Abstract
TANK-binding kinase 1 (TBK1) and IκB kinase ε (IKKε) regulate the production of Type 1 interferons during bacterial and viral infection, but the lack of useful pharmacological inhibitors has hampered progress in identifying additional physiological roles of these protein kinases and how they are regulated. Here we demonstrate that BX795, a potent and relatively specific inhibitor of TBK1 and IKKε, blocked the phosphorylation, nuclear translocation, and transcriptional activity of interferon regulatory factor 3 and, hence, the production of interferon-β in macrophages stimulated with poly(I:C) or lipopolysaccharide (LPS). In contrast, BX795 had no effect on the canonical NFκB signaling pathway. Although BX795 blocked the autophosphorylation of overexpressed TBK1 and IKKε at Ser-172 and, hence, the autoactivation of these protein kinases, it did not inhibit the phosphorylation of endogenous TBK1 and IKKε at Ser-172 in response to LPS, poly(I:C), interleukin-1α (IL-1α), or tumor necrosis factor α and actually enhanced the LPS, poly(I:C), and IL-1α-stimulated phosphorylation of this residue. These results demonstrate that the phosphorylation of Ser-172 and the activation of TBK1 and IKKε are catalyzed by a distinct protein kinase(s) in vivo and that TBK1 and IKKε control a feedback loop that limits their activation by LPS, poly(I:C) and IL-1α (but not tumor necrosis factor α) to prevent the hyperactivation of these enzymes.
Invading bacteria and viruses are sensed by the host pattern recognition receptors, which bind components of these organisms, called pathogen-associated molecular patterns. The binding of pathogen-associated molecular patterns to pattern recognition receptors activates signaling cascades that culminate in the production of proinflammatory cytokines, chemokines, and interferons, which are released from immune cells into the circulation, where they mount responses to combat the invading pathogen (1). The interaction between pathogen-associated molecular patterns and pattern recognition receptors leads invariably to the activation of the mitogen-activated protein (MAP)3 kinases, termed p38 MAP kinases and c-Jun N-terminal kinases 1 and 2 (JNK1/2) and the IκB kinase (IKK) complex. The latter contains the protein kinases IKKα and IKKβ, which switch on the transcription factor NFκB and, hence, NFκB-dependent gene transcription, by phosphorylating IκBα and other IκB isoforms (2). IKKβ also activates the protein kinase Tpl2 by phosphorylating its p105 regulatory subunit, leading to the activation of two other MAP kinases, termed extracellular signal-regulated kinase 1 (ERK1) and ERK2 (3, 4). Together, the MAP kinases and NFκB regulate the production of many proinflammatory cytokines and chemokines.
A subset of pattern recognition receptors, namely Toll-like receptors 3 and 4 (TLR3, TLR4) and the cytosolic receptors RIG-I (retinoic acid-inducible gene I) and MDA-5 (melanoma differentiation-associated gene 5), also activate a distinct signaling pathway requiring the IKK-related kinases, IKKε and TANK-binding kinase 1 (TBK1) (5, 6). Early studies, largely based on overexpression experiments, suggested that a major role of TBK1 and IKKε was to activate NFκB and NFκB-dependent gene transcription, and for this reason, TBK1 has also been called NFκB-activating kinase (7–9). However, later studies using cells from mice that do not express TBK1 and/or IKKε failed to support this conclusion (10, 11). Instead, they indicated that these protein kinases play an essential role in regulating the production of type I interferons (IFNs) by phosphorylating the transcription factor, termed interferon regulatory factor 3 (IRF3) (10, 11). Under basal conditions IRF3 is cytosolic, but after the TBK1/IKKε-mediated phosphorylation of its C terminus, IRF3 dimerizes and translocates to the nucleus, where it activates a gene transcription program leading to the production of IFN-β (12, 13). The production of IFN-β may additionally require the TBK1/IKKε-catalyzed phosphorylation of other proteins, such as the Dead-box RNA-helicase DDX3 (14, 15) and MITA (16). IKKε has also been implicated in the phosphorylation of the STAT1 transcription factor at Ser-708 in a pathway that protects cells against infection by influenza A virus (17).
However, mouse knock-out studies are not always definitive because the complete loss of a protein kinase(s) may be compensated for by other protein kinases, whereas the prolonged absence of a protein kinase may result in long term changes in gene transcription programs so that the effects observed may be indirect. The embryonic lethality of the TBK1 knock-out mouse also limits its use in understanding the physiological roles of this protein kinase. Moreover, papers continue to be published proposing roles for TBK1 and IKKε in phosphorylating defined sites on the RelA and c-Rel components of the NFκB transcription complex that are thought to control the expression of a subset of NFκB-dependent genes (18–20). Finally, there is considerable evidence that TBK1 and IKKε play additional roles in cells. For instance, TBK1 is activated by TNF, and TBK1 knock-out mice die just before birth because the fetal hepatocytes undergo TNFα-induced apoptosis (21). These observations imply that TBK1 plays a key role in preventing apoptosis in the fetal hepatocytes of wild type mice. TBK1 is also reported to be activated by hypoxia and to control the production of angiogenic factors, such as vasoendothelial growth factor (22), whereas the overexpression of IKKε in breast cancer lines is reported to contribute a survival signal to the transformed cells (23). The direct substrates of TBK1 and IKKε or molecular pathways underlying any of these responses and the possible roles of these protein kinases in the pathogenesis of human cancer are unknown.
The identification of the physiological substrates and biological roles of protein kinases has been greatly aided by the use of relatively specific, small cell-permeable inhibitors of these enzymes. These compounds can be used simply and rapidly and provide a complementary approach to the use of mouse knockouts or RNA interference technology, avoiding the potential drawbacks in ablating the expression of a protein kinase that were mentioned above. The compound BX795 was originally developed as a small molecule inhibitor of 3-phosphoinositide-dependent protein kinase 1 (PDK1) (24), but we recently found that it also inhibited TBK1 and IKKε at low nanomolar concentrations in vitro (25). Moreover, BX795 only inhibited a few other protein kinases significantly out of 70 tested and, importantly, did not inhibit IKKβ (25). These findings suggested that BX795 might be the first pharmacological inhibitor suitable for studying the regulation and roles of TBK1 and IKKε in cells. In this paper we demonstrate the utility of this compound and exploit it to show that, unexpectedly, the activation of TBK1 and IKKε is not an autophosphorylation event but is mediated by a distinct “upstream” protein kinase.
EXPERIMENTAL PROCEDURES
Materials—BX795 was synthesized as described (25), dissolved in DMSO, and stored as a 10 mm solution at –20 °C. Poly(I:C) and LPS were from InvivoGen, and mouse IL-1α and TNFα were from Sigma.
DNA Constructs—TBK1 (NCBI NP_037386.1) was amplified from IMAGE EST 5492519 (Geneservice) using KOD Hot Start DNA Polymerase (Novagen). The PCR product was cloned into pSC-b (Stratagene) and sequenced to completion. The insert was excised using BamHI and NotI and inserted into pCMV-FLAG-1 or pEBG6P to generate FLAG-TBK1 and GST-TBK1, respectively. IKKε (NCBI NP_054721.1) was cloned in a similar manner using IMAGE EST 3062062. Point mutations were created using the QuikChange mutagenesis kit (Stratagene) but using KOD Hot Start DNA Polymerase. IRF3 (GenBank™ CAA91227.1) was amplified by PCR using IMAGE EST 5494536, ligated with pSC-b, and sequenced. The insert was subcloned into NotI sites of pGEX6P-2. PRDII (NFκB) and PRDIII-I (IRF3) elements from the IFN-β promoter cloned into the pLuc-MCS vector were a kind gift from Katherine Fitzgerald (University of Massachusetts). pTK-RL was obtained from Stratagene.
