Abstract
Estrogen receptor-related receptor γ (ERRγ/ERR3/NR3B3) is a member of the orphan nuclear receptor with important functions in development and homeostasis. Recently it has been reported that ERRα is involved in osteoblast differentiation and bone formation. In the present study we examined the role of ERRγ in osteoblast differentiation. Here, we showed that ERRγ is expressed in osteoblast progenitors and primary osteoblasts, and its expression is increased temporarily by BMP2. Overexpression of ERRγ reduced BMP2-induced alkaline phosphatase activity and osteocalcin production as well as calcified nodule formation, whereas inhibition of ERRγ expression significantly enhanced BMP2-induced osteogenic differentiation and mineralization, suggesting that endogenous ERRγ plays an important role in osteoblast differentiation. In addition, ERRγ significantly repressed Runx2 transactivity on osteocalcin and bone sialoprotein promoters. We also observed that ERRγ physically interacts with Runx2 in vitro and in vivo and competes with p300 to repress Runx2 transactivity. Notably, intramuscular injection of ERRγ strongly inhibited BMP2-induced ectopic bone formation in a dose-dependent manner. Taken together, these results suggest that ERRγ is a novel negative regulator of osteoblast differentiation and bone formation via its regulation of Runx2 transactivity.
Bone formation is a series of well orchestrated lineage-specific differentiation events (1). Osteoblasts, which play key roles in bone formation, are derived from pluripotent mesenchymal stem cells that have the capacity to differentiate into myocytes, adipocytes, and chondrocytes (2). Osteoblasts possess the necessary components to form bone matrix, which allows subsequent mineralization. Several hormones, growth factors, cytokines, and nuclear receptor proteins regulate these sequential events to trigger a complex network of signaling pathways.
Bone morphogenetic proteins (BMPs)5 are members of the transforming growth factor β family and were originally identified by their capacity to induce ectopic bone formation (3, 4). Among the BMP family members, the action of BMP2 has been studied extensively in embryonic skeletal development, postnatal bone remodeling, and bone repair (5, 6). BMP2 promotes the commitment of pluripotent mesenchymal cells to the osteoblast lineage by regulating the signals that stimulate the specific transcriptional programs required for bone formation (5, 7).
A master regulator of osteoblasts, Runx2, is indispensable for a skeletal development and maturation. Targeted disruption of Runx2 results in a complete lack of functional osteoblasts (8, 9). Runx2 directly regulates osteoblast-specific genes such as osteocalcin (OC), bone sialoprotein (BSP), osteopontin, and type I collagen through binding to specific DNA enhancer elements of its target gene promoters (4). In addition, Runx2 interacts with a variety of transcription factors (10) and recruits both co-activators (11–13) and co-repressors (14, 15) to form a complex on its target promoter. Therefore, it is important to identify the possible partners of Runx2 to understand the mechanism of Runx2-dependent osteoblast differentiation.
Estrogen receptor-related receptors (ERRs) are closely related to estrogen receptor (ER) without binding to the classical ER ligand but share high homology in their DNA binding domain (16). To date, three subtypes of ERR have been identified as ERRα,-β, and -γ, based on their sequence similarity to ERα, and have been shown to regulate a broad spectrum of genes in their target cells. Recently, it has been reported that ERRα is involved functionally in bone differentiation. It is strongly expressed throughout the osteoblast differentiation process and plays a physiological role in differentiation and bone formation (17). It also regulates osteopontin expression through a non-canonical ERRα response element (18). ERRγ is the most recently described member of the ERR subfamily. It differs from the other family members in that it is a constitutively active nuclear receptor with high basal transcriptional activity (19).
In this study we examined the role of ERRγ in osteoblast differentiation in vitro and ectopic bone formation in vivo, and our results show that orphan nuclear receptor ERRγ plays an inhibitory role in BMP2-induced osteoblast differentiation and bone formation.
EXPERIMENTAL PROCEDURES
Recombinant Proteins—Recombinant human BMP2 (rhBMP2) was obtained from R&D Systems (Minneapolis, MN). The stock solution (1 mg/ml) was prepared in phosphate-buffered saline containing 0.1% bovine serum.
Plasmids and Adenoviruses—The ERRγ promoter was PCR-amplified from mouse genomic DNA (Novagen) and inserted into the pGL3 basic vector (Promega) using the MluI and XhoI restriction enzyme site (ERRγ-Luc). pcDNA3/HA-ERRγ and GST-ERRγ are previously described (20). 6×OSE-Luc reporter construct, pcDNA3/Runx2, GST-Runx2, and pcDNA3/p300 are provided by Dr. K. Y. Lee (Chonnam National University, Republic of Korea). The constructs for OG2-Luc (21) and BSP-Luc (22) are described previously. Adenovirus BMP2 (Ad-BMP2) (4) and ERRγ (Ad-ERRγ) (20) are described previously. Adenovirus short hairpin ERRγ (Ad-shERRγ) was generated with pAd-easy system as described (23). The target sequence of shERRγ is GAACGGACTGGACTCGCCACCTCTCTA.
Cell Cultures and Viral Infection—Murine myoblast (C2C12) and human embryonic kidney (HEK)-293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen). The pre-osteoblast cells (MC3T3-E1) were cultured in α-minimal essential medium (Invitrogen) and the same antibiotics. The cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C. For the in vitro adenovirus infection, the cells were plated at a density of 50,000 cells/cm2. After virus infection, the culture medium was changed to a mineralizing medium (50 μg/ml ascorbic acid and 5 mm β-glycerophosphate), which was replaced every other day unless otherwise indicated.
Preparation of Primary Osteoblasts—Calvariae were isolated from 10-day-old neonatal mice and digested with 0.1% collagenase (Roche Applied Science) at 37 °C for 30 min. The collagenase digest was then discarded and replaced with other fresh collagenase solution. After 30 min the cells were collected by a sedimentation step and centrifuged twice at 400 × g for 10 min. Finally, cells in the pellet fraction were used for primary culture.
