Abstract
The gene that encodes the ATM protein kinase is mutated in ataxia-telangiectasia (A-T). One of the prominent features of A-T is progressive neurodegeneration. We have previously reported that primary astrocytes isolated from Atm-/- mice grow slowly and die earlier than control cells in culture. However, the mechanisms for this remain unclear. We show here that intrinsic elevated intracellular levels of reactive oxygen species (ROS) are associated with the senescence-like growth defect of Atm-/- astrocytes. This condition is accompanied by constitutively higher levels of ERK1/2 phosphorylation and p16Ink4a in Atm-/- astrocytes. We also observe that ROS-induced up-regulation of p16Ink4a occurs correlatively with ERK1/2-dependent down-regulation and subsequent dissociation from chromatin of Bmi-1. Furthermore, both mitogen-activated protein kinase (MAPK)/ERK inhibitor PD98059 and antioxidant N-acetyl-l-cysteine restored normal proliferation of Atm-/- astrocytes. These results suggest that ATM is required for normal astrocyte growth through its ability to stabilize intracellular redox status and that the inability to control ROS is the molecular basis of limited cell growth of Atm-/- astrocytes. This defect may be mediated by a mechanism involving ERK1/2 activation and Bmi-1 derepression of p16Ink4a. These data identify new potential targets for therapeutic intervention in A-T neurodegeneration.
A prominent feature of ataxia-telangiectasia (A-T)2 is neurodegeneration that is caused by mutations in the Atm (ataxia-telangiectasia mutated) gene (1–3). The Atm gene product, ATM protein kinase, regulates the cell cycle in response to DNA damage and to oxidative stress (4, 5). Within the central nervous system (CNS), proper responses to ROS are required to prevent neurodegeneration (6–9). Others have shown that exogenous antioxidants prevent Purkinje cell death (10) and correct neurobehavioral deficits in Atm-/- mice (11, 12). These findings suggest that abnormalities in redox status contribute to the A-T phenotype (13–16). Although increased oxidative stress has been shown to characterize ATM deficiency in the CNS, the mechanisms by which oxidative stress promotes neuronal cell loss in A-T remain unclear.
A-T neurodegeneration is primarily due to a loss of cerebella Purkinje neurons and to malfunction of other neuronal cells as well (3). Neurons in the CNS depend upon astrocytes for structural, functional, and thiol support (17, 18). For example, Purkinje neurons in the cerebellum rely heavily on their supporting Bergmann astrocytes (19). We have shown that Bergmann astrocytes of Atm-/- mice are under oxidative stress (20), implying that oxidative stress resulting from a loss of ATM may promote astrocyte dysfunction. Others have also shown that cultured astrocytes from Atm-/- mice have cell cycle and growth defects (21) and that astrocytes isolated from Atm-/- mouse embryos grow poorly in culture and readily undergo senescence (15, 20). These observations imply that ATM is essential for normal astrocyte growth and maturation. In light of the importance of astrocytes in supporting neurons against oxidative stress, we hypothesize that aberrant growth and function of Atm-/- astrocytes may contribute to neuron loss in A-T.
We show here that Atm-/- astrocytes exhibit senescence-like growth arrest, compared with the growth characteristics of their wild type (Atm+/+) counterparts. We also show that elevated ROS levels are one of the causes of proliferation defects in Atm-/- astrocytes. This conclusion is based on the observation that defective cell growth of Atm-/- astrocytes is restored to normal by the antioxidant N-acetyl-l-cysteine (NAC). This in turn implies that the role of ATM in normal astrocytic proliferation involves stabilization of redox status in the cell. We also observe that growth arrest of Atm-/- astrocytes is associated with chronic maintenance of high levels of the cyclin-dependent kinase inhibitors and phosphorylation of ERK1/2 under conditions in which intracellular ROS levels are elevated. In addition, we identify an intermediate player, Bmi-1, that links ERK1/2 to up-regulation of p16Ink4a. Bmi-1, apolycomb group protein, has been implicated in proliferation of several cell types, through its transcriptional repression of p16Ink4a (22, 23). We observe that ROS-induced Bmi-1 down-regulation and dissociation from chromatin are both ERK1/2-dependent. This suggests that the up-regulation of p16Ink4a is mediated by a mechanism involving ERK1/2 activation and Bmi-1 loss-of-function as transcription repressor of p16Ink4a. Finally, we show that the MEK inhibitor PD98059 reverses p16Ink4a up-regulation and chromatin dissociation of Bmi-1. Furthermore, knockdown of p16Ink4a greatly corrected the cell growth defect in Atm-/- astrocytes. These studies identify a role of ATM in controlling normal astrocyte growth and maturation. This is carried out by suppression of oxidative stress-dependent signaling pathways.
EXPERIMENTAL PROCEDURES
Mice—Atm-/- mice were originally generated by C. Barlow (24). For this study, they were purchased from the Jackson Laboratories (Bar Harbor, ME). We genotyped offspring (Atm+/+, Atm+/-, or Atm-/-) by real time polymerase chain reaction-based assays of mouse tail DNA. Littermates were used as controls in all of the experiments. Animal care was in accordance with the University of Texas M. D. Anderson Cancer Center guidelines for animal experiments.
Cells and Cell Culture—Immortalized murine C1 astrocytes were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin) (25). The cells were passaged biweekly and used for experiments while in the exponential growth phase. Primary astrocytes were isolated from 1–2-day-old newborn mouse pups by a method described previously (20, 26). Whole brain of each newborn mouse was removed and minced separately in ice-cold Dulbecco's modified Eagle's medium/F-12 medium. A single-cell suspension was obtained by passing the minced tissue through a 70-m nylon mesh cell strainer. The cells were plated onto poly-l-lysine-coated 10-mm flasks and grown in Dulbecco's modified Eagle's medium/F-12 medium, supplemented with 10% fetal bovine serum, 5 units/ml penicillin, 5 μg/ml streptomycin, and 2.5 g/ml Fungizone. The cells were cultured for 4 days in an incubator with 5% CO2 atmosphere at 37 °C until reaching confluence. They were passaged by trypsinization. Cells from passage 4 or 5 were used for all experiments. Astrocytes by the fourth or fifth passage were >99% glial fibrillary acidic protein (GFAP)-positive (27).
Chemical Reagents—ATM inhibitor KU55933 (10 μm) was purchased from Sigma. MEK inhibitor PD98059 (50 μm) was purchased from BD Biosciences, and antioxidant NAC (1 mm) was kindly provided by M. Yan (M. D. Anderson Cancer Center) (28).
Analysis of Cell Senescence—SA β-galactosidase assay kit (Cell Signaling) was used to visualize senescent cells. Astrocytes were cultured in 6-well plates for 2 days and then fixed after being washed twice with PBS. The cells were further incubated for 24 h in the presence of staining solution-containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal). Senescent cells were distinguished from viable cells using β-galactosidase, which is blue under the microscope.
Analysis of Intracellular ROS—Intracellular ROS accumulation was monitored by using 2′-7′-dichlorofluorescene diacetate (H2DCFDA), which forms a fluorescent compound, dichlorofluorescein, upon oxidation with ROS. After 30 min of incubation in 10 μm H2DCFDA, the cells were rinsed twice with PBS. Fluorescence was monitored by scanning the whole well using a NucleoCounter (New Brunswick Scientific Co., Ltd.) set at excitation and emission wavelengths of 485 and 535 nm.