Cell Culture—HEK293 cells stably expressing TLR3-FLAG (termed HEK293-TLR3 cells) were provided by Katherine Fitzgerald (University of Massachusetts), immortalized mouse embryonic fibroblasts (MEFs) were from wild type mice, and mice expressing a truncated, inactive form of TAK1 (26) were provided by Professor Shizuo Akira (Osaka University, Japan). HEK293-TLR3, RAW264.7 cells (hereafter termed RAW cells) and MEFS were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mm glutamine, 10% fetal calf serum, and the antibiotics penicillin and streptomycin. Bone-marrow derived macrophages were generated from mice as described (27). Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Antibodies—Antibodies were raised in sheep against the human TBK1 protein expressed in insect Sf21 cells (Sheep S041C, bleed 2) (25) and the C-terminal peptide of mouse IKKε (NRLIERLHRVPSAPDV) (Sheep S277C, bleed 2) and used for immunoprecipitation. The phosphopeptide CEKFVS*VYGTE (where S* indicates phosphoserine) corresponding to the sequence surrounding Ser-172 of TBK1 and IKKε was used to generate an antibody that immunoprecipitated the phosphorylated forms of these protein kinases (Sheep S051C, bleed 2). The phosphopeptide was coupled separately to keyhole limpet hemocyanin and bovine serum albumin, then mixed and injected into sheep at Diagnostics Scotland (Edinburgh, UK). The antisera were purified by affinity chromatography on immobilized TBK1 or the peptide antigen, respectively, in the Division of Signal Transduction Therapy (University of Dundee. The phospho-specific antibody was incubated with the unphosphorylated form of the peptide immunogen (10 μg of peptide per μg of antibody) before use to neutralize any antibodies recognizing the unphosphorylated forms of TBK1 and IKKε. The following antibodies were used for immunoblotting: anti-glyceraldehyde-3-phosphate dehydrogenase (Research Diagnostics Inc.), anti-GST (Division of Signal Transduction Therapy, University of Dundee), horseradish peroxidase-conjugated secondary antibodies (Pierce), anti-IRF3, anti-TAK1 (Santa Cruz), anti-FLAG, anti-IKKε (Sigma), anti-TBK1, anti-TANK and anti-IκBα (Cell Signaling Technology). Antibodies recognizing phosphorylated Ser-933 (Ser(P)-933) of p105 (NFκB1), Ser(P)-32/Ser(P)-36 of IκBα, Ser(P)-468 of RelA, Ser(P)-536 of RelA, Ser(P)-396 of IRF3, the Thr(P)-Glu-Tyr(P) sequence of ERK1 and ERK2, the Thr(P)-Gly-Tyr(P) sequence of p38 MAP kinases and a pan-PDK1 phosphorylation site antibody which recognizes the phosphorylated activation loop (Thr(P)-229) of S6 kinase 1 (28) were also from Cell Signaling Technology. The antibody recognizing the Thr(P)-Pro-Tyr(P) sequence of JNK1/2 was from BIOSOURCE, whereas that recognizing Ser(P)-172 of TBK1 was from BD Biosciences.
Immunoprecipitation and Immunoblotting—Pharmacological inhibitors dissolved in DMSO or an equivalent volume of DMSO for control incubations were added to the culture medium of cells grown as monolayers. After 1 h at 37 °C, the cells were stimulated with LPS, poly(I:C), IL-1α, or TNFα as described in all the figure legends. Thereafter, the cells were rinsed in ice-cold phosphate-buffered saline and extracted in lysis buffer (50 mm Tris/HCl, pH 7.4, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 5 mm sodium pyrophosphate, 10 mm sodium β-glycerol 1-phosphate, 1 mm dithiothreitol, 1 mm sodium orthovanadate, 0.27 m sucrose, 1% (v/v) Triton X-100, 1 mg/ml aprotinin, 1 mg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride). Cell extracts were clarified by centrifugation at 14,000 × g for 10 min at 4 °C, and protein concentration was determined using the Bradford assay. Proteins were immunoprecipitated by incubating 1 mg of cell extract protein with 10 μg of antibody for 90 min at 4 °C followed by the addition of Protein G-Sepharose. After mixing for 15 min at 4 °C and brief centrifugation, the immunocomplexes were washed 3 times in lysis buffer, denatured in SDS, and subjected to SDS-PAGE. To detect proteins in cell lysates, 40 μg of protein extract was separated by SDS-PAGE. After transfer to polyvinylidene difluoride membranes, proteins were detected by immunoblotting and visualized by treating the blots with ECL (Amersham Biosciences) followed by autoradiography.
Protein Kinase Assays—IRF3 was expressed in Escherichia coli as a GST fusion protein and purified by affinity chromatography on glutathione-Sepharose. Endogenous TBK1 was immunoprecipitated from 1 mg of cell lysate protein using the sheep anti-TBK1 (sheep S041C, bleed 2) antibody and overexpressed FLAG-TBK1 with an anti-FLAG M2-agarose (Sigma). Immunocomplexes were washed 3 times in lysis buffer and twice in 50 mm Tris-HCl, pH 7.5, 0.1% (v/v) 2-mercaptoethanol, 0.1 mm EGTA, 10 mm magnesium acetate and then resuspended in the same buffer containing 2 μm GST-IRF3 (endogenous TBK1) or the peptide KKKKERLLDDRHDSGLDSMKDEE (0.3 mm), corresponding to the sequence surrounding Ser-32 and Ser-36 of IκBα, plus four lysine residues at the N terminus to facilitate binding to phosphocellulose paper (termed IκBα peptide substrate). Assays were initiated by adding [γ-32P]ATP (1000 cpm/pmol) to a final concentration of 0.1 mm. When GST-IRF3 was used as the substrate, the reactions were terminated after 30 min at 30 °C by the addition of SDS containing 40 mm EDTA, pH 7.0, heated for 5 min at 100 °C and separated by SDS-PAGE, and phosphorylated proteins were detected by autoradiography. Quantification was performed by phosphorimaging analysis. For assays with the IκBα substrate peptide, reactions were terminated after 10 min at 30 °C by spotting an aliquot of the reaction on to a 2 × 2-cm2 piece of phosphocellulose P81 paper (Whatman) followed by immersion in 75 mm phosphoric acid. After washing six times in phosphoric acid and once in acetone, the papers were dried and counted. One unit of TBK1 activity was defined as that amount of enzyme catalyzing the incorporation of 1 nmol of phosphate into substrate in 1 min.
Luciferase Assays—Cells were co-transfected with PRD(II)2 or PRD(III-I)3-pLuc-MCS and pTK-RL plasmid DNA. 24 h later cells were stimulated and extracted in Passive Lysis Buffer (Promega). Luciferase activity was measured with a dual-luciferase assay system (Promega) according to the manufacturer's instructions.
Measurement of IFN-β Production—Cells were treated for 1 h with or without inhibitors then stimulated for 6 h with 100 ng/ml LPS or 10 μg/ml poly(I:C). The cell culture medium was removed and clarified by centrifugation for 10 min at 14,000 × g, and the concentration of mouse IFN-β was measured using an enzyme-linked immunosorbent assay kit (R&D Systems) according to manufacturer's protocol.
Microscopy—Cells seeded on glass coverslips were serum-starved overnight before stimulation with poly(I:C). The cells were fixed, permeabilized, and stained as described (29) using anti-IRF3 (1:100; Santa Cruz) followed by incubation with Alexa546-conjugated anti-rabbit IgG (1:500; Molecular Probes). The cells were then visualized using a Zeiss-LSM 510-meta microscope fitted with an alpha Plan-Fluar 100x/1.45 oil objective.
Statistical Analysis—Quantitative data are presented as the mean ± S.E. Statistical significance of differences between experimental groups was assessed with Student's t test. Differences in means were considered significant if p < 0.05.
RESULTS
Specificity of BX795 in Vitro—We reported previously that six protein kinases were inhibited by >90% in vitro in the presence of 0.1 μm BX795, namely TBK1, IKKε, PDK1, Aurora B, ERK8, and MARK3, but 60 other protein kinases tested were unaffected or only slightly inhibited at this concentration (25). The IC50 values for these six protein kinases are shown in Table 1. MARK3 is a member of the subgroup of protein kinases that include the AMP-activated protein kinase. In the present study we found that the other MARK isoforms (MARK1, MARK2, and MARK4) and another AMP-activated protein kinase-related kinase (NUAK1) were inhibited with similar potency to MARK3 (Table 1). We also studied the effect of BX795 on a number of additional kinases not examined previously. These experiments showed that BX795 did not inhibit the following protein-tyrosine kinases at 1 μm: ephrin receptors A2 and B3, Syk, Bruton's tyrosine kinase, and fibroblast growth factor receptor 1. However, the vascular endothelial growth factor receptor was inhibited, albeit much less potently than TBK1 (Table 1). We reported previously that BX795 did not inhibit IKKβ (25), and in the present study we found that the IKKα isoform is also unaffected at 1 μm in vitro (results not shown). For reasons discussed later, we also examined the effect of BX795 on a number of MAP kinase kinases (MKKs) and MAP kinase kinase kinases (termed MAP3Ks). At 1 μm, BX795 did not inhibit MKK1, MKK3, MKK4, MKK6, and MKK7, or the MAP3Ks Tpl2 and c-Raf (results not shown) but did potently inhibit the mixed lineage kinases, termed MLK1 (MAP3K9), MLK2 (MAP3K10), and MLK3 (MAP3K11)) (Table 1). MAP3K7, also called transforming growth factor β-activated kinase-1 (TAK1), and MAP3K5, also called apoptosis-stimulating kinase-1 (ASK1), were inhibited >100-fold less potently than TBK1 (Table 1). The ability of BX795 to inhibit the TBK1-catalyzed phosphorylation of IRF3 at Ser-396 declined as the ATP concentration in the assay was increased (supplemental Fig. S1), indicating that BX795 is an ATP competitive inhibitor of TBK1 as is the case for PDK1 (24).
