Abstract
We have previously shown that the C-terminal cystathionine β-synthase (CBS) domains of the nucleotide-binding domains of the ABC transporter OpuA, in conjunction with an anionic membrane surface function, act as sensor of internal ionic strength (Iin). Here, we show that a surface-exposed cationic region in the CBS module domain is critical for ion sensing. The consecutive substitution of up to five cationic residues led to a gradual decrease of the ionic strength dependence of transport. In fact, a 5-fold mutant was essentially independent of salt in the range from 0 to 250 mm KCl (or NaCl), supplemented to medium of 30 mm potassium phosphate. Importantly, the threshold temperature for transport was lowered by 5–7 °C and the temperature coefficient Q10 was lowered from 8 to ∼1.5 in the 5-fold mutant, indicating that large conformational changes are accompanying the CBS-mediated regulation of transport. Furthermore, by replacing the anionic C-terminal tail residues that extend the CBS module with histidines, the transport of OpuA became pH-dependent, presumably by additional charge interactions of the histidine residues with the membrane. The pH dependence was not observed at high ionic strength. Altogether the analyses of the CBS mutants support the notion that the osmotic regulation of OpuA involves a simple biophysical switching mechanism, in which nonspecific electrostatic interactions of a protein module with the membrane are sufficient to lock the transporter in the inactive state.
In their natural habitats microorganisms are often exposed to changes in the concentration of solutes in the environment (1). A sudden increase in the medium osmolality results in loss of water from the cell, loss of turgor, a decrease in cell volume, and an increase in intracellular osmolyte concentration. Osmoregulatory transporters such as OpuA in Lactococcus lactis, ProP in Escherichia coli, and BetP in Corynebacterium glutamicum diminish the consequences of the osmotic stress by mediating the uptake of compatible solutes upon an increase in extracellular osmolality (2–4). For the ATP-binding cassette (ABC)5 transporter OpuA, it has been shown that the system, reconstituted in proteoliposomes, is activated by increased concentrations of lumenal ions (increased internal ionic strength) (2, 5, 6). This activation is instantaneous both in vivo and in vitro and only requires threshold levels of ionic osmolytes. Moreover, the ionic threshold for activation is highly dependent of the ionic lipid content (charge density) of the membrane and requires the presence of so-called cystathionine β-synthase (CBS) domains, suggesting that the ionic signal is transduced to the transporter via critical interactions of the protein with membrane lipids.
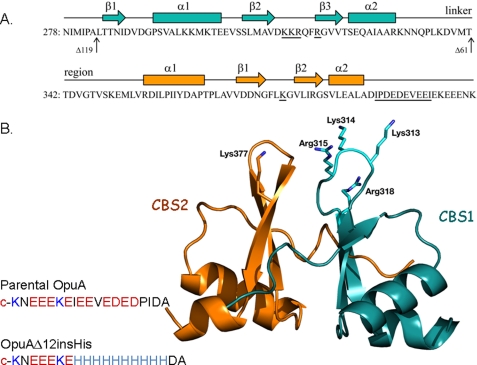
The ABC transporter OpuA consists of two identical nucleotide-binding domains (NBD) fused to CBS domains and two identical substrate-binding domains fused to transmembrane domains. The NBD-CBS and substrate-binding domain-transmembrane domain subunits are named OpuAA and OpuABC, respectively. Two tandem CBS domains are linked to the C-terminal end of the NBD; each domain (CBS1 and CBS2) has a β-α-β-β-α secondary structure (5) (Fig. 1A). The CBS domains are widely distributed in most if not all species of life but their function is largely unknown. Most of the CBS domains are found as tandem repeats but data base searches have also revealed tetra-repeat units (5). The crystal structures of several tandem CBS domains have been elucidated (7–9, 32), and in a number of cases it has been shown that two tandem CBS domains form dimeric structures with a total of four CBS domains per structural module (hereafter referred to as CBS module). The crystal structures of the full-length MgtE Mg2+ transporter confirm the dimeric configuration and show that the CBS domains undergo large conformational changes upon Mg2+ binding or release (10, 11). In general, ABC transporters are functional as dimers, which implies that two tandem CBS domains are present in the OpuA complex. Preliminary experiments with disulfides engineered at the interface of two tandem CBS domains in OpuA suggest that large structural rearrangements (association-dissociation of the interfaces) play a determining role in the ionic strength-regulated transport. Finally, a subset of CBS-containing proteins has a C-terminal extension, which in OpuA is highly anionic (sequence: ADIPDEDEVEEIEKEEENK) and modulates the ion sensing activity (6).
FIGURE 1.
Domain structure of CBS module of OpuA. A, sequence of tandem CBS domains. The predicted secondary structure is indicated above the sequence. The residues modified in this study are underlined. The amino acid sequence end-points of OpuAΔ61 and OpuAΔ119 are indicated by vertical arrows. B, homology model of tandem CBS domain of OpuA. The CBS domains were individually modeled on the crystal structure of the tandem CBS protein Ta0289 from T. acidophilum (PDB entry 1PVM), using Phyre. Ta0289 was used for the initial modeling, because its primary sequence was more similar to the CBS domains of OpuA than those of the other crystallized CBS proteins. The individual domain models were then assembled with reference to the atomic coordinates of the tandem CBS domains of IMPDH from Streptococcus pyogenes (PDB entry 1ZFJ) to form the tandem CBS pair, using PyMOL (DeLano). The positions of the (substituted) cationic residues are indicated.
In this study, we have engineered the surface-exposed cationic residues of the CBS module and the C-terminal anionic tail of OpuA (Fig. 1B). The ionic strength and lipid dependence of the OpuA mutants were determined in vivo and in vitro. We show that substitution of five cationic residues for neutral amino acids is sufficient to inactivate the ionic strength sensor and convert OpuA into a constitutively active transporter. Moreover, by substituting six anionic plus four neutral residues of the C-terminal anionic tail for histidines, the transport reaction becomes strongly pH-dependent.
MATERIALS AND METHODS
Plasmid Construction
Site-specific mutations in the CBS part of the opuAA gene were made via the megaprimer approach. Briefly, specific megaprimers were obtained by using a specific forward oligonucleotide (Table 1) and the general reverse primer EOL0404 5′ → 3′ and plasmid pNZOpuA(PstI)His as template. pNZOpuA(PstI)His was created previously (5) and contains an additional PstI site at the 3′ end of opuAA to facilitate the swapping of a 798-bp ClaI-PstI fragment. The corresponding protein, hereafter referred to as OpuA(parental), has two mutations at the very end of the C terminus (Table 1), but its activity is identical to that of wild type OpuA. The megaprimer was used as reverse primer in a second amplification reaction with the forward primer EOL0606 5′ → 3′. After digestion with ClaI and PstI, the parental gene fragment of pNZOpuA-(PstI)His was exchanged for the mutated fragment, yielding the spe-cific CBS domain mutant (Table 1). To create OpuA[K1], the plasmid carrying opuA(parental) was used as template; for OpuA[K2], the plasmid carrying opuA[K1] was used, and so forth.