Protein Analysis—The cells (1–5 × 106) were seeded into 10-cm dishes, grown to 70% confluence, and treated with H2O2 at different concentrations. At 1, 4, or 16 h after H2O2 treatment, the cells were washed twice with ice-cold PBS and then scraped directly into lysis buffer containing 150 mm NaCl, 0.5% w/v SDS, 0.5% v/v Nonidet P-40, 0.5% w/v sodium deoxycholate, 1 mm EGTA, and a mixture of protease inhibitors (Complete Mini tablets; Roche Applied Science). Protein concentrations were determined using a Bradford reagent (Bio-Rad). The protein lysates (30 μg) were separated by SDS-polyacrylamide gel electrophoresis on a 12% gel and transferred to polyvinylidene difluoride membrane. Antibodies used for Western blotting analysis were anti-ERK1/2, anti-phospho-ERK1/2 (Thr202/Tyr204), anti-phospho-Rb (Ser807/811), anti-PCNA, and anti-p27kip1 (Cell Signaling Technology); anti-Bmi-1 (Upstate Biotechnology); anti-p21cip1, anti-p16Ink4a, and anti-actin (Santa Cruz Biotechnology).
siRNA-mediated Knockdown—For siRNA knockdown of Atm, the cells were transfected for 48 h with 20 nm siRNA (mouse-specific Atm) in medium containing 10% (v/v) fetal bovine serum using Lipofectamine reagent (Invitrogen) according to the manufacturer's protocol for cells. Validated siRNA (SMARTpool) was obtained from Dharmacon. The negative control siRNA used was siCONTROL, which contains at least four mismatches to all known human, mouse, and rat genes. For mock transfection, we used siRNA suspension buffer and Lipofectamine containing no siRNA. For siRNA knockdown of p16Ink4a, mouse-specific p16Ink4a siRNA was purchased from Santa Cruz Biotechnology.
Immunofluorescence—The cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature and then permeabilized with 0.1% Triton X-100 in PBS for 15 min. For staining with antibodies, the cells were incubated for 2 h at 37 °C with 10% fetal bovine serum in PBS. Primary antibodies were added, and the cells were incubated at 4 °C overnight. The cells were then washed three times in PBS, 0.1% Triton X-100 for 5 min each, and fluorescent secondary antibodies were added for 1 h at 37 °C. The nuclei were stained with 4′,6-diamidino-2-phenylindole, and the slides were mounted in Slowfade (Molecular Probes). The cells were imaged under an Olympus IX2-SL microscope equipped with a ×400 objective and an Olympus DP 70 digital camera connected to a PC computer.
Statistics—For each experiment, the data are presented as the means ± S.D. of values. Each experiment was repeated at least three times. Statistical comparisons of values for Atm+/+ versus Atm-/- mice and for untreated control versus treated samples were made using the Mann-Whitney U test. The differences were considered significant when p < 0.05.
RESULTS
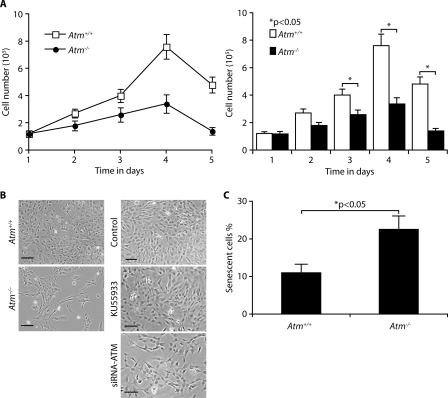
Loss of ATM Impairs Proliferation in Astrocytes—We have reported previously that ATM-deficient primary astrocytes grow slowly, become senescent, and die in culture (20). These results are consistent with those reported by Gosink et al. (21). To compare the rate of proliferation of Atm+/+ and Atm-/- astrocytes, we analyzed their cell growth curves. As shown in Fig. 1A, Atm-/- astrocytes proliferated much more slowly than Atm+/+ astrocytes. In particular, Atm+/+ astrocytes show 2.5–3 times higher rates of proliferation than Atm-/- astrocytes at 4 and 5 days after subculturing. When monolayer of Atm+/+ and Atm-/- astrocytes were observed under the microscope following further incubation for 4 days, Atm-/- astrocytes showed lower densities than wild type control cells with a decrease of ∼50% in cell number (Fig. 1B, left panel). This is consistent with our findings for C1 astrocytes, a murine immortalized astrocyte cell line (25). Although the ATM inhibitor KU55933 slightly decreases astrocytes proliferation, the loss of ATM by ATM siRNA reduced cell proliferation by ∼40% (Fig. 1B, right panel). To obtain direct evidence that ATM deficiency causes cell senescence, a specific SA β-galactosidase assay was performed after 2 days of culture. Fig. 1C shows that senescent cells are present two times more in Atm-/- astrocyte cultures than Atm+/+ cultures. These data indicate that ATM deficiency causes the senescent-like cell growth defect in Atm-/- astrocytes.
FIGURE 1.
Loss of ATM impairs proliferation in astrocytes. A, astrocytes were isolated from the brains of newborn Atm+/+ and Atm-/- mice, and their growth curves were determined by trypan blue staining at indicated days after initial seeding of cells. The means ± S.D. of three independent experiments are shown. *, p < 0.05 when Atm-/- astrocytes were compared with Atm+/+ astrocytes. B, phase contrast photomicrographs of Atm+/+ and Atm-/- astrocytes following further incubation for 4 days (left panel). ATM in C1 astrocytes was inactivated by ATM inhibitor KU55933, and knocked down using ATM siRNA (right panel). Scale bars, 50 μm. C, the proportions of senescent cells in Atm+/+ and Atm-/- astrocytes were determined by SA β-galactosidase expression 2 days after cultivation. The means ± S.D. of three independent experiments are shown. *, p < 0.05 when Atm-/- astrocytes were compared with Atm+/+ astrocytes.
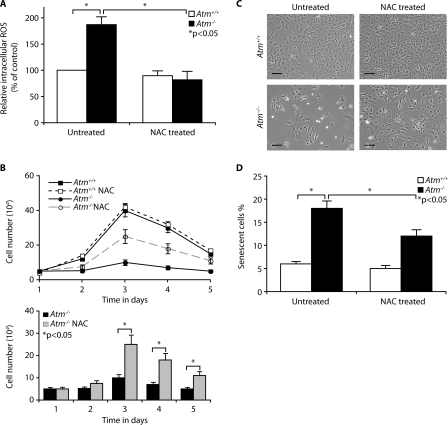
Decreased Proliferation in Atm-/- Astrocytes Is Caused by Elevated ROS Levels—ATM monitors cellular redox status in addition to its monitoring of DNA damage and genomic instability (26), and cells lacking ATM show ROS accumulation (16). Although we have previously shown that a loss of Atm results in oxidative damage in the brain (20), the involvement of oxidative stress in defective astrocyte growth in the ATM-deficient mouse has not been tested. To address this issue, we compared intracellular ROS levels in Atm+/+ and Atm-/- astrocytes by H2DCFDA staining of intracellular H2O2. The data show that the intracellular levels of ROS are significantly higher in Atm-/- astrocytes than in Atm+/+ astrocytes and that antioxidant NAC reduces the elevated ROS levels of Atm-/- astrocytes to normal levels (Fig. 2A). This result suggests that elevated ROS levels are intrinsic characteristics of cells lacking ATM. This is consistent with our results from C1 astrocytes treated with ATM-specific inhibitor, KU55933, which show higher levels of intracellular ROS compared with untreated control cells (data not shown). For direct evidence that defective proliferation of Atm-/- astrocytes is due to elevated ROS levels, we treated Atm-/- astrocytes with NAC and compared their proliferation rates to those of untreated cells. Fig. 2B shows that proliferation rates for Atm-/- astrocytes were increased by treatment with NAC, showing nearly 2–2.5 times higher rates than untreated Atm-/- counterpart at 3 and 4 days after subculturing. This effect of NAC was confirmed by photomicrograph, which disclosed increased cell density by NAC (Fig. 2C). This is consistent with our findings that NAC decreases the proportion of senescent cells in Atm-/- astrocyte cultures (Fig. 2D). These results provide strong evidence linking defective growth of Atm-/- astrocytes to elevated intracellular ROS.