TABLE 1.
I50 values for the inhibition of selected protein kinases by BX795
Data are presented as the average of duplicate determinations. VEGFR, vasoendothelial growth factor receptor.
| Kinase | IC50 | ATP |
|---|---|---|
| μm | μm | |
| TBK1 | 0.006 ± 0.001 | 100 |
| IKKε | 0.041 ± 0.001 | 100 |
| PDK1 | 0.111 ± 0.013 | 100 |
| Aurora B | 0.031 ± 0.002 | 100 |
| ERK8 | 0.140 ± 0.027 | 100 |
| MARK3 | 0.081 ± 0.008 | 100 |
| MARK1 | 0.055 ± 0.004 | 100 |
| MARK2 | 0.053 ± 0.005 | 100 |
| MARK4 | 0.019 ± 0.001 | 100 |
| NUAK1 | 0.005 ± 0.001 | 100 |
| VEGFR | 0.157 ± 0.011 | 100 |
| MLK1 | 0.050 ± 0.003 | 100 |
| MLK2 | 0.046 ± 0.011 | 100 |
| MLK3 | 0.042 ± 0.005 | 100 |
| TAK1 | 0.70 ± 0.14 | 100 |
| ASK1 | 0.620 ± 0.004 | 100 |
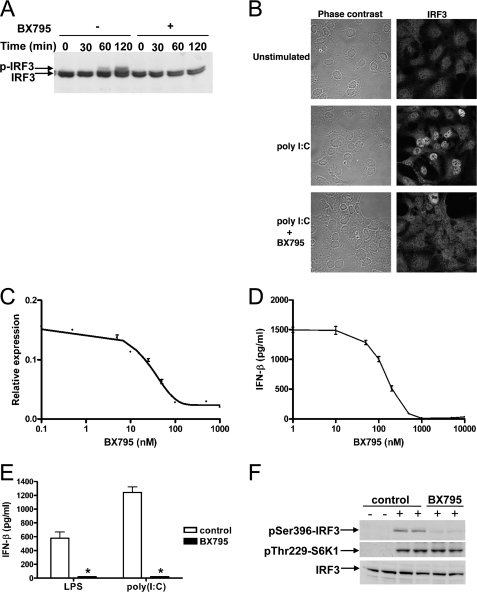
BX795 Blocks TBK1- and IKKε-mediated Activation of IRF3 and Production of IFN-β—To examine whether BX795 inhibited TBK1 and IKKε when added to mammalian cells in culture, we used HEK293 cells that stably overexpress TLR3 (termed HEK293-TLR3 cells) for initial studies. IRF3 migrated as a doublet in unstimulated cells. Stimulation with poly(I:C), a synthetic TLR3 agonist, led to the appearance of a more slowly migrating species whose level became maximal after 2 h (Fig. 1A). The appearance of this species, which has been shown by others to result from phosphorylation (30), was prevented by prior incubation with BX795 (Fig. 1A). We also observed that IRF3 accumulated in the nucleus on a similar time scale after poly(I:C) treatment, which was blocked by BX795 (Fig. 1B). The next step in this pathway is IRF3-stimulated gene transcription. As expected from the results presented above, BX795 inhibited IRF3-dependent gene transcription (Fig. 1C). Finally, BX795 blocked the secretion of IFN-β from macrophages whether stimulated by LPS, a TLR4 agonist (Fig. 1, D and E), or poly(I:C) (Fig. 1E).
FIGURE 1.
BX795 blocks the phosphorylation, nuclear translocation, and transcriptional activity of IRF3 and production of interferon β in response to TLR3 and TLR4 agonists. A, HEK293-TLR3 cells were incubated for 60 min without (–) or with (+)1 μm BX795 and subsequently stimulated with 50 μg/ml poly(I:C) for the times indicated. Cell extract protein (40 μg) was subjected to SDS-PAGE and immunoblotted for IRF3. B, HEK293-TLR3 cells were stimulated for 2 h with poly(I:C) as in A. The cells were fixed, stained for IRF3, and visualized by confocal microscopy. C, HEK293-TLR3 cells were co-transfected with DNA encoding an IRF3 luciferase reporter construct and pTK-Renilla luciferase plasmid DNA. 24 h post-transfection cells were incubated for 1 h with varying concentrations of BX795 before stimulation for 6 h with 50μg/ml poly(I:C). Luciferase activity was measured and normalized to Renilla luciferase activity (mean ± S.E., n = 4). D, RAW264.7 cells were incubated for 1 h at various concentrations of BX795 and then stimulated for 6 h with 100 ng/ml LPS. The concentration of IFN-β released into the culture medium was measured by enzyme-linked immunosorbent assay. (mean ± S.E., n = 4). E, the experiment was carried out as in D except that cells were incubated without (white bars) or with (black bars)1 μm BX795 and stimulated with either 100 ng/ml LPS or 10 μg/ml of poly(I:C). (mean ± S.E., n = 3; *, p < 0.01). F, the effects of BX795 on TLR signaling do not result from inhibition of PDK1. RAW264.7 macrophages were treated for 30 min without (control) or with 1 μm BX795 then stimulated for 45 min without (–) or with (+) 100 ng/ml LPS. Cell extracts (40 μg protein) were immunoblotted with antibodies recognizing IRF3, IRF3 phosphorylated at Ser-396, and S6 kinase 1 (S6K1) phosphorylated at Thr-229.
BX795 was originally developed as an inhibitor the protein kinase PDK1, but BX795 had no effect on the LPS-stimulated phosphorylation of p70 ribosomal S6 kinase 1 at Thr-229, the site that is targeted by PDK1 (31), under conditions where it completely blocked the LPS-stimulated phosphorylation of IRF3 at Ser-396 (Fig. 1F). This experiment demonstrated that TBK1/IKKε was inhibited much more potently than PDK1 by BX795 in cells and excluded the possibility that BX795 suppressed the activation of IRF3 and production of IFN-β by inhibiting PDK1.
BX795 Does Not Affect Activation of the IKKα/β Complex or NFκB-dependent Gene Transcription by LPS, poly(I:C), IL-1α, or TNFα—The question of whether TBK1 and IKKε play a role in regulating NFκB-dependent gene transcription is an issue that is still not fully resolved (see the Introduction). We, therefore, studied the effect of BX795 on the activation of NFκB and NFκB-dependent transcriptional activity. Under conditions where BX795 completely blocked the LPS (Fig. 2A)- or poly(I:C)-stimulated (Fig. 2B) phosphorylation of IRF3 at Ser-396, this compound did not affect the phosphorylation at Ser-32 and Ser-36 or degradation of the IκBα inhibitory component of the NFκB transcription complex or the phosphorylation at Ser-933 of the p105 (NFκB1) regulatory subunit of the protein kinase Tpl2 (Fig. 2, A and B), which are established physiological substrates of the IKKα/β complex. These results demonstrated that BX795 did not inhibit any of the steps involved in the LPS- or poly(I:C)-stimulated activation of the IKKα/β complex or the ability of IKKα/β to phosphorylate downstream substrates. Nor did BX795 inhibit the LPS- or poly(I:C)-stimulated phosphorylation of the RelA component of the NFκB transcription factor at Ser-468 and Ser-536 (Fig. 2, A and B), amino acid residues reported to become phosphorylated when TBK1 and/or IKKε were overexpressed in cells (18, 19). BX795 did not inhibit NFκB-dependent gene transcription in response to poly(I:C) in HEK293-TLR3 cells (Fig. 2C), which is consistent with the failure of BX795 to suppress the activation or activity of the IKKα/β complex or the phosphorylation of RelA. BX795 also had no effect on the activation of the canonical NFκB pathway by IL-1α and TNFα (Fig. 2, D and E), although TBK1 and IKKε are activated as discussed later.
FIGURE 2.