TABLE 1.
List of OpuA modifications and primers used in this study
| Protein | Modifications | Primers/Reference |
|---|---|---|
| OpuA | Wild type | Van der Heide and Poolman (12) |
| OpuA(PstI) (Parental) | E402A, K403A | Biemans-Oldehinkel et al. (5) |
| OpuAΔ12 | Δ392–401, E402A, K403A | Biemans-Oldehinkel et al. (5) |
| OpuAΔ12insHis | D391A, #392–401H, E402A, K403A | EOL0416 5′–GAGCATCACCATCACCATCACCATCACCATCACGCTGCA–3′ and EOL0417 5′–GCGTGATGGTGATGGTGATGGTGATGGTGATGCTCTGCA–3′ |
| OpuAΔ61 | Deletion of CBS2 | Biemans-Oldehinkel et al. (2006) |
| OpuAΔ119 | Deletion of CBS1 and 2 | Biemans-Oldehinkel et al. (2006) |
| OpuA[K1] | K313Q, E402A, K403A | EOL0608 5′–GCTGTTGACCAAAAGCGTCAATTCCGTGGT–3′ |
| OpuA[K2] | K313Q, K314N, E402A, K403A | EOL0607 5′–GCTGTTGACCAAAACCGTCAATTCCGTGGT–3′ |
| OpuA[K2R] | K313Q, K314N, R315N, E402A, K403A | EOL0609 5′–GTTGACCAAAACAACCAATTCCGTGGTGTT–3′ |
| OpuA[K2R2] | K313Q, K314N, R315N, R318L, E402A, K403A | NA0601 5′–CAAAACAACCAATTCCTTGGTGTTGTTACGAGTG–3′ |
| OpuA[K3R2] | K313Q, K314N, R315N, R318L, K377V, E402A, K403A | NA0602 5′–TGATAATGGATTCCTTgttGGTGTATTGATTCGAG–3′ |
To replace part of the negatively charged C terminus of OpuAA with histidines, pNZOpuA(Δ12)His was digested with PstI and an annealed oligonucleotide, encoding 10 histidine residues and containing PstI compatible ends, was inserted, resulting in pNZOpuA(insHis)His. pNZOpuA(Δ12)His lacks the main part of the C-terminal anionic tail of OpuAA (5). Primers used to create the annealed oligonucleotide were EOL0416 5′-GAGCATCACCATCACCATCACCATCACCATCACGCTGCA-3′ and EOL0417 5′-GCGTGATGGTGATGGTGATGGTGATGGTGATGCTCTGCA-3′. All mutants were verified by DNA sequencing.
Bacterial Strains, Growth Conditions, and Vesicle Preparation
L. lactis strain Opu401 (4) was cultivated semianaerobically at 30 °C in a medium containing 2% (w/v) gistex LS (Strik BV, Eemnes, NL) and 65 mm sodium phosphate, pH 6.5, supplemented with 1.0% (w/v) glucose and 5 μg/ml chloramphenicol when carrying pNZOpuAHis or derivatives. For isolation of membrane vesicles, cells were grown in a 2-liter pH-regulated bioreactor to an A600 of 2, after which transcription from the nisA promoter was switched on by the addition of 0.1% (v/v) culture supernatant of the nisin A-producing strain NZ9700. The cells were harvested and membrane vesicles were prepared according to standard procedures (12). The protein concentration was determined using the DC Protein Assay (Bio-Rad).
Synthesis of Hybrid Membranes
Membrane vesicles of the L. lactis Opu401/pNZOpuA derivative were isolated as described (13), and fused 1:10 with preformed liposomes, composed of dioleoyl-phosphatidylethanolamine (DOPE), dioleoyl-phosphatidylglycerol (DOPG), and dioleoyl-phosphatidylcholine (DOPC). The hybrid membranes were obtained by two freeze/thaw cycles, followed by extrusion through a polycarbonate filter (200 nm pore size); for details on the preparation of liposomes and the freeze-thaw-extrusion steps, we refer to Geertsma et al. (14). The fraction of DOPE was always 50 mol % and the mole fractions of DOPG and DOPC were varied reciprocally; for most experiments we used either 38 mol % DOPG, 12 mol % DOPC or 12 mol % DOPG, 38 mol % DOPC. The synthetic lipids were obtained from Avanti Polar Lipids, AL.
Purification and Membrane Reconstitution of OpuA
Purified OpuA and mutant derivatives were reconstituted in liposomes composed of synthetic lipids (see “Synthesis of Hybrid Membranes”), essentially as described previously (14). The final protein to lipid ratio was 1:100 (w/w), unless stated otherwise.
Transport Assays
In Vivo Uptake—Opu401 cells carrying the pNZOpuA-derived plasmids were grown in M17 supplemented with 0.5% glucose and 5 μg/ml chloramphenicol to an A600 of 0.8. For induction of the opuA genes, 2 × 10-4 % (v/v) culture supernatant of the nisin A producing strain NZ9700 was used. After induction, the cells were washed twice with ice-cold 50 mm K-HEPES, pH 7.3. Prior to initiation of transport, cells were pre-energized for 5 min with 10 mm glucose at 30 °C. Uptake of [14C]glycine betaine was assayed in 50 mm K-HEPES, pH 7.3, supplemented with 50 μg/ml chloramphenicol and 10 mm glucose, with or without sucrose as indicated. The concentration of cells in the uptake assay was ∼0.4 mg of total protein/ml.
Hybrid Membranes—For ATP-driven uptake of glycine betaine in hybrid membranes, the ATP-regenerating system was enclosed during the fusion of membrane vesicles with liposomes (14); the ATP-regenerating system corresponds to 10 mm Na2-ATP + 10 mm MgSO4 + 24 mm Na2-creatine phosphate + 2.4 mg/ml creatine kinase in 50 mm potassium Pi, pH 7.0. The fused and loaded hybrid membranes were washed twice with 90 mm potassium Pi, pH 7.0 (osmolality of approximately 240 mosmol/kg), and resuspended in the same buffer to a concentration of 60 mg of lipids/ml. For osmotically activated transport, the hybrid membranes were diluted to a lipid concentration of 4.5–12 mg/ml into assay buffer (90 mm potassium Pi, pH 7.0 (unless specified otherwise), supplemented with different concentrations of KCl). Following incubation for 2 min at 30 °C, the transport reaction was initiated by the addition of [14C]glycine betaine (Amersham Biosciences) to a final concentration of 31 μm (more than 10-fold above the Km for transport and KD for binding) (13). At given time intervals, 40-μl samples were taken and diluted with 2 ml of ice-cold isotonic assay buffer. The samples were filtered rapidly through 0.45-μm pore-size cellulose nitrate filters (Schleicher & Schuell) and washed twice with 2 ml of assay buffer. The radioactivity on the filters was determined by liquid scintillation counting.
