Abstract
Oxidative stress is a hallmark of metabolism-related diseases and a risk factor for atherosclerosis. FoxO factors have been shown to play a key role in vascular endothelial development and homeostasis. Foxo3a can protect quiescent cells from oxidative stress through the regulation of detoxification genes such as sod2 and catalase. Here we show that Foxo3a is a direct transcriptional regulator of a group of oxidative stress protection genes in vascular endothelial cells. Importantly, Foxo3a activity requires the transcriptional co-activator PGC-1α, because it is severely curtailed in PGC-1α-deficient endothelial cells. Foxo3a and PGC-1α appear to interact directly, as shown by co-immunoprecipitation and in vitro interaction assays, and are recruited to the same promoter regions. The notion that Foxo3a and PGC-1α interact directly to regulate oxidative stress protection genes in the vascular endothelium is supported by the observation that PGC-1α transcriptional activity at the sod2 (manganese superoxide dismutase) promoter requires a functional FoxO site. We also demonstrate that Foxo3a is a direct transcriptional regulator of PGC-1α, suggesting that an auto-regulatory cycle regulates Foxo3a/PGC-1α control of the oxidative stress response.
A central question in cardiovascular medicine is how metabolic dysfunctions affect the vascular system and in particular how they give rise to endothelial oxidative stress. Reactive oxygen species (ROS)6 are normally produced in the mitochondria as a byproduct of aerobic metabolism. Low levels of ROS have been shown to play a role in cell signaling (reviewed in Ref. 1) and to be required for cell proliferation (2). However, oxygen-free radicals are highly reactive and can damage cellular constituents including proteins, lipids, and DNA, with mitochondrial structures being the most susceptible. Excessive generation of ROS can thus result in altered cellular function, leading eventually to apoptotic cell death or oncogenic transformation (3).
Excessive generation of mitochondrial ROS has been linked to aging; neurodegenerative diseases such as Alzheimer disease, epilepsy, and Parkinson disease; cancer; and vascular disease. In the vasculature, oxidative stress is associated with metabolic alterations (diabetes, obesity, and high cholesterol) and results in endothelial dysfunction. Endothelial dysfunction has in turn been identified as an early sign of atherosclerosis and its clinical complications (coronary disease, hypertension, and heart failure) and is thus considered a common risk factor for many cardiovascular diseases (4, 5).
Cells have evolved complex antioxidant systems to prevent excessive accumulation of ROS and consequent cell damage. Well known examples are catalase and the glutathione system. Within mitochondria, ROS production is suggested to be prevented by uncoupling proteins (UCP) 2 and 3, the latter mainly expressed in muscle, and several detoxification enzymes have been identified, including manganese superoxide dismutase (MnSOD), peroxiredoxin 3 (Prx3), peroxiredoxin 5 (Prx5), thioredoxin 2 (Trx2), thioredoxin reductase 2 (TrxR2), glutaredoxin 2a, and glutathione peroxidase 4 (3).
The transcriptional co-activator peroxisome proliferator-activated receptor γ co-activator 1-α (PGC-1α) is a well characterized positive regulator of mitochondrial function and oxidative metabolism (6). PGC-1α regulates the transcription of a group of genes involved in ROS detoxification (7). Overexpression of PGC-1α in primary endothelial cells reduces ROS levels and prevents endothelial dysfunction and apoptotic cell death in response to oxidative stress conditions (8). Consistently, alterations in PGC-1α levels or activity have been described in several pathologies associated with oxidative stress conditions including diabetes, heart disease, and neurological disorders (9–11).
Another key determinant of ROS homeostasis and endothelial function is the O subfamily of forkhead transcription factors (FoxO). FoxO factors regulate hormonal, nutrient, and stress responses and play a key role in endothelial homeostasis, being necessary for endothelial development and for the induction of apoptosis to limit angiogenesis (12). A major regulator of FoxO activity is protein kinase B (Akt), which directly phosphorylates and inactivates FoxO factors, triggering their translocation from the nucleus to the cytoplasm (13). FoxO family members activate transcription by specifically binding to apparently shared binding sites (the consensus sequence is GTAAACA) in the promoters of target genes (14).
Several lines of evidence suggest a role for FoxO factors in ROS homeostasis. FoxO-deficient hematopoietic stem cells contain high concentrations of ROS and show reduced expression of genes involved in ROS detoxification (12). In particular, the FoxO factors Foxo3a and Foxo4 have been shown to protect quiescent cells in vitro from oxidative stress (15) and, in response to elevated ROS, translocate to the nucleus (16). Foxo3a has been shown to directly activate transcription of three important antioxidant enzymes: MnSOD, catalase, and Prx3 (15, 17, 18).
