Abstract
Axonal degeneration is a key component of many neurodegenerative diseases. Injured axons undergo a program of self-destruction termed Wallerian degeneration that is an active, well-regulated process. The pathways leading to axon fragmentation are uncharacterized, but experiments with wlds mutant mice led to the discovery that overexpression of nicotinamide mononucleotide adenylyltransferase (Nmnat1) or treatment with NAD+ can inhibit axonal degeneration. Here, we show that the purine nucleosides adenosine and guanosine, but not inosine, inhibit injury-induced axonal degeneration in cultured DRG neurons. Axons can be preserved by adding adenosine within 6 hr of the axonal injury. The presence of adenosine was required continuously after the injury to maintain axonal protection. Together these results suggest that adenosine does not alter the neuronal response to injury, but instead inhibits a local axonal pathway necessary for the commitment and/or execution of the axon destructive program.
Keywords: Axonal Degeneration, adenosine, guanosine, purine nucleosides, Wallerian degeneration
Introduction
Neurodegenerative diseases including Parkinson’s Disease, Amyotrophic Lateral Sclerosis as well as peripheral neuropathies caused by genetic mutations, trauma, or chemotherapy (Coleman 2005, Coleman & Perry 2002). Unfortunately, the molecular mechanisms that underlie axonal degeneration and the factors that regulate it are poorly understood. Studies of the wlds mutant mouse, which manifests slowed axonal degeneration (Lunn et al. 1989), have shown that axonal degeneration is an active process reminiscent of, but distinct from, apoptosis (Raff et al. 2002). Axonal degeneration in these mutant mice is delayed in response to a variety of genetic and toxin-induced injuries (Ferri et al. 2003, Samsam et al. 2003, Wang et al. 2002, Sajadi et al. 2004, Gillingwater et al. 2006, Hasbani & O’Malley K 2006, Mi et al. 2005). Axonal damage from a wide variety of insults can be inhibited in wlds mice, suggesting that there is a common pathway leading to axonal destruction that could be therapeutically targeted (Coleman 2005).
The mutation responsible for the wlds phenotype is a tandem triplication of a gene fusion containing the N-terminal 70 amino acids of ubiquitination factor 4b (Ube4b/Ufd2a), an 18 unique amino acid linking region and the full length coding region of nicotinamide mononucleotide adenylyltransferase 1 (Nmnat1) (Conforti et al. 2000). The axonal sparing effects of this Wlds fusion protein have been replicated using in vitro cultures of mouse sympathetic and dorsal root ganglia (DRG) (Conforti et al. 2006, Deckwerth & Johnson 1994, Wang et al. 2005, Araki et al. 2004). Nmnat1 (or Nmnat3) overexpression alone is sufficient to slow axonal degeneration and this protection is dependent on Nmnat enzymatic activity (Araki et al. 2004, Wang et al. 2005, Press & Milbrandt 2008, Sasaki et al. 2006).
Nmnat1 converts nicotinamide mononucleotide (NMN) to nicotinamide adenine dinucleotide (NAD+) using NMN and ATP as substrates (Berger et al. 2005). Interestingly, NAD+ as well as NAD+ precursors like NMN or nicotinamide riboside, can provide axonal protection in vitro and in vivo (Sasaki et al. 2006, Kaneko et al. 2006). It is unclear how NAD+ mediates axonal protection, but one possibility is that some of its metabolites may play a role. Indeed, purine nucleosides like adenosine can delay neuronal cell death and increase neurite outgrowth (Bocklinger et al. 2004, Benowitz et al. 1998, Irwin et al. 2006). To explore the axonal protective potential of these derivatives, we used an in vitro DRG axotomy assay. Adenosine was found to slow axonal degeneration in a dose-dependent fashion. It provided axonal protection when present prior to axotomy or when added several hours after injury. Similar protective activity was provided by guanosine, but not inosine. Finally, we found that the continued presence of adenosine was required for maintaining the integrity of injured axons, suggesting that it affects an ongoing degenerative process.
