Abstract
In neuroscience, combining patch-clamping with protein identification in the same cell is becoming increasingly important to define which subtype or developmental stage of a neuron or glial cell is being recorded from, and to attribute measured membrane currents to expressed ion channels or receptors. Here we describe a protocol to achieve this when studying cells in acute brain slices, which antibodies penetrate poorly into, and for which detergent permeabilization cannot be used when using antibodies that recognize lipid components such as O4 sulfatide. The method avoids the need for resectioning of the electrophysiologically recorded slices. It employs filling of the cell with a fluorescent dye during whole-cell recording, to allow subsequent localization of the cell, followed by fixation and free floating section labelling with up to 3 antibodies, which may recognize membrane, nuclear or cytosolic proteins. With practice, ∼80% of patch-clamped cells can be retrieved and have their proteins identified in this way. The entire protocol can be completed in 3-4 days.
INTRODUCTION
Diversity in the set of neurons in the brain is defined partly by differences in the proteins that they express: for example cortical and hippocampal inhibitory interneurons can be divided into ∼15 subclasses defined by their anatomy, electrophysiological properties dictated by their expression of ion channel proteins, and expression of different calcium binding proteins and neuropeptides1,2. Furthermore, during the development of the nervous system, neurons and glial cells change the pattern of proteins they express. This occurs in the nucleus where changes in transcription factors or calcium-binding protein expression control the production of different cell lineages3,4, in the cytoplasm where calcium-binding proteins become increasingly important to buffer activity-induced rises of [Ca2+]i during development5, and in the cell membrane where the expression of voltage-gated currents6, neurotransmitter transporters7 and transmitter-gated channels8 is altered to support the function of the cell. It is often important, therefore, to define the subtype or developmental stage of a cell being recorded from by characterizing which proteins it expresses.
Single cell PCR, in which mRNA is recovered from a whole-cell pipette used to record from a cell, has been used to correlate cell phenotype with protein expression9-12, but suffers from four disadvantages. First, the technique is technically challenging, owing to the small amount of mRNA retrieved and the fact that during prolonged recording mRNA breakdown may occur. Second, mRNA level may not correlate well with the expression level of proteins. Third, neurons in brain slices are often wrapped by glia, and it is hard to avoid the possibility that some glial cytoplasm is harvested with the neuronal cytoplasm, which may generate false positive results. Finally, false negative results may occur if insufficient mRNA is harvested when the cytoplasm is sucked into the pipette13. An alternative approach is to make mice expressing a fluorescent protein, such as eGFP, under the control of a cell-specific promoter, so that the experimenter can choose to record only from cells of a particular type14-17. However, such mice take time to generate and, unless the construct used to drive eGFP expression recapitulates exactly the control sequences regulating the promoter in vivo, then the eGFP may be expressed in cells in which the promoter is normally inactive18,19.
To overcome these problems it is desirable to combine electrophysiological recording of cells in brain slices with post-recording antibody labelling of the proteins that they express20-22. This offers a major advantage over single cell PCR in that, using specific antibodies, it provides unambiguous identification of protein expression in the recorded cell, and avoids the possibility of contamination from proteins expressed in neighbouring cells. However, although immunolabelling of cells in culture or in thin cryostat sections is straightforward, it is more difficult in the brain slices used for electrophysiology because of their thickness (200-300μm), which limits antibody penetration and can require cryostat resectioning for labelling of cells more than ∼10μm below the surface of the slice20. Furthermore, although detergent such as Triton X-100 is often employed to increase antibody penetration into slices, even when the epitope is extracellular, this is not possible when using antibodies that recognize lipid epitopes such as the sulfatide O4 that defines a developmental stage of oligodendrocytes. Here we describe a protocol which we have applied successfully23, after whole-cell clamping oligodendrocytes, astrocytes and their precursors in brain slices, to label neurotransmitter receptors and myelin basic protein in the cell membrane, the lipid sulfatide O4, the membrane proteoglycan NG2, the cytoplasmic structural protein glial fibrillary acidic protein (GFAP) and the nuclear transcription factor Olig2. The protocol typically allows labelling at a depth of up to 50μm below the surface of the slice.
Although we have so far only employed this protocol to define cell identity and study changes in brain cell properties during development, it could easily be extended to investigate changes induced by pathological conditions such as ischaemia, and applied to other tissues in which electrical recording of cells is performed in tissue slices.
PROTOCOL
MATERIALS
REAGENTS
Pre-made phosphate buffered saline (PBS) tablets to dissolve in distilled water to make 0.1M PBS (Oxoid).
Sodium azide (Sigma).! CAUTION Highly toxic, avoid inhalation and skin contact (as it will compete with O2 for haemoglobin binding sites).
Paraformaldehyde (Sigma).! CAUTION This is a known toxin and carcinogen, avoid inhalation, contact with eyes, skin and mucous membranes.
Triton X-100 (BDH)! CAUTION Triton is a strong detergent, avoid contact with skin and eyes, use gloves.
Serum from the animal species that the secondary antibody was raised in, e.g. goat serum (Vector).
Primary and secondary antibodies (various suppliers, according to antibody used).
Antifade glycerol-based mounting medium, such as Citifluor (Citifluor).
Nail varnish, preferably that which dries in a few seconds (available at the nearest pharmacy).
DAPI (4’,6-diamidino-2-phenylindole, Molecular Probes).
Non-specific immunoglobulins: antibodies not raised against a particular protein, but purified from the blood of the host animal in which the primary antibodies used to label particular proteins are raised (Vector).
Equipment
Borosilicate glass capillaries, 1.5mm outside diameter x 0.86mm inside diameter (i.e. thick walled glass) for small cells, or 1.5mm outside diameter x 1.17mm inside diameter (thin walled glass) for larger cells (Harvard Apparatus), to make pipettes used for patch clamp recordings.
Slice storage chamber, a 150mL glass beaker with a gauze platform in the middle for the slices to be kept on, bubbled gently with 95% O2/5%CO2.
Motorized vibrotome slicer with cooling unit (Campden instruments or Leica).
Cut off glass Pasteur pipette (with the thin end removed) with rubber teat attached to the cut end, used for slice transfer.