RNA Preparation and Semi-quantitative RT-PCR—Total RNA was extracted from the cells using the TRIzol reagent (Invitrogen) and RNase-free DNase (Qiagen) according to the manufacturer's instructions. The complementary DNA was synthesized from the total RNA using a random primer and reverse transcriptase (Invitrogen). Each reaction consisted of initial denaturation at 94 °C for 1 min followed by three-step cycling: denaturation at 94 °C for 30 s, annealing at a temperature optimized for each primer pair for 30 s, and extension at 72 °C for 1 min. After the requisite number of cycles (27–30 cycles), the reactions underwent a final extension at 72 °C for 5 min. The following primer sequences, designed based on published cDNA sequences, were used for PCR: ERRα, forward 5′-CAGGAAAGTGAATGCCCAGG-3′ and reverse 5′-CTTTGCAGCAAATATACATT-3′; ERRβ, forward 5′-TGGACTCGCCGCCTATGTTCG and reverse 5′-ACTTGCGCTCCGTTTGGTGA-3′; ERRγ, forward 5′-ACCATGAATGGCCATCAGAA-3′ and reverse 5′-ACCAGCTGAGGGTTCAGGTAT-3′; OC, forward 5′-CTCCTGAGAGTCTGACAAAGCCTT-3′ and reverse 5′-GCTGTGACATCCATTACTTGC-3′; ALP, forward 5′-GATCATTCCCACGTTTTCAC-3′ and reverse 5′-TGCGGGCTTGTGGGCCTGC-3′.
Transient Transfection and Luciferase Assay—C2C12 and MC3T3-E1 cells were transiently transfected with the indicated plasmids using FuGENE 6 (Roche Applied Science). Approximately 48 h after transfection, the cells were lysed and assayed using the Dual Luciferase reporter assay system (Promega). The luciferase activity was normalized to the β-galactosidase activity.
Determination of Osteogenic Differentiation—MC3T3-E1 and C2C12 cells were infected with Ad-ERRγ or Ad-shERRγ. After 24 h the medium was changed to an osteogenic medium containing ascorbic acid (50 μg/ml) and β-glycerophosphate (5 mm) with or without BMP2 (200 ng/ml). At the designated time point, the cell homogenates were reacted with the ALP assay mixture containing 0.1 m 2-amino-2-methyl-1-propanol (Sigma), 1 mm MgCl2, and 8 mm p-nitrophenyl phosphate. After 5 min of incubation at 37 °C, the reaction was quenched by adding 0.1 n NaOH, and the absorbance was measured at 405 nm. The quantitative double-stranded DNA in solution, which was used to normalize the ALP activity, was measured using a Picogreen® double-stranded DNA quantification kit (Molecular Probes). The amount of OC was detected in cell supernatants by a commercially available enzyme-linked immunosorbent assay kit (Biomedical Technologies, Stoughton, MA). For alizarin red staining, cells were fixed with 70% ethanol and treated with a 40 mm alizarin red stain (AR-S) solution (pH 4.2) for 10 min to stain the calcium deposits. The stained cultures were photographed, and the AR-S was extracted using 10% (w/v) cetylpyridinium chloride in 10 mm sodium phosphate (pH 7.0) for quantification. The AR-S concentration was determined by measuring the absorbance at 540 nm on a multiplate reader using an AR-S standard curve in the same solution.
Glutathione S-Transferase (GST) Pulldown Assay—The Runx2 and p300 cDNA in pcDNA3 were transcribed and translated in vitro using a coupled rabbit reticulocyte system (Promega) in the presence of [35S]methionine according to the manufacturer's instructions. The indicated GST fusion proteins or GST alone were expressed in Escherichia coli BL21 (DE3) pLys using 0.2 mm isopropyl-β-d-thiogalactopyranoside. The GST fusion proteins were pre-bound to a 30-μl aliquot of glutathione-Sepharose beads (Amersham Biosciences) and then incubated with the in vitro translated proteins for 4 h at 4 °C. The beads were then washed three times with a washing buffer, analyzed by SDS-PAGE, and visualized using a phosphorimaging analyzer (BAS-1500, Fuji).
Western Blot Analysis—Total cell extracts were lysed, subjected to 10% SDS-PAGE, and transferred to polyvinylidene difluoride membranes. After blocking in 5% skim milk in Tris-buffered saline with 0.1% Tween 20, the membrane was incubated with specific antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Antigen-antibody interactions were visualized by incubation with ECL chemiluminescence reagent (Amersham Biosciences).
Coimmunoprecipitation Analysis—HEK-293T cells were transfected with pcDNA3/Runx2 and pcDNA3/HA-ERRγ. Whole cell extracts were lysed with a buffer containing 1% Triton X-100, 1% deoxycholate, 50 mm Tris (pH 7.4), 100 mm NaCl, 25 mm sodium fluoride, 10 mm sodium pyrophosphate, 2 mm sodium orthovanadate, 2 mm EDTA, and a protease inhibitor mixture (Roche Applied Science). The lysates were precleared with 50 μl of protein A/G-agarose beads (Invitrogen) for 2 h, which were removed by centrifugation. A total of 2 μgof antibody (polyclonal) against Runx2 (Santa Cruz Biotechnology) were added to the precleared lysates. After centrifugation, the immunoprecipitated complexes were separated by SDS-PAGE, transferred to polyvinylidene difluoride, and immunoblotted with specific antibodies.
Ectopic Bone Formation in Vivo—C57BL/6J mice (male, 8-week-old) were purchased from Daehan Biolink (Chungbuk, Korea) and assigned randomly to each experimental group. The studies were carried out under the guidelines of the Chonnam National University Animal Care and Use Committee. The mice were injected with a designated dose of particle number (PN) of the adenovirus in the thigh muscles; 5 × 1010 PN of Ad-LacZ (MOCK, n = 4), 5 × 1010 PN of Ad-BMP2 (n = 4), 5 × 1010 PN (n = 4), or 15 × 1010 PN (n = 4) of Ad-ERRγ diluted in phosphate-buffered saline. The amount of ectopic bone formation in the two-dimensional image was monitored using a microradiographic apparatus (Hi-Tex, Japan; exposure at 35 kV for 45 s) and x-ray film (Eastman Kodak Co.). MicroCT (μCT) (Skyscan 1172, Skyscan, Belgium) was used for three-dimensional observations of bone formation. The scanned images and samples were collected at 50 kV and 200 μA and reconstructed using NRecon software and three-dimensional CT analyzer software (Skyscan). For histological analysis, the mice were sacrificed at 5 weeks after virus injection. The injected thigh muscles were removed and fixed in 10% neutral-buffered formalin. The samples were decalcified in 10% EDTA, embedded in paraffin, and cut into sections (4 μm). The sections were stained with hematoxylin and eosin.