Proteoliposomes—ATP-driven uptake of glycine betaine by right-side in reconstituted OpuA was performed as described by van der Heide and Poolman (12), with some modifications. Briefly, proteoliposomes were loaded with 9 mm Mg-ATP (prepared from 9 mm MgSO4 plus 9 mm Na2-ATP), 10 mm potassium Pi, pH 7.0, unless stated otherwise. The inclusion of the Mg-ATP/potassium Pi was done via two cycles of freeze-thawing. After extrusion of the proteoliposomes through a polycarbonate filter (200 nm pore size), the proteoliposomes loaded with Mg-ATP were washed twice with 30 mm potassium Pi, pH 7.0, and resuspended in the same buffer unless specified otherwise (30 mm potassium Pi, pH 7.0, is isoosmolar with the luminal contents and corresponds to an osmolality of ∼70 mosmol/kg as determined by freezing point depression) to a concentration of 80 mg of lipids/ml.
For osmotically activated transport, the proteoliposomes were diluted to a lipid concentration of 5 mg/ml into assay buffer (30 mm potassium Pi, pH 7.0, supplemented with different concentrations of KCl). Following incubation for 2 min at 30 °C, the transport reaction was initiated by the addition of [14C]glycine betaine (Amersham Biosciences) to a final concentration of 42.5 μm. At given time intervals, 40-μl samples were taken and diluted with 2 ml of ice-cold isotonic assay buffer and processed further as described under “Hybrid Membranes.”
The initial rates of uptake were calculated from the linear part of the transport curves, using linear regression. Radiolabeled N-[methyl-14C]choline chloride (55 mCi/mmol) was from Amersham Biosciences, and was converted to N-[methyl-14C]glycine betaine as described (15).
RESULTS
Engineering Strategy
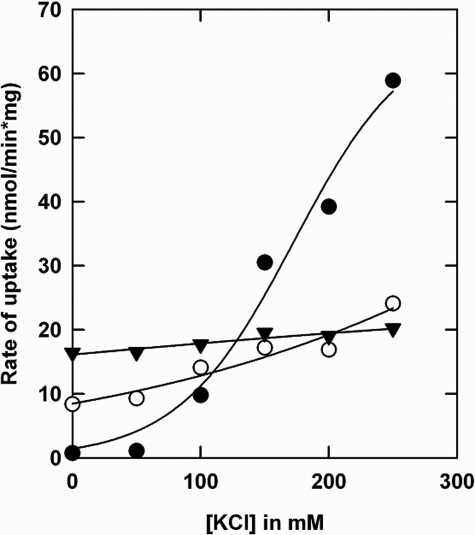
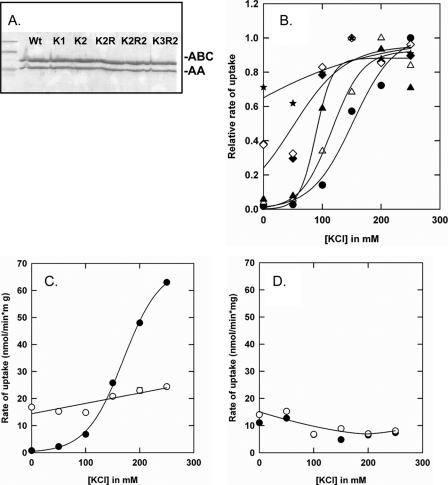
We have previously shown that deletion of the CBS2 domain (OpuAΔ61) is sufficient to abolish most of the ionic strength dependence of OpuA (5). A thorough analysis of the mutant with the entire CBS module deleted (OpuAΔ119) has not been reported. We now show that the activity of OpuAΔ119 is independent of salt in the 0–250 mm KCl range (or NaCl), present in the basal assay medium of 30 mm potassium Pi, pH 7.0 (Fig. 2). For comparison, the ionic activation profiles of parental OpuA and OpuAΔ61 are shown. For OpuAΔ61 and OpuAΔ119, part of the OpuAA subunit was lost in the reconstitution process (observed as lower ratio of AA/ABC subunits on SDS-PAGE), which explains at least part of the lower activity (i.e. at 250 mm KCl) of these mutants. To gain further insight into the role of the CBS domains in the mechanism of ionic activation, a series of more subtle mutants were designed and constructed: (i) surface-exposed cationic residues in the CBS module were substituted for neutral amino acids; and (ii) anionic residues in the C-terminal tail of the CBS domains were changed to histidines.
FIGURE 2.
Comparison of glycine betaine uptake for parental OpuA and CBS deletion mutants: OpuA (•), OpuAΔ119 (▾), and OpuAΔ61 (○). The purified proteins were reconstituted in proteoliposomes, composed of 50 mol % DOPE, 12 mol % DOPC, and 38 mol % DOPG. The lumen of the vesicles contained 9 mm Na2-ATP, 9 mm MgSO4, and 10 mm potassium Pi, pH 7.0, which was enclosed by 2 cycles of freeze-thawing. Uptake of [14C]glycine betaine was assayed in 30 mm potassium Pi, pH 7.0, with or without added KCl as indicated on the x axis.
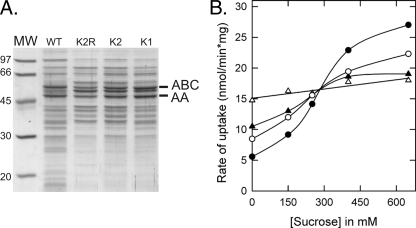
On the basis of a homology-based structure model of the OpuA CBS domains (using a multiple sequence alignment and the crystal structure of the tandem CBS protein Ta0289 from Thermoplasma acidophilum), we identified five cationic residues (Lys313, Lys314, Arg315, Arg318, and Lys377) as potential candidates for mediating the anionic lipid-dependent ionic strength response. The cationic residues 313–315 and Arg318 are located in the loop between β2 and β3 of CBS1 and Lys377 is located in the corresponding loop of CBS2. Single [K1], double [K2], triple [K2R], quadruple [K2R2], and quintuple [K3R2] mutants were constructed and Lys and/or Arg residues were substituted for neutral amino acids (Table 1). The mutants were made and characterized in two rounds of mutagenesis, i.e. K1, K2, and K2R were constructed first and based on the in vivo and in vitro activity data, K2R2 and K3R2 were designed and analyzed subsequently. Each of the mutants expressed to levels of at least 50% of parental OpuA (e.g. Fig. 3A).