FIGURE 2.
Decreased proliferation in Atm-/- astrocytes is caused by elevated ROS levels. A, Atm+/+ and Atm-/- astrocytes were either left untreated or pretreated with NAC (1 mm) for 2 h. Fluorescent H2DCFDA levels were determined for these cells. The means ± S.D. of three independent experiments are shown. *, p < 0.05 when untreated Atm-/- culture were compared with untreated Atm+/+ astrocytes or when NAC-treated Atm-/- astrocytes were compared with untreated Atm-/- astrocytes. B, growth curves of Atm-/- astrocytes in the presence or absence of NAC (1 mm) were analyzed for 5 days and then compared with those of Atm+/+ astrocytes. The means ± S.D. of three independent counting are shown. *, p < 0.05 when NAC-treated Atm-/- astrocytes were compared with untreated Atm-/- astrocytes. C, photomicrographs of Atm+/+ and Atm-/- astrocytes in the presence or absence of NAC. Scale bars, 50 μm. D, senescence of Atm+/+ and Atm-/- astrocytes in the presence or absence of NAC was compared by SA β-galactosidase expression 2 days after cultivation. The means ± S.D. of three independent experiments are shown. *, p < 0.05 when untreated Atm-/- culture was compared with untreated Atm+/+ astrocytes or when NAC-treated Atm-/- astrocytes was compared with untreated Atm-/- astrocytes.
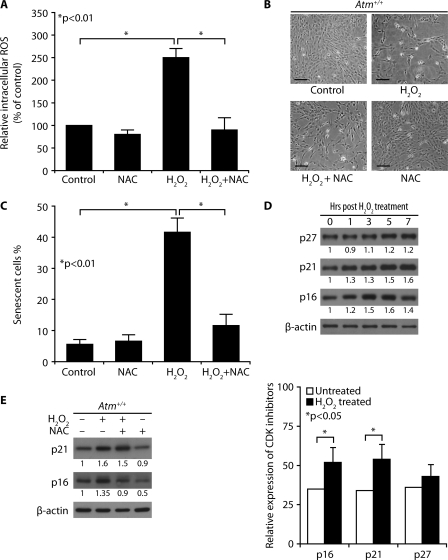
H2O2-treated Atm+/+ Astrocytes Show Reduced Proliferation and Up-regulated Levels of CDK Inhibitors—To determine whether H2O2 decreases Atm+/+ astrocyte proliferation and whether antioxidant inhibits ROS-mediated defective proliferation in normal Atm+/+ astrocytes subjected to exogenous oxidative stress, we exposed Atm+/+ astrocytes to H2O2 and treated the same H2O2-exposed cells with NAC. Fig. 3A shows that H2O2 elevated intracellular ROS levels in Atm+/+ astrocytes and that treatment of Atm+/+ astrocytes with NAC reduced their intracellular ROS to normal levels. Atm+/+ astrocytes also showed significantly decreased proliferation 2 days after H2O2 treatment, but H2O2-induced defective proliferation was partially restored by NAC (Fig. 3B). Treatment with H2O2 for 2 days followed by 1 day of incubation in H2O2-free medium induced SA β-galactosidase expression in ∼40% of Atm+/+ astrocytes, but NAC significantly prevented H2O2-induced senescence (Fig. 3C). These results indicate that H2O2 inhibits astrocytes proliferation through elevating intracellular ROS, regardless of their ATM status.
FIGURE 3.
H2O2-treated Atm+/+ astrocytes show reduced proliferation and up-regulated levels of CDK inhibitors. A, Atm+/+ astrocytes were pretreated with 1 mm NAC before treatment with 100 μm H2O2. Intracellular ROS levels were then measured in cultures. The means ± S.D. of three independent experiments are shown. *, p < 0.01 when H2O2-treated Atm+/+ astrocytes was compared with untreated Atm+/+ culture or when NAC- and H2O2-treated Atm+/+ astrocytes were compared with H2O2-treated Atm+/+ culture. B, Atm+/+ astrocytes were pretreated with 1 mm NAC before treating with 100 μm H2O2. Photomicrographs were taken following a subsequent 4-day incubation. Scale bars, 50 μm. C, SA β-galactosidase expressions in cultures from the experiment shown in A. The means ± S.D. of three independent experiments are shown. *, p < 0.01 when H2O2-treated Atm+/+ culture was compared with untreated Atm+/+ culture or when NAC- and H2O2-treated Atm+/+ culture was compared with H2O2-treated Atm+/+ culture. D, Atm+/+ astrocytes were treated with 100 μm H2O2, and then levels of p27kip1, p21cip1, and p16Ink4a were determined. The means ± S.D. of three independent experiments are shown. *, p < 0.05 when H2O2-treated Atm+/+ astrocytes were compared with untreated Atm+/+ astrocytes. E, the effects of NAC on p21cip1 and p16Ink4a expressions were determined by direct Western blotting analysis after H2O2 treatment in the presence of NAC.
We then investigated the effects of ROS on several known key intermediates associated with cell proliferation. The inhibitors of cyclin-dependent kinases (CDK) are considered to play an important role in cell cycle arrest and premature senescence (30). Others have shown that the CDK inhibitor p21cip1 is up-regulated in Atm-/- astrocytes (26). Therefore, we investigated the effects of ROS on levels of CDK inhibitors, including p16Ink4a, p19Arf, p21cip1, and p27kip1 in Atm+/+ astrocytes. H2O2 treatment of Atm+/+ astrocytes stimulated expression of p21cip1 and p16Ink4a, but not p27kip1 in Atm+/+ astrocytes (Fig. 3D, upper and lower panels). However, no p19Arf band was present in the lysates from those cells. These alterations indicate that activation of the p16Ink4a and/or p19Arf-p21cip1 senescence pathway(s) may be involved in ROS-mediated senescence in astrocytes. Furthermore, activation of p16Ink4a was attenuated by NAC (Fig. 3E). These findings suggest that intracellular ROS-mediated oxidative stress induces senescence in astrocytes via activation of the p16Ink4a pathway.