Electrophysiology set up: Upright fixed stage differential interference contrast microscope (e.g. Olympus or Zeiss) with 4x and 40x or 60x objectives, halogen lamp and filter sets suitable for the excitation and emission wavelengths of the fluorescence dye used, recording chamber with 1mL volume which fits the stage of the microscope, peristaltic pump or gravity fed perfusion system, patch clamp head stage and amplifier (e.g. Axon), micro-manipulators (e.g. Luigs and Neumann).
Two stage electrode puller, either vertical or horizontal, to make patch pipettes. We use a vertical puller (Narishige, PC-10).
24- or 48-well plates, depending upon the size of the tissue slices. It is better to use the smallest possible well as it will reduce the cost of the experiment. (Cellstar and Costar).
Rotary shaker that can go as slow as 60 rpm.
Paint brushes, as many as the number of antibodies used. These should preferably be made from acrylic fibres as these tend to stick less to the tissue. The size of the paintbrush should be correlated with the size of the tissue. For example, we use brushes from numbers 0-2 for the small cerebellar slices and 1/8” flat ones for cerebral cortical slices.
Confocal microscope (e.g. Zeiss, LSM 510), microscope slides and coverslips (use appropriate thickness for the objective used, VWR).
An opaque (black) box in which the 24- or 48-well plate can be placed. If no suitable opaque box is available, a transparent box can be covered in aluminium foil to reduce bleaching of the dyes used for cell filling and antibody labelling.
REAGENT SETUP
Intracellular dye-filling recording solution
Add a fixable fluorescent dye into an internal solution that fits your experimental purpose the best, typically a K+ -based internal for mimicking physiological conditions or a Cs+ -based internal to improve voltage-clamp quality23. The dye that we have mostly used is a fixable Lucifer yellow (Lucifer yellow CH), as it is economical and works satisfactorily. However, we have also used biocytin (Vector, especially to study gap junctional coupling) or the Alexa dyes (Alexa 488 or 568, Molecular Probes) with equal success. When using 2mM Lucifer yellow CH (1 mg/ml), add to the internal solution of interest. Sonicate to dissolve the dye, and filter if necessary. The Alexa dyes from Molecular Probes are expensive but very bright, and so can be used at low concentration (0.2 mg/ml); it is possible to just fill the tip of the electrode with the dye-filled solution (see Procedure, Day 1, for how to do this), and then backfill the pipette with the same internal solution but lacking the dye. Alternatively neurobiotin can be used at 1.5 mg/ml for dye filling, but to study gap junctional coupling use a higher concentration of neurobiotin, e.g. 5-10 mg/ml. CRITICAL The composition of the electrode solution needs to be designed taking into account the osmotic pressure added by the neurobiotin or dye present.
4% paraformaldehyde (PFA) solution
Dissolve 20g PFA in 500 ml of 0.1M PBS slowly on a heated magnetic stirrer, wait till the solution reaches 60°C, then add a few drops of 10M NaOH solution until the PFA solution goes clear. Then filter the solution. Aliquot into 5 ml tubes and freeze at -20°C for up to a year. Thaw on the day of use, and throw away any PFA remaining unused from that aliquot.
1% NaN3 stock solution
Dissolve 10g of sodium azide in 1 l of distilled water.! CAUTION Highly toxic, avoid inhalation or skin contact (as it will compete with O2 for haemoglobin binding sites).
0.1M PBS without and with 0.05% NaN3
Dissolve Oxoid PBS tablets in distilled water (1 tablet per 100 ml). For 0.1M PBS with NaN3, dilute the NaN3 stock solution 20-fold with distilled water and than dissolve the Oxoid tablets in that.
5% Triton X-100 stock solution in PBS
Add 1 ml of Triton X-100 (it is very viscous, so pipette slowly to not generate bubbles) into 19 ml of 0.1M PBS and 0.05% NaN3 solution.! CAUTION Triton is a strong detergent, avoid contact with skin and eyes, use gloves.
Blocking and permeabilizing solution: 10% goat (or other animal) serum, 0.5% Triton X-100, 0.05% NaN3 and 0.1M PBS
For 20 ml add 2 ml of 5% Triton stock solution and 2 ml of goat serum (or the serum from the animal that your secondary antibody was raised in) to 16 ml of 0.05% NaN3/0.1M PBS solution. When labelling lipid antigens like the sulfatide O4 omit the Triton (use 18ml of 0.05% NaN3/0.1M PBS solution). CRITICAL Make up fresh before use.
PROCEDURE
DAY 1: Whole cell recordings, dye filling and fixation
-
1.
Prepare for the electrophysiological part of the experiment, whole-cell recording in acute slices, as normal (e.g. as described in Refs. 24 and 25). Briefly, humanely sacrifice an animal (we use Sprague-Dawley rats, aged postnatal day 7-28) in accordance with local animal welfare regulations. Immerse the head immediately in ice-cold oxygenated slicing medium (normal Krebs’ solution gassed with 95% O2/5% CO2, with the addition of 1mM kynurenic acid to block glutamate receptor-mediated cell damage during the slicing process). Open the skull by cutting between the eyes, then making lateral cuts on either side of the head from the anterior to the posterior. The excised region of the skull is then lifted with forceps, and the whole brain is quickly removed and placed in ice-cold oxygenated slicing medium. Then the brain area of interest is dissected out and superglued onto a block for cutting. Vibrotome sections of 225μm are cut (the vibration amplitude and frequency, and speed of advancing the brain, vary between slicers, and optimal values need to be chosen for each slicer). As soon as the slices are cut, transfer them (with the cut off Pasteur pipette) into the slice storage chamber filled with oxygenated slicing medium, where they are allowed to settle for an hour prior to recording. The slices can be used up to 9 hours after slicing.
-
2.
Take an aliquot of PFA from the freezer and thaw it at room temperature.
-
3.
Pull electrodes that match your purpose the best, making the resistance as large as you think you can get away with without prejudicing satisfactory recording of the membrane currents.