BX795 selectively blocks IRF3 but not NFκB signaling. A, RAW264.7 cells were incubated without (–) or with (+)1 μm BX795 and then either left unstimulated (–) or stimulated (+) for 30 min with 100 ng/ml LPS. Cell extracts (40 μg protein) were then immunoblotted with the antibodies used in Fig. 1 and with antibodies that recognize TBK1, RelA phosphorylated at Ser-468 or Ser-536, IκBα phosphorylated at Ser-32 and Ser-36, or p105 phosphorylated at Ser-933. B, same as A except that the RAW264.7 cells were stimulated with 10 μg/ml poly(I:C) for 60 min, and immunoblotting for IκBα was carried out using an antibody recognizing all forms of IκBα instead of the antibody recognizing phosphorylated IκBα. C, HEK293-TLR3 cells were co-transfected with DNA encoding an IRF3 or a NFκB luciferase reporter construct and pTK-Renilla luciferase plasmid DNA. 24 h post-transfection cells were treated without (white bars) or with (black bars)1 μm BX795 and then stimulated for 6 h with 50μg/ml poly(I:C). Luciferase activity was measured and normalized to Renilla luciferase activity (mean ± S.E., n = 4; *, p < 0.001). D and E, MEFs were serum-starved overnight and incubated without (–) or with (+)1 μm BX795 for 60 min before stimulation with 10 ng/ml IL-1α (D) or TNF-α (E). Cell extracts were immunoblotted as described in A and B.
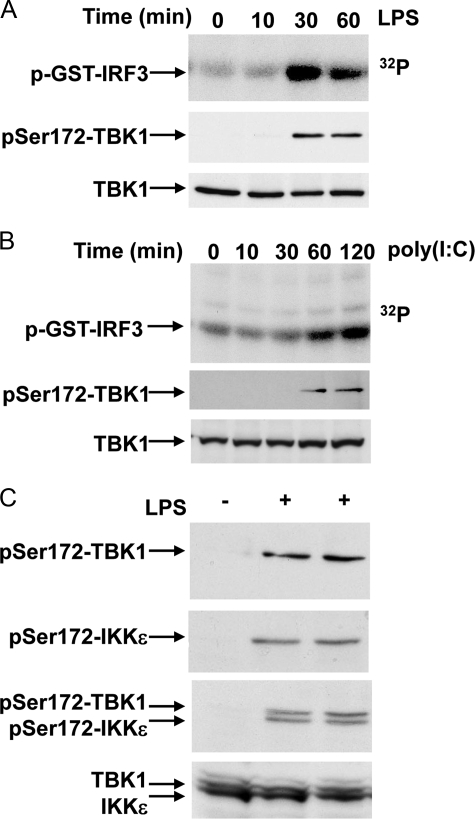
Activation of TBK1 and IKKε Correlates with the Phosphorylation of Ser-172—The molecular mechanisms that lead to the activation of TBK1 and IKKε are still rather poorly defined, although the activation of these protein kinases is known to require their phosphorylation at Ser-172 within the activation loop (9, 15, 32). In the experiments described below we initially monitored the increase in the catalytic activity of TBK1 and IKKε by direct enzyme assay as well as by the phosphorylation of Ser-172 (Fig. 3). LPS stimulation of RAW macrophages increased the catalytic activity of TBK1, which was maximal after 30 min, persisted until 60 min, and correlated with the phosphorylation of Ser-172 (Fig. 3A). Similarly, poly(I:C) stimulation of HEK293-TLR3 cells led to an increase in TBK1 activity after 60 min that was maximal after 120 min and again correlated with the phosphorylation of Ser-172 (Fig. 3B).
FIGURE 3.
Phosphorylation of TBK1 and IKKε at Ser-172 correlates with catalytic activity. A, RAW264.7 macrophages were stimulated with 100 ng/ml LPS for the times indicated. Catalytic activity was measured by immunoprecipitating TBK1 and incubating the immunocomplexes with GST-IRF3 in presence of Mg[γ-32P]ATP. Proteins were resolved by SDS-PAGE and stained with Coomassie Blue, and phosphorylated IRF3 detected by autoradiography (top panel). Phosphorylation of Ser-172 on TBK1 was monitored by immunoprecipitating the phosphorylated TBK1 with the anti-IKKε Ser(P)-172 antibody and immunoblotting with an antibody recognizing all forms of TBK1 (middle panel). A further aliquot of cell extract was subjected to SDS-PAGE and immunoblotted with the same TBK1 antibody. B, same as A, except that HEK293-TLR3 cells were stimulated for the times indicated with 50 μg/ml poly(I:C). C, RAW264.7 macrophages were left unstimulated (–) or stimulated (+) for 45 min with 100 ng/ml LPS. The Ser-172-phosphorylated forms of TBK1 and IKKε were immunoprecipitated from cell extracts using the anti-Ser(P)-172 IKKε antibody, and the presence of TBK1 and/or IKKε was revealed by immunoblotting using antibodies recognizing all forms of TBK1 (top panel) or IKKε (second panel from top) or a mixture of the two antibodies (third panel from the top). In the bottom panel, 40 μg of extract protein was immunoblotted without immunoprecipitation using a mixture of the antibodies recognizing all forms of TBK1 and IKKε.
The sequence surrounding Ser-172 is conserved between TBK1 and IKKε. The phospho-specific antibody raised against the sequence surrounding Ser-172 should, therefore, recognize the phosphorylated forms of both protein kinases. We found that the anti-phospho-Ser-172 antibody that we generated immunoprecipitated phosphorylated TBK1 and IKKε, allowing the phosphorylation of these protein kinases to be monitored by subsequent immunoblotting with antibodies that recognize all forms of TBK1 and IKKε. This method also enabled changes in the phosphorylation of TBK1 and IKKε to be studied simultaneously, as TBK1 migrated more slowly than IKKε during SDS-PAGE (Fig. 3C). These studies revealed that TBK1 and IKKε were activated in parallel in response to LPS (Fig. 3C).
Transfected Catalytic Subunits of TBK1 and IKKε Are Activated by Autophosphorylation—It was reported that the transfected catalytic subunit of TBK1 became phosphorylated at Ser-172 but catalytically inactive mutants did not, which has led to the assumption that phosphorylation of Ser-172 is an autophosphorylation event (9, 15). These observations with transfected TBK1 were confirmed in the present study (Fig. 4A), where we also found that BX795 prevented the phosphorylation of Ser-172 (Fig. 4B) and the activation of transfected TBK1 (Fig. 4C). Additionally, we found that the phosphorylation of TBK1 at Ser-172 is at least partly an intermolecular autophosphorylation event, because wild type TBK1 associated with and phosphorylated the catalytically inactive mutant TBK1-(K38A) in co-transfection experiments (Fig. 4D). Moreover, TBK1-(K38A) also interacted with and could be phosphorylated by wild type IKKε (Fig. 4D).
FIGURE 4.
The overexpression of TBK1 and IKKε leads to autophosphorylation and transphosphorylation of Ser-172. A, wild type (WT) and two different catalytically-inactive FLAG-tagged mutants of TBK1 (TBK1-(K38A) and TBK1[D157A]) were expressed in HEK293 cells. Cell extract (40 μg protein) was then subjected to SDS-PAGE and immunoblotted using anti-Ser(P)-172 TBK1 and anti-FLAG. B, FLAG-WT-TBK1 was transfected into HEK293 cells. 24 h later the cells were treated without (–) or with (+)1 μm BX795 for the times indicated. Cell extracts (40 μg protein) were then immunoblotted using anti-FLAG and anti-Ser(P)-172 TBK1 as in A. C, a vector encoding FLAG WT-TBK1 was transfected into HEK293 cells, then incubated for 1 h without (–) or with (+)1 μm BX795. TBK1 was then immunoprecipitated from 0.1 mg of cell extract protein with anti-FLAG (washed) and assayed for activity using the IκBα peptide substrate (mean ± S.E., n = 4; *, p < 0.001), D, a vector encoding FLAG-tagged TBK1-(K38A) was co-expressed in HEK293 cells with an empty vector (–), a vector encoding GST (+), or a vector encoding GST-WT-TBK1 (TBK1) GST-WT-IKKε (IKKε). The cells were lysed, and 40 μg of cell extract protein was immunoblotted (WB) using anti-Ser(P)-172 TBK1/IKKε or anti-FLAG as in A (upper two panels). A further 0.5 mg of cell extract protein was incubated for 1 h at 4 °C with 0.01 ml of glutathione-Sepharose beads with continuous mixing. The beads were collected by centrifugation and washed three times in lysis buffer, and bound proteins were released with SDS and immunoblotted with anti-GST or anti-FLAG antibodies (lower two panels). E, wild type and catalytically-inactive mutants of FLAG-TBK1 and FLAG-IKKε were expressed in HEK293 cells, and the lysates were immunoblotted using anti-FLAG as in A. F, WT-FLAG-IKKε was expressed in HEK293 cells. 24 h later the cells were treated with 1 μm BX795 for the times indicated. Cell extracts (40 μg protein) were then immunoblotted using anti-FLAG as in A. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Although the transfected wild type and catalytically inactive mutants of TBK1 were expressed at similar levels, we noticed that wild type IKKε accumulated to levels that were far greater than the catalytically inactive mutants (Fig. 4E). Moreover, unlike TBK1 (Fig. 4B), incubation of the cells expressing wild type Flag-IKKε with BX795 led to a rapid decrease in the protein to the levels observed when a catalytically inactive mutant was expressed (Fig. 4F). Furthermore, incubation with BX795 did not decrease the expression of the catalytically inactive mutant of IKKε further (results not shown). Taken together, these experiments demonstrate that the phosphorylation and/or the catalytic activity of IKKε is required for the stability of the transfected protein kinase.