FIGURE 3.
In vivo characterization of CBS surface charge mutants of OpuA. A, visualization of the expression of the CBS mutants in membrane vesicles. Parental OpuA and CBS mutants were expressed in L. lactis Opu401 and 6.5 μg of membrane vesicles was loaded per lane on a 12.5% SDS-PAGE gel. B, in vivo activation profiles of parental OpuA and single (K1), double (K2), and triple (K2R) surface charge mutants. Transport of [14C]glycine betaine by wild type OpuA (•), OpuA[K1] (○), OpuA[K2] (▴), and OpuA[K2R] (▵) was assayed in 50 mm K-HEPES, pH 7.3, supplemented with 10 mm glucose, with or without sucrose as indicated.
Assay Strategy
Transport activity was assessed in vivo, using whole cells of L. lactis osmotically stressed with sucrose, and in vitro, using hybrid membranes or proteoliposomes. Although the internal ionic strength in whole cells can be increased by increasing the osmotic stress, the absolute values are difficult to quantify and low values of internal ionic strength cannot be achieved, that is, without compromising the physiological well being of the organism. In hybrid membranes and proteoliposomes, the internal ionic strength can be set precisely and varied by the addition of salt or sucrose to the external medium. Hybrid membranes offer the advantage that the mutant proteins do not need to be purified, which can be desirable for mutants that are less stable. L. lactis membrane vesicles contain 50–60 mol % anionic lipids (16), and, after a 1 to 10 fusion with liposomes composed of 38 mol % DOPG, the fraction of anionic lipids was ∼40 mol %. Full control over lipid and protein composition and ionic strength was obtained in proteoliposomes, in which purified OpuA was incorporated into membranes composed of synthetic lipids.
Surface-exposed Cationic Residues
Single, Double, and Triple Mutants—In vivo OpuA[K1], OpuA[K2], and OpuA[K2R] displayed different activation profiles compared with parental OpuA (Fig. 3B). Removing a single cationic residue, OpuA[K1], increased the activity at low osmotic stress, that is, conditions corresponding to lower values of intracellular ionic strength, which is indicative of a lower degree of inhibition by anionic lipids. Removing two cationic residues, OpuA[K2], led to a further increase in activity at low osmotic stress, and by removing all three cationic residues, OpuA[K2R], the transporter became essentially independent of the imposed osmotic stress. The activity of the three mutants at high osmotic stress (0.65 m sucrose in Fig. 3B) was lower than that of the parental transporter, but this point was not studied further in vivo.
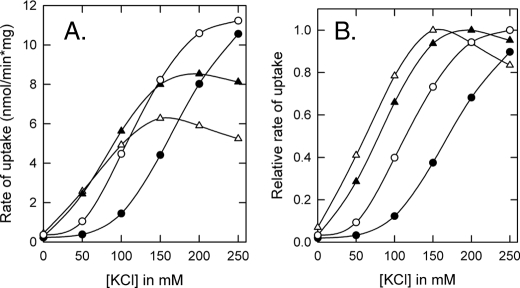
Next, membrane vesicles bearing parental OpuA, OpuA[K1], OpuA[K2], or OpuA[K2R] were isolated and fused 1:10 (w/w in lipids) with liposomes containing 38 mol % DOPG, 12 mol % DOPC plus 50% mol % DOPE (Fig. 4). It has been previously shown that for ionic strength control of transport, OpuA requires a high fraction of anionic lipids (>25–30 mol %) (5). Fig. 4, A and B, show that in the hybrid membranes with ∼40 mol % anionic lipids, the activation profiles of OpuA[K1], OpuA[K2], and OpuA[K2R] were shifted to lower Iin when compared with wild type or parental OpuA. The more positive charges were neutralized the further the shift to lower Iin became, suggesting that the cationic residues mediate the inhibition of the transporter by anionic lipids. However, OpuA[K2R] was still dependent on ionic strength, which was not observed in the whole cell assays (Fig. 3). These differences can be explained by the fact that in vivo the internal salt concentrations are much higher than the ionic strength range that is probed in vitro. The in vitro measurements suggest that additional charged residues participate in the interaction of the CBS module with the membrane. Likely candidates are Arg318 in CBS1 and Lys377 in CBS2 (Fig. 1B).
FIGURE 4.
In vitro characterization of CBS surface charge mutants of OpuA in hybrid membranes. Membrane vesicles of L. lactis bearing OpuA mutants were fused with liposomes composed of 50 mol % DOPE, 12 mol % DOPC, and 38 mol % DOPG. The ATP-regenerating system was enclosed inside the hybrid membranes. Uptake of [14C]glycine betaine by parental OpuA (•), OpuA[K1] (○), OpuA[K2] (▴), and OpuA[K2R] (▵) in hybrid membranes was assayed in 90 mm potassium Pi, pH 7.0, with or without added KCl as indicated on the x axis. Activation profiles in A show the measured values, whereas activation profiles in B represent normalized rates; data were normalized relative to the highest activity in the concentration range of 0 to 0.3 m KCl.
Quadruple and Quintuple Mutants—On the basis of the in vivo and in vitro activity measurements of the K1, K2, and K2R mutants, OpuA[K2R2] and OpuA[K3R2] were constructed (Table 1). In whole cell transport assays, the activity of both OpuA[K2R2] and OpuA[K3R2] was independent of the imposed osmotic stress (data not shown) and similar to that of OpuA[K2R] (Fig. 3B). Each of the mutants was then purified (Fig. 5A) and reconstituted into proteoliposomes with 38 mol % of DOPG. Fig. 5B shows the activities of parental and mutant OpuA (K1, K2, K2R, K2R2, and K3R2; Table 1). Clearly, by decreasing the number of positively charged residues further, the OpuA activity became less dependent of the ionic strength. In fact, the inactivation of transport at low ionic strength was no longer observed in K3R2, indicating that this mutant is virtually completely deregulated.
FIGURE 5.