Choice of whole-cell pipette size There is a trade off between obtaining a good access resistance (which requires a large pipette diameter) and keeping the cell soma intact and attached to the rest of the cell after removal of the pipette (which is easiest using small diameter pipettes). For cells which have a soma diameter of ∼5-10 μm (like oligodendrocytes and small neurons), the highest success rate comes from having pipettes with a resistance of 6-7 MΩ. However, cells with larger somata (neurons like Purkinje and pyramidal cells, as well as large astrocytes) can be successfully recorded from and subsequently labelled using larger pipettes, around 2-4 MΩ. When a low series resistance is essential to record large membrane currents, it is possible to use lower resistance pipettes and still leave the cell soma attached to the cell after electrode removal, although the success rate for satisfactory electrode removal is reduced.
-
4.
For recording and dye filling, take a brain slice out of the slice storage chamber, which contains all the cut slices, using the cut off Pasteur pipette, and put it into a recording chamber under the microscope. Continuously perfuse the bath with oxygenated artificial cerebral fluid solution (Krebs’ solution), using gravity or a pump driven perfusion system. The slice can be held in place in the bath by placing on top of it a “harp”, made of a parallel array of nylon threads (separated from each other by 0.5-1.0 mm) strung on a U shape platinum frame. Look at the slice through the microscope at low magnification (4x), make a note of the orientation of the slice for your records (for an example see Fig. 1a, b, and step 8 below) and find your area of interest in the slice. Change to high magnification (40x or 60x) and find the cell of interest in the slice.
-
5.
Fill a pipette with dye-containing solution. If using an Alexa dye or neurobiotin, put the pipette upside down (i.e. pointed end upwards) in an Eppendorf tube with 20μl of the dye solution in it, and the tip will fill up by capillary pressure. Then, by using a cannula attached to a syringe containing internal solution without dye, backfill the pipette with internal solution lacking the dye. For fixable Lucifer yellow (Lucifer yellow CH) just fill the pipette normally, i.e. the dye is in all of the internal solution.
-
6.
Approach cells in the same way as normal, blowing solution out of the pipette to clean the cell surface: we have never found a high background from dye that is blown out of the pipette while approaching the cell of interest, as it is not taken up significantly by neighbouring cells. When the pipette touches the cell of interest, stop blowing and suck a little and at the same time depolarize the cell membrane. Wait until at least a gigohm seal has formed, then apply negative pressure to rupture the cell membrane beneath the pipette and go into whole cell mode. The dye will now diffuse into the cell. In experiments where the wish is just to dye-fill the cell and then proceed with the immunohistochemistry, one should try to keep the recordings short, ∼5 min should be enough. However, in most cases one wants to conduct longer recordings to study the cell’s electrophysiological properties in detail: this is possible, but the longer the recording is, the lower is the success rate for the dye being retained in the cell after removing the pipette from the cell, perhaps because the membrane is more leaky after long recordings. We have, however, kept the success rate quite high even with 1 hour recordings involving exposing the tissue to ischaemia23.
-
7.
Remove the pipette from the cell. CRITICAL STEP The seal resistance determines the best protocol for removing the pipette from the cell soma. The higher the seal resistance, the better is the success rate for removing the pipette without destroying the soma. To start removing the electrode, depolarize the cell to 0mV. With a seal resistance of 10-30 GΩ, the best way to remove the pipette from the soma is by pulling away and lifting the pipette upward at the same time, slowly, as if you were making an outside-out patch. Looking at the cell you should see a patch of membrane sprouting out of the soma and adhering to the pipette, and this thread of membrane will then detach from the soma leaving the cell soma intact. In the case of a lower seal resistance, it is often good to apply some positive pressure to the pipette, as well as wiggling the pipette sideways, and up and down, to loosen the pipette from the soma. In a bad scenario, the cell soma is well attached to the electrode, and the “thread” does not appear: in these extreme cases it is possible to tap lightly on the stage or on the pipette holder to shake the pipette off, however this often leads to the cell body detaching from the cell with the pipette. In some cases with a low seal resistance it is possible to remove the pipette quickly from the cell soma, instead of slowly, however this is risky as it can make the dye leak out. One should try these alternative methods when the first approach is not working.
-
8.
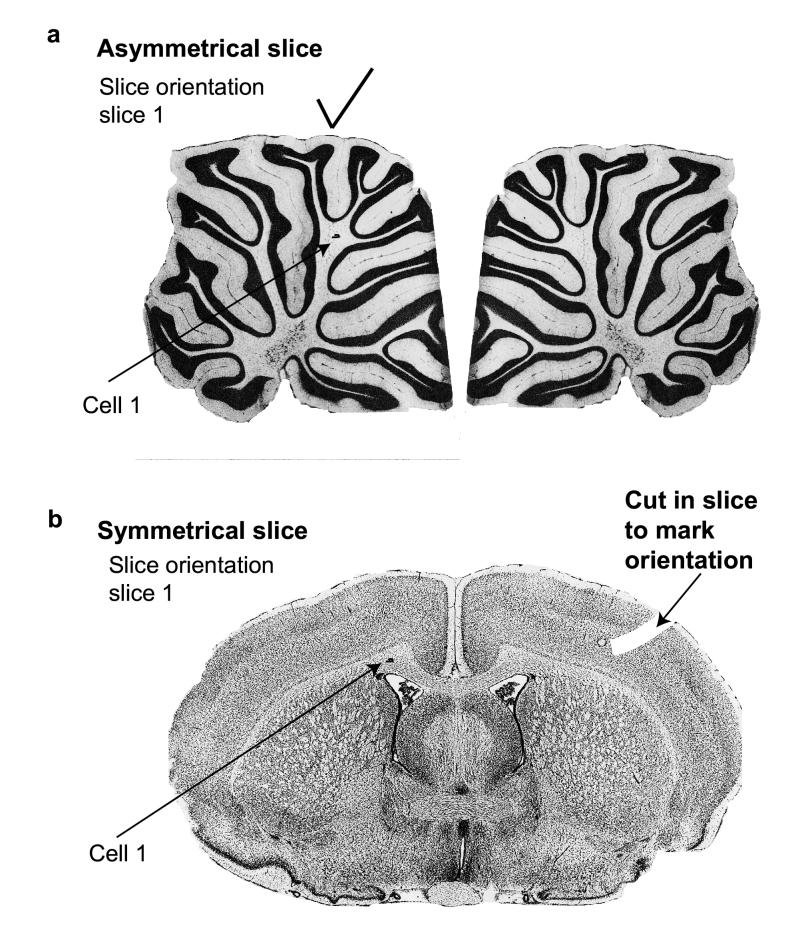
Mark the location of the cell on a map of the tissue slice (for an example see Fig. 1a, b) so that it can easily be found later. The location is best specified using a low power objective. In addition, if recording from more than one cell in the same slice, it is important to sketch the morphology of the cell (as revealed by the dye fill, observed by fluorescence with the slice still on the recording microscope), since marking the location in the slice does not always give enough information to define which cell was which. To be able to locate the cell in the tissue later, it is also very important to mark the orientation of the tissue slice, to know which of the two slice surfaces the cell was recorded from. In the case of an asymmetrical slice, like the cerebellum, mark on a diagram like that in Fig. 1a which side is upwards (i.e. the side from which the electrode was attached) by noting which of the two possible slice shapes is seen when looking down the microscope. This cannot be done for symmetrical slices (e.g. the transverse forebrain slice in Fig. 1b), and then it is important to cut the tissue on one side (for example with a pipette) to introduce an asymmetry, and to mark on a diagram for each slice where the cut is, as seen down the microscope, to define the surface orientation correctly (see Fig. 1b).