The level of expression of the endogenous IKKε is increased by prolonged exposure to inflammatory stimuli, and for this reason, it is also called IKK-inducible (IKKi) (9). We found that the LPS-induced increase in the expression of endogenous IKKε was not decreased by BX795 (Fig. 5A), demonstrating that it is not dependent on IKKε catalytic activity. Moreover, in contrast to the overexpressed catalytic subunits, immunoprecipitation of endogenous IKKε did not coimmunoprecipitate TBK1 or vice versa (Fig. 5B). Immunoprecipitation of IKKε or TBK1 also led to the coimmunoprecipitation of TANK (Fig. 5B), as expected from earlier reports (8). Taken together, these results indicate that the endogenous TBK1 and IKKε are present in RAW macrophages as separate complexes.
FIGURE 5.
Endogenous IKKε protein expression is unaffected by treatment with BX795 and does not form a complex with TBK1. A, RAW264.7 cells were treated for 1 h without (–) or with (+)1 μm BX795 before stimulation for 16 h without (–) or with (+) 100 ng/ml LPS. Cell extracts were immunoblotted with antibodies raised against IKKε, TBK1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B, endogenous TBK1 and IKKε were immunoprecipitated (IP) from cell extracts of RAW264.7 cells. IgG isolated from a non-immunized sheep served as a negative control. Proteins present in the immunoprecipitates were detected by immunoblotting with antibodies that recognize IKKε, TBK1, and TANK.
Phosphorylation of Endogenous TBK1 and IKKε at Ser-172 Is Mediated by a Distinct Protein Kinase(s)—We next studied the activation (Fig. 6A) and Ser-172 phosphorylation (Fig. 6B) of the endogenous TBK1 and IKKε in LPS-stimulated RAW macrophages. Surprisingly, and in complete contrast to the transfection experiments described above, BX795 not only failed to reduce the phosphorylation of the endogenous protein kinases at Ser-172 but actually enhanced phosphorylation (Fig. 6, B and C) and activation (Fig. 6A) by about 2-fold. Similar results were obtained in primary bone marrow-derived macrophages stimulated with LPS (Fig. 6D) or poly(I:C) (Fig. 6E).
FIGURE 6.
BX795 increases the phosphorylation of Ser-172 and the catalytic activity of TBK1 and IKKε in response to LPS and poly(I:C). A, RAW264.7 cells were treated for 1 h without (–) or with (+)1 μm BX795 before stimulation for 45 min without (–) or with (+) 100 ng/ml LPS. TBK1 was immunoprecipitated from 1 mg of cell extract protein, and its catalytic activity was measured by incubation for 30 min at 30 °C with GST-IRF3 and Mg[γ-32P]ATP. Reactions were terminated in SDS, proteins were resolved by SDS-PAGE, and the gel was autoradiographed (top panel). The gel was subjected to phosphorimaging analysis, and the catalytic activity of TBK1 was quantitated and normalized to that observed in the unstimulated cells (mean ± S.E., n = 3; *, p < 0.05). B, an aliquot of the cell extract in A was used to analyze the phosphorylation of TBK1 and IKKε at Ser-172 as in Fig. 3C (top panel). As a loading control, cell extract (40 μg of protein) was also immunoblotted with antibodies recognizing all forms of TBK1 and IKKε (lower panel). This antibody also recognizes a nonspecific band migrating slower than TBK1 and IKKε. C, RAW264.7 cells were incubated for 1 h with the indicated concentrations of BX795 and then either left unstimulated (–) or stimulated (+) for 45 min with 100 ng/ml LPS. The phosphorylation of TBK1 and IKKε at Ser-172 was then examined by immunoprecipitation/immunoblotting as in Fig. 3C. D, bone marrow-derived macrophages were incubated for 1 h without (–) or with (+)1 μm BX795 followed by stimulation for 30 or 60 min with 100 ng/ml LPS. Cell extract (20 μg protein) was then immunoblotted with the antibodies indicated. E, the experiment was performed as in D, but the cells were stimulated for 60 min with 10 μg/ml poly(I:C). F, RAW264.7 cells were stimulated for 45 min with 100 ng/ml LPS, TBK1 was immunoprecipitated from 0.5 mg of cell extract protein, and its ability to phosphorylate GST-IRF3 (2 μm) was measured at varying concentrations of BX795 using Mg[γ-32P]ATP. Reactions were terminated in SDS, proteins were resolved by SDS-PAGE, and the gel was autoradiographed (top panel). The gel was subjected to phosphorimaging analysis, and the catalytic activity of TBK1 was quantitated and normalized to that measured in the absence of inhibitor (mean ± S.E., n = 4).
The different effects of BX795 on the phosphorylation of Ser-172 of endogenous and transfected TBK1/IKKε raised the question of whether the endogenous kinases were insensitive to BX795. Although this seemed unlikely, as BX795 suppresses the phosphorylation of the TBK1/IKKε substrate IRF3 in response to LPS and poly(I:C) (Fig. 6), we tested this possibility after immunoprecipitating endogenous TBK1 from LPS-stimulated RAW macrophages. These experiments confirmed that the endogenous TBK1 was potently inhibited by BX795 (Fig. 6F).
We also found that IL-1α (Fig. 7A) and TNFα (Fig. 7B) activated TBK1 and IKKε in immortalized MEFs, activation again correlating with the phosphorylation of Ser-172. The IL-1α-stimulated activation peaked after 5–10 min and declined thereafter. Activation by TNFα peaked at 10 min but had disappeared by 60 min. Similar to LPS and poly(I:C), BX795 enhanced the activation of TBK1 and the phosphorylation of TBK1/IKKε at Ser-172 by IL-1α (Fig. 7C). BX795 did not decrease the TNFα-stimulated activation of TBK1 or the phosphorylation of TBK1/IKKε, but in contrast to LPS, poly(I:C), and IL-1α, did not increase it (Fig. 7D).
FIGURE 7.
Activation and phosphorylation of TBK1 and IKKε by IL-1α and TNFα. A and B, kinetics of activation of TBK1 and IKKε. MEFs were serum-starved overnight and stimulated for the indicated times using 10 ng/ml IL-1α (A) or TNFα (B). Catalytic activity was measured by immunoprecipitating TBK1 and incubating the immune complexes with GST-IRF3 in the presence of Mg[γ-32P]ATP. Proteins were resolved by SDS-PAGE, and phosphorylated proteins were detected by autoradiography (top panel). Phosphorylation of Ser-172 on TBK1 was monitored by immunoprecipitating the phosphorylated TBK1 and IKKε with the anti-IKKε Ser(P)-172 antibody (S051C) and immunoblotting with an antibody recognizing all forms of TBK1 and IKKε (middle panel). C and D, effect of BX795 on the activation of TBK1 and IKKε. MEFs were incubated for 1 h without (–) or with (+)1μm BX795 before stimulation for 10 min with 10 ng/ml IL-1α (C) or TNFα (D). Catalytic activity and phosphorylation of TBK1 and IKKε were measured as in A and B.
Activation of TBK1/IKKε by TNFα but Not IL-1α Is Reduced in TAK1-deficient MEFs—TBK1 and IKKε are members of the IKK subfamily of protein kinases. To start to address the question of which protein kinase(s) activates TBK1 and IKKε in cells, we therefore investigated whether the activation of TBK1/IKKε was affected in MEFs that express a truncated inactive form of TAK1 (MAP3K7) (26), a MAP3K that has been implicated in the activation of IKKα/β by inflammatory stimuli. These experiments showed that the activation of TBK1/IKKε by IL-1α was unaffected (Fig. 8A), but a reduction in the extent of activation after stimulation by TNFα was observed consistently (Fig. 8B). As expected, the phosphorylation of p105, an established physiological substrate of IKKβ, was abolished in the TAK1-deficient MEFs (Fig. 8).
