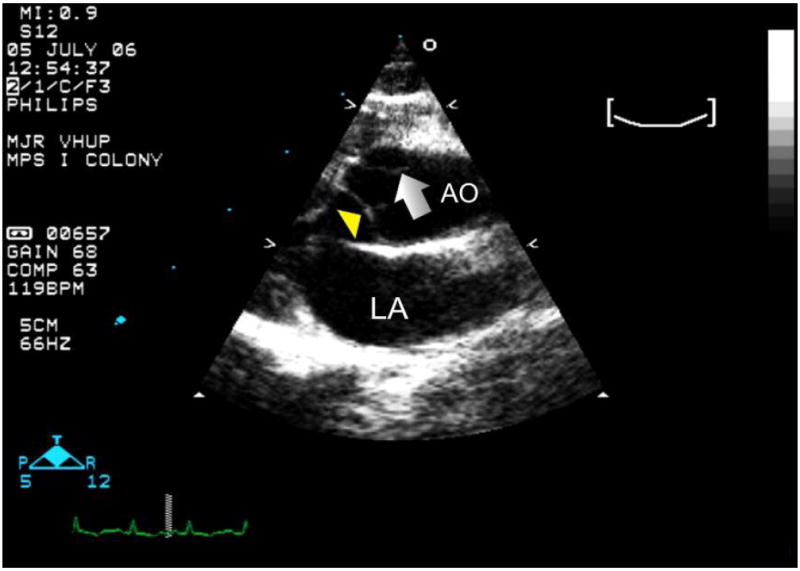
A long axis right parasternal echocardiogram obtained at the heart base which demonstrates an aortic aneurysm (arrow) detected in a 2.5 year old cat with MPS I. AO= aorta; LA= left atrium; arrow head = aortic valve.
Conclusions
The mucopolysaccharidoses (MPS) are a family of lysosomal storage diseases resulting from defective catabolism of glycosaminoglycans (GAGs). The disease occurs naturally in the mouse, rat, dog, cat, goat, and emu (5). Cardiovascular abnormalities seen by echocardiography have been reported previously in a colony of MPS VII (ß–glucuronidase-deficient) dogs and included: mitral valve thickening and regurgitation, increased aortic dimensions in both the long and short axes, and thickening of the aortic valves (12,14). Because the disease phenotype is very similar to that seen in children, dogs and cats represent excellent models to assess therapeutic efficacy of various techniques. Studies have already evaluated therapeutic approaches (11,13,15) and physical, biochemical, and pathological findings in affected cats have been previously described (7,9,10). One study suggests that the feline model may be particularly useful for predicting an approach around a potential immune response to gene therapy (13). Moreover, cats affected with MPS I and VI survive comfortably for more than five years, even without treatment, which allows their use in chronic therapeutic trials (7). However, although several advantages have been recognized in the feline model and clinical cardiovascular abnormalities have been thoroughly reported in dogs with MPS VII, this study represents the first complete description regarding the cardiovascular lesions present in MPS I and VI cats.
Although the measured PR interval was shorter in the young MPS I group compared to the young MPS VI and unaffected groups (table 1), it and all other measured intervals were within the normal range for the species. The difference is believed to be clinically insignificant and the difference was not present in older animals. Noted significant ECG abnormalities in affected cats related to intra-cardiac conduction and included the presence of sinus arrhythmia and aberrant ventricular conduction (notched QRS morphology). The significance of these changes is unclear. Aberrant conduction was not associated with obvious echocardiographic or gross pathologic myocardial thickening or scarring, but it is possible myocardial GAG storage affected the cardiac conduction system resulting in aberrant conduction. Further histological evaluation, particularly focusing on the cardiac conduction pathway, is warranted. Electrocardiographic changes have been reported in one relatively large study of MPS VI children with 74% having ECG changes (11% with a sinus arrhythmia, and 54% with ECG changes consistent with aberrant conduction) (1). The reasons for ECG changes in affected humans have not been elucidated.
Echocardiographic findings are consistent with previous pathologic reports of affected cats (7,10). Specifically, aortic and mitral valve thickening (Figure 5A and B) and aortic dilation was often present and appeared to increase in severity with age. Similar findings have also been reported in MPS VII dogs (8,12). The only cat noted to have an aortic aneurysm was an MPS 1 animal examined at a relatively older age. Aortic aneurysms in the pediatric population are rare; however, they have been reported in a child affected with an unclassified MPS (6). MPS was presumed because chondroitin sulfate and heparan sulfate were detected with urine electrophoresis, but a definitive diagnosis of MPS type was not reported. Ours is the first aortic aneurysm reported in a dog or cat with MPS.
Figure 5.
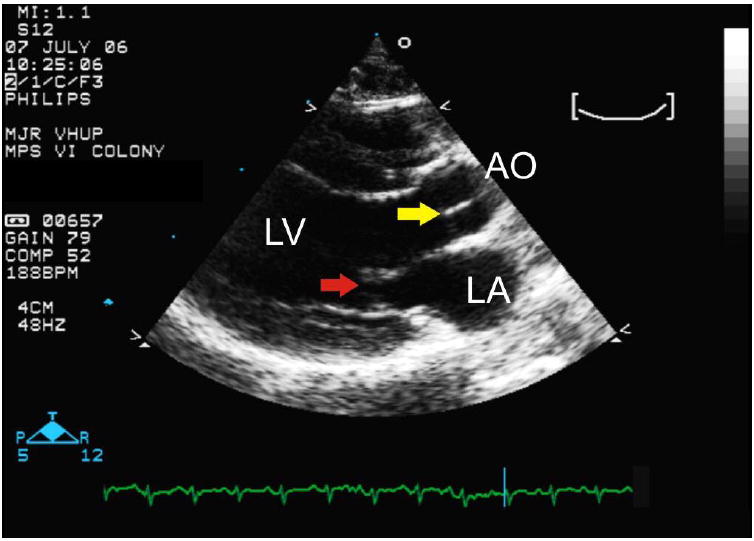
- A right parasternal short axis echocardiogram obtained at the heart base. Note the severely thickened aortic valve (arrow) compared to the unaffected cat in figure 1A. AO= aorta; LA= left atrium.