The role of FoxO factors, in particular Foxo3a, in endothelial function is an active area of research (12, 19). However, despite the well established physiological relevance of ROS production to vascular function, the role of FoxO factors in endothelial oxidative stress protection has not been characterized. Here, we show that FoxO3a is a direct transcriptional regulator of a group of oxidative stress protection genes in primary endothelial cells and that this regulation requires PGC-1α. We also show that Foxo3a and PGC-1α interact directly in endothelial cells and propose that these factors cooperate through this interaction to regulate mitochondrial oxidative stress protection.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents—Human umbilical vein endothelial cells (HUVEC), bovine aortic endothelial cells (BAEC), mouse aortic endothelial cells (MAEC), and mouse embryonic fibroblasts (MEF) were isolated and cultured as previously described (20–23). Umbilical cords were obtained from Ruber International Hospital (Madrid, Spain) with the approval of the donors and the institutional ethics committee. All of the protocols used conform to the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (publication number 85-23).
Adenoviral Vectors and Infection—Replication-deficient adenoviruses expressing PGC-1α or a constitutively active form of Foxo3a (TM-Foxo3a) under the control of the cytomegalovirus promoter have been previously described (8, 24). Adenovirus generation and purification were as described (8). HUVEC, MAEC, and MEF were infected with adenoviral vectors at a multiplicity of infection of 1–50 over 6 h. The viruses were then washed off, and the cells were harvested 48 h post-infection. mRNA and protein were analyzed by real time PCR of reverse transcribed cDNA (qRT-PCR) and Western blot as described (25, 26).
Small Interference RNA—Adenovirus expressing small hairpin RNAs were prepared as described (8) using the pSilencer adeno 1.0 cytomegalovirus promoter system from ABI. The cells were infected o/n at a multiplicity of infection of 50 and harvested 24 h post-infection. The oligonucleotides used were: control: sense, 5′-TCGAGCACCAGAGAGCTGCCATCCTTCAAGAGAGGATGGCAGCTCTGTGGTGTTA-3′, and antisense, 5′-CTAGTAACACCACAGAGCTGCCATCCTTTCTCTTGAAAAGGATGGCAGCTCTGTGGTGTT-3′; and Foxo3a, sense, 5′-TCGAGGAGCTCTTGGTGGATCATCTTCAAGAGAGATGATCCACCAAGAGCTCTTA-3′, and antisense, 5′-CTAGTAAGAGCTCTTGGTGGATCATCTCTCTTGAAGATGATCCACCAAGAGCTCC-3′.
Co-immunoprecipitation—Preparation of whole cell extracts and immunoprecipitation were carried out as described (25).
Chromatin Immunoprecipitation (ChIP)—Experimental conditions for ChIP were as described previously (8). PGC-1α was immunoprecipitated with a polyclonal α-PGC-1α antibody from Santa Cruz Biotechnology and Foxo3a with a mixture of polyclonal antibodies from Santa Cruz and Upstate. Co-precipitated DNA was analyzed by qPCR, using the primer pairs described (8) and the primers listed below. The β-actin gene was used as a negative control. Catalase, forward, 5′-gctgtgtaactttgggcaagttatt-3′, and reverse, 5′-cctcccaacaaccctatgagttag-3′; Prx3, forward, 5′-acagaagccggaagtctctacct-3′, and reverse, 5′-tcccactgttttgttaaccttgtg-3′; and Trx2, forward, 5′-gcaaaatccaaaaccacggg-3′, and reverse, 5′-ccctagaggagggaccggaag-3′.
Reactive Oxygen Species Analysis—ROS levels were determined by flow cytometry using two different fluorochromes. In the first method, the cells were labeled with 5-chloromethyl-2′ 7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) as described (8). To exclude interference by GFP, the signal with GFP alone was subtracted from the total signal obtained for CM-H2DCFDA. In the second method, the cells were labeled with dihydrorhodamine 6G (DhR6G) as described (27). To control for changes in the DhR6G signal caused by variation in mitochondrial membrane potential (ΔΨm), ΔΨm was monitored by flow cytometry analysis of cells labeled with tetramethylrhodamine methyl ester as described (8).