In vitro characterization of CBS surface charge mutants of OpuA proteoliposomes. A, visualization of the proteoliposomes on SDS-PAGE gel. 35 μl of proteoliposomes were treated with 2% (v/v) SDS plus 5× sample buffer (3% (w/v) Tris-HCl, 50% (v/v) glycerol, 0.005% (v/v) bromphenol blue, 1% (v/v) SDS, 3% (v/v) β-mercaptoethanol). 30 μl of the mixture was applied to a 12.5% SDS-PAGE gel and electrophoresed for 90 min at constant current of 15 mA. B, in vitro transport assay, using purified protein reconstituted in proteoliposomes. The proteoliposomal membranes were composed of 50 mol % DOPE, 12 mol % DOPC, and 38 mol % DOPG at 1:50 of protein to lipid ratio. Proteoliposomes were loaded with 9 mm MgSO4, 9 mm Na2ATP plus 10 mm potassium Pi, which is equiosmolar with 30 mm potassium Pi. Uptake of [14C]glycine betaine by parental OpuA (•), OpuA (K2) (▴), OpuA (K2R) (♦), OpuA (K1) (▵), K2R2 (⋄), and OpuA (K3R2) (234). Uptake of [14C]glycine betaine was assayed in 30 mm potassium Pi, pH 7.0, with or without added KCl (0–250 mm). Normalized rates of transport are shown; the actual uptake rates at 0.25 m KCl were 45, 30, 22, 20, 21, and 19 nmol/min per mg of protein for parental OpuA, K1, K2, 2R, K2, R2, and K3R2, respectively. C and D, lipid and salt dependence. Panel C, proteoliposomes composed of 50 mol % DOPE, 12 mol % DOPC, and 38 mol % DOPG; parental OpuA (•) and OpuA (K3R2) (○). Panel D, proteoliposomes composed of 50 mol % DOPE, 38 mol % DOPC, and 12 mol % DOPG; parental OpuA (•) and OpuA (K3R2) (○). Further conditions are as described under B.
The deregulated activity could be due to a lower degree of inhibition by anionic lipids. To address this point, we determined the anionic lipid dependence of OpuA by comparing proteoliposomes with either 38 (panel C) or 12 (panel D) mol % of DOPG (Fig. 5). The fraction of the zwitterionic lipid DOPC was varied reciprocally with DOPG, and the fraction of DOPE was kept constant at 50%. At low (12 mol %) DOPG, the activities of parental OpuA and OpuA[K3R2] were similar and decreased slightly with increasing ionic strength (Fig. 5D). For comparison, the same batches of protein in liposomes with 38 mol % of DOPG revealed the strong ionic strength-dependence of parental OpuA and the ionic strength-independence of OpuA[K3R2] (Fig. 5C). Taken together, these data strongly suggest that the cationic amino acid residues participate in interaction of the CBS module with the membrane, the more cationic residues, the tighter the binding and thus the more salt one needs to screen the electrostatic interactions.
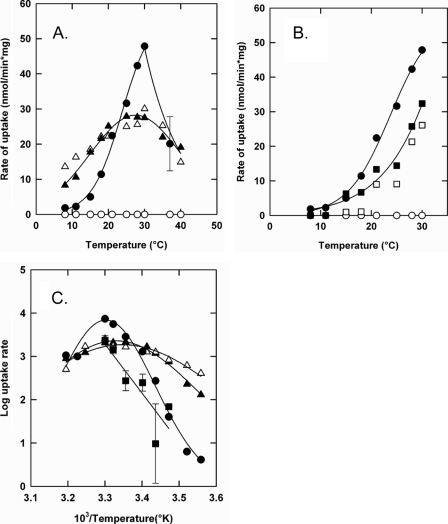
We also measured the temperature dependence of parental OpuA, OpuAΔ119, and OpuA[K3R2] to determine the activation energies for (the regulation of) transport. Fig. 6 shows the initial rates of glycine betaine uptake from 7 to 40 °C (panel A, parental OpuA and OpuA[K3R2]) and from 7 to 30 °C (panel B, parental OpuA and OpuAΔ119); the corresponding Arrhenius plots are shown in Fig. 6C. Parental OpuA was only active in the presence of salt (Iin ∼ 0.6 in this particular experiment; closed circles) and above a temperature of 13–14 °C; the low salt concentration at which the system was not active corresponded to Iin ∼ 0.06 (open circles). The temperature coefficient Q10 of parental OpuA was about 8 between 15 and 30 °C, yielding a activation energy (EA) of 148 kJ/mol. As anticipated, OpuAΔ119 was equally active at low and high salt and had a somewhat lower EA of 119 kJ/mol compared with parental OpuA. Importantly, significant activity of OpuA[K3R2] was already observed at temperatures below 10 °C and the activity was relatively insensitive to temperature both at high (Iin ∼ 0.6) and low (Iin ∼ 0.06) ionic strength. The Q10 of OpuA[K3R2] was only ∼1.5 and corresponded to an EA of 29 kJ/mol. The temperature dependences of the parental OpuA, OpuAΔ119, and the 5-fold mutant OpuA[K3R2] indicate that deletion of the CBS module or substitution of the surface-exposed cationic residues, both leading to ionic strength-independent transport, have different consequences for the activation energy of the translocation reaction. Depending on the modification made to the CBS module, the transporter can be in a deregulated-low (OpuAΔ119) or deregulated-high energy state (OpuA[K3R2]).
FIGURE 6.
Temperature dependence of the transport activity of parental OpuA (panels A and B), OpuA[K3R2] (panel A), and OpuAΔ119 (panel B) at low and high internal ionic strength. Proteoliposomes with parental OpuA (○, •), OpuA (K3R2) (▵, ▴), and OpuAΔ119 (□, ▪) were composed of 50 mol % DOPE, 12 mol % DOPC, and 38 mol % DOPG and were loaded with 9 mm MgSO4, 9 mm Na2ATP plus 10 mm potassium Pi. Uptake of [14C]glycine betaine was assayed in 30 mm potassium Pi, pH 7.0, with (closed symbols) or without (open symbols) 250 mm KCl. Error bars show standard deviations of average rates from 3 independent experiments. Panel C is the Arrhenius transformation of the data from panels A and B.
C-terminal Tail
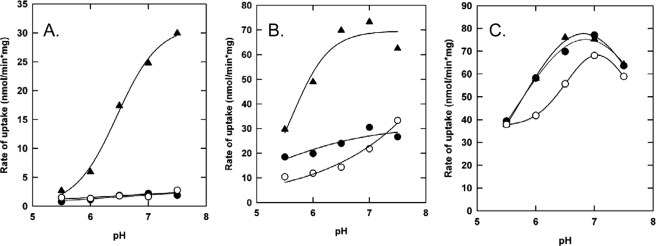
It has been previously shown that deleting 12 amino acids of the anionic tail C-terminal of the CBS domains shifts the Iin dependence of OpuA to higher values (OpuAΔ12 mutant) (4), indicating that the anionic tail attenuates the ion sensing function. The role of the anionic C terminus of OpuAA was further explored by substituting 6 of the acidic plus 4 neutral residues of the anionic C terminus for 10 histidines, yielding OpuAΔ12insHis (Table 1). The pH dependence of transport of parental OpuA, OpuAΔ12, and OpuAΔ12insHis were determined as a function of pH at low (Iin ∼ 0.06), intermediate (Iin ∼ 0.3), and high (Iin ∼ 0.5) internal ionic strength. Assuming an average pKa of about 6.4, the decahistidine sequence will have a charge of +9 to +10 at pH 5.5 and be lower than +1 at pH 7.5. The pH will thus strongly affect the overall charge of the CBS module. To avoid possible interference of the pH on the regeneration of lumenal ATP (i.e. creatine kinase activity), MgATP instead of the ATP-regenerating system was incorporated in the vesicle lumen.