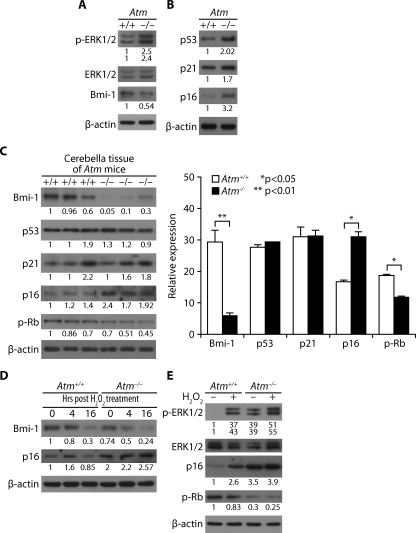
In Atm-/- Astrocytes, ERK1/2 Is Constitutively Activated, and CDK Inhibitors Are Up-regulated—We have previously reported that ERK1/2 is activated in both Atm-/- astrocytes in culture and in Bergmann glia in the cerebella of Atm-/- mice (20). We consistently observed higher level of phospho-ERK1/2 in Atm-/- astrocytes than in Atm+/+ astrocytes. In addition, Bmi-1, a polycomb ring finger oncogene product, is down-regulated in Atm-/- cells (Fig. 4A), which is consistent with the fact that Bmi-1 promotes cell proliferation via repression of CDK inhibitors. In Fig. 3D, we have shown that intracellular ROS levels increased in Atm-/- astrocytes, and H2O2 up-regulated CDK inhibitors. We therefore reasoned that CDK inhibitors might be up-regulated to decrease proliferation in Atm-/- astrocytes. We thus compared the basal levels of CDK inhibitors in Atm-/- astrocytes with those in wild type control cells. Fig. 4B shows that the basal expression levels of p53, p21cip1, and p16Inka were higher in Atm-/- cells than in the wild type cells. p16Ink4a was up-regulated Atm-/- cerebella tissues. Not surprisingly, Atm-/- astrocytes show lower levels of retinoblastoma (Rb) phosphorylation than do wild type control cells. Moreover, Bmi-1 levels were significantly down-regulated in the Atm-/- tissues (Fig. 4C, left and right panels).
FIGURE 4.
In Atm-/- astrocytes, ERK1/2 is constitutively activated and CDK inhibitors are up-regulated. A and B, for Atm+/+ and Atm-/- astrocytes, levels of phospho-ERK1/2, ERK1/2, and Bmi-1 (A) and levels of p53, p21cip1, and p16Ink4a (B) were assessed by direct Western blotting analysis. C, levels of Bmi-1, p53, p21cip1, p16Ink4a, and phospho-Rb were compared for Atm+/+ and Atm-/- cerebella tissues by direct Western blotting analysis. The means ± S.D. of three different mice tissues are shown. *, p < 0.05; **, p < 0.01 when Atm-/- tissues was compared with Atm+/+ tissues. D, Atm+/+ and Atm-/- astrocytes were treated with 100 μm H2O2. The cells were then harvested to access levels of Bmi-1 and p16Ink4a at the indicated times after H2O2 treatment. E, Atm+/+ and Atm-/- astrocytes were either left untreated or treated with 100 μm H2O2. Levels of phospho-ERK1/2, ERK1/2, p16Ink4a, and phospho-Rb were determined by direct Western blotting analysis.
We next compared p16Ink4a levels in Atm-/- astrocytes and Atm+/+ astrocytes, to which exogenous H2O2 had been added. Interestingly, the addition of H2O2 caused a brief up-regulation of p16Inka at 4 h in wild type control cells but then reversed itself down to the untreated basal level at 16 h post-treatment. However, when Atm-/- astrocytes were exposed to H2O2, the level of p16Ink4a expression was further elevated, and this up-regulation persisted from 4 to 16 h post-H2O2 treatment. This means that oxidative stress caused by elevated ROS is reversible to normal levels when ATM kinase is present. In both Atm+/+ and Atm-/- astrocytes, Bmi-1 was down-regulated in response to H2O2, but its level was lower in Atm-/- astrocytes than in Atm+/+ astrocytes (Fig. 4D). In Atm+/+ astrocytes, the brief expression of p16Ink4a may shut down cell cycling, allowing time for the cells to repair any damage. Once the job is done, their levels return to normal, as a result of the redox balancing action of ATM.
Fig. 4E shows that in both Atm+/+ and Atm-/- astrocytes, H2O2 treatment also causes phosphorylation of ERK1/2 and up-regulation of p16Ink4a. Phospho-ERK1/2 and p16Ink4a changes that occurred in H2O2-treated Atm+/+ astrocytes were less pronounced than in H2O2-treated Atm-/- astrocytes. Atm-/- astrocytes also show lower levels of Rb phosphorylation than do wild type control cells. Moreover, Rb phosphorylation was further reduced after H2O2 exposure. These results indicate that Atm-/- astrocytes are differentially hypersensitive to oxidative stress and maintain high expression levels of phospho-ERK1/2 and p16Ink4a. If oxidative stress is prolonged, as it is in cells lacking ATM, up-regulation of phospho-ERK1/2 persistently may increase p16Ink4a expression, resulting in prolonged cell cycle arrest and retardation of cell proliferation. These data strongly implicate the involvement of ERK1/2-p16Ink4a signaling pathway in ROS-induced cell growth arrest of Atm-/- astrocytes.
ERK1/2 Signaling Mediates Bmi-1 Down-regulation and Chromatin Dissociation in Atm-/- Astrocytes— We next set out to identify the molecular mechanisms underlying the effects of ROS on the cell growth defects of Atm-/- astrocytes, focusing on the mechanisms for phospho-ERK1/2 and p16Ink4a up-regulation. p16Ink4a expression is known to be regulated by the MAPK pathways, including activation of ERK1/2 (31). Furthermore, exposure to H2O2 activates MAPKs in many cell types (32, 33). Therefore, we tested the effect of ROS on ERK1/2 downstream mediators. Upon phosphorylation at two amino acids (Thr202/Tyr204), ERK1/2 translocates into the nucleus, where it phosphorylates its substrates. Because p16Ink4a expression level does not depend on phosphorylation by ERK1/2, it is not a direct substrate of activated ERK1/2. Instead, p16Ink4a expression is negatively regulated by Bmi-1 (34). Amino acid sequence analysis indicates that Bmi-1 has two predicted consensus motifs for ERK1 phosphorylation. We thus asked whether ROS-induced ERK1/2 signaling has effects on Bmi-1 function as a transcription repressor for p16Ink4a.