FIGURE 8.
TNFα, but not IL-1α, induced phosphorylation of TBK1 and IKKε is reduced in TAK1-deficient MEFs. Wild type (WT) MEFs and MEFs expressing a truncated inactive form of TAK1 (26) were stimulated with 10 ng/ml IL-1α (A) or TNFα (B) for 10 min. The phosphorylation of TBK1 and IKKε was monitored using the immunoprecipitation/immunoblotting assay described in Fig. 3C. The phosphorylation of p105 as well as total TAK1 and TBK1/IKKε was detected in total protein extracts (40 μg) by immunoblotting with the respective antibodies. TAK1Δ is an inactive form of TAK1, which lacks 37 amino acid residues in a region critical for ATP binding. Similar results were obtained in two independent experiments. KO, knockout.
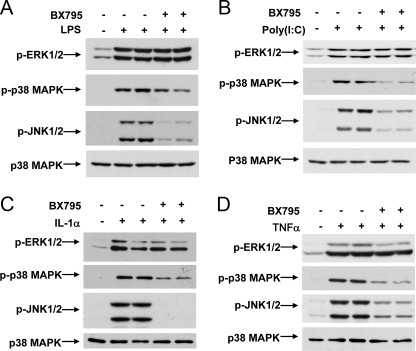
Effect of BX795 on Activation of MAP Kinases by LPS, poly(I: C), IL-1α, or TNFα—BX795 had no effect on the LPS (Fig. 9A)- or poly(I:C)-stimulated (Fig. 9B) phosphorylation of the activating Thr-Glu-Tyr motif of ERK1/ERK2. In contrast, BX795 strongly suppressed the LPS (Fig. 9A)- or poly(I:C)-stimulated (Fig. 9B) phosphorylation of the activating Thr-Pro-Tyr motif on JNK1/2 and partially inhibited the phosphorylation of the activating Thr-Gly-Tyr motif on p38α MAP kinase. BX795 also suppressed the phosphorylation of JNK1/2 and p38α MAPK in MEFs stimulated with IL-1α or TNFα (Fig. 9, C and D). As discussed below, the effect of BX795 on the activation of JNK1/2 and p38α MAPK does not result from the inhibition of TBK1/IKKε.
FIGURE 9.
Effect of BX795 on the activation of MAP kinases. A and B, RAW264.7 cells were treated without (–) or with (+)1 μm BX795 and either left unstimulated (–) or stimulated (+) with 100 ng/ml LPS (A) or 10 μg/ml poly(I:C) (B). The cells were lysed, and aliquots of the lysates were subjected to SDS-PAGE and immunoblotting with antibodies that recognize the phosphorylated forms of ERK1/2, p38α MAPK, and JNK1/2. As a loading control, cell extracts were immunoblotted with an antibody recognizing all forms of p38α MAP kinase. C and D, same as A and B, except that MEFs were deprived of serum overnight and then incubated without (–) or with (+)1 μm BX795 for 60 min before stimulation with 10 ng/ml IL-1α (C) or TNFα (D).
DISCUSSION
Small cell-permeable inhibitors of protein kinases have proved to be valuable reagents for identifying novel substrates of these enzymes and for studying their physiological roles, but more compounds with the requisite potency and specificity to be really useful are needed. Here, we introduce BX795 as the first pharmacological inhibitor suitable for studying the function and regulation of TBK1 and IKKε in cells.
TBK1 and IKKε are known to be required for the activation of the transcription factor IRF3 and production of Type 1 interferons (10–13). However, when overexpressed in mammalian cells, these protein kinases also activate NFκB-dependent gene transcription (7–9, 18–20) (see the Introduction). In the present study we found that BX795 suppressed the activation of IRF3 and the production of interferon β but had no effect on the activation of NFκB or NFκB-dependent gene transcription by any of the stimuli that we studied. These results, which do not support a role for TBK1 and IKKε in activating NFκB in cells, are consistent with the phenotype of the mouse knock-out, where the absence of TBK1 and IKKε expression also had no effect on NFκB-dependent gene transcription by inflammatory stimuli (10, 11). We, therefore, conclude that despite their similarity to IKKα and IKKβ, the other members of the IKK subfamily of protein kinases, TBK1 and IKKε, are not rate-limiting for the activation of NFκB by TLR3 and TLR4 agonists or by TNFα and IL-1α.
The present study is the first to show that TBK1 and IKKε can be activated by IL-1α. We found that the activation of TBK1 and IKKε by IL-1α and TNFα was rapid (5–10 min) but transient. This contrasted with the slower and more sustained activation of these protein kinases by poly(I:C) and LPS and might explain, at least in part, why TNFα and IL-1α did not induce the phosphorylation of IRF3 or the production of interferon β (results not shown). The inability of TNFα and IL-1α to couple to TRAF3, which is located in the endosomal compartment of cells (33), may also account for the failure of TNFα and IL-1α to activate the interferon pathway. The substrates of TBK1 and IKKε in the TNFα and IL-1α signaling pathways remain to be identified, although TBK1 knock-out mice die just before birth from TNF-induced apoptosis of the liver (21). This suggests that TBK1 normally plays a role in suppressing TNF-stimulated apoptosis, at least in embryonic liver.
Before the present study, others had proposed that the phosphorylation of TBK1 and IKKε at Ser-172 and the activation of these protein kinases was an autophosphorylation event (9, 15). These conclusions were based on overexpression studies, and indeed, we confirmed that the phosphorylation of Ser-172 and activation of the overexpressed TBK1 catalytic subunit was blocked by BX795. However, BX795 failed to suppress the phosphorylation and activation of endogenous TBK1 and IKKε in response to poly(I:C), LPS, TNFα, and IL-1α. These results demonstrate that although TBK1 and IKKε can autophosphorylate at Ser-172 and autoactivate when they are overexpressed in cells, the activation of the endogenous protein kinases is not mediated by autophosphorylation but by a distinct upstream activating kinase(s). The molecular mechanisms that prevent the autophosphorylation of endogenous TBK1 and IKKε are unclear. However, endogenous TBK1 and IKKε are present as inactive and separate complexes in unstimulated cells, presumably due to their interaction with other proteins, such as TANK, NAP-1, SINTBAD, and Optineurin (8, 34–36) and/or their dephosphorylation by protein phosphatases.
It will clearly be important to identify the protein kinase(s) responsible for phosphorylating TBK1 and IKKε at Ser-172, the most likely candidates being one or more of the 22 members of the MAP3K subfamily and the 12 members of the MAP4K subfamily. LPS- and poly(I:C)-stimulated IRF3-dependent gene transcription was not affected in MEFs that do not express TAK1 (MAP3K7) (37) and the overexpression of a dominant negative mutant of TAK1 blocked NFκB-dependent gene transcription without affecting IRF3-dependent gene transcription in response to poly(I:C) and TLR3 agonists (37, 38). Thus, TAK1 does not seem to be required for the activation of TBK1/IKKε by LPS or poly(I:C). In the present study we showed that the IL-1α-mediated phosphorylation of TBK1 and IKKε at Ser-172 in MEFs that express a truncated, inactive form of TAK1 instead of the wild type protein is similar to wild type MEFs. Interestingly, the TNFα-induced phosphorylation of TBK1/IKKε at Ser-172 was reduced significantly in the TAK1-deficient MEFs (Fig. 8), indicating that TAK1 contributes to the activation of TBK1/IKKε by this agonist. Nevertheless, TAK1 might not be a direct activator of TBK1/IKKε but is, instead, required for the synthesis or activation of another protein kinase that directly phosphorylates and activates TBK1/IKKε under these conditions. In conclusion, these experiments in TAK1-deficient MEFs indicate that distinct protein kinases may mediate the activation of TBK1/IKKε by TNFα and IL-1α.
The MLK isoforms are potently inhibited by BX795 (Table 1), which may underlie the suppression of JNK1/2 and p38α MAPK activation by BX795, as discussed later. However, as BX795 does not inhibit the phosphorylation of TBK1/IKKε at Ser-172, the MLKs can probably also be excluded as activators of TBK1/IKKε.