Materials and Methods
Reagents
All reagents were obtained from Sigma-Aldrich (St. Louis, Missouri) unless otherwise noted. The purity of the purine reagents used in these studies were all of highest purity (>99%). Their purity was confirmed by LC-MS using standard methods.
Culture of DRGs
Tissue culture plates were coated with poly-D-lysine and laminin (Invitrogen, Carlsbad, California). The plates were initially coated with 0.1 mg/ml poly-D-lysine solution for 16 hr, washed twice with water, and air-dried in a sterile hood. Laminin (2μg/ml) was added for 1–2 hr. The plates were then again air-dried prior to plating the neurons.
DRGs were collected from CD1 mouse embryos at the gestation days between embryonic day 12.5 (E12.5) and E13.5 based on a previously described method (Chen et al. 2008). DRGs from 6 embryos were collected into a 1.5 ml microfuge tube containing DMEM, treated with trypsin, and triturated to make a single cell suspension. Cells were pelleted and resuspended (at 50 μl/embryo). Two microliters (~1000 neurons/μl) of the cell suspension were placed as a drop slightly below the center of each coated well (in a 24-well plate) and incubated at 37°C with 5% CO2 for 1 hr. Under these conditions, the DRG cell bodies clustered within a 3 to 5 mm diameter circle and axons extend radially. Axons are severed by a micro-scalpel (Fine Science Tools, Foster City, CA) after 14 days in vitro (DIV).
Preparation of purine nucleoside solutions
Stock solutions of adenosine and inosine were dissolved in water at a concentration of 200 mM after gentle heating in a 100 degree heating block with repeated vortexing. Guanosine was more limited in solubility, thus, the stock solution was made at 50 mM by the same method. All solutions were rapidly added to culture media immediately after their preparation.
Quantification of axonal degeneration
Axonal degeneration was quantified as described elsewhere (Sasaki et al. 2009). Briefly, after axonal severing, phase contrast images were taken with an inverted microscope with a 20x objective (Eclipse TE 300; Nikon). For each well, 3–4 random fields of distal axons were imaged by using CCD camera (Cool SNAP ES; Nikon) and Metamorph software (Molecular Devices) with 40 ms exposure time. Images were adjusted for brightness and background intensity by the auto-level function and converted to 8-bit in Adobe Photoshop (Adobe, San Jose, CA) and analyzed by Image J (NIH). To obtain the total area of axon, images were binarized. Non-degenerated axons have continuous tracts, while unhealthy degenerating axons that were fragmented and beaded were detected as aggregates. The total axonal area was determined by the total number of detected pixels after the imaged was binarized. Degenerated axons were detected using the particle analyzer of Image J as small particles, while healthy axonal area was represented by large continuous areas. The degeneration index (DI) is the ratio of fragmented axon area to total axon area. ≥20 fields were evaluated per condition combined from multiple independent experiments and the data presented are adjusted representative images from repeated experiments.
Statistics
All comparisons were analyzed by student’s t-tests with Bonferroni’s correction for multiple comparisons. Values are reported as the average ± SEM.