Statistical Analysis—All experiments were repeated at least twice, and a Student's t test was used to examine the differences between the two groups of data. Differences with a p < 0.05 were considered statistically significant. The results are expressed as mean ± S.D.
RESULTS
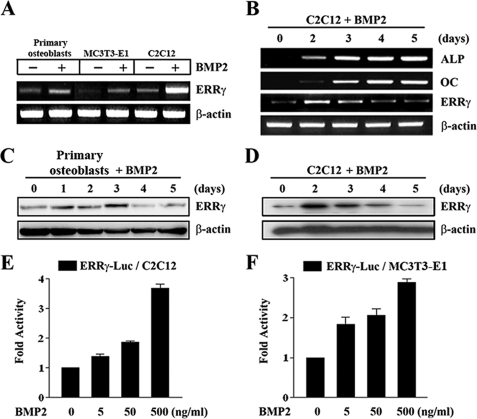
Expression Profiles of ERRγ during Osteogenic Differentiation—To evaluate the potential role of ERRγ in BMP2-mediated osteogenic differentiation, we examined the expression levels of ERRγ in various murine progenitor cells after BMP2 treatment. As shown in Fig. 1A, ERRγ gene expression was significantly regulated by BMP2 in the tested cells. During osteoblast differentiation, which was determined by ALP and OC expression, ERRγ mRNA and proteins were significantly but temporarily increased at day 2 or 3 and then were restored to the basal level thereafter in C2C12 and primary osteoblasts, respectively (Fig. 1, B–D). To investigate whether the expression of ERRγ is dependent on BMP2, we examined the effect of BMP2 on ERRγ gene promoter. As shown in Fig. 1, E and F, the promoter activity of ERRγ was significantly increased by BMP2 in a dose-dependent manner in both C2C12 and MC3T3-E1 cells, suggesting that BMP2 is a potential regulator of ERRγ gene expression. These results suggest that ERRγ, which is partially induced by BMP2, has a functional role in osteoblast differentiation.
FIGURE 1.
Expression profiles of the ERRγ in osteoblast progenitor cells. A, expression of ERRγ mRNA in osteoblast progenitor cells. Primary osteoblasts, MC3T3-E1, and C2C12 cells were cultured in the absence or presence of BMP2 (200 ng/ml) for 24 h. RT-PCR was carried out with the indicated primers. B, expression of ERRγ during osteoblast differentiation by BMP2. C2C12 cells were cultured in osteogenic medium containing ascorbic acid (50 μg/ml) and β-glycerophosphate (5 mm) in the presence of BMP2 (200 ng/ml) for 5 days. At the designated time points, cells were harvested for total RNA isolation, and RT-PCR was performed with the indicated primers. C and D, Western blot analysis of ERRγ expression. Primary osteoblasts and C2C12 cells were cultured in osteogenic medium with BMP2 for 5 days. At the indicated time points, the protein extracts were used for Western blot analysis with the indicated antibody. E and F, transactivation of ERRγ promoter by BMP2. C2C12 and MC3T3-E1 cells were transfected with 200 ng of ERRγ-Luc reporter plasmid and 100 ng of pCMV-β-galactosidase as an internal control with the indicated amounts of BMP2, respectively. Data are expressed as the mean ± S.D. of three independent experiments.
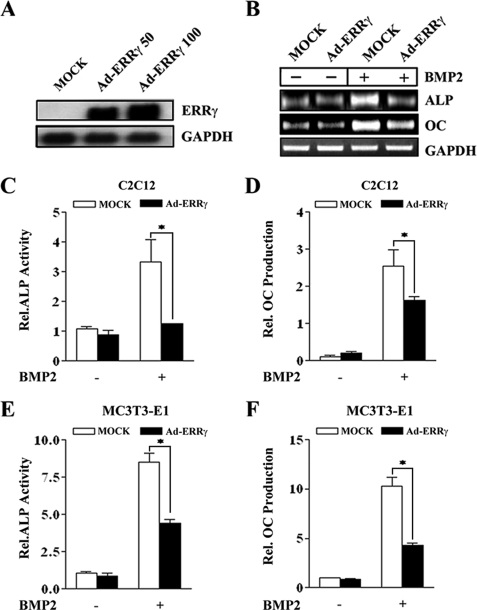
ERRγ Negatively Regulates BMP2-induced Osteogenic Differentiation—To clarify the potential role of ERRγ in osteogenic differentiation, we generated adenoviral vector expressing ERRγ to obtain a high level of expression of this factor (Fig. 2A). As shown in Fig. 2B, adenovirus-mediated overexpression of ERRγ in C2C12 cells significantly repressed BMP2-induced osteoblast-specific marker genes, ALP and OC. As a functional analysis, we further confirmed the changes in ALP activity and OC production by Ad-ERRγ in C2C12 (Fig. 2, C and D) and MC3T3-E1 (Fig. 2, E and F) cells, respectively. BMP2-induced ALP activity and OC production were significantly suppressed by Ad-ERRγ.
FIGURE 2.
Overexpression of ERRγ inhibits BMP2-induced osteogenic differentiation. A, adenovirus-mediated overexpression of ERRγ. C2C12 cells were infected with Ad-LacZ (MOCK) and Ad-ERRγ (multiplicity of infection = 50 and 100) for 24 h, and Northern blot analysis was performed with the indicated probes. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. B, osteoblastic marker gene expression by ERRγ. C2C12 cells were infected with MOCK virus or Ad-ERRγ (multiplicity of infection = 100) in the absence or presence of BMP2 (200 ng/ml) for 4 days, and RT-PCR was performed with the indicated primers. C–F, BMP2-induced ALP activity and OC production by ERRγ overexpression in C2C12 and MC3T3-E1 cells. Cells were infected with MOCK virus or Ad-ERRγ in the absence or presence of BMP2 (200 ng/ml). Five days later cells were harvested, and the lysates and culture medium were used for ALP activity and OC production assays, respectively, as described under “Experimental Procedures.” Data are expressed as the mean ± S.D. of triplicate samples (*, p < 0.05).