Our finding that the activation of endogenous TBK1 and IKKε is not an autophosphorylation event, as believed previously, has more general implications, because it raises the possibility that other protein kinases currently believed to be activated by autophosphorylation based on overexpression experiments alone may also be activated by separate upstream kinases in vivo. This issue will need to be revisited when specific pharmacological inhibitors of these protein kinases become available.
Interestingly, BX795 enhanced the phosphorylation and activation of TBK1 and IKKε by poly(I:C), LPS, and IL-1α but not by TNFα. This finding implies that a feedback control mechanism exists in which TBK1 and IKKε phosphorylate an upstream component(s) of this signaling pathway to limit the extent to which TBK1/IKKε can be activated, thereby avoiding the overproduction of Type 1 interferons. Which upstream component is the target for feedback control is unknown, but if it was the protein kinase that activates TBK1/IKKε in response to LPS, poly(I:C), and IL-1α, this may explain why the feedback loop does not operate in the pathway leading to the activation of TBK1/IKKε by TNFα, which appears to require a separate and/or additional upstream activating protein kinase.
MAPK cascades play important roles in regulating the production of inflammatory mediators during bacterial and viral infection, which led us to find that BX795 suppresses the activation of p38α MAPK and JNK1/2 by LPS, poly(I:C), IL-1α, and TNFα. Two lines of evidence indicate that this effect of BX795 is independent of TBK1/IKKε. First, the activation of these MAPKs by poly(I:C) is not impaired in MEFs from TBK1/IKKε double knock-out mice (10). Second, novel chemical entities have recently been derived from BX795 by our collaborators at MRC Technology, which retain their ability to inhibit TBK1/IKKε and to suppress the phosphorylation of IRF3 and production of interferon β but no longer inhibit the activation of p38α MAPK or JNK1/2 in cells.4 The immediate upstream activators of JNK1/2 are MKK4 and MKK7 and for p38 MAP kinases are MKK3 and MKK6, but these four MKKs were unaffected by BX795 in vitro at concentrations (1 μm) that suppress the activation of JNK1/2 and p38α MAPK in cells. This suggests that the target of BX795 in the p38α MAPK and JNK1/2 pathways is upstream of the MKKs.
LPS, IL-1α, and TNFα are unable to activate the IKKα/β complex, JNK1/2, and p38 MAPKs in TAK1-deficient MEFs (26, 37), and these findings have led to a widespread acceptance that TAK1 is the direct activator of IKKα/β as well as the MKKs that activate JNK1/2 and p38 MAPK, in response to these stimuli. However, in the present study, we found that BX795 suppressed the activation of JNK1/2 but had no effect on the activation of the IKKα/β complex by inflammatory stimuli. These observations are inconsistent with the notion that the same MAP3K activates both signaling pathways but are consistent with the work of Zhong and Kyriakis (39, 40). These investigators reported that LPS and some other inflammatory stimuli activate JNK1/2 and p38 MAPK by stabilizing MAP4K2, also termed germinal centre kinase (39). This led to a rapid rise in the expression of germinal centre kinase, enabling it to activate MLK2 (MAP3K10) and MLK3 (MAP3K11), which then activated the MKKs that switch on JNK1/2 and p38 MAPK. In contrast, the germinal centre kinase-MLK2/3 pathway does not activate the IKKα/β complex (40). These findings led us to discover that BX795 is a potent inhibitor of MLK isoforms in vitro (Table 1). Moreover, the novel compounds that have recently been derived from BX795 by MRC Technology and do not suppress the activation of JNK1/2 and p38α MAPK in cells are far less potent inhibitors of MLK3 in vitro than BX795.4 These results suggest that inhibition of one or more MLK isoforms may underlie the inhibition of JNK1/2 and p38α MAPK by LPS, poly(I:C), IL-1α, and TNFα. In contrast, BX795 was found to be a much weaker inhibitor of TAK1 in vitro (Table 1), which could explain why BX795 does not suppress the activation of IKKα/β complex. Why inflammatory stimuli cannot activate JNK1/2 and p38α MAPK in TAK1-deficient cells is unknown, although one possible explanation is that TAK1 controls the expression of one or more germinal centre kinase and/or MLK isoforms.
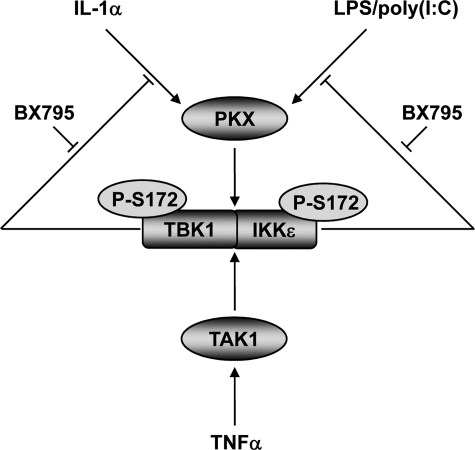
In summary, we have used the pharmacological inhibitor BX795 to study the function and regulation of TBK1 and IKKε. Most importantly, our results have demonstrated that the activation of endogenous TBK1 and IKKε, which correlates with the phosphorylation of Ser-172, is not an autophosphorylation event. Our current paradigm for the activation of TBK1 and IKKε in response to proinflammatory stimuli is summarized in Fig. 10. Upon stimulation of cells with LPS, poly(I:C), IL-1α, and TNFα, protein kinases are activated that phosphorylate Ser-172 in the activation loop of TBK1 and IKKε. LPS, poly(I:C), and IL-1α control a negative feedback loop that prevents the hyperactivation of TBK1 and IKKε by phosphorylating one or more upstream components of these pathways. Alternatively, or in addition, the feedback control loop might involve the TBK1/IKKε-mediated activation of a protein phosphatase(s) that dephosphorylates Ser-172. This feedback control loop does not operate in the TNFα signaling pathway in MEFs, which seems to employ a distinct upstream protein kinase for the activation of TBK1 and IKKε that is dependent on TAK1 activity.
FIGURE 10.
Model for the activation and feedback control of TBK1 and IKKε by proinflammatory stimuli. The binding of IL-1α, LPS, and poly(I:C) to their respective receptors induces the activation of an unidentified protein kinase (PKX). PKX subsequently phosphorylates TBK1 and IKKε at Ser-172, thereby triggering their activation. TBK1 and IKKε can then phosphorylate substrates such as the transcription factor IRF3. As shown in this study, TBK1 and IKKε also exert a negative feedback control on their activation by IL-1α, LPS, and poly(I:C) presumably by phosphorylation and inhibition of an upstream component of the pathway and/or by activating a Ser-172 protein phosphatase (not illustrated). The TNFα-stimulated activation of TBK1 and IKKε appears to require a separate or additional protein kinase that is at least partially dependent on TAK1 activity, and this arm of the pathway is not subject to feedback control.
Acknowledgments
We thank Dr. Katherine Fitzgerald for generously providing IRF3 and NFκB reporter constructs and the 293-TLR3 cells. We also thank Professor Shizuo Akira for kindly providing immortalized MEFs expressing a truncated, inactive form of TAK1 and control MEFs, Dr. Alan Prescott for assistance with microscopy, Dr. Natalia Shpiro for synthesizing BX795, and the Protein and Antibody Production Teams of the Division of Signal Transduction Therapy at the University of Dundee (coordinated by Hilary McLauchlan and James Hastie) for proteins and antibodies.
The work was supported by the United Kingdom Medical Research Council, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck-Serono, and Pfizer.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: MAP, mitogen-activated protein; MAPK, MAP kinase; MKK, MAPK kinase; MAP3K, MAPK kinase kinase; ASK1, apoptosis-stimulating kinase-1; ERK, extracellular signal-regulated kinase; IFN, interferon; IKK, IκB kinase; IRF3, interferon regulatory factor 3; JNK1/2, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MLK, mixed lineage kinase; PDK1, 3-phosphoinositide-dependent protein kinase 1; TAK1, transforming growth factor β-activated kinase 1; TBK1, TANK-binding kinase 1; TLR, Toll-like receptor; TNF, tumor necrosis factor; IL, interleukin; MEF, mouse embryonic fibroblast; GST, glutathione S-transferase.