Results
Adenosine inhibits axonal degeneration following axotomy
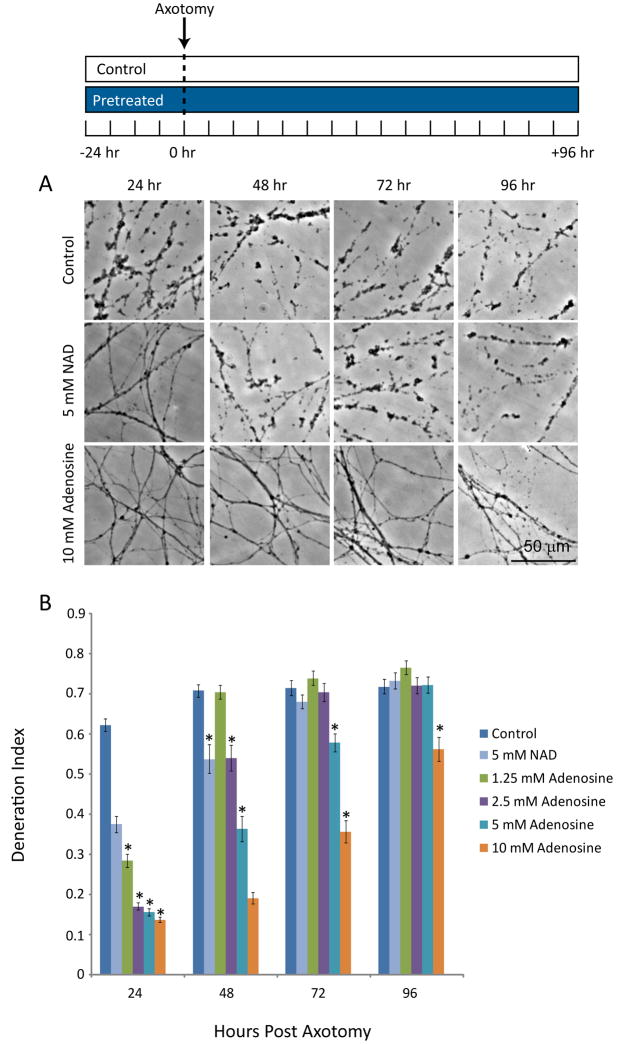
Purine nucleosides prevent neuronal apoptosis and support axonal growth (Benowitz et al. 1998, Bocklinger et al. 2004, Irwin et al. 2006). To explore whether these nucleosides could inhibit axonal degeneration, we employed an in vitro axotomy assay. Mouse DRGs from E13.5 embryos were cultured as dissociated neuronal drop cultures on laminin and poly-D-lysine coated plates such that axons extend outward to form an axonal halo. After 14 days in vitro (DIV), the axons were severed using a micro-scalpel and axonal degeneration was monitored daily using phase contrast microscopy. A degeneration index (DI) was calculated using the fraction of total axonal area that was composed of axonal fragments (i.e. a higher number indicates more axonal degeneration). Under these conditions, axons exhibit a significant amount of swelling, beading and fragmentation 24 hr after injury (DI=0.62±0.02) (Fig. 1). To determine the effect of adenosine on axonal degeneration we added 1.25 to 10 mM adenosine to neuronal cultures 24 hr prior to axotomy, and axonal degeneration was monitored for 96 after axotomy. Adenosine promoted robust axonal protection that extended for up to 96 hr at high doses (Fig. 1). We compared directly, using these dissociated cultures, the level of protection provided by adenosine vs. NAD+. We found that under these culture conditions (see Materials and Methods) that adenosine provided greater axonal protection than NAD+ at 48 hr after injury (NAD+: DI=0.54±0.04 vs. adenosine: DI=0.19±0.01) (Fig. 1).
Figure 1. Adenosine inhibits axonal degeneration in a dose-dependent manner.
A) DRG cultures were incubated with adenosine at various concentrations (1.25–10 mM), or NAD+ (5 mM), for 24 hr prior to axotomy. Axonal degeneration was monitored for up to 96 hr using phase contrast microscopy. B) The degeneration index was determined for each condition (see Materials and Methods). (*=p<0.05 compared to control at each timepoint, n≥20 fields from 2–3 independent experiments, blue bars indicate the presence of adenosine).