-
9.
After drawing the cell and marking the diagram of the slice, put the slice (in extracellular solution) in an empty well of the 24-well plate. Then fill another well in the same plate with 4% PFA solution, and remove the slice from the external solution with a paint brush and put it gently into the PFA. Insert the 24-well plate into the “black box” (see above: so the slice is not exposed to light) and then put it on a rotary shaker with the speed of 60-80 rpm for one hour. Now it is possible to repeat steps 4-9 if recording from more slices on the same day.
CRITICAL STEP Mark the lid of the 24-well plate with the cell number and date over each well where the slices are.
-
10.
At the end of the day, fix some of the remaining (not recorded from) slices that have no filled cells in them to be used as control slices for the immunohistochemistry (see Box 2, and Table 1 for the number of unfilled slices to fix). Mark on the lid of the plate over the well where the control slices are.
PAUSE POINT The slices in PFA can be put in a fridge overnight.
-
11.
After an hour or after overnight in the fridge in PFA, wash the slice(s) 3x15 min in 0.1M PBS made freshly that day. Use a paint brush (dipped previously in PBS, so it is wet and without air bubbles) to transfer the slices between wells.
PAUSE POINT After the last wash, slices can be put in the fridge for up to 5-7 days in 0.05% NaN3 in 0.1M PBS. The longer the slices are kept, the worse the tissue condition gets and this often reduces immunoreactivity and increases background for the antibody labelling. However we found it to be fine for up to 5-7 days. Remember to keep the plate with the slices in the “black box” always.
Figure 1.
Documenting cell position in asymmetrical and symmetrical slices. These figures are examples of those that we use for documenting the exact location of each recorded cell in slices. The patched cell will be near one surface of the slice, which must be identified for correct mounting of the slice for subsequent confocal microscopy. a, For an asymmetrical slice, like the cerebellar slices shown here, the surface orientation (i.e. which side is “up” and nearest the patch electrode) is defined by the shape of the slice, and all that is needed is to choose which of the two possible shapes is relevant: place a tick on the appropriate diagram (e.g. the left one as here) and mark the cell position on the figure so that locating it in the confocal microscope is faster. b, For a symmetrical slice like this transverse forebrain slice, it is necessary to introduce an asymmetry to define the surface orientation – this is done by making a cut in the slice with an electrode. Then the cell position is marked as before. Brain pictures from Ref. 28.
BOX 2: Testing for artefacts: control experiments for antibody non-specificity.
Specificity of the secondary antibodies
a) Are the secondary antibodies specific, i.e. are you sure they do not attach to sites other than the primary antibody? This is checked using a blank control, i.e. labelling the slice without a primary antibody (see Table 1). If this shows the same immunolabelling as the slice with the primary antibody, then the secondary antibody is binding to something other than the primary. There should be very little binding in the blank control. However, in slices fixed after recording (i.e. not from perfusion-fixed animals) the secondary antibodies often (almost always) label blood vessels.
b) The use of more than one primary antibody calls for extra control experiments. In addition to the slices containing filled cells being treated with all the primary antibodies, a set of slices that were not recorded from are labelled with each single primary antibody alone but then treated with all the secondaries. This is to make sure that one secondary antibody is not binding to more than one primary antibody. In that situation one would erroneously get an apparently “perfect” co-localization of the labelling of two antibodies. (Of course, when using multiple primaries, one has to have them raised in different animals, for example mouse, rabbit and sheep, to prevent the secondary recognizing more than one primary).
Specificity of the primary antibodies
c) Antibodies are purified immunoglobulins which have been raised to recognize a certain epitope, however these are big proteins and there is always scope for the protein to get “stuck” to some area in the tissue other than the desired epitope, leading to unspecific staining by the primary antibody. This can be tested for in two ways. First, examine the labelling obtained after preabsorbing the antibody with the peptide to which it was raised (if this is available). We preabsorb in an Eppendorf tube (on a rotary shaker at 60-80 rpm for 2-8 hours), using the peptide at a 5 to 10-fold higher concentration (in g/l) than the antibody: no labelling should be seen after the preabsorption. Alternatively, if no peptide is available, buy purified immunoglobulins (from the same host animals) which have not been deliberately raised against any protein, i.e. just purified IgG or IgM from the blood of the animals. Using these immunoglobulins at the same concentration as the primary antibody tests whether there is some non-specific binding that is inherent to immunoglobulins. If labelling in this slice comes out to be the same as the one with the primary, but the blank slice (to which no primaries were applied) is not the same, then that shows that the primary antibody is not specific (note that similar labelling in the IgG/IgM slice and the experimental slice could reflect non-specificity of either the primary or the secondary antibody, and comparison with the blank slice is necessary to establish where the problem lies).
d) To further test for specificity of the primary antibody one can perform the immunohistochemistry on a tissue where the expression of the protein of interest is known. If the antibody does not label where it should then there is something suspicious going on. In addition, one should test the antibody on a tissue where it is not supposed to be expressed and see if there is still detectable staining, ideally in an animal in which the protein of interest has been knocked out. To start to understand cross-reactivity of primary antibodies, if the antibody has been raised to a defined peptide sequence, then searching for other proteins containing the same peptide sequence in a protein database may provide an explanation for the labelling seen. For further consideration of this issue see Ref. 27.