To further confirm the role of endogenous ERRγ in BMP2-induced osteogenic differentiation, we used adenoviral short hairpin RNA (Ad-shRNA) for ERRγ to inhibit the expression of ERRγ. As shown in Fig. 3A, Ad-shERRγ significantly inhibited the gene expression of ERRγ in MC3T3-E1 cells. Moreover, inhibition of ERRγ gene expression significantly increased BMP2-induced ALP activity and OC production in MC3T3-E1 cells (Fig. 3, B and C). Taken together, these results demonstrate that ERRγ plays a crucial role in BMP2-induced osteogenic differentiation.
FIGURE 3.
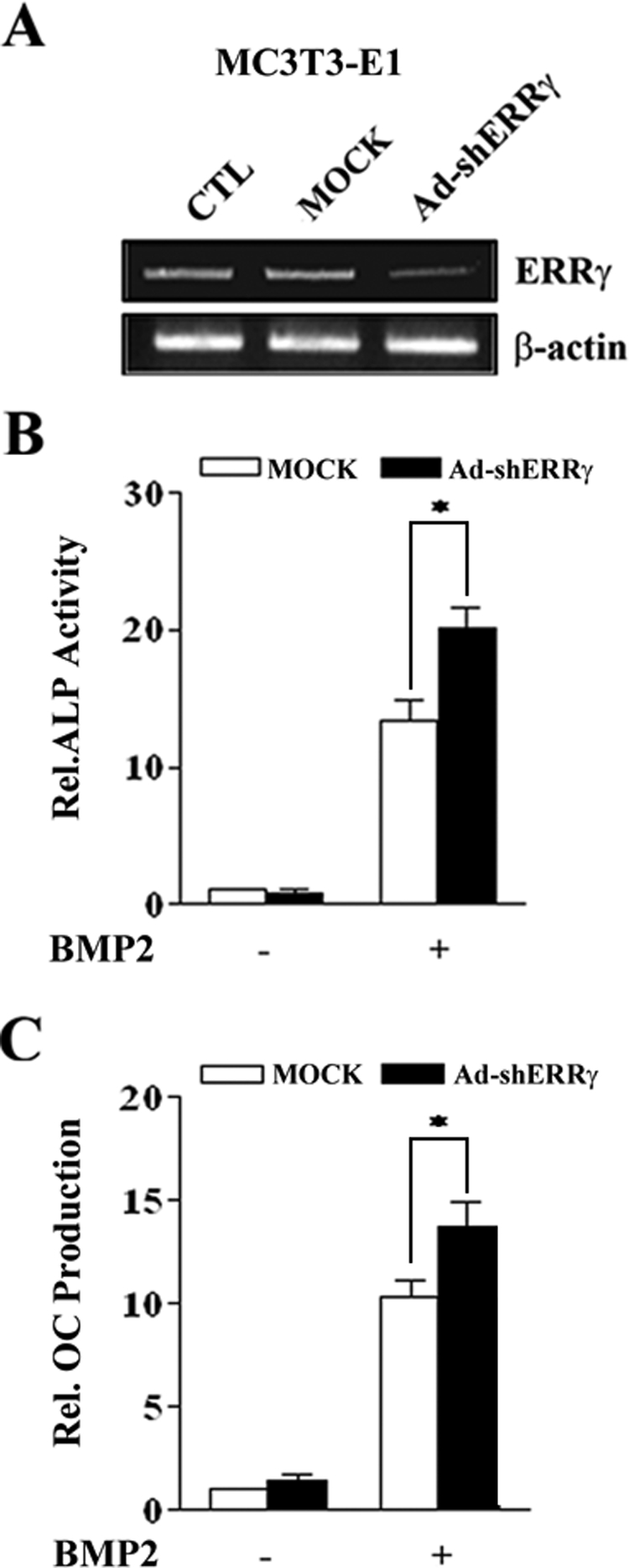
Inhibition of endogenous ERRγ expression enhances BMP2-induced osteogenic differentiation. A, inhibition of ERRγ expression by Ad-shERRγ. MC3T3-E1 cells were infected with MOCK virus or Ad-shERRγ. 24 h after virus infection, the cells were harvested, and RT-PCR was performed. CTL, control. B and C, BMP2-induced ALP activity and OC production after Ad-shERRγ infection. MC3T3-E1 cells were infected with shERRγ in the absence or presence of BMP2 (200 ng/ml). 4 days later cells were harvested, and the lysates and culture medium were used for ALP activity and OC production assays, respectively (*, p < 0.05).
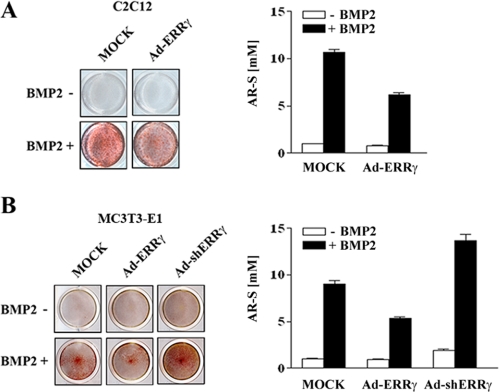
ERRγ Inhibits BMP2-induced Mineralized Nodule Formation—Extracellular matrix mineralization is the most important phenomenon in bone formation (24). To verify the functional influence of ERRγ on BMP2-induced mineralization, we either induced or inhibited the expression of ERRγ using Ad-ERRγ or Ad-shERRγ, respectively, in the presence or absence of BMP2 and assessed the amount of mineralization by alizarin red staining. As shown in Fig. 4A, overexpression of ERRγ significantly decreased BMP2-induced mineralized nodule formation in C2C12 cells. In MC3T3-E1 cells, Ad-ERRγ also significantly decreased the BMP2-induced calcified nodule formation, whereas Ad-shERRγ strongly increased the nodule formation (Fig. 4B). These results indicate that ERRγ prevents mineralization during osteoblast maturation.