In proteoliposomes with 38 mol % of DOPG, the activity and ionic activation of parental OpuA and OpuAΔ12 was not very sensitive to pH in the range of 5.5 to 7.5. OpuAΔ12insHis, on the other hand, was highly dependent on pH. These effects are best observed when the activities of wild type OpuA, OpuAΔ12, and OpuAΔ12insHis are compared as a function of pH at Iin ∼0.06 (Fig. 7A), Iin ∼ 0.3 (Fig. 7B), and Iin ∼ 0.5 (Fig. 7C). At Iin ∼ 0.06 and 38 mol % of DOPG in the membrane, parental OpuA and OpuAΔ12 were inactive over the entire pH range (Fig. 7A). OpuAΔ12insHis, on the contrary, was inactive at pH 5.5 but the activity increased with an apparent pKa of ∼6.4. At intermediate ionic strength (Iin ∼ 0.3), the differences in pH dependence of the various proteins were still observable (Fig. 7B), but at Iin ∼ 0.5 the pH dependences became nearly identical (Fig. 7C). Thus, a high ionic strength abrogates the pH control of OpuAΔ12insHis. In proteoliposomes with 18 mol % of DOPG, the pH-dependent activation was no longer observed and the pH profiles of OpuA, OpuAΔ12, and OpuAΔ12insHis were very similar at every Iin (data not shown), suggesting that pH-dependent switching observed in Fig. 7A (and to a lesser extent Fig. 7B) requires the interaction of the (modified) CBS module with the membrane, either directly or via an additional factor in the core domains of OpuA.
FIGURE 7.
The effect of pH on the activity and osmotic activation of parental OpuA, OpuAΔ12, and OpuAΔ12insHis. Proteoliposomes were loaded with 9 mm MgSO4, 9 mm Na2ATP plus 50 mm potassium Pi at the corresponding pH, which is equiosmolar with 70 mm potassium Pi of the outside medium. Uptake of [14C]glycine betaine by OpuA (•), OpuAΔ12 (○), and OpuAΔ12insHis (▴) in 70 mm potassium Pi and supplemented with 0 mm KCl (A), 100 mm KCl (B), and 200 mm KCl (C) is shown. Proteoliposomes were composed of 50 mol % DOPE, 12 mol % DOPC, and 38 mol % DOPG.
DISCUSSION
We have previously shown that the ABC transporter OpuA is activated when the intracellular ionic strength reaches a threshold value. Below the threshold, the transporter is kept in the inactive “electrostatically locked” state, presumably through interactions of a protein domain with the anionic membrane surface. This mechanism is based on the following observations: (i) the ionic strength needed for activation increases with the fraction of anionic lipids in the membrane; (ii) at 40–60 mol % of DOPG, the transporter is inactive below 100–200 mm salt, which nicely matches the physiological conditions at which the system needs to be “on” or “off”; (iii) OpuA is no longer regulated by ionic strength when the CBS module is deleted; (iv) deletion of the anionic C-terminal tail reinforces the ionic regulation of transport, indicating that this amino acid sequence attenuates the interaction of a protein domain (presumably the CBS module) with the membrane surface.
CBS Module and Role of Anionic Lipids—Homology modeling suggested that residues Lys313, Lys314, Arg315, Arg318, and Lys377 form a cationic patch at the surface of the CBS module, which raised a testable hypothesis: the salt-dependent activation of OpuA involves screening of electrostatic interactions formed by these surface-exposed residues. In accordance with the postulate, the salt dependence of transport decreased with the number of cationic residues substituted for neutral ones. In the 5-fold mutant (K3R2) the transport activity was essentially independent of ionic strength, both at low and high anionic charge at the membrane surface. By dissecting translocation catalysis from regulation of transport in OpuA[K3R2], we can thus conclude that anionic lipids serve a dual role in the transport protein: (i) anionic lipids are needed for maximal activity (saturating levels are reached at 25–30 mol % of DOPG or DOPS, see also Ref. 4); and (ii) anionic lipids together with the CBS module mediate the ionic regulation of transport.
The putative interaction of the CBS module with the membrane surface could also be influenced by changing the overall charge of the C-terminal tail as shown for OpuAΔ12insHis. The activity of OpuAΔ12insHis was dependent of three parameters: (i) the membrane surface charge, (ii) internal ionic strength, and (iii) pH. At 18 mol % of DOPG (low surface charge), the CBS module presumably did not interact with the membrane, irrespective of the pH (i.e. the charge of the C-terminal tail). At 38 mol % of DOPG (high surface charge), pH 5.5, and a low Iin, OpuAΔ12insHis was in the inactive state. The engineered transporter, however, was switched on by increasing Iin or pH; both parameters are expected to diminish the electrostatic interactions with the membrane surface. Thus, by engineering a completely artificial peptide sequence C-terminal of the CBS domains, we have introduced a new functionality (pH control) in the transporter. These findings imply a simple biophysical switching mechanism as the basis for osmotic (= ionic) regulation of transport, which involves unspecific and reversible electrostatic interactions of a protein module with the membrane.
The observation that OpuA-Δ12insHis is active at alkaline pH, whereas parental OpuA and OpuAΔ12 are not (Fig. 7A), suggests that at alkaline pH the mere presence of the decahistidine sequence is sufficient for activation. Upon lowering of the pH, the decahistidine tail reinforces the electrostatic interaction of the CBS module with the membrane, which inactivates OpuAΔ12insHis. We emphasize that the decahistidine sequence is most likely unstructured and modulates the transporter without being an inherent part of the CBS ion-sensing module, in line with the concept of a unspecific electrostatic switching mechanism.