When should each specificity test be performed?
For each primary antibody, c and d only need to be done once, however a and b should always be performed for every experiment.
Table 1.
Number of extra slices needed for control experiments on secondary antibody specificity (to be done every day)
| Number of antigens to be tested (i.e number of primary antibodies to be used) | Number of unfilled (not recorded from) slices to fix to test the specificity of the secondary antibodies - blank with no primary antibodies added, but all the secondary antibodies added | Number of unfilled slices to fix to test for non-specific binding of the secondary antibodies to the “wrong” primary antibody: with each primary antibody present alone, all the secondary antibodies are added |
|---|---|---|
| 1 | 1 | 0 |
| 2 | 1 | 2 |
| 3 | 1 | 3 |
| 4 | 1 | 4 |
DAY 2: Primary antibody labelling of the slices
-
12.
If continuing without storing slices in the fridge, go straight to step 13. When taking slices from the fridge, wash each slice once in fresh 0.1M PBS for 15 min.
-
13.
For small tissue slices use a 48-well plate and place, into the same number of wells as you have slices, 200-500μl of the blocking and permeabilizing solution, made up fresh. For larger slices use a 24-well plate and place up to 1ml of solution in each well. To transfer the slices from the washing 0.1M PBS to the blocking and permeabilizing solution use a paint brush. Dip the paint brush in PBS to wet the brush, removing all air bubbles that form (as the tissue will adhere to the brush if there are some air bubbles). Then gently fish the slice out of the PBS and immerse it in the well with the blocking solution; continue like this for all the slices. Write on the lid of the plate the date and number of each cell in the slice, also mark where the control slices (described below) are. Put the plate in the “black box” and on a shaker for 4-6 hrs (at 60-80 rpm), depending on the thickness of the slice. For 225μm slices use about 5 hrs.
-
14.
Dilute the primary antibody in 0.1M PBS with 0.05% NaN3 at the concentration for which reliable results have been found (see Box 1 for ?TROUBLESHOOTING the concentration of an antibody). Calculate the amount of antibody containing solution as follows: each well in a 48-well plate needs a minimum of 200μl and each well in a 24-well plate needs at least 500μl of the antibody solution.
-
15.
Prepare also the solutions for the control experiments. Have one blank control, i.e. a slice with no primary antibody to which you will only apply secondary antibody in step 22 (apply all the secondary antibodies if using more than one primary antibody). In addition, when using more than one primary antibody, make a control slice for each primary antibody (to check the specificity of the secondary antibodies), to which that primary and all the secondary antibodies are applied in step 22 (see Box 2, and for more details on the number of control slices to use see Table 1). These controls should be carried out every time an experiment is done. CRITICAL STEP When using several primary antibodies, they have to come from different host animals.
-
16.
If you have not done so before, prepare and include controls for the specificity of the primary antibody. These need only to be done once for a given primary antibody. If the peptide to which the antibody was raised is available, pre-absorb the antibody with that peptide; and/or check whether similar labelling is produced by a non-specific immunoglobulin (i.e. not deliberately raised against any protein of interest in your experiment) which is from the same species as your primary antibody of interest and at the same concentration. This controls for unspecific binding of the immunoglobulin (IgG or IgM) molecule: for more details see Table 2 and Box 2.
-
17.
Add the antibody mixture into each well where a dye-filled slice will go; for the negative control with no primary antibodies use only 0.05% NaN3 in 0.1M PBS.
-
18.
After incubating the slices in the blocking and permeabilizing solution (step 13), transfer the slices with brushes into a new 24-/48-well plate containing the primary antibody solutions, starting on the negative controls and changing the brush between antibodies if using more than one. Remember to wet the brushes first in PBS. Afterwards, wash the brushes in hot water and rinse in distilled water. Wrap Parafilm around the edges of the 24-/48-well plate to minimize evaporation.
-
19.
Incubate the primary antibody in the “black box” on a shaker (60-80rpm) at room temperature overnight or for 12-15hrs.
BOX 1: Antibody dilutions.
When starting to use a new antibody, first try to either find a previous paper where the antibody has been used on fixed tissue, or get information from your supplier on a suitable dilution range.
a) Based on a previous paper: make 3 dilutions, one 5-10 times more diluted than that which the paper used, one the same, and the third less diluted (e.g. 0.5 times less). If the more diluted solution works, try to dilute it further (as this will save money).
b) Based on supplier information: normally suppliers give a range of dilutions, such as from 1:100-1:500. Again make 3 dilutions, one at the highest of the range of dilution, one at the lowest of the range, and then one 2-fold more diluted than the supplier’s range.
c) Sometimes no information about useful dilutions exists for fixed tissue. If there is information for Western blotting, then use double that concentration and try also two dilutions around that value. So if Western blotting uses 1:1000, then make dilutions of 1:100, 1:500 and 1:1000.
d) If no information exists in any usable form, start with dilutions of 1:50-1:100, 1:500 and 1:1000.
In general when a dilution that works has been found, one could try to dilute slightly, for example if 1:500 worked but not 1:1000, then 1:700 could be tried.
Similar dilution trials can be made for secondary antibodies, but normally we have used the concentrations that the companies specify, although it is possible to dilute them further.
Dilutions and time are interrelated, so with a longer incubation time one can get away with lower concentration of antibodies.
Table 2.
Control experiments to be done once per primary antibody to test its specificity
Suppose you are using 3 primary antibodies , which happen to be an IgG raised in rabbit, an IgM from mouse and an IgG from guinea pig. Obtain peptides for preabsorption and/or non-specific immunoglobulins of the same type and species, and label 3 slices each with them as follows.
| Primary antibody (Ab) | Peptide or immunoglobulin | No. of slices to label |
|---|---|---|
| IgG rabbit | Label after preabsorbing with peptide to Ab | 3 |
| IgM mouse | Label after preabsorbing with peptide to Ab | 3 |
| IgG guinea pig | Label after preabsorbing with peptide to Ab | 3 |
| IgG rabbit | Label with unspecific IgG rabbit | 3 |
| IgM mouse | Label with unspecific IgM mouse | 3 |
| IgG guinea pig | Label with unspecific IgG guinea pig | 3 |
DAY 3: Secondary antibody labelling of the slices
-
20.