Adenosine is protective when administered after axonal injury
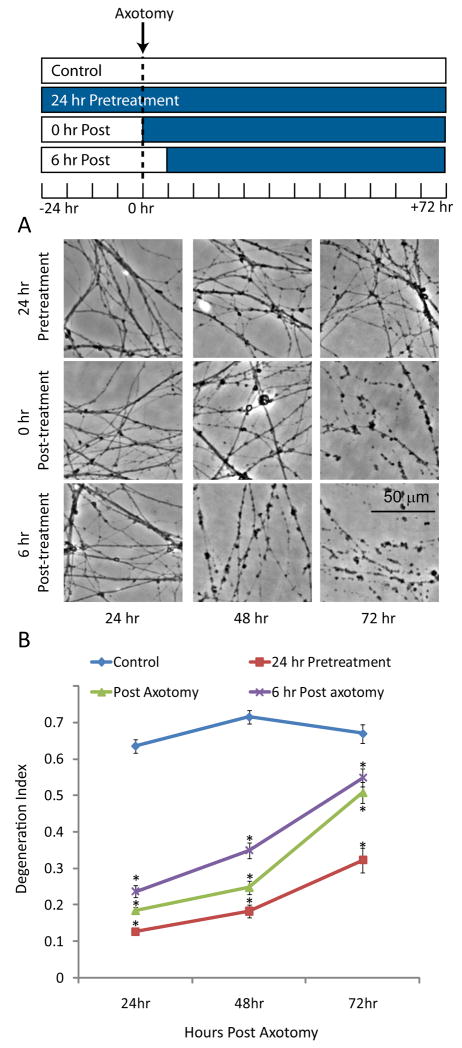
Axonal degeneration can be divided into three phases: 1) a competence phase that includes expression of the machinery required for the degeneration program; 2) a commitment phase that includes signaling generated in response to axonal injury; and, 3) an execution phase that results in the physical breakdown of the axon (Saxena & Caroni 2007). To investigate when neurons required adenosine to maintain axonal integrity after injury, DRG cultures were treated with 10 mM adenosine 24 hr prior to axotomy, immediately following axotomy, or 6 hr after the injury. The extent of degeneration was measured for up to 72 after the injury. At 24 hr, both pre- and post-axotomy administration of adenosine was protective (Fig. 2). However, at longer times it became apparent that the level of protection was dependent on the length of treatment, with 24 hr pretreatment giving the strongest protection. Importantly, adenosine delivered at the time of injury or 6 hr later provided significant protection. The ability of adenosine to alter degeneration after axonal severing demonstrates that adenosine likely acts at the commitment or execution phase of axonal degeneration by affecting local processes within the axon. However, because pre-treatment with adenosine provided the most robust protection, we cannot exclude the possibility that somal or nuclear processes (i.e. transcription) are also involved.
Figure 2. Adenosine is protective when added after the injury.
A) DRG cultures were incubated with adenosine either 24 hr prior to, immediately after, or 6 hr after axotomy. The degeneration was monitored for 72 hr by phase contrast microscopy. B) The degeneration index was determined for each condition. (*=p<0.05 compared to control at each timepoint, n≥20 fields from 2–3 independent experiments, blue bars indicate the presence of adenosine).
Adenosine is necessary after injury to maintain protection
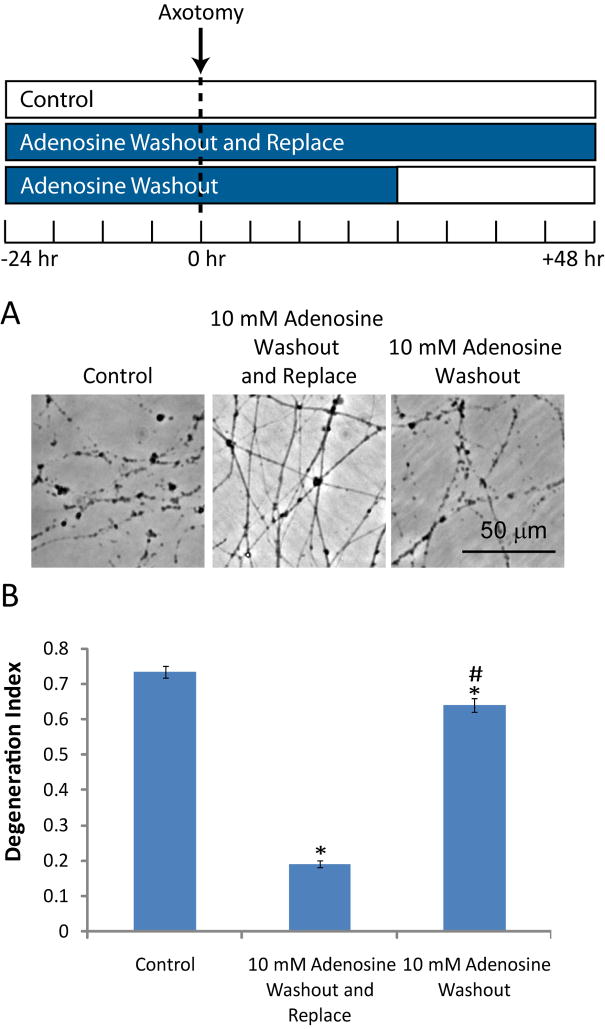
While adenosine clearly has a local effect on axonal preservation, it is unclear whether it is required continuously or only during a post-injury critical window when a potential “initiating event” could occur (i.e. during the first 3–6 hr after injury). To examine this question, we treated cultures with adenosine (10 mM) prior to injury and for 24 hr after injury, then we replaced the media with media either lacking or containing adenosine. The axonal degeneration index was measured 24 hr after the media exchange and showed that removing adenosine after injury resulted in rapid axonal fragmentation (Fig. 3). The cultures in which adenosine was removed had only slightly less axonal degeneration than untreated cultures, indicating that continuous exposure to adenosine after injury is required for axonal maintenance.