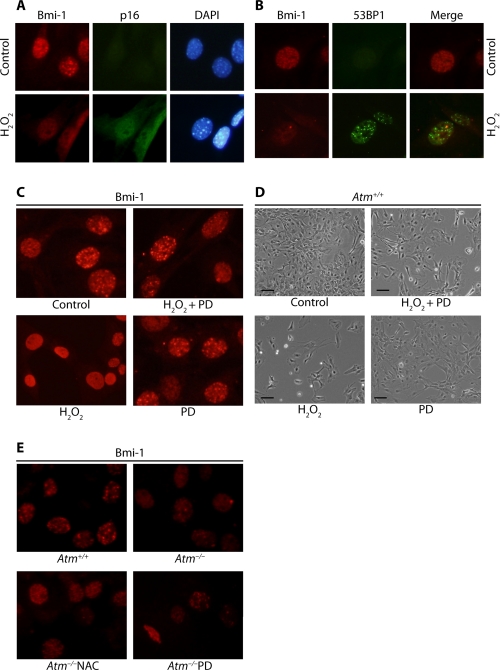
The polycomb group proteins including Bmi-1 function as transcription repressors. Chromatin association of these proteins can be demonstrated by puncta in the nucleus in previous study (35). Immunofluorescence imaging of endogenous Bmi-1 in Atm+/+ astrocytes indicates that Bmi-1 localizes mainly in chromatin and that it dissociates from chromatin after cells are exposed to H2O2 with a concomitant up-regulation of p16Ink4a (Fig. 5A). Bmi-1/chromatin association correlates with trimethylation at the lysine 27 residue of histone H3 (H3K27me3; data not shown), because H3K27me3 is required for polycomb/histone H3 interaction (36). However, it was also noted that Bmi-1/chromatin association does not correlate with sites of DNA double strand breaks, because fluorescence images of 53BP1 did not colocalize with Bmi-1 in astrocytes treated with H2O2 (Fig. 5B).
FIGURE 5.
ERK1/2 signaling mediates Bmi-1 down-regulation and chromatin dissociation in Atm-/- astrocytes. A, Atm+/+ astrocytes were synchronized by contact inhibition after subculture, at which time cells were treated with 100 μm H2O2. Bmi-1 association with chromatin was analyzed by fluorescence microscope for the presence of puncta in the nucleus. p16Ink4a up-regulation and Bmi-1/chromatin dissociation was tested using anti-p16Ink4a antibody. B, colocalization of Bmi-1 and 53BP1 was tested in the cells after H2O2 treatment using the same methods. C, Atm+/+ astrocytes were left untreated or pretreated with 50 μm PD98059 (PD) overnight, before treating with 100 μm H2O2. The effects of 50 μm PD98059 on Bmi-1 localization in astrocytes were analyzed. D, photomicrographs of Atm+/+ astrocytes culture from the experiment shown in C. Scale bars, 50 μm. E, Atm-/- astrocytes were treated with 1 mm NAC or 50 μm PD98059 for 2 days. Bmi-1 expression and localization in Atm+/+ and Atm-/- astrocytes were determined. DAPI, 4′,6-diamidino-2-phenylindole.
We next asked whether H2O2-induced Bmi-1/chromatin dissociation is dependent on ERK1/2 signaling. We thus pretreated Atm+/+ astrocytes with MEK inhibitor PD98059 before H2O2 treatment, because MEK is known as the upstream of ERK1/2 (32). Fig. 5C shows that H2O2-induced Bmi-1/chromatin dissociation is significantly inhibited by PD98059. This suggests that Bmi-1 dissociation from chromatin occurs via ERK1/2 signaling. In addition, H2O2 inhibits astrocyte proliferation, but PD98059 partially rescues it (Fig. 5D). Taken together with increased proliferation by PD98059, reversion of chromatin dissociation and restoration of Bmi-1 by PD98059 indicate that H2O2-induced proliferation decrease is associated to Bmi-1 dysfunction by ERK1/2 signaling.
Next, we assessed the effect of ATM deficiency on endogenous Bmi-1 localization using Atm-/- astrocytes. Fig. 5E shows that Bmi-1 is down-regulated, and less Bmi-1 associate with chromatin in Atm-/- astrocytes than in Atm+/+ astrocytes. (NAC and PD98059 partially restored levels of Bmi-1 expression and chromatin association in Atm-/- astrocytes). These results are consistent with previous findings of Bmi-1 down-regulation and p16Ink4a up-regulation that occur in Atm-/- astrocytes (Fig. 4A). The data link p16Ink4a up-regulation to Bmi-1 down-regulation in Atm-/- astrocytes and in turn also link these two events to elevated intracellular ROS levels. Because PD98059 also restored normal Bmi-1 and p16Ink4a levels in Atm-/- astrocytes, we conclude that ERK1/2 mediates Bmi-1 down-regulation and chromatin dissociation, as well as p16Ink4a up-regulation.
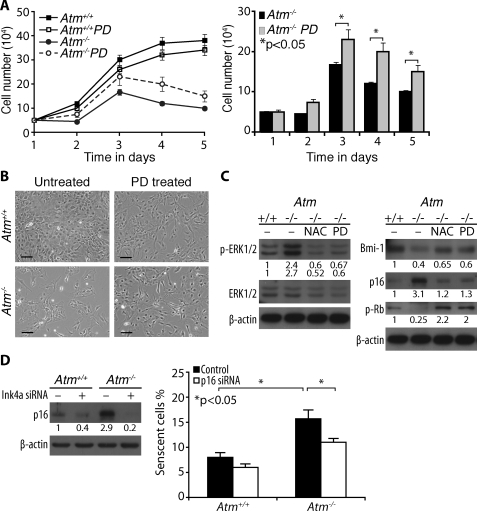
Restoration of Normal Proliferation of Atm-/- Astrocytes by Inactivation of ERK1/2 Signaling or Knockdown of p16Ink4a-—We next asked whether activation of ERK1/2 impairs survival and proliferation of Atm-/- astrocytes and whether inhibiting ERK1/2 signaling restores defective cell growth. We established cultures of Atm-/- astrocytes in the presence of PD98059. Growth curve analysis for 5 days showed that PD98059 partially rescued Atm-/- astrocytes from defective proliferation (Fig. 6A). The restorative actions of PD98059 were also evident in culture, as shown by increase in cell density (Fig. 6B). However, the positive effect of PD98059 on proliferation of Atm-/- astrocytes was not comparable with that of the antioxidant NAC. In addition, PD98059 appeared to inhibit mitogenic pathways in Atm+/+ astrocytes, because it reduced growth rate and proliferation in these cells. In normal Atm+/+ mice, most astrocytes are quiescent, and thus ERK1/2 signaling is suspended in an inactive state. However, Atm-/- astrocytes maintain constitutive ERK1/2 signaling associated with oxidative stress conditions.
FIGURE 6.
Restoration of normal proliferation of Atm-/- astrocytes by inactivation of ERK1/2 signaling or knockdown of p16Ink4a. A, growth curves of Atm+/+ and Atm-/- astrocytes, cultured either in the presence or absence of 50 μm PD98059. PD98059 partially rescued Atm-/- astrocytes from defective proliferation. The means ± S.D. of three independent countings are shown. *, p < 0.05 when PD98059-treated Atm-/- astrocytes were compared with untreated Atm-/- astrocytes. B, photomicrographs of Atm+/+ and Atm-/- astrocytes culture in the presence or absence of PD98059. Scale bars, 50 μm. C, Atm+/+ astrocytes were left untreated. Atm-/- astrocytes were either untreated left or treated with 1 mm NAC or 50 μm PD98059 for 2 days. The levels of phospho-ERK1/2, ERK1/2, Bmi-1, p16Ink4a, and phospho-Rb were determined by direct Western blotting analysis. D, Atm+/+ and Atm-/-astrocytes were transfected with Ink4a siRNA. p16Ink4a levels were determined by direct Western blotting analysis (left panel). Proportions of senescent cells were determined by SA β-galactosidase expression 2 days after cultivation (right panel). The means ± S.D. of three independent experiments are shown. *, p < 0.05 when untransfected Atm-/-astrocytes were compared with untransfected Atm+/+ culture or when Ink4a siRNA-transfected Atm-/-astrocytes were compared with untransfected Atm-/- astrocytes.