Wash the slices 4x20 min in 0.1M PBS. Use a brush to transfer the slices, remembering always to change brush between antibodies and to start on the negative controls, so that there is no contamination of different antibodies between wells. Wash the brushes well between washes, first with hot water and then rinse in distilled water.
-
21.
During the washing make up the secondary antibody solution. See Box 1 for suitable antibody dilutions. Normally when using secondary antibodies from Molecular Probes we use a dilution of 1:200-500. Incubation time and concentration are interlinked, so a lower concentration implies a longer incubation time and vice versa. Secondary antibodies should go in every well, i.e. including those for the controls (see Box 2 and Table 1).
-
22.
After the washing, transfer the slices into a new 24-/48-well plate containing the secondary antibody solutions. Do this carefully with a brush, starting with the negative controls and changing brush between primary antibodies. Seal the plate with Parafilm to reduce evaporation. Put in the “black box” and on the shaker for 4-8hrs at room temperature.
PAUSE POINT After half an hour’s shaking you can put the slices in the fridge overnight or for up to 48hrs; if using a long incubation in this way, reduce the concentration of secondary antibody.
-
23.
Wash 4x20 min in 0.1M PBS. Use a brush to transfer the slices, remembering always to change brush between primary antibodies and start on the negative controls, so there is no contamination between wells of different primary antibodies. Wash the brushes well between washes, first with hot water and then rinse in distilled water
PAUSE POINT After 2 washes slices can be put in 0.05% NaN3/0.1M PBS and kept in the fridge for few days until it is a suitable time to mount the slices.
When using more than one primary antibody, one can apply them all simultaneously as they are raised in different animals, providing the antibodies are used at low concentrations. Alternatively, apply them sequentially by going through steps 14-23 for each one: this is preferred when using antibodies at high concentrations.
-
24.
If testing whether a nuclear protein is expressed (if not go to step 26), label the slices also for DAPI. Make up 300nM DAPI solution in 0.1M PBS and incubate the slices for 10-20 mins on the shaker (60-80rpm) at room temperature, in the “black box”. Transfer slices using brushes and be careful to use a new brush for each antibody.
CRITICAL STEP This is essential because often, when taking the pipette away from the cell, the nucleus may be removed, making it impossible to conclude whether the cell is negative for the nuclear protein because the nucleus is not labelled, or if the nucleus is just missing.
-
25.
Wash slices 3x15 min in 0.1M PBS. Use brushes to transfer the slices carefully.
-
26.
Mount the slices on microscope slides using a stereomicroscope. Use the pictures of the slices made in step 8 to find the right orientation, i.e. with the recorded cell on the appropriate side of the slide to face the microscope lens. If the slice is upside down, use two paint brushes to flip the slice over and to flatten it out. Dry away all excess PBS solution by suction with a pipette first, and then use a narrow angled piece of tissue to carefully dry most of the liquid away. Then put a drop of Citifluor on the slide and place a coverslip over it. Press lightly with forceps and seal around the edge of the cover slip with nail varnish. View with a confocal microscope.
PAUSE POINT Slides can be put in a dark box and in the fridge for a few days, until the confocal session can start.
TIMING
Electrophysiology preparation, patching cells, dye-filling, fixing and washing require one day (steps 1-11).
Blocking and permeabilizing steps require 30 mins preparation and slice transfer, but 4 hrs incubation (steps 12-13).
Primary antibody solutions require 20 mins for slice transfer, but overnight incubation (steps 14-19).
Washing after the primary antibody incubation requires 4x20 mins plus a slice transfer time for each wash of 10 mins, giving a total of ∼2 hrs (step 20).
Applying secondary antibody solutions takes 15 mins, but then 4-8 hrs incubation (steps 21-22). Washing after the secondary antibody incubation takes 4x20 mins plus a slice transfer time of 10 mins per wash, giving a total of ∼2 hrs (step 23).
DAPI staining and transfer takes 20mins (step 24, optional).
Washing after DAPI takes 3x15 mins plus a slice transfer time of 10 mins per wash, giving a total of ∼1 hr (step 25, optional).
Time to mount slides depends on number of slices: allow 10 mins per slice (step 26).
? TROUBLESHOOTING See Table 3
Table 3.
? TROUBLESHOOTING
| Problem | Possible reason | Solution |
|---|---|---|
| Dye leaks out of the cell and disappears | Removal of the pipette was too fast. | Next time go slower and be more careful. |
| The cell died. | Recording was too long: try to shorten the recordings. | |
| Unhealthy cell. | Try selecting very healthy good looking cells, without a highly refractile granular appearance. | |
| Not possible to locate the filled cell after immunohistochemistry. | Not using fixable Lucifer yellow. | Make sure to buy fixable version of Lucifer Yellow, marked CH (Alexa dyes fix well). |
| Using too much Triton X-100. | Lower the incubation time in the blocking and permeabilizing solution. | |
| Perhaps it leaked during the tissue processing or bleached. | Happens to about 10% of cells, unfortunately! | |
| Cells look blurred under the microscope after immunohistochemistry. | Slice mounted up side down. | Take care in marking the orientation of the slice and orientate it accordingly when mounting. |
| High background noise in the 488nm channel. | PFA autofluoresces at this excitation wavelength. | Increase washes after fixation. |
| Brain slices too old, i.e. recorded from too long after slicing | Record earlier after slicing | |
| Kept for too long in fridge after fixation. | Shorten the time the slices are kept in the fridge. | |
| The slices look “foggy” under the epifluorescence microscope. | Too much PBS left on slice before adding Citifluor. | Remove as much excess PBS from the slides as possible before mounting. |
| Slices kept for too long in fridge without NaN3, hence got contaminated. | When incubating slices for a long time, always have NaN3 present. | |
| Low antibody penetration. | Increase incubation time of blocking and permeabilizing solution, and keep on the shaker during incubation. | |
| Increase concentration of both primary and secondary antibodies | ||
| Increase incubation time of primary antibodies and keep on the shaker during incubation. | ||
| No labelling. | Too low a concentration of antibodies. | Increase either the concentration of primary antibody or the incubation time. |
| Tissue overfixed. | Try shortening the fixation period to 45min at room temperature. | |
| Tissue overfixed | Try 1% PFA instead. | |
| Epitope masked by fixative. | Try antibody retrieval technique. See Ref. 26. | |
| “Strange” labelling, clusters of fluoresence. | Too long Triton X-100 incubation. | Reduce Triton X-100 incubation time or its concentration. |
| Triton X-100 dissolves antigen. | Remove Triton X-100 from the blocking solution. |
ANTICIPATED RESULTS
By following the protocol above you should expect to recover most whole-cell clamped dye-filled cells with a well-maintained morphology, and satisfactorily label their membrane proteins at least 50μm into the slice, i.e. covering the depth used for most visually-guided patch clamp recording. As examples of the use of the technique, here we show the use of antibodies to label membrane proteins (lipid sulfatide O4, NG2, NMDA receptors), cytoplasmic structural proteins (GFAP) and nuclear proteins (the transcription factor Olig2), to define cell type (astrocytes versus oligodendrocytes in this case), to define the developmental stage of recorded cells (oligodendrocyte lineage cells in this case) and to define the presence of neurotransmitter receptors.