Figure 3. Adenosine is continuously required to maintain injured axons.
A) DRG cultures were treated with adenosine for 24 hr prior to axotomy. Twenty-four hr after axotomy, the media was replaced either with media containing or lacking adenosine. Axonal degeneration was monitored 48 hr after axotomy by phase contrast microscopy. B) The degeneration index was determined for each condition. (*=p<0.05 compared to control at each timepoint, #=p<0.05 compared to adenosine containing media, n≥20 fields from 2–3 independent experiments, blue bars indicate the presence of adenosine).
Guanosine, but not inosine slows Wallerian degeneration
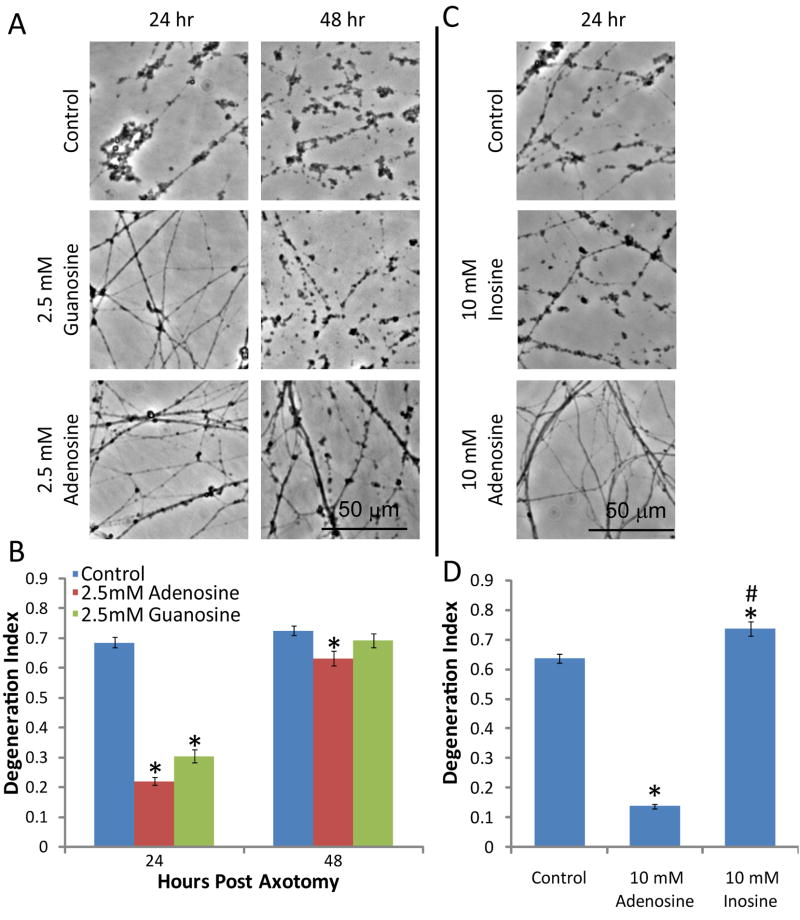
The axonal protection promoted by adenosine encouraged us to test other purine nucleosides including guanosine and inosine. We compared the effects of treating DRG neurons prior to injury with guanosine, which could only be tested at 2.5 mM due to poor solubility, with adenosine. Axonal degeneration was assessed and showed that like adenosine at this concentration, it provided significant protection at 24 hr but failed to protect at longer time points (Fig. 4A, B). Similar experiments were performed with inosine at 10 mM; however, no axonal protection was observed, indicating that the protective effect is not a general effect of purine nucleosides (Fig. 4C).