Despite its inhibitory effect on Atm+/+ astrocytes proliferation, PD98059 partially restored Bmi-1 levels in Atm-/- astrocytes. In addition, both NAC and PD98059 reduced levels of p21cip1 and p16Ink4a in Atm-/- astrocytes to levels similar to those in Atm+/+ astrocytes. In accordance with these results, level of phospho-Rb was also increased by NAC and PD98059 (Fig. 6C). These results provide direct evidence linking defective growth of Atm-/- astrocytes to activation of ERK1/2 signaling as a result of oxidative stress. However, PD98059 has modest and partial rescuing effect on Atm-/- astrocytes proliferation, indicating that there may be additional pathways associated with defective proliferation in Atm-/- astrocytes.
Because increases in cell senescence and p16Ink4a level were observed in Atm-/- astrocytes, we next decided to access whether p16Ink4a up-regulation is responsible for inducing cell senescence and whether inhibition of p16Ink4a expression would reverse the defective growth phenotype of Atm-/- astrocytes. Ink4a was knocked down in both Atm+/+ and Atm-/- astrocytes using siRNA against mouse p16Ink4a. Fig. 6D shows that more senescent cells were observed in Atm-/- astrocytes than in Atm+/+ astrocytes. However, Atm-/- astrocytes when p16Ink4a was knocked down had fewer senescent cells than did the cells whose p16Ink4a was intact.
DISCUSSION
In A-T patients, Purkinje neuron loss in the cerebellum is the most critical feature of the neuropathological phenotype (37). Up to now, therefore, most studies have focused on the effects of ATM deficiency in neurons, with the role(s) of astrocytes going unexplored. However, accumulating evidence now suggests that astrocytes are key elements serving pivotal functions in the central nervous system, including structural and redox support for neuron, neurotransmitter synthesis, and transport of nutrients and metabolic precursors to neurons (17, 19, 38–40). Previous studies have reported that transgenic and knock-out mouse models for certain astrocyte-specific proteins result in neurodegenerative disorders (41, 42). This implies that abnormalities in astrocytes might also cause neuropathophysiology of A-T. This is consistent with earlier observations by others that Purkinje cell survival in Atm-deficient mice is partially reversed when these cells are cultured in the presence of Atm+/+ astrocyte-conditioned medium (18). It will be important now to determine whether Atm-/- astrocytes have cytotoxic effects for neurons in coculture. Studies now in progress in our lab will address the effects of Atm-/- astrocytes on neurons in this context.
At normal concentrations, ROS play a role in cellular functions involving signal transduction (43, 44). However, an imbalance between generation of ROS and capacity of antioxidants to neutralize ROS can result in disruption of cellular redox status, leading to oxidative stress (45–47). Mammalian cells have therefore evolved a wide range of mechanisms, including ATM, for maintaining normal levels of intracellular ROS. Neuronal cells require large quantities of oxygen to function because of their high metabolic rate. As a result, neurons generate high levels of ROS. For this reason, the CNS is very vulnerable to ROS imbalance and accumulation. In general, low doses of H2O2 promote cell proliferation, whereas high levels of ROS elicit cell cycle arrest, senescence, and apoptosis (48). Our data show that Atm-/- astrocytes have a lower proliferation rate than that of normal cells. We previously observed increased spontaneous DNA synthesis in cultured Atm-/- astrocytes relative to Atm+/+ astrocyte despite their decreased proliferation (20). The reason for this discrepancy is unknown. However, it is possible that abnormally elevated DNA synthesis in Atm-/- astrocytes may occur without cell division. This is in turn could elevate ROS production in the cells (15).
Our observations that p21cip1 is up-regulated in Atm-/- astrocytes (Fig. 4B) and that PD98059 inhibits p21cip1 up-regulation (data not shown) are consistent with those from a previous study (26). The Ink4a/Arf locus encodes two proteins, p16Ink4a and p19Arf, by alternative splicing (49–51). Ink4a and Arf are important components in Rb and p53 pathways, respectively, and both proteins function in cell cycle regulation (52, 53). Bmi-1 is a potent repressor of both Ink4a and Arf. Our data suggest that Bmi-1 down-regulation and dissociation from chromatin with resultant p16Ink4a up-regulation may contribute to the defective proliferation and premature senescence of Atm-/- astrocytes. A very interesting observation made by other researchers is that Bmi-1 knock-out mice progressively develop A-T-like motor abnormalities, such as ataxia (22). This information substantiates the observations reported above and supports the idea that CNS pathology is similar if either ATM or Bmi-1 is absent. Because derepression of the Ink4a/Arf gene locus has been correlated with Bmi-1-deficient phenotypes in the nervous system, it is important to determine whether the deletion of both Ink4a and Arf genes restores functions of Atm-/- astrocytes. Notably, we observed that knockdown of Ink4a restores proliferation of Atm-/- astrocytes (Fig. 6D). Furthermore, work by others has also shown that the A-T like motor abnormalities observed in Bmi-1 knock-out mice restored in Bmi-1-/-/p16Ink4a-/- double knock-out mice (54). This result further substantiates that p16Ink4a plays a role in Bmi-1-deficient conditions.
We have shown that Bmi-1/chromatin association is disrupted by H2O2 (Fig. 5, A–C). Previous studies on Bmi-1 have reported that its function as transcription repressor is regulated by its phosphorylation status (35). Because Bmi-1 contains two predicted ERK1 phosphorylation sites, we asked whether Bmi-1 phosphorylation is induced by oxidative stress and whether it is ERK1/2 dependent. We reasoned that ROS-induced phosphorylation of Bmi-1 may directly or indirectly contribute to its chromatin dissociation, which in turn would result in derepression of p16Ink4a. Because no specific antibody is available to detect the phosphorylated form of Bmi-1, its phosphorylation was determined by mobility shift on gels in the presence of phosphatase inhibitors. These experiments demonstrated that H2O2 treatment causes the mobility shift of Bmi-1 (data not shown). However, even though down-regulation and chromatin dissociation of Bmi-1 occur in Atm-/- astrocytes, no intrinsic difference in Bmi-1 phosphorylation is observed between Atm+/+ and Atm-/- astrocytes in Western blotting analysis (Fig. 4A). This might be explained by the fact that the mobility shift of Bmi-1 is able to be detected for only short time after H2O2 treatment, after which time Bmi-1 levels decreased (Fig. 4D). Therefore, it is possible that phosphorylation of Bmi-1 may lead to its degradation. It will be interesting to determine whether inhibition of proteasomal degradation restores functions of Atm-/- astrocytes by preventing Bmi-1 degradation.