FIGURE 4.
ERRγ decreases BMP2-induced mineralized nodule formation. A, C2C12 cells were infected with MOCK virus or Ad-ERRγ and cultured in osteogenic medium containing ascorbic acid (50 μg/ml) and β-glycerophosphate (5 mm) in the absence or presence of BMP2 (200 ng/ml) for 7 days. B, MC3T3-E1 cells were infected with MOCK virus or Ad-ERRγ or Ad-shERRγ and maintained in osteogenic medium in the absence or presence of BMP2 for 9 days. In both A and B, cells were fixed with 70% ethanol and stained with AR-S solution, and the AR-S concentration was measured to determine the level of mineralization as described under “Experimental Procedures.” The right panels of A and B depict the eluted AR-S concentrations, indicating the level of mineralization.
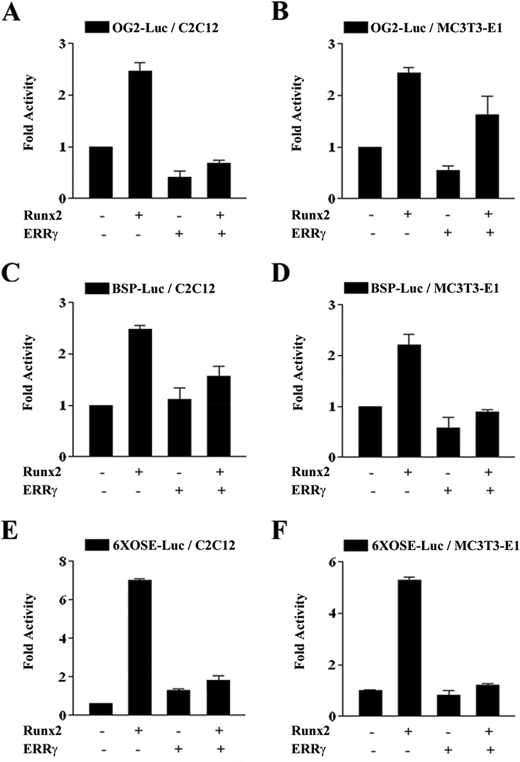
ERRγ Suppresses Runx2 Transactivity for Osteoblast-specific Genes—To elucidate the inhibitory mechanism of ERRγ in osteoblast differentiation, we hypothesized that Runx2 might be involved in the regulation of osteoblast differentiation by ERRγ as BMP2-Runx2 cascade is important for the differentiation of mesenchymal stem cells into osteoblast lineage (10). Moreover, many osteoblast-specific marker genes such as OC and BSP are reported to be regulated by Runx2 (22, 25). Therefore, we examined the effect of ERRγ on the transcriptional activity of Runx2 on osteoblast-specific genes. As shown in Fig. 5, A–D, Runx2-dependent promoter activities of osteocalcin (OG2-Luc) and bone sialoprotein (BSP-Luc) were significantly repressed by ERRγ overexpression in C2C12 and MC3T3-E1, respectively. To further confirm the specificity for Runx2, we used a 6×OSE-Luc reporter gene, which harbors six copies of the Runx2 binding region. As shown in Fig. 5, E and F, ERRγ strongly repressed the reporter activity in both cell lines. These results further support our hypothesis that the inhibition of osteoblast differentiation by ERRγ occurs through the repression of Runx2 transactivity.
FIGURE 5.
ERRγ represses Runx2 transcriptional activity. A–F, C2C12 and MC3T3-E1 cells were cotransfected with 200 ng of the indicated luciferase reporter constructs and 100 ng of pcDNA3/Runx2 constructs together with 100 ng of pcDNA3/HA-ERRγ. 48 h after transfection, cells were lysed, and luciferase activity was measured and normalized to β-galactosidase activity. Data are expressed as the mean ± S.D. of three independent experiments.
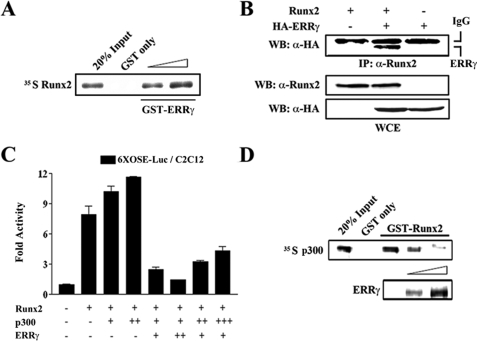
ERRγ Physically Interacts with Runx2 and Competes with p300 to Suppress Runx2 Transactivity—To gain insight into the molecular mechanism by which ERRγ represses Runx2 transactivity, we investigated the possibility of physical interaction between ERRγ and Runx2. To evaluate this possibility, we carried out in vitro and in vivo interaction assays. As shown in Fig. 6A, GST-ERRγ, but not GST alone, interacts with the in vitro translated Runx2 in a dose-dependent manner. To further examine whether ERRγ binds to Runx2 in vivo, we performed coimmunoprecipitation assays in HEK-293 cells. As shown in Fig. 6B, ERRγ indeed interacted with Runx2 in the cells. These results suggest that ERRγ physically interacts with Runx2 in vitro and in vivo.
FIGURE 6.
ERRγ physically interacts with Runx2 and competes with p300 to repress the Runx2 transactivity. A, direct interaction of ERRγ with Runx2. In vitro translated 35S-labeled Runx2 was incubated with a different amounts of GST-ERRγ proteins. Bound proteins were detected by autoradiography after SDS-PAGE. B, in vivo interaction of ERRγ with Runx2. HEK-293T cells were transfected with pcDNA3/HA-ERRγ and/or pcDNA3/Runx2. 48 h after transfection the cell extracts were immunoprecipitated (IP) with the anti-Runx2 antibody. The protein complexes were separated by SDS-PAGE and analyzed by immunoblotting (WB) with anti-HA antibody. The expression levels of each protein were verified by Western blot using antibodies specific for HA and Runx2 (bottom). WCE, whole cell extracts. C, competition of ERRγ with p300 for Runx2 transactivity. C2C12 cells were cotransfected with OSE-Luc and pcDNA3/Runx2 together with the indicated amounts of p300 and ERRγ expression vectors (+, 100 ng; ++, 200 ng; +++, 300 ng). 48 h after transfection cells were lysed, and luciferase activity was measured and normalized to β-galactosidase activity. Data are expressed as the mean ± S.D. of three independent experiments. D, ERRγ interferes a direct interaction of p300 with Runx2. In vitro translated 35S-labeled p300 and ERRγ were incubated with purified GST alone or GST-Runx2. The protein complexes were then resolved on a 10% SDS-PAGE and analyzed by autoradiography.