Figure 4. Guanosine, but not inosine, inhibits axonal degeneration.
A) DRG cultures were treated with guanosine (2.5 mM) or adenosine (2.5 mM) 24 hr prior to axotomy. The degeneration was monitored for up to 48 hr by phase contrast microscopy. B) The degeneration index was determined for each condition. (*=p<0.05 compared to control at each timepoint, n≥20 fields from 2–3 independent experiments). C) DRG cultures were treated with inosine (10 mM) or adenosine (10 mM) 24 hr prior to axotomy and degeneration was monitored after 24 hr by phase contrast microscopy. D) The degeneration index was determined for each condition. (*=p<0.05 compared to control at each timepoint, n≥20 fields from 2–3 independent experiments).
Discussion
The observations that NAD+ and its precursors are able to slow axonal degeneration (Sasaki et al. 2006) along with evidence that purine nucleosides prevent neuronal apoptosis and support axonal growth (Bocklinger et al. 2004, Irwin et al. 2006, Benowitz et al. 1998) prompted us to test additional NAD+ related compounds for axonal protective activity. Adenosine as well as guanosine treatment of cultured DRG neurons robustly delayed axonal degeneration in response to axotomy. Axonal preservation was observed even when adenosine was added after the injury, indicating that it must modulate local events within the injured axon. The enhanced protection observed when adenosine was added prior to injury indicates that additional somal or nuclear events could also be involved in adenosine-mediated axonal protection. These results indicate that extracellular purine nucleosides can influence axonal degeneration. Adenosine metabolism is altered after peripheral injury and it accumulates to high levels after neuronal injury, thus purine nucleosides may represent endogenous axonal protective agents (Stone et al. 2007, Sawynok & Liu 2003).
Adenosine has at least two major modes of action 1) receptor-mediated effects and 2) intracellular interactions via membrane transport (Fredholm et al. 2001). Adenosine has four known G-coupled protein receptors, A1, A2A, A2B, and A3 receptors. The A1 and A3 receptors appear to be inhibitory and act through Gi and Go, whereas the A2 receptors are excitatory and act through Gs, Golf, and Gq (Sawynok & Liu 2003). The best studied adenosine receptors are A1 and A2A. A1 receptor agonists and A2A receptor antagonists were neuroprotective when tested in the MPTP model of Parkinson’s Disease (Lau & Mouradian 1993, Pierri et al. 2005), and analgesic effects of adenosine after spinal cord injury are dependent on A1 stimulation (Sawynok & Liu 2003). While the role for these receptors in adenosine-mediated axonal degeneration should be explored, the high millimolar concentrations required in our in vitro assays, suggests that these receptors, with nanomolar affinities (Cunha 2001) are unlikely to mediate these effects.
Purine nucleosides are transported into cells via two classes of receptors that operate under either equilibrative (ENT1 - ENT4 receptors) or concentrative (CNT1 – CNT5 receptors) mechanisms (Podgorska et al. 2005). Purines enhance neurite outgrowth in a number of cellular systems that involve equilibrative transport and activation of protein kinase N (PKN) or Ste20-like protein kinase-3b (Mst3b) (Benowitz et al. 1998, Irwin et al. 2006, Bocklinger et al. 2004). In these experiments, inosine was a potent inducer of neurite outgrowth and the effects of adenosine were exerted through its conversion to inosine via adenosine deaminase. In contrast, our results on axonal degeneration demonstrated that inosine was totally ineffective, suggesting that adenosine can affect multiple pathways. Interestingly work done with adenosine and apoptosis suggests that intracellular adenosine also plays a role in neuronal survival and cell death (Wakade et al. 2001).