In recent years, accumulating evidence has indicated that protein kinase activities are redox-sensitive because key cysteine residues in these proteins can undergo post-translational modifications by oxidants. H2O2-induced formation of disulfide bonds in protein kinases has been documented, suggesting that kinases function as redox sensors and that H2O2-induced conformational changes or formation of dimers from disulfide bonds triggers kinase activation (45, 55). This is consistent with our observation that ATM is activated in response to H2O2 (data not shown). Although at present the mechanism of how H2O2-induced ERK1/2 activation is unclear, we speculate that a candidate upstream mediator, such as receptor tyrosine kinase may be responsible for H2O2-induced ERK1/2 activation, because others have shown that upstream receptor tyrosine kinase may act as a trigger for such events (29). Our further studies will address the role of receptor tyrosine kinase in modulating H2O2-induced ERK1/2 signaling.
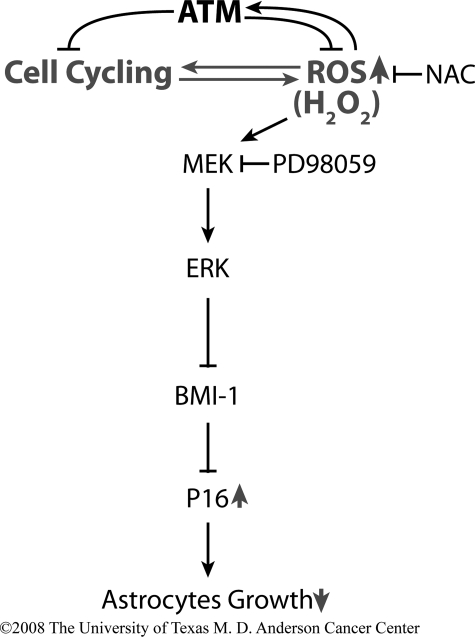
In conclusion, our data demonstrate that ATM is required to maintain normal growth of astrocytes by controlling intracellular levels of ROS. The absence of ATM limits the growth of astrocytes through ERK1/2-dependent mechanisms, including down-regulation and chromatin dissociation of Bmi-1 and subsequent up-regulation of p16Ink4a. The events are prevented by antioxidants and inhibited by inactivation of MEK, which is upstream of ERK1/2 (Fig. 7). These data therefore identify a novel mechanism by which ATM deficiency leads to the astrocytes growth arrest, and they identify new targets for effective pharmacological intervention in A-T patients.
FIGURE 7.
Proposed model of a role for ATM in the maintenance of normal astrocyte proliferation. ATM is necessary to control intracellular levels of ROS. In the absence of ATM, elevated levels of ROS elicit oxidative stress and limited growth of astrocytes, through multiple ERK1/2-dependent mechanisms, including down-regulation and chromatin dissociation of Bmi-1, as well as up-regulation of p16Ink4a. Abnormal proliferation of Atm-/- astrocytes may lead to oxidative stress in entire central nervous system and eventually to neurodegeneration. Oxidative stress-mediated growth defects of Atm-/-astrocytes may be prevented by antioxidants, and normal astrocyte proliferation may be partially restored by inhibition of ERK1/2 signaling. The arrows denote an increase or decrease when ATM is absent.
Acknowledgments
We thank Lifang Zhang for providing excellent technical support for the astrocyte cultures and Atm mouse preparation and Mingshan Yan (M. D. Anderson Cancer Center) for kindly providing advice and the antioxidant NAC. We also thank Virginia Scofield for careful reading of the manuscript.
This work was supported by a fellowship from the Odyssey Program of M. D. Anderson Cancer Center (to J. K.) and by funds from the Longevity Foundation and Ataxia-telanglectasia (A-T) Children's Project.
Footnotes
The abbreviations used are: A-T, ataxia-telangiectasia; H2DCFDA, 2′-7′-dichlorofluorescene diacetate; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; NAC, N-acetyl-l-cysteine; SA, senescence-associated; ROS, reactive oxygen species; MEK, MAPK/ERK kinase; CNS, central nervous system; PBS, phosphate-buffered saline; siRNA, small interfering RNA; CDK, cyclin-dependent kinase(s); Rb, retinoblastoma.
References
- 1.Savitsky, K., Bar-Shira, A., Gilad, S., Rotman, G., Ziv, Y., Vanagaite, L., Tagle, D. A., Smith, S., Uziel, T., Sfez, S., Ashkenazi, M., Pecker, I., Frydman, M., Harnik, R., Patanjali, S. R., Simmons, A., Clines, G. A., Sartiel, A., Gatti, R. A., Chessa, L., Sanal, O., Lavin, M. F., Jaspers, N. G., Taylor, A. M., Arlett, C. F., Miki, T., Weissman, S. M., Lovett, M., Collins, F. S., and Shiloh, Y. (1995) Science 268 1749-1953 [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen, T. J., and Shiloh, Y. (1996) Int. J. Radiat. Biol. 69 527-537 [DOI] [PubMed] [Google Scholar]
- 3.Crawford, T. O. (1998) Semin. Pediatr. Neurol. 5 287-294 [DOI] [PubMed] [Google Scholar]
- 4.Shiloh, Y., and Rotman, G. (1996) J. Clin. Immunol. 16 254-260 [DOI] [PubMed] [Google Scholar]
- 5.Shiloh, Y. (2003) Nat. Rev. Cancer 3 155-168 [DOI] [PubMed] [Google Scholar]
- 6.Lucius, R., and Sievers, J. (1996) Brain Res. 743 56-62 [DOI] [PubMed] [Google Scholar]
- 7.Campese, V. M., Ye, S., Zhong, H., Yanamadala, V., Ye, Z., and Chiu, J. (2004) Am. J. Physiol. 287 695-703 [DOI] [PubMed] [Google Scholar]
- 8.Leutner, S., Schindowski, K., Frölich, L., Maurer, K., Kratzsch, T., Eckert, A., and Müller, W. E. (2005) Parmacopsychiatry 38 312-315 [DOI] [PubMed] [Google Scholar]
- 9.Jiang, Y., Scofield, V. L., Yan, M., Qiang, W., Liu, N., Reid, A. J., Lynn, W. S., and Wong, P. K. (2006) J. Virol., 80 4557-4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, P., Peng, C., Luff, J., Spring, K., Watters, D., Bottle, S., Furuya, S., and Lavin, M. F. (2003) J. Neurosci. 23 11453-11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne, S. E., Roberts, L. J., II, Dennery, P. A., Doctrow, S. R., Beal, M. F., Barlow, C., and Levine, R. L. (2004) Free. Radic. Biol. Med. 36 938-942 [DOI] [PubMed] [Google Scholar]
- 12.Gueven, N., Luff, J., Peng, C., Hosokawa, K., Bottle, S. E., and Lavin, M. F. (2006) Free Radic. Biol. Med. 41 992-1000 [DOI] [PubMed] [Google Scholar]
- 13.Rotman, G., and Shiloh, Y. (1997) Cancer Surv. 29 285-304 [PubMed] [Google Scholar]
- 14.Reichenbach, J., Schubert, R., Schindler, D., Muller, K., Bohles, H., and Zielen, S. (2002) Antioxid. Redox Signal. 4 465-469 [DOI] [PubMed] [Google Scholar]