Previous studies have reported that various cofactors interact with Runx2 to regulate its transactivity (11–13). Among the regulators, p300 is a potential coactivator which interacts with Runx2, and the interaction is critical for the cooperative action of Runx2-dependent osteogenesis (13). To further characterize the mechanism whereby ERRγ inhibits Runx2 transactivity, we examined whether ERRγ competes with p300, which results in repression of Runx2 transactivity. As shown in Fig. 6C, ERRγ competes with p300 for Runx2 transactivity. However, increasing amounts of p300 marginally recovers Runx2 transactivity, indicating that the repressive effect of ERRγ on Runx2 transactivity is predominant as compared with the co-activation of p300. To further examine whether ERRγ interferes with a direct interaction of p300 and Runx2, we performed a GST pulldown assay as shown in Fig. 6D. 35S-Labeled p300 bound to GST-Runx2 as previously reported, and ERRγ specifically disrupted this interaction in a dose-dependent manner. These results demonstrate that ERRγ represses the transcriptional activity of Runx2 via competition with p300.
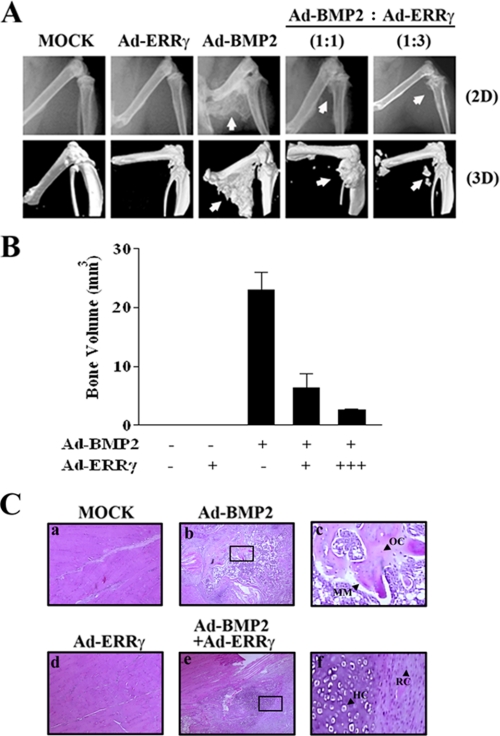
ERRγ Inhibits BMP2-induced Ectopic Bone Formation in Vivo—The preceding results revealed that ERRγ may play a substantial role in BMP2-induced osteogenic differentiation. A previous study reported that subcutaneous or intramuscular administration of BMP2 induces ectopic endochondral bone formation (3). Therefore, we determined the effect of ERRγ on BMP2-induced ectopic bone formation in vivo. To analyze the effects of ERRγ on intramuscular bone formation, mice were injected with Ad-ERRγ and/or Ad-BMP2 into the thigh muscle. At 5 weeks after injection, animals were sacrificed and subjected to x-ray (two-dimensional) and μCT (three-dimensional) imaging. As shown in Fig. 7, A and B, high resolution μCT scans showed that Ad-ERRγ alone had no effect on intramuscular bone formation. However, BMP2 profoundly induced ectopic bone formation in the thigh muscle, whereas ERRγ significantly decreased the ectopic bone formation by BMP2 in a dose-dependent manner. In addition, histology analysis showed that BMP2 induces newly formed mineralized matrix, which has plentiful amounts of osteocytes and bone marrow, whereas overexpression of ERRγ with BMP2 showed only immature mineralized areas and retained cartilage stage (Fig. 7C). These results are in agreement with the μCT results and indicate that ERRγ decreases BMP2-induced ectopic bone formation. Taken together, our findings suggest that ERRγ down-regulates the expression of BMP-induced osteogenic genes and mineralization of potent osteoblastic cells, which results in repressed bone formation.
FIGURE 7.
ERRγ inhibits BMP2-induced ectopic bone formation in vivo. A, representative radiograph (two-dimensional (2D)) and high resolution of μCT imaging (three-dimensional (3D)) on bone formation. MOCK virus (5 × 1010 PN), Ad-BMP2 (5 × 1010 PN), or/and Ad-ERRγ (5 × 1010 PN or 15 × 1010 PN) were injected intramuscularly into the thighs of mice. 5 weeks after injection, radiographs and μCT analysis were performed as described under “Experimental Procedures.” The white arrow indicates newly formed ectopic bone. B, quantitative analysis of newly formed bone. C, histology analysis of intramuscular ectopic bone formation. Each sample was formalin-fixed and paraffin-embedded, and then the sections were stained with hematoxylin and eosin. Panels c and f are magnified pictures of the squared areas in panels b and e, respectively. Black arrowheads indicate mineralized matrix (MM), osteocytes (OC), hypertrophied chondrocytes (HC), resting chondrocytes (RC).
DISCUSSION
Osteoblast differentiation and bone formation are regulated by various transcription factors. To date, several orphan nuclear receptors such as ERRα and Nurr1 were known to participate in osteoblast differentiation by regulating the expression of osteoblast specific genes (26–28). However, the involvement of ERRγ in bone formation has not yet been demonstrated. Based on this fact, we examined the role of ERRγ, the newest member of the orphan nuclear receptor ERR family, in osteogenic differentiation and bone formation. In this study we observed the expression of ERRs in potent osteoblastic cells such as mouse primary calvarial cells and MC3T3 and C2C12 cells. ERRγ mRNA expression is strongly induced by BMP2 (Fig. 1A), whereas the expression of ERRα and ERRβ was not significantly increased by BMP2 (data not shown). A previous study demonstrated that ERRα is expressed throughout the osteoblast differentiation process and plays a physiological role in differentiation and bone formation (17). However, in this report the expression of ERRα was BMP2-independent. Rather, ERRα was expressed subsequently during osteoblast differentiation. Therefore, it is possible that ERRα has a functional role in osteoblast differentiation through a BMP2-independent signal pathway.