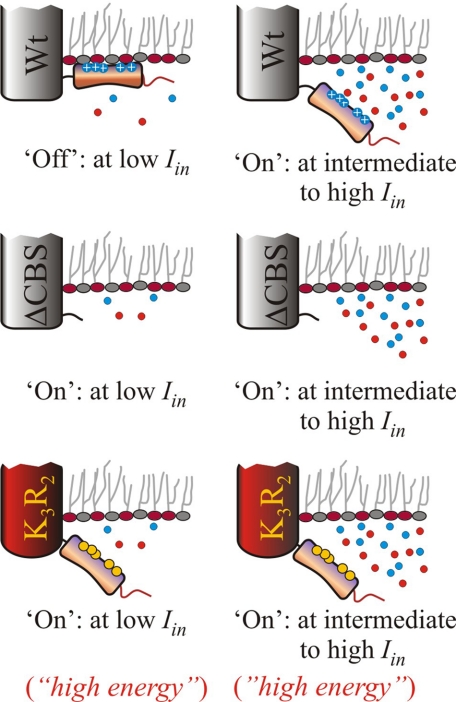
Activation Energy for Transport— The temperature dependence of transport revealed an unexpected mechanistic feature of the ionic regulation of OpuA. The activation energy for transport by parental OpuA in the activated state was relatively high (Q10 ∼ 8), indicative of large conformational transitions in the catalytic cycle of transport. To the best of our knowledge, activation energies for translocation by ABC transporters have not been reported. For secondary transporters, Q10 values of 2–3 have been described, whereas for flux through channel proteins Q10 is <1.5 (17–19). The high Q10 values suggest that the driving force for the transition between different states must have a large enthalpic contribution (e.g. Brownian motion of protein domains). Surprisingly, even though OpuAΔ119 and OpuA-[K3R2] are completely deregulated in terms of ionic activation, the temperature dependences and activation energies were very different. OpuAΔ119, lacking the CBS module, had a Q10 ∼ 5. OpuA[K3R2] had a highly reduced activation energy (Q10 ∼ 1.5), suggesting that the transporter is in a state where the proposed large conformational transition is bypassed. Thus, OpuAΔ119 and OpuA[K3R2] seem to reflect different energy states, with the K3R2 mutations trapping the system in an activated state (Fig. 8).
FIGURE 8.
Schematic representation of the activity of OpuA at low (12 mol %) and high (38 mol %) of DOPG. The transporter (ligand binding receptor, translocator including ATP binding cassette) is depicted by the main cylinder, the CBS module in orange, the surface-exposed residues in blue (cationic) or yellow (neutral substitutions), and the anionic C terminus in red; the curled tail indicates the deletion of the tandem CBS domain. Anionic lipids are depicted by red circles and neutral lipids are in gray. Top, wild type OpuA; middle, OpuAΔ119 (=ΔCBS); bottom, OpuA[K3R2].
The range of screened electrostatic interactions is defined by Debye length, usually denoted by 1/κ (20, 21). Because the Debye length is essentially independent of temperature (20, 21), it is not likely that the temperature dependence is related to the electrostatic interaction of the ion-sensing module with the membrane. Thus, at 250 mm KCl plus 30 mm potassium Pi, pH 7.0, in the external medium (yielding an Iin ∼ 0.6), the electrostatic interactions are sufficiently screened and similar at every temperature. The substitution of five cationic residues thus seems to have dual effects: the interaction of the ion sensing module with the membrane is decreased (transport has become independent of Iin and is no longer anionic lipid-regulated) and the activation barrier for transport is lowered. Although both phenomena are most likely interrelated, the ionic activation of transport must involve more than a release of the CBS module from the membrane. Whatever the precise structural rearrangements may be, the CBS module in conjunction with anionic lipids play a central role in osmotic, i.e. ionic strength, sensing.
How Universal Is “Simple” Electrostatic Control of Membrane Transport?—Within the potassium channels, human TREK-1 and the archaeal MJK2 (22, 23), protein-lipid interaction serves to regulate the activity in response to membrane stretch and cell swelling (hypo-osmotic stress). The sensing domains in these channels consist of a positively charged C-terminal cluster, which interacts with the membrane surface. This interaction can be modified by introducing either polylysine (a cationic molecule used to mask the charge of anionic lipids) or changing the fraction of phosphatidylinositol biphosphate (PIP2) in the membrane. PIP2 has a valence of -4 at pH 7.0 and changing the fraction of PIP2 will have a large effect on the surface potential of the membrane (24).
Electrostatic interactions between protein and membrane surfaces are also of major importance for the regulation of the natively unstructured protein myristoylated alanine-rich C-kinase substrate (MARCKS) (25). In this protein, a basic and hydrophobic region has been identified to be responsible for concentrating the signaling lipid PIP2, which constitutes about 1% of the total lipids in the plasma membrane. The hydrophobic residues penetrate into the lipid bilayer and the basic residues attract three PIP2 molecules by means of nonspecific electrostatic interactions. Calmodulin, complexed with Ca2+, has the ability to wrap around the basic cluster of MARCKS, reversing its charge from positive to negative and repelling the complex from the membrane. The interaction of MARCKS with the membrane regulates PIP2 sequestration and connects the local intracellular Ca2+ concentration to PIP2, allowing interaction of these signaling molecules with other biologically important molecules.
Next to PIP2, the bioactive lipids phosphatidic acid (PA) and lysophosphatidic acid (LPA) are important signaling lipids in the membrane. Although their abundance in the membrane is low, they are involved in many intracellular processes and are important intermediates in lipid biosynthesis (26). Recently, it has been shown that the phosphomonoester headgroups of L(PA) can form inter- and intramolecular hydrogen bonds, thereby increasing the protonation state of L(PA) (27). These results suggest that hydrogen bonds between L(PA) and basic residues in proteins play an important role in protein-lipid interactions. An example is the CTP:phosphocholine cytidylyl-transferase, the key regulatory enzyme in the synthesis of phosphatidylcholine. The activity of cytidylyltransferase is dependent on the content of anionic lipids in the membrane, including PA and phosphatidylglycerol (28). Cytidylyltransferase contains an amphipatic helix that has been identified to be responsible for sensing the physical properties of the membrane (29). A similar amphitropic effect has been observed on the myristoylated alanine-rich C-kinase MARCKS (25). Cytidylyltransferase and MARCKs are both amphitropic proteins that bind to membranes in a two-step process. One step involves electrostatic interactions and serves to localize the protein to the surface, the other step constitutes the hydrophobic interaction. For the CBS module of OpuA, it is not clear whether hydrophobic interactions occur between the protein domains and the membrane. Knowing that CBS tandem domains have a distinct hydrophobic core and a more hydrophilic exterior, as indicated by the various crystal structures of CBS homologues (8, 11, 30, 31), hydrophobic interactions with the membrane seem less important than electrostatic interactions.
In conclusion, the surface-exposed cationic residues of the CBS module play an important role in osmotic (i.e. ionic) regulation of OpuA. We propose that the CBS module may interact (directly or indirectly) with the anionic membrane surface and lock the transporter in an inactive state at low ionic strength. Above threshold values of ionic strength, the electrostatic interactions are screened and OpuA is activated. The strength of the electrostatic interaction is tuned by the C-terminal tail, which does not require a specific structure but simply depends on the number of co- and counterions in the protein and on the membrane. By introducing histidines in the C-terminal, the transporter became responsive to the internal pH.
Acknowledgments
We thank Ria Duurkens and Fabrizia Fusetti for assistance with size-exclusion chromatography experiments and modeling of CBS domains.
This work was supported by “Top-subsidie” NWO-CW Grants 700-50-302 and 700-56-302 and funds from the Netherlands Proteomics Centre and European Union E-MeP (504601) programs.