- 15.Watters, D. J. (2003) Redox Rep. 8 23-29 [DOI] [PubMed] [Google Scholar]
- 16.Barzilai, A., and Yamamoto, K. (2004) DNA Repair 3 1109-1115 [DOI] [PubMed] [Google Scholar]
- 17.Wong, P. K. Y., and Lynn, W. S. (1997) J. Immunol. Immunophamacol. 17 30-35 [Google Scholar]
- 18.Wang, X. F., and Cynader, M. S. (2000) J. Neurochem. 74 1434-1442 [DOI] [PubMed] [Google Scholar]
- 19.Takuma, K., Baba, A., and Matsuda, T. (2004) Prog. Neurobiol. 72 111-127 [DOI] [PubMed] [Google Scholar]
- 20.Liu, N., Stoica, G., Yan, M., Scofield, V. L., Qiang, W., Lynn, W. S., and Wong, P. K. Y. (2005) Lab. Investig. 85 1471-1480 [DOI] [PubMed] [Google Scholar]
- 21.Gosink, E. C., Chong, M. J., and McKinnon, P. J. (1999) Cancer Res. 59 5294-5298 [PubMed] [Google Scholar]
- 22.Molofsky, A. V., He, S., Bydon, M., Morrison, S. J., and Pardal, R. (2005) Genes Dev. 19 1432-1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardal, R., Molofsky, A. V., He, S., and Morrison, S. J. (2005) Cold Spring Harbor Symp. Quant. Biol. 70 177-185 [DOI] [PubMed] [Google Scholar]
- 24.Barlow, C., Hirotsune, S., Paylor, R., Liyanage, M., Eckhaus, M., Collins, F., Shiloh, Y., Crawley, J. N., Ried, T., Tagle, D., and Wynshaw-Boris, A. (1996) Cell 86 159-171 [DOI] [PubMed] [Google Scholar]
- 25.Lin, Y. C., Chow, C. W., Yuen, P. H., and Wong, P. K. Y. (1997) J. Neurovirol. 3 28-37 [DOI] [PubMed] [Google Scholar]
- 26.Lee, Y., Chong, M. J., and McKinnon, P. J. (2001) J. Neurosci. 21 6687-6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shikova, E., Lin, Y. C., Saha, K., Brooks, B. R., and Wong, P. K. Y. (1993) J. Virol. 67 1137-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan, M., Zhu, C., Liu, N., Jiang, Y., Scofield, V. L., Riggs, P. K. Y., Qiang, W., Lynn, W. S., and Wong, P. K. Y. (2006) Free. Radic. Biol. Med. 41 640-648 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd, A. C. (2006) J. Biol. (Bronx N. Y.) 5 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collado, M., Blasco, M. A., and Serrano, M. (2007) Cell 130 223-233 [DOI] [PubMed] [Google Scholar]
- 31.Wen-Sheng, W. (2003) Oncogene 22 955-963 [DOI] [PubMed] [Google Scholar]
- 32.Bartov, O., Sultana, R., Butterfield, D. A., and Atlas, D. (2006) Brain Res. 1069 198-206 [DOI] [PubMed] [Google Scholar]
- 33.Moors, M., Cline, J. E., Abel, J., and Fritsche, E. (2007) Toxicol. Appl. Pharmacol. 221 57-67 [DOI] [PubMed] [Google Scholar]
- 34.Vonlanthen, S., Heighway, J., Altermatt, H. J., Gugger, M., Kappeler, A., Borner, M. M., van Lohuizen, M., and Betticher, D. C. (2001) Br. J. Cancer 84 1372-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voncken, J. W., Schweizer, D., Agaard, L., Sattler, L., Jantsch, M. F., and von Lohuizen, M. (1999) J. Cell Sci. 112 4627-4639 [DOI] [PubMed] [Google Scholar]
- 36.Min, J., Zhang, Y., and Xu, R. M. (2003) Genes Dev. 17 1823-1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, Y., and Herrup, K. (2005) J. Neurosci. 25 2522-2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maragakis, N. J., and Rothstein, J. D. (2006) Nat. Clin. Pract. Neurol. 2 679-689 [DOI] [PubMed] [Google Scholar]
- 39.Vargas, M. R., Johnson, D. A., Sirkis, D. W., Messing, A., and Johnson, J. A. (2008) J. Neurosci. 28 13574-13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai, M., Re, D. B., Nagata, T., Chalazonitis, A., Jessell, T. M., Wichterle, H., and Przedborski, S. (2007) Nat. Neurosci. 10 615-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otani, N., Nawashiro, H., Fukui, S., Oigawa, H., Ohsumi, A., Toyooka, T., Shima, K., Gomi, H., and Brenner, M. (2006) J. Clin. Neurosci. 13 934-938 [DOI] [PubMed] [Google Scholar]
- 42.Pardo, A. C., Wong, V., Benson, L. M., Dykes, M., Tanaka, K., Rothstein, J. D., and Maragakis, N. J. (2006) Exp. Neurol. 201 120-130 [DOI] [PubMed] [Google Scholar]
- 43.Gamaley, I. A., and Klyubin, I. V. (1999) Int. Rev. Cytol. 188 203-255 [DOI] [PubMed] [Google Scholar]
- 44.Kamsler, A., Daily, D., Hochman, A., Stern, N., Shiloh, Y., Rotman, G., and Barzilai, A. (2001) Cancer Res. 61 1849-1854 [PubMed] [Google Scholar]
- 45.Rhee, S. G. (2006) Science 312 1882-1883 [DOI] [PubMed] [Google Scholar]
- 46.Yu, B. P. (1994) Physiol. Rev. 74 139-162 [DOI] [PubMed] [Google Scholar]
- 47.Biswas, S., Chida, A. S., and Rahman, I. (2006) Biochem. Pharmacol. 71 551-564 [DOI] [PubMed] [Google Scholar]
- 48.Benhar, M., Engelberg, D., and Levitzki, A. (2002) EMBO Rep. 3 420-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyslop, P. A., Zhang, Z., Pearson, D. V., and Phebus, L. A. (1995) Brain Res. 671 181-186 [DOI] [PubMed] [Google Scholar]
- 50.Gottfried, C., Tramontina, F., Goncalves, D., Gonçalves, C. A., Moriguchi, E., Dias, R. D., Wofchuk, S. T., and Souza, D. O. (2002) Mech. Ageing Dev. 123 1333-1340 [DOI] [PubMed] [Google Scholar]
- 51.Serrano, M., Lee, H., Chin, L., Cordon-Cardo, C., Beach, D., and DePinho, R. A. (1996) Cell 85 27-37 [DOI] [PubMed] [Google Scholar]
- 52.Quelle, D. E., Cheng, M., Ashmun, R. A., and Sherr, C. J. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 669-673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ivanchuk, S. M., Mondal, S., Dirks, P. B., and Rutka, J. T. (2001) J. Neurooncol. 51 219-229 [DOI] [PubMed] [Google Scholar]
- 54.Bruggeman, S. W., Valk-Linbeek, M. E., van der Stoop, P. P., Jacobs, J. J., Kieboom, K., Tanger, E., Hulsman, D., Leung, C., Arsenijevic, Y., Marino, S., and van Lohuizen, M. (2005) Genes Dev. 19 1438-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgoyne, J. R., Madhani, M., Cuello, F., Charles, R. L., Brennan, J. P., Schröder, E., Browning, D. D., and Eaton, P. (2007) Science 317 1393-1397 [DOI] [PubMed] [Google Scholar]