- A right parasternal long axis echocardiogram. Note the thickening of the mitral (red arrow) and aortic (yellow arrow) valves compared to the unaffected cat in figure 1B. AO= aorta; LA= left atrium; LV= left ventricle.
As previously reported, affected cats can survive to an age which allows them to be useful models for therapy of lysosomal storage diseases. Affected animals may also be useful to study therapeutic and interventional approaches to primary valve disease because of the relatively slow progression of systemic disease and the unique involvement of valves which commonly are affected in humans. However, further studies evaluating progression of MPS in older cats are necessary to determine if affected cats are a good model for valve repair approaches.
Acknowledgments
NIH grants DK25759, RR02512.
References
- 1.Azevedo AC, Schwartz IV, Kalakun L, Brustolin S, Burin MG, Beheregaray AP, Leistner S, Giugliani C, Rosa M, Barrios P, Marinho D, Esteves P, Valadares E, Boy R, Howovitz D, Mabe P, da Silva LC, de Souza IC, Ribeiro M, Martins AM, Palhares D, Kim CA, Giugliani R. Clinical and biochemical study of 28 patients with mucopolysaccharidosis type VI. Clin Genet. 2004;66(3):208–213. doi: 10.1111/j.1399-0004.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 2.Boon JA. Manual of Veterinary Echocardiography. 1. Williams and Wilkins; Philadelphia: 1998. pp. 35–128. [Google Scholar]
- 3.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47(8):1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dangel JH. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders. Acta Paediatr. 1998;157:534–538. doi: 10.1007/s004310050872. [DOI] [PubMed] [Google Scholar]
- 5.Ellinwood NM, Vite CH, Haskins ME. Gene therapy for lysosomal storage diseases: The lessons and promise of animal models. J Gene Med. 2004;6(5):481–506. doi: 10.1002/jgm.581. [DOI] [PubMed] [Google Scholar]
- 6.Engle J, Safi HJ, Abbassi O, Iliopoulos DC, Dorsay D, Cartwright J, Jr, Weilbaecher D. Mucopolysaccharidosis presenting as pediatric multiple aortic aneurysm: First reported case. J Vasc Surg. 1997;26(4):704–710. doi: 10.1016/s0741-5214(97)70074-0. [DOI] [PubMed] [Google Scholar]
- 7.Haskins ME, Aguirre GD, Jezyk PF, Desnick RJ, Patterson DF. The pathology of the feline model of mucopolysaccharidosis I. Am J Pathol. 1983;112(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Haskins ME, Jezyk PF, Desnick RJ, McGovern MM, Vine DT, Patterson DF. Animal models of mucopolysaccharidosis. Prog Clin Biol Res. 1982;94:177–201. [PubMed] [Google Scholar]
- 9.Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson D F. Alpha-L-iduronidase deficiency in a cat: A model of mucopolysaccharidosis I. Pediatr Res. 1979;13(11):1294–1297. doi: 10.1203/00006450-197911000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson DF. Mucopolysaccharidosis in a domestic short-haired cat--a disease distinct from that seen in the siamese cat. J Am Vet Med Assoc. 1979;175(4):384–387. [PubMed] [Google Scholar]
- 11.Kakkis ED, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, Hasson CW, O’Malley T, Weil MA, Aguirre GA, Brown DE, Haskins ME. Enzyme replacement therapy in feline mucopolysaccharidosis I. Mol Genet Metab. 2001;72(3):199–208. doi: 10.1006/mgme.2000.3140. [DOI] [PubMed] [Google Scholar]
- 12.Ponder KP, Melniczek JR, Xu L, Weil MA, O’Malley TM, O’Donnell PA, Knox VW, Aguirre GD, Mazrier H, Ellinwood NM, Sleeper M, Maguire AM, Volk SW, Mango RL, Zweigle J, Wolfe JH, Haskins ME. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci USA. 2002;99(20):13102–13107. doi: 10.1073/pnas.192353499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponder KP, Wang B, Wang P, Ma X, Herati R, Wang B, Cullen K, O’Donnell P, Ellinwood NM, Traas A, Primeau TM, Haskins ME. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-ig. Mol Ther. 2006;14(1):5–13. doi: 10.1016/j.ymthe.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Sammarco C, Weil M, Just C, Weimelt S, Hasson C, O’Malley T, Evans SM, Wang P, Casal ML, Wolfe J, Haskins M. Effects of bone marrow transplantation on the cardiovascular abnormalities in canine mucopolysaccharidosis VII. Bone Marrow Transplant. 2000;25(12):1289–1297. doi: 10.1038/sj.bmt.1702448. [DOI] [PubMed] [Google Scholar]
- 15.Sleeper MM, Fornasari B, Ellinwood NM, Weil MA, Melniczek J, O’Malley TM, Sammarco CD, Xu L, Ponder KP, Haskins ME. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation. 2004;110(7):815–820. doi: 10.1161/01.CIR.0000138747.82487.4B. [DOI] [PubMed] [Google Scholar]
- 16.Weyman AE. Principles and Practice of Echocardiography. 2. Lea and Febiger; Philadelphia: 1994. p. 127. [Google Scholar]