K. Clark, L. Plater, and P. Cohen, unpublished results.
References
- 1.Akira, S., Uematsu, S., and Takeuchi, O. (2006) Cell 124 783–801 [DOI] [PubMed] [Google Scholar]
- 2.Hayden, M. S., and Ghosh, S. (2008) Cell 132 344–362 [DOI] [PubMed] [Google Scholar]
- 3.Beinke, S., Robinson, M. J., Hugunin, M., and Ley, S. C. (2004) Mol. Cell. Biol. 24 9658–9667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterfield, M., Jin, W., Reiley, W., Zhang, M., and Sun, S. C. (2004) Mol. Cell. Biol. 24 6040–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai, T., and Akira, S. (2006) Nat. Immunol. 7 131–137 [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi, O., and Akira, S. (2008) Curr. Opin. Immunol. 20 17–22 [DOI] [PubMed] [Google Scholar]
- 7.Tojima, Y., Fujimoto, A., Delhase, M., Chen, Y., Hatakeyama, S., Nakayama, K., Kaneko, Y., Nimura, Y., Motoyama, N., Ikeda, K., Karin, M., and Nakanishi, M. (2000) Nature 404 778–782 [DOI] [PubMed] [Google Scholar]
- 8.Pomerantz, J. L., and Baltimore, D. (1999) EMBO J. 18 6694–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimada, T., Kawai, T., Takeda, K., Matsumoto, M., Inoue, J., Tatsumi, Y., Kanamaru, A., and Akira, S. (1999) Int. Immunol. 11 1357–1362 [DOI] [PubMed] [Google Scholar]
- 10.Hemmi, H., Takeuchi, O., Sato, S., Yamamoto, M., Kaisho, T., Sanjo, H., Kawai, T., Hoshino, K., Takeda, K., and Akira, S. (2004) J. Exp. Med. 199 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry, A. K., Chow, E. K., Goodnough, J. B., Yeh, W. C., and Cheng, G. (2004) J. Exp. Med. 199 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McWhirter, S. M., Fitzgerald, K. A., Rosains, J., Rowe, D. C., Golenbock, D. T., and Maniatis, T. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald, K. A., McWhirter, S. M., Faia, K. L., Rowe, D. C., Latz, E., Golenbock, D. T., Coyle, A. J., Liao, S. M., and Maniatis, T. (2003) Nat. Immunol. 4 491–496 [DOI] [PubMed] [Google Scholar]
- 14.Schroder, M., Baran, M., and Bowie, A. G. (2008) EMBO J. 27 2147–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soulat, D., Burckstummer, T., Westermayer, S., Goncalves, A., Bauch, A., Stefanovic, A., Hantschel, O., Bennett, K. L., Decker, T., and Superti-Furga, G. (2008) EMBO J. 27 2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong, B., Yang, Y., Li, S., Wang, Y. Y., Li, Y., Diao, F., Lei, C., He, X., Zhang, L., Tien, P., and Shu, H. B. (2008) Immunity 29 538–550 [DOI] [PubMed] [Google Scholar]
- 17.Tenoever, B. R., Ng, S. L., Chua, M. A., McWhirter, S. M., Garcia-Sastre, A., and Maniatis, T. (2007) Science 315 1274–1278 [DOI] [PubMed] [Google Scholar]
- 18.Buss, H., Dorrie, A., Schmitz, M. L., Hoffmann, E., Resch, K., and Kracht, M. (2004) J. Biol. Chem. 279 55633–55643 [DOI] [PubMed] [Google Scholar]
- 19.Mattioli, I., Geng, H., Sebald, A., Hodel, M., Bucher, C., Kracht, M., and Schmitz, M. L. (2006) J. Biol. Chem. 281 6175–6183 [DOI] [PubMed] [Google Scholar]
- 20.Harris, J., Oliere, S., Sharma, S., Sun, Q., Lin, R., Hiscott, J., and Grandvaux, N. (2006) J. Immunol. 177 2527–2535 [DOI] [PubMed] [Google Scholar]
- 21.Bonnard, M., Mirtsos, C., Suzuki, S., Graham, K., Huang, J., Ng, M., Itie, A., Wakeham, A., Shahinian, A., Henzel, W. J., Elia, A. J., Shillinglaw, W., Mak, T. W., Cao, Z., and Yeh, W. C. (2000) EMBO J. 19 4976–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korherr, C., Gille, H., Schafer, R., Koenig-Hoffmann, K., Dixelius, J., Egland, K. A., Pastan, I., and Brinkmann, U. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 4240–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehm, J. S., Zhao, J. J., Yao, J., Kim, S. Y., Firestein, R., Dunn, I. F., Sjostrom, S. K., Garraway, L. A., Weremowicz, S., Richardson, A. L., Greulich, H., Stewart, C. J., Mulvey, L. A., Shen, R. R., Ambrogio, L., Hirozane-Kishikawa, T., Hill, D. E., Vidal, M., Meyerson, M., Grenier, J. K., Hinkle, G., Root, D. E., Roberts, T. M., Lander, E. S., Polyak, K., and Hahn, W. C. (2007) Cell 129 1065–1079 [DOI] [PubMed] [Google Scholar]
- 24.Feldman, R. I., Wu, J. M., Polokoff, M. A., Kochanny, M. J., Dinter, H., Zhu, D., Biroc, S. L., Alicke, B., Bryant, J., Yuan, S., Buckman, B. O., Lentz, D., Ferrer, M., Whitlow, M., Adler, M., Finster, S., Chang, Z., and Arnaiz, D. O. (2005) J. Biol. Chem. 280 19867–19874 [DOI] [PubMed] [Google Scholar]
- 25.Bain, J., Plater, L., Elliott, M., Shpiro, N., Hastie, C. J., McLauchlan, H., Klevernic, I., Arthur, J. S., Alessi, D. R., and Cohen, P. (2007) Biochem. J. 408 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato, S., Sanjo, H., Takeda, K., Ninomiya-Tsuji, J., Yamamoto, M., Kawai, T., Matsumoto, K., Takeuchi, O., and Akira, S. (2005) Nat. Immunol. 6 1087–1095 [DOI] [PubMed] [Google Scholar]
- 27.Ananieva, O., Darragh, J., Johansen, C., Carr, J. M., McIlrath, J., Park, J. M., Wingate, A., Monk, C. E., Toth, R., Santos, S. G., Iversen, L., and Arthur, J. S. (2008) Nat. Immunol. 9 1028–1036 [DOI] [PubMed] [Google Scholar]
- 28.Collins, B. J., Deak, M., Murray-Tait, V., Storey, K. G., and Alessi, D. R. (2005) J. Cell Sci. 118 5023–5034 [DOI] [PubMed] [Google Scholar]
- 29.Clark, K., Langeslag, M., van Leeuwen, B., Ran, L., Ryazanov, A. G., Figdor, C. G., Moolenaar, W. H., Jalink, K., and van Leeuwen, F. N. (2006) EMBO J. 25 290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, R., Heylbroeck, C., Pitha, P. M., and Hiscott, J. (1998) Mol. Cell. Biol. 18 2986–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pullen, N., Dennis, P. B., Andjelkovic, M., Dufner, A., Kozma, S. C., Hemmings, B. A., and Thomas, G. (1998) Science 279 707–710 [DOI] [PubMed] [Google Scholar]
- 32.Kishore, N., Huynh, Q. K., Mathialagan, S., Hall, T., Rouw, S., Creely, D., Lange, G., Caroll, J., Reitz, B., Donnelly, A., Boddupalli, H., Combs, R. G., Kretzmer, K., and Tripp, C. S. (2002) J. Biol. Chem. 277 13840–13847 [DOI] [PubMed] [Google Scholar]
- 33.Kagan, J. C., Su, T., Horng, T., Chow, A., Akira, S., and Medzhitov, R. (2008) Nat. Immunol. 9 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita, F., Taniguchi, Y., Kato, T., Narita, Y., Furuya, A., Ogawa, T., Sakurai, H., Joh, T., Itoh, M., Delhase, M., Karin, M., and Nakanishi, M. (2003) Mol. Cell. Biol. 23 7780–7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryzhakov, G., and Randow, F. (2007) EMBO J. 26 3180–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morton, S., Hesson, L., Peggie, M., and Cohen, P. (2008) FEBS Lett. 582 997–1002 [DOI] [PubMed] [Google Scholar]
- 37.Shim, J. H., Xiao, C., Paschal, A. E., Bailey, S. T., Rao, P., Hayden, M. S., Lee, K. Y., Bussey, C., Steckel, M., Tanaka, N., Yamada, G., Akira, S., Matsumoto, K., and Ghosh, S. (2005) Genes Dev. 19 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang, Z., Mak, T. W., Sen, G., and Li, X. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong, J., and Kyriakis, J. M. (2004) Mol. Cell. Biol. 24 9165–9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong, J., and Kyriakis, J. M. (2007) J. Biol. Chem. 282 24246–24254 [DOI] [PubMed] [Google Scholar]