BMPs regulate osteoblast differentiation and bone formation with the balance between the positive and negative factors. For example, BMPs stimulate the expression of several positive regulators of osteoblast differentiation, such as Runx2 and Dlx5 (21, 29). It has been well demonstrated that the BMP2-Runx2 cascade is important for the differentiation of mesenchymal stem cells into the osteoblast lineage (10). Runx2 positively and negatively regulates the osteoblast-specific genes by binding to its target promoters (15, 30–32). For example, bone-specific expression of OC is regulated principally by Runx2 (25). BMP also induces the expression of the negative regulator such as Tob (33). Thus, the tentative expression of ERRγ by BMP2 (Fig. 1, B–D) indicated that ERRγ may be a novel negative regulator which is involved in a finely tuned regulation of osteoblast differentiation. Meanwhile, we observed that BMP2 promoter activity was significantly decreased by ERRγ expression (data not shown). This result implies that ERRγ may regulate BMP2 expression negatively. As a further study, it is important to identify a key signal transduction pathway to regulate BMP2-dependent ERRγ expression.
Like other nuclear receptors, ERRγ consists of a DNA binding domain and a ligand binding domain (34). The DNA binding domain of ERRγ recognizes ERRγ response elements in the promoter region of its target genes (e.g. DAX-1, SHP) (19, 20), and its binding activates the transcriptional machinery (20). We could not identify the putative ERRγ response elements on OC or BSP proximal promoter sequences. Our results revealed that ERRγ reduces osteoblast marker gene expression as well as osteoblast differentiation. These results suggest that ERRγ works with a regulator for BMP2-induced osteogenic differentiation, and we identified Runx2 as a partner for ERRγ. Moreover, our results revealed that ERRγ significantly repressed Runx2-dependent promoter activities of OC and BSP, and ERRγ physically interacts with Runx2, suggesting that the functional outcome of ERRγ activity depends on specific protein-protein interactions.
Meanwhile, Runx2 by itself is not sufficient to induce its target gene expression (10). To date, various coregulators of Runx2 have been identified. Among those regulators, p300, a member of histone acetyltransferase, is a well known co-regulator of Runx2 (13), suggesting that chromatin remodeling is essential for regulating the osteoblast-specific genes. Interestingly, in this study, ERRγ competitively inhibited the association of p300 with Runx2 (Fig. 6, C and D). These results indicate that ERRγ exploits a cofactor competition mechanism to down-regulate Runx2 transactivity, which results in repression of osteoblast differentiation. However, the presence of additional and more complex mechanisms of cross-talk between ERRγ and other co-regulators of Runx2 cannot be ruled out.
In this study we examined the effect of ERRγ on BMP2-induced ectopic bone formation, and the in vivo results revealed that ERRγ attenuates BMP2-dependent de novo bone formation (Fig. 7). Interestingly, Ad-ERRγ-treated groups showed larger cartilage regions and smaller bone marrow cavity even in the presence of BMP2, which results in substantial loss of mature bone. Hence, it is interpreted that ERRγ may hold the transition from cartilage stage to mineralized bone formation stage. In conclusion, the present study clearly suggests that the orphan nuclear receptor ERRγ, which is temporally induced by BMP2, inhibited not only BMP2-induced osteoblast differentiation, in part by interfering with a coregulator of Runx2 in vitro, but also ectopic bone formation in vivo. Pathological bone diseases such as myositis ossificans traumatica, osteogenic sarcoma, and vascular calcification result from an excessive BMP expression in inappropriate places (35, 36). Therefore, in vivo regulation of ERRγ expression or activity by its synthetic or natural ligand can be a novel therapeutic approach to treat such bone-related disorders. Further studies using ERRγ-deficient mice would improve our understanding of the importance of ERRγ in bone homeostasis.
This work was supported by National Research Laboratory Grant M1-0500-4705J-4710 (to H.-S. C.) and the Korea Research Foundation Grant, funded in part by Korean Government Ministry of Education and Human Resource Development Grant KRF-2007-313-E00469 (to J.-T. K.).
Footnotes
The abbreviations used are: BMP, bone morphogenetic protein; OC, osteocalcin; BSP, bone sialoprotein; ERR, estrogen receptor-related receptors; Ad, adenovirus; AR-S, alizarin red stain; GST, glutathione S-transferase; PN, particle number; μCT, microCT; sh-, short hairpin-; RT, reverse transcription; ALP, alkaline phosphatase; HA, hemagglutinin.