The current studies suggest that purine nucleosides and their analogs could be useful in treating disorders where axonopathy is a major component. Under normal steady-state conditions extracellular adenosine concentrations are in the nanomolar range. However, after injury, such as ischemia, the concentration of extracellular adenosine is thought to increase dramatically, well into the micromolar range (Latini & Pedata 2001, Pedata et al. 2001, Rathbone et al. 1999, Snyder 1985). It is possible that millimolar concentrations are achievable in vivo with exogenous adenosine administration and could be therapeutically useful. In this regard, intrathecal administration of adenosine at millimolar concentrations provides neuroprotection in stroke models (Kitagawa et al. 2002). Unfortunately, due to the extremely short half life of adenosine in the bloodstream (Lerman & Belardinelli 1991), it is not possible to test the effects of adenosine on peripheral axonal degeneration in vivo via intravenous administration.
Guanosine on the other hand can be used in vivo and has been tested as an anti-apoptotic agent in models of stroke and spinal cord injury. Interestingly, guanosine treatment decreased the area of ischemia and level of disability after middle cerebral artery occlusion (MCAO); however, it had no effect on the number of apoptotic cells in the ischemic penumbra (Chang et al. 2008). Guanosine also reduced the severity of injury after spinal cord compression, with a reduction in apoptosis in the spinal cord and preservation of myelinated fibers (Jiang et al. 2007). Oral administration of guanosine has been used successfully to decrease seizure activity in mice (de Oliveira et al. 2004). These results suggest that guanosine or perhaps adenosine analogs may be useful in promoting axonal protection in vivo. Further exploration of the mechanism of action for purine nucleoside-mediated axonal protection may result in new therapeutic strategies for a wide range of neurological disorders.
Acknowledgments
We would like to thank T. Scott Isbell and Cheryl Lichti for their invaluable help with LC-MS to verify compound purity. We would also like to thank Eugene Johnson, Bradley Miller, Biplab Dasgupta, Jason Gustin, Yo Sasaki, Leah Press and Bhupinder Vohra for fruitful discussions and reading of the manuscript. This work was supported by an NIH Neuroscience Blueprint Core Grant NS057105 to Washington University, the HOPE Center for Neurological Disorders, the Muscular Dystrophy Association (JM), and National Institutes of Health Grants AG013730 and NS040745 to JM.
Abbreviations
- DI
Degeneration Index
- Nmnat1
nicotinamide mononucleotide adenylyltransferase 1
- Nmnat3
nicotinamide mononucleotide adenylyltransferase 3
- NGF
Nerve growth factor
- DIV
Days in vitro
- ADA
Adenosine deaminase
References
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Jing Y, Tabibiazar R, Jo SA, Petrausch B, Stuermer CA, Rosenberg PA, Irwin N. Axon outgrowth is regulated by an intracellular purine-sensitive mechanism in retinal ganglion cells. The Journal of biological chemistry. 1998;273:29626–29634. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. The Journal of biological chemistry. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- Bocklinger K, Tomaselli B, Heftberger V, Podhraski V, Bandtlow C, Baier-Bitterlich G. Purine nucleosides support the neurite outgrowth of primary rat cerebellar granule cells after hypoxia. European journal of cell biology. 2004;83:51–54. doi: 10.1078/0171-9335-00362. [DOI] [PubMed] [Google Scholar]
- Chang R, Algird A, Bau C, Rathbone MP, Jiang S. Neuroprotective effects of guanosine on stroke models in vitro and in vivo. Neuroscience letters. 2008;431:101–105. doi: 10.1016/j.neulet.2007.11.072. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW. NS21: redefined and modified supplement B27 for neuronal cultures. Journal of neuroscience methods. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Conforti L, Fang G, Beirowski B, et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2006;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochemistry international. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- de Oliveira DL, Horn JF, Rodrigues JM, Frizzo ME, Moriguchi E, Souza DO, Wofchuk S. Quinolinic acid promotes seizures and decreases glutamate uptake in young rats: reversal by orally administered guanosine. Brain research. 2004;1018:48–54. doi: 10.1016/j.brainres.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev Biol. 1994;165:63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- Ferri A, Sanes JR, Coleman MP, Cunningham JM, Kato AC. Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr Biol. 2003;13:669–673. doi: 10.1016/s0960-9822(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological reviews. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Ingham CA, Parry KE, Wright AK, Haley JE, Wishart TM, Arbuthnott GW, Ribchester RR. Delayed synaptic degeneration in the CNS of Wlds mice after cortical lesion. Brain. 2006;129:1546–1556. doi: 10.1093/brain/awl101. [DOI] [PubMed] [Google Scholar]