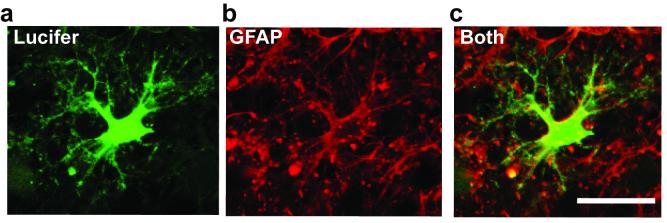
Figure 2 shows an astrocyte filled with Lucifer yellow from the whole-cell pipette and labelled after recording for GFAP23. The morphology of the cell revealed by Lucifer yellow together with the GFAP labelling defines the cell as an astrocyte.
Figure 2.
Combining whole-cell clamping with antibody labelling to GFAP. a, Lucifer yellow fill (green) of a cell in a rat cerebellar slice which showed a low resistance ohmic I-V relation with a resting potential near the potassium reversal potential (a presumed astrocyte from its electrophysiology). b, Post-recording labelling for GFAP (red). c, Overlay of a and b shows the cell labels for GFAP. Scale bar 20 μm.
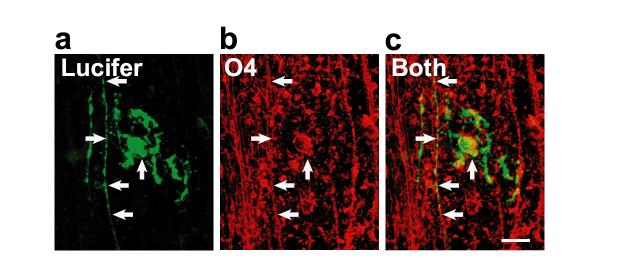
Figure 3 shows an oligodendrocyte lineage cell with a morphology revealed by Lucifer filling in which some processes align with axons, and some do not. This, together with the labelling by antibody to the lipid sulfatide O4, define the cell as being an immature oligodendrocyte that is only starting to myelinate axons23. This figure shows that even with no Triton X-100 permeabilization (which would destroy the lipid where the O4 epitope resides) good labelling can be obtained because the antibody is recognizing an extracellular epitope, and because prolonged antibody incubation with tissue shaking is employed (see protocol above).
Figure 3.
Combining whole-cell clamping with antibody labelling to the lipid sulfatide O4. a, Lucifer yellow fill (green) of a cell in a rat cerebellar slice shows a morphology appropriate to an immature oligodendrocyte, with some processes aligned with axons and some still seeking axons to myelinate. b, Post-recording labelling for O4 (red). c, Overlay of a and b shows the cell labels for O4 (arrows). Scale bar 20 μm. From ref. 23.
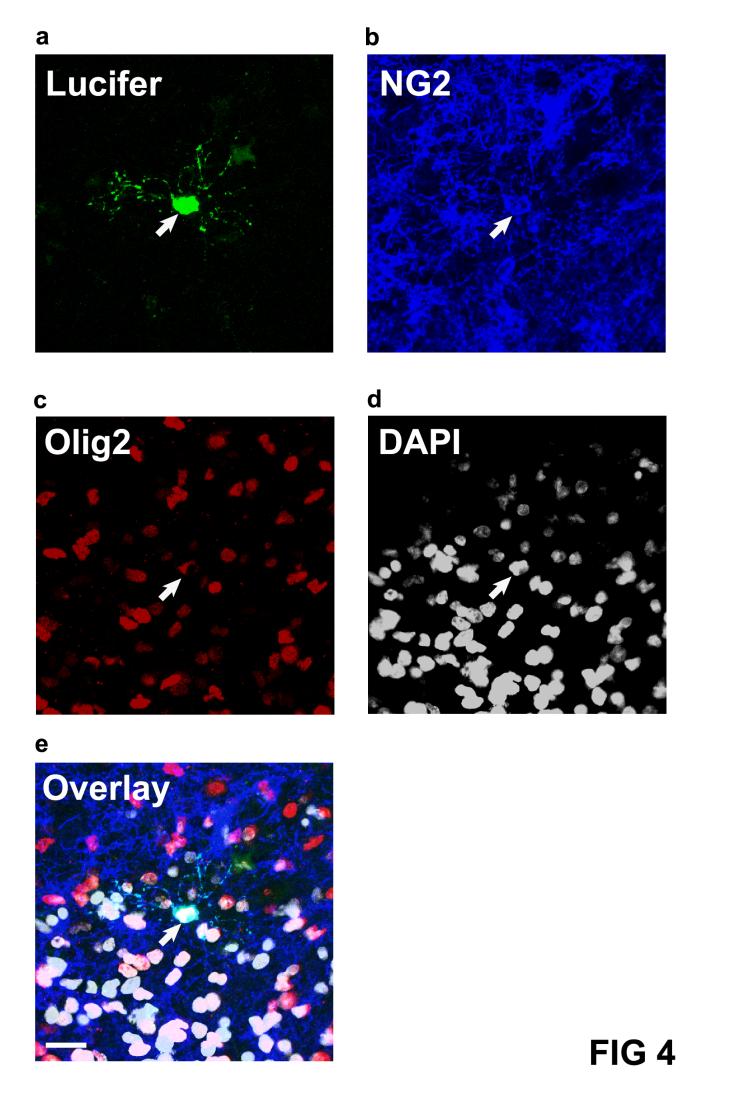
Figure 4 shows the use of 2 antibodies and DAPI to label the nucleus, on the same recorded cell. The Lucifer yellow fill reveals a small cell with processes radiating in all directions. This, together with the antibody labelling to the proteoglycan NG2, and to the oligodendrocyte specific transcription factor Olig2, establish that the cell is an oligodendrocyte precursor. Cells which are positive for Olig2 and not NG2 are immature and mature oligodendrocytes.