References
- 1.Olsen, B. R., Reginato, A. M., and Wang, W. (2000) Annu. Rev. Cell Dev. Biol. 16 191–220 [DOI] [PubMed] [Google Scholar]
- 2.Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S., and Marshak, D. R. (1999) Science 284 143–147 [DOI] [PubMed] [Google Scholar]
- 3.Wozney, J. M., Rosen, V., Celeste, A. J., Mitsock, L. M., Whitters, M. J., Kriz, R. W., Hewick, R. M., and Wang, E. A. (1988) Science 242 1528–1534 [DOI] [PubMed] [Google Scholar]
- 4.Koh, J. T., Zhao, Z., Wang, Z., Lewis, I. S., Krebsbach, P. H., and Franceschi, R. T. (2008) J. Dent. Res. 87 845–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mundy, G., Garrett, R., Harris, S., Chan, J., Chen, D., Rossini, G., Boyce, B., Zhao, M., and Gutierrez, G. (1999) Science 286 1946–1949 [DOI] [PubMed] [Google Scholar]
- 6.Koh, J. T., Ge, C., Zhao, M., Wang, Z., Krebsbach, P. H., Zhao, Z., and Franceschi, R. T. (2006) Mol. Ther. 14 684–691 [DOI] [PubMed] [Google Scholar]
- 7.Chen, D., Ji, X., Harris, M. A., Feng, J. Q., Karsenty, G., Celeste, A. J., Rosen, V., Mundy, G. R., and Harris, S. E. (1998) J. Cell Biol. 142 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducy, P., Zhang, R., Geoffroy, V., Ridall, A. L., and Karsenty, G. (1997) Cell 89 747–754 [DOI] [PubMed] [Google Scholar]
- 9.Ducy, P. (2000) Dev. Dyn. 219 461–471 [DOI] [PubMed] [Google Scholar]
- 10.Lee, K. S., Kim, H. J., Li, Q. L., Chi, X. Z., Ueta, C., Komori, T., Wozney, J. M., Kim, E. G., Choi, J. Y., Ryoo, H. M., and Bae, S. C. (2000) Mol. Cell. Biol. 20 8783–8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yagi, R., Chen, L. F., Shigesada, K., Murakami, Y., and Ito, Y. (1999) EMBO J. 18 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLarren, K. W., Lo, R., Grbavec, D., Thirunavukkarasu, K., Karsenty, G., and Stifani, S. (2000) J. Biol. Chem. 275 530–538 [DOI] [PubMed] [Google Scholar]
- 13.Sierra, J., Villagra, A., Paredes, R., Cruzat, F., Gutierrez, S., Javed, A., Arriagada, G., Olate, J., Imschenetzky, M., Van Wijnen, A. J., Lian, J. B., Stein, G. S., Stein, J. L., and Montecino, M. (2003) Mol. Cell. Biol. 23 3339–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javed, A., Guo, B., Hiebert, S., Choi, J. Y., Green, J., Zhao, S. C., Osborne, M. A., Stifani, S., Stein, J. L., Lian, J. B., van Wijnen, A. J., and Stein, G. S. (2000) J. Cell Sci. 113 2221–2231 [DOI] [PubMed] [Google Scholar]
- 15.Westendorf, J. J., Zaidi, S. K., Cascino, J. E., Kahler, R., van Wijnen, A. J., Lian, J. B., Yoshida, M., Stein, G. S., and Li, X. (2002) Mol. Cell. Biol. 22 7982–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giguere, V. (2002) Trends Endocrinol. Metab. 13 220–225 [DOI] [PubMed] [Google Scholar]
- 17.Bonnelye, E., Merdad, L., Kung, V., and Aubin, J. E. (2001) J. Cell Biol. 153 971–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zirngibl, R. A., Chan, J. S., and Aubin, J. E. (2008) J. Mol. Endocrinol. 40 61–73 [DOI] [PubMed] [Google Scholar]
- 19.Sanyal, S., Kim, J. Y., Kim, H. J., Takeda, J., Lee, Y. K., Moore, D. D., and Choi, H. S. (2002) J. Biol. Chem. 277 1739–1748 [DOI] [PubMed] [Google Scholar]
- 20.Park, Y. Y., Ahn, S. W., Kim, H. J., Kim, J. M., Lee, I. K., Kang, H., and Choi, H. S. (2005) Nucleic Acids Res. 33 6756–6768 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Phimphilai, M., Zhao, Z., Boules, H., Roca, H., and Franceschi, R. T. (2006) J. Bone Miner. Res. 21 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roca, H., Phimphilai, M., Gopalakrishnan, R., Xiao, G., and Franceschi, R. T. (2005) J. Biol. Chem. 280 30845–30855 [DOI] [PubMed] [Google Scholar]
- 23.Koo, S. H., Flechner, L., Qi, L., Zhang, X., Screaton, R. A., Jeffries, S., Hedrick, S., Xu, W., Boussouar, F., Brindle, P., Takemori, H., and Montminy, M. (2005) Nature 437 1109–1111 [DOI] [PubMed] [Google Scholar]
- 24.Puleo, D. A. (1997) J. Cell. Physiol. 173 93–101 [DOI] [PubMed] [Google Scholar]
- 25.Makita, N., Suzuki, M., Asami, S., Takahata, R., Kohzaki, D., Kobayashi, S., Hakamazuka, T., and Hozumi, N. (2008) Gene (Amst.) 413 8–17 [DOI] [PubMed] [Google Scholar]
- 26.Lammi, J., Huppunen, J., and Aarnisalo, P. (2004) Mol. Endocrinol. 18 1546–1557 [DOI] [PubMed] [Google Scholar]
- 27.Lee, M. K., Choi, H., Gil, M., and Nikodem, V. M. (2006) J. Cell. Biochem. 99 986–994 [DOI] [PubMed] [Google Scholar]
- 28.Bonnelye, E., and Aubin, J. E. (2002) J. Bone Miner. Res. 17 1392–1400 [DOI] [PubMed] [Google Scholar]
- 29.Miyama, K., Yamada, G., Yamamoto, T. S., Takagi, C., Miyado, K., Sakai, M., Ueno, N., and Shibuya, H. (1999) Dev. Biol. 208 123–133 [DOI] [PubMed] [Google Scholar]
- 30.Thomas, D. M., Carty, S. A., Piscopo, D. M., Lee, J. S., Wang, W. F., Forrester, W. C., and Hinds, P. W. (2001) Mol. Cell 8 303–316 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida, C. A., Furuichi, T., Fujita, T., Fukuyama, R., Kanatani, N., Kobayashi, S., Satake, M., Takada, K., and Komori, T. (2002) Nat. Genet. 32 633–638 [DOI] [PubMed] [Google Scholar]
- 32.Schroeder, T. M., Kahler, R. A., Li, X., and Westendorf, J. J. (2004) J. Biol. Chem. 279 41998–42007 [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, Y., Tanaka, S., Umemori, H., Minowa, O., Usui, M., Ikematsu, N., Hosoda, E., Imamura, T., Kuno, J., Yamashita, T., Miyazono, K., Noda, M., Noda, T., and Yamamoto, T. (2000) Cell 103 1085–1097 [DOI] [PubMed] [Google Scholar]
- 34.Huppunen, J., Wohlfahrt, G., and Aarnisalo, P. (2004) Mol. Cell. Endocrinol. 219 151–160 [DOI] [PubMed] [Google Scholar]
- 35.Shafritz, A. B., and Kaplan, F. S. (1998) Clin. Orthop. Relat. Res. 346 46–52 [PubMed] [Google Scholar]
- 36.Hruska, K. A., Mathew, S., and Saab, G. (2005) Circ. Res. 97 105–114 [DOI] [PubMed] [Google Scholar]