- Hasbani DM, O’Malley KL. Wld(S) mice are protected against the Parkinsonian mimetic MPTP. Experimental neurology. 2006;202:93–99. doi: 10.1016/j.expneurol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Irwin N, Li YM, O’Toole JE, Benowitz LI. Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci U S A. 2006;103:18320–18325. doi: 10.1073/pnas.0605135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Bendjelloul F, Ballerini P, D’Alimonte I, Nargi E, Jiang C, Huang X, Rathbone MP. Guanosine reduces apoptosis and inflammation associated with restoration of function in rats with acute spinal cord injury. Purinergic signalling. 2007;3:411–421. doi: 10.1007/s11302-007-9079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Wang J, Kaneko M, Yiu G, Hurrell JM, Chitnis T, Khoury SJ, He Z. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J Neurosci. 2006;26:9794–9804. doi: 10.1523/JNEUROSCI.2116-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Mori A, Shimada J, Mitsumoto Y, Kikuchi T. Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurological research. 2002;24:317–323. doi: 10.1179/016164102101199819. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. Journal of neurochemistry. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Lau YS, Mouradian MM. Protection against acute MPTP-induced dopamine depletion in mice by adenosine A1 agonist. Journal of neurochemistry. 1993;60:768–771. doi: 10.1111/j.1471-4159.1993.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Lerman BB, Belardinelli L. Cardiac electrophysiology of adenosine. Basic and clinical concepts. Circulation. 1991;83:1499–1509. doi: 10.1161/01.cir.83.5.1499. [DOI] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Mi W, Beirowski B, Gillingwater TH, et al. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain. 2005;128:405–416. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann N Y Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Pierri M, Vaudano E, Sager T, Englund U. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology. 2005;48:517–524. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Podgorska M, Kocbuch K, Pawelczyk T. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochim Pol. 2005;52:749–758. [PubMed] [Google Scholar]
- Press C, Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J Neurosci. 2008;28:4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Sajadi A, Schneider BL, Aebischer P. Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Curr Biol. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Samsam M, Mi W, Wessig C, Zielasek J, Toyka KV, Coleman MP, Martini R. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J Neurosci. 2003;23:2833–2839. doi: 10.1523/JNEUROSCI.23-07-02833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BPS, Lund FE, Milbrandt J. Nmnat-mediated axonal protection requires enzymatic activity but not increased levels of neuronal NAD+ J Neuroscience. 2009 doi: 10.1523/JNEUROSCI.5469-08.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Adenosine as a neuromodulator. Annual review of neuroscience. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Stone TW, Forrest CM, Mackay GM, Stoy N, Darlington LG. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab Brain Dis. 2007;22:337–352. doi: 10.1007/s11011-007-9064-3. [DOI] [PubMed] [Google Scholar]
- Wakade AR, Przywara DA, Wakade TD. Intracellular, nonreceptor-mediated signaling by adenosine: induction and prevention of neuronal apoptosis. Molecular neurobiology. 2001;23:137–153. doi: 10.1385/MN:23:2-3:137. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MS, Davis AA, Culver DG, Glass JD. WldS mice are resistant to paclitaxel (taxol) neuropathy. Ann Neurol. 2002;52:442–447. doi: 10.1002/ana.10300. [DOI] [PubMed] [Google Scholar]