Figure 4.
Combining whole-cell clamping with antibody labelling to the proteoglycan NG2 and the nuclear transcription factor Olig2, and nuclear labelling with DAPI. a, Lucifer yellow fill (green) of a cell in a rat cerebellar slice which showed the morphology of an oligodendrocyte precursor. b, Post-recording labelling for NG2 (blue, Lucifer filled cell is arrowed). c, Post recording labelling for Olig2 (red). d, Post-recording labelling for DAPI (white). e, Overlay of a-d showing the cell expresses NG2 and Olig2 and the nucleus is labelled by DAPI. Scale bar 20um.
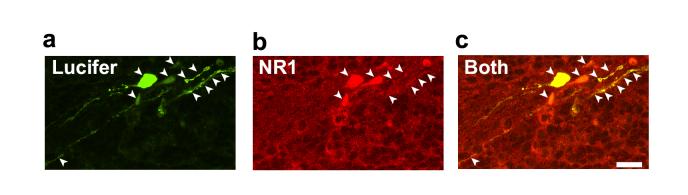
Finally in Figure 5 we show the use of the protocol to define the presence of a current generating membrane protein, the NMDA receptor. The Lucifer yellow fill reveals the morphology of a fairly mature oligodendrocyte, while the subsequent labelling for the NR1 subunit of NMDA receptors demonstrates that NMDA receptors are present in the cell and so could account for NMDA-evoked currents recorded while whole-cell clamping23.
Figure 5.
Combining whole-cell clamping with antibody labelling to the NMDA receptor subunit NR1. a, Lucifer yellow fill (green) of a cell in a rat cerebellar slice which showed the morphology of a myelinating oligodendrocyte. b, Post-recording labelling for NR1 (red). c, Overlay of a and b shows that the cell expresses NR1 (arrows). Scale bar 20um. From Ref 23.
Acknowledgments
Acknowledgements: We thank D. Rowitch, C.D. Stiles & J. Alberta for Olig2 antibody, W. Stallcup for NG2 antibody, F.A. Stephenson, R.J. Wenthold & O.P. Ottersen for NR1 antibody, and W. Andrews, M. Catsicas, I. Hans, K. Jessen, R. Mirsky, P. Mobbs, S. Rakic and W. Richardson for advice. Supported by the Wellcome Trust.
References
- 1.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 2.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessell TM, Sanes JR. The decade of the developing brain. Curr. Opin. Neurobiol. 2000;10:599–611. doi: 10.1016/s0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 4.Deloulme JC, Raponi E, Gentil BJ, Bertacchi N, Marks A, Labourdette G, Baudier J. Nuclear expression of S100β in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes’ maturation. Mol. Cell. Neurosci. 2004;27:453–465. doi: 10.1016/j.mcn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Grateron L, et al. Postnatal development of calcium-binding proteins immunoreactivity (parvalbumin, calbindin, calretinin) in the human entorhinal cortex. J. Chem. Neuroanat. 2003;26:311–316. doi: 10.1016/j.jchemneu.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Gurantz D, Lautermilch NJ, Watt SD, Spitzer NC. Sustained upregulation in embryonic spinal neurons of a Kv3.1 potassium channel gene encoding a delayed rectifier current. J. Neurobiol. 2000;42:347–356. [PubMed] [Google Scholar]
- 7.Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur. J. Neurosci. 1997;9:1646–1655. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J. Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9,:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- 12.Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 13.Guyon A, Laurent S, Paupardin-Tritsch D, Rossier J, Eugene D. Incremental conductance levels of GABAA receptors in dopaminergic neurones of the rat substantia nigra pars compacta. J. Physiol. 1999;516:719–737. doi: 10.1111/j.1469-7793.1999.0719u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikawa M, Kominami K, Yoshimura Y, Tanaka K, Nishimune Y, Okabe M. Green fluorescent protein as a marker in transgenic mice. Dev. Growth Differ. 1995;37:455–459. doi: 10.1046/j.1440-169X.1995.t01-2-00012.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhuo L, Sun B, Zhang C-L, Fine A, Chiu S-Y, Messing A. Live astrocytes visualised by green fluorescent protein in transgenic mice. Dev. Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- 16.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. J. Neurosci.Res. 2002;70:529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- 18.Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat. Rev. Neurosci. 2001;2:861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- 19.Monyer H, Markram H. Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci. 2004;27:90–97. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J. Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- 21.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 22.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J. Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Káradóttir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakmann B, Stuart G. Patch-pipette recordings from the soma, dendrites, and axon of neurons in brain slices. In: Sakmann, Neher, editors. Single-Channel Recording. 2nd edition New York: Plenum; 1995. [Google Scholar]
- 25.Gibb AJ. Patch-clamp recording. In: Ashley RH, editor. Ion Channels:a practical approach. Oxford: Oxford University Press; 1995. [Google Scholar]
- 26.Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J. Neurosci. Methods. 1999;93:149–162. doi: 10.1016/s0165-0270(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 27.Holmseth S, Lehre KP, Danbolt NC. Specificity controls for immunocytochemistry. Anat. Embryol. (Berl.) 2006;Jan 25:1–10. doi: 10.1007/s00429-005-0077-6. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1982. [Google Scholar]