Footnotes
The abbreviations used are: ABC, ATP-binding cassette; CBS, cystathionine β-synthase; NBD, nucleotide-binding domain; DOPE, dioleoyl-phosphatidylethanolamine; DOPG, dioleoyl-phosphatidylglycerol; DOPC, dioleoylphosphatidylcholine; PIP2, phosphatidylinositol biphosphate; MARCKS, myristoylated alanine-rich protein kinase C substrate; PA, phosphatidic acid; LPA, lysophosphatidic acid.
References
- 1.Wood, J. M. (1999) Mol. Biol. Rev. 63 230-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heide, T., Stuart, M. C., and Poolman, B. (2001) EMBO J. 20 7022-7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Racher, K. I., Culham, D. E., and Wood, J. M. (2001) Biochemistry 40 7324-7333 [DOI] [PubMed] [Google Scholar]
- 4.Rübenhagen, R., Morbach, S., and Kramer, R. (2001) EMBO J. 20 5412-5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biemans-Oldehinkel, E., Mahmood, N. A. B. N., and Poolman, B. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 10624-10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmood, N. A., Biemans-Oldehinkel, H., Patzlaff, J., Schuurman-Wolters, G. K., and Poolman, B. (2006) J. Biol. Chem., 281 29830-29839 [DOI] [PubMed] [Google Scholar]
- 7.Bateman, A. (1997) Trends Biochem. Sci. 22 12-13 [DOI] [PubMed] [Google Scholar]
- 8.Miller, M. D., Schwarzenbacher, R., von Delft, F., Abdubek, P., Ambing, E., Biorac, T., Brinen, L. S., Canaves, J. M., Cambell, J., Chiu, H. J., Dai, X., Deacon, A. M., DiDonato, M., Elsliger, M. A., Eshagi, S., Floyd, R., Godzik, A., Grittini, C., Grzechnik, S. K., Hampton, E., Jaroszewski, L., Kariak, C., Klock, H. E., Koesema, E., Kovarik, J. S., Kreusch, A., Kuhn, P., Lesley, S. A., Levin, I., McMullan, D., McPhillips, T. M., Morse, A., Moy, K., Ouyang, J., Page, R., Quijano, K., Robb, A., Spraggon, G., Stevens, R. C., van den Bedem, H., Velasquez, J., Vincent, J., Wang, X., West, B., Wolf, G., Xu, Q., Hodgson, K. O., Wooley, J., and Wilson, I. A. (2004) Proteins 57 213-21715326606 [Google Scholar]
- 9.Ragunathan, P., Kumarevel, T., Agari, Y., Shinkai, A., Kuramitsu, S., Yokoyama, S., and Ponnuraj, K. (2008) Biochem. Biophys. Res. Commun. 375 124-128 [DOI] [PubMed] [Google Scholar]
- 10.Ishitani, R., Sugita, Y., Dohmae, N., Furuya, N., Hattori, M., and Nureki, O. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 15393-15398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori, M., Tanaka, Y., Fukai, S., Ishitani, R., and Nureki, O. (2007) Nature 448 1072-1076 [DOI] [PubMed] [Google Scholar]
- 12.van der Heide, T., and Poolman, B. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 7102-7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biemans-Oldehinkel, E., and Poolman, B. (2003) EMBO J. 22 5983-5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geertsma, E. R., Mahmood, N. A. B., Schuurman-Wolters, G. K., and Poolman, B. (2008) Nature Prot. 3 256-266 [DOI] [PubMed] [Google Scholar]
- 15.Landfald, B., and Strom, A. R. (1986) J. Bacteriol. 165 849-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driessen, A. J., Zheng, T., In't Veld, G., Op den Kamp, J. A., and Konings, W. N. (1988) Biochemistry 27 865-872 [DOI] [PubMed] [Google Scholar]
- 17.Law, C. J., Yang, Q., Soudant, C., Maloney, P. C., and Wang, D.-N. (2007) Biochemistry 46 12190-12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pusch, M., Ludewig, U., and Jentsch, T. J. (1997) J. Gen. Physiol. 109 105-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chraibi, A., and Horisberger, J.-D. (2002) J. Gen. Physiol. 120 133-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poolman, B., Spitzer, J. J., and Wood, J. (2004) Biochim. Biophys. Acta 1666 88-104 [DOI] [PubMed] [Google Scholar]
- 21.Poolman, B., and Spitzer, J. (2009) Microbiol. Mol. Biol. Rev., in press [DOI] [PMC free article] [PubMed]
- 22.Chemin, J., Patel, A. J., Duprat, F., Lauritzen, I., Ladzunski, M., and Honoré, E. (2005) EMBO J. 24 44-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ptak, C. P., Cuello, L. G., and Perozo, E. (2005) Biochemistry 44 62-71 [DOI] [PubMed] [Google Scholar]
- 24.Mclaughlin, S., Wang, J., Gambhir, A., and Murray, D. (2002) Annu. Rev. Biophys. Biomol. Struct. 31 151-175 [DOI] [PubMed] [Google Scholar]
- 25.Mclaughlin, S., and Murray, D. (2005) Nature 438 605-611 [DOI] [PubMed] [Google Scholar]
- 26.Athenstaedt, K., and Daum, G. (1999). Eur. J. Biochem. 266 1-6 [DOI] [PubMed] [Google Scholar]
- 27.Kooijman, E. E., Carter, K. M., van Laar, E. G., Chupin, V., Burger, K. N., and de Kruijff, B. (2005) Biochemistry 44 17007-17015 [DOI] [PubMed] [Google Scholar]
- 28.Arnold, R. S., and Cornell, R. B. (1996) Biochemistry 35 9917-9924 [DOI] [PubMed] [Google Scholar]
- 29.Johnson, J. E., Aebersold, R., and Corneel, R. B. (1997) Biochim. Biophys. Acta 1324 273-284 [DOI] [PubMed] [Google Scholar]
- 30.Meijer, S., and Dutzler, R. (2006) Structure 14 199-207 [Google Scholar]
- 31.Zhang, R., Evans, G., Rotella, F. J., Westbrook, E. M., Beno, D., Huberman, E., Jochiamiak, A., and Collart, F. R. (1999) Biochemistry 38 4691-4700 [DOI] [PubMed] [Google Scholar]
- 32.Proudfoot, M., Sanders, S. A., Singer, A., Zhang, A., Brown, G., Binkowski, A., Xu, L., Lukin, J. A., Murzin, A. G., Joachimiak, A., Arrowsmith, C. H., Edwards, A. M., Savchenko, A. V., and Yakunin, A. F. (2008) J. Mol. Biol. 375 301-315 [DOI] [PMC free article] [PubMed] [Google Scholar]