Promoter Constructs and Luciferase Assays—The 2-kb sod2 promoter construct was generated by PCR amplification of human genomic DNA, and the product was subcloned into the XhoI site of pGL3basic (Promega). The point mutation at the IRS site was introduced with the QuikChange® XL site-Directed mutagenesis kit. The amplification primers were: Sod2-X/f, 5′-accgctcgaggacttttgtccttccccttgc-3′; and Sod2-X/r, 5′-accgctcgagaagcaacggaaacggttcagc-3′. The mutagenesis primers were: Sod2m1/f, 5′-cttctgacgtctgtaaaGaagcccagcccttcc-3′; and Sos2m1/r, 5′-ggaagggctgggcttCttctttacagacgtcagaag-3′.
The following plasmid constructs have been described previously: expression vector encoding TM-Foxo3a (non Akt phosphorylatable Foxo3a) (24); expression vector encoding PGC-1α (24, 25); the FoxO reporter (PrFx3X) (28); and plasmids 5×UAS-luc and G4-PGC-1 (29). Plasmids were transfected into BAEC with Lipofectamine 2000™ for 8 h. At 24 h post-transfection, luciferase activity was determined with the Dual-luciferase® reporter assay system (Promega).
In Vitro Interaction Assays—PGC-1α domains (amino acids 1–186, 1–350, 350–580, and 561–797) were inserted in frame between the XhoI and NotI sites of pGEX-6P-2 (GE Healthcare) to generate GST-PGC-1α fusions. Recombinant GST fusion proteins were expressed from pGEX expression vectors in Escherichia coli Bl21DE3 and were purified using glutathione-agarose (GE Healthcare) according to the manufacturer's instructions. The vector containing Foxo3a cDNA under the control of the T7 promoter has been described (30).
The proteins were labeled with [35S]methionine by in vitro transcription and translation using the TnT system (Promega). Labeled proteins were incubated with 1 μg of GST fusion proteins immobilized on 20 μl of agarose beads in binding buffer (20 m Tris-HCl, pH 8.0, 10% glycerol, 5 mm MgCl2, 0.5 mm EDTA, 0.5 mm EGTA, 2 mm dithiothreitol, 0.1–0.4% Nonidet P-40, 100 mm KCl). Incubation was for 1 h at room temperature, in binding buffer (final volume, 200 μl). The beads were washed three times with 1 ml of binding buffer. Bound proteins were eluted with Laemmli sample buffer, boiled, and resolved by SDS-PAGE.
Statistics—The data are expressed as the means ± S.D. values. Statistical significance was evaluated by analysis of variance or a nonparametric test, as appropriate. The values were considered to be statistically significant at p < 0.05. n ≥ 3inall experiments.
RESULTS
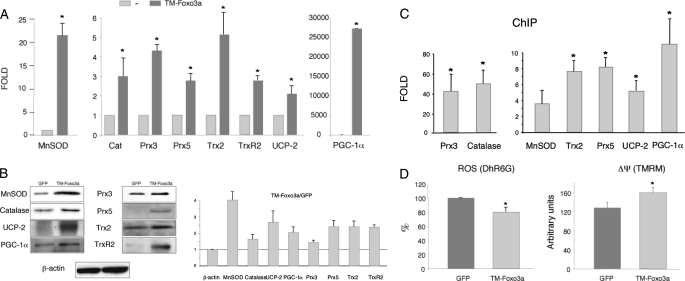
Foxo3a Regulates Oxidative Stress Protection Genes in HUVEC—To investigate the role of Foxo3a in the regulation of oxidative stress protection genes in endothelial cells, we infected HUVEC with a recombinant adenovirus encoding a constitutively active Foxo3a (TM-Foxo3a) or a GFP control adenovirus (GFP). Analysis of mRNA and protein expression showed that TM-Foxo3a induced the expression of all oxidative stress genes tested, including well known targets (MnSOD, catalase, and Prx3) and other previously uncharacterized targets such as Prx5, UCP-2, Trx2, and TrxR2 (Fig. 1A). Importantly, TM-Foxo3a also induced the expression of PGC-1α, a general transcriptional regulator of the oxidative stress protection system. In all cases, increased gene expression in response to TM-Foxo3a resulted in corresponding increases in protein content (Fig. 1B). To test whether all these genes were direct targets of Foxo3a, we carried out ChIP assays. The primers were directed to promoter regions that are either “rich” in predicted transcription factor-binding sites or contain conserved FoxO-like boxes. These experiments showed association of Foxo3a with the promoter regions of MnSOD, catalase, Prx3, Prx5, UCP-2, Trx2, and PGC-1α. (Fig. 1C). These data suggest that Foxo3a induces a whole set of mitochondrial antioxidant genes in endothelial cells and also induces the expression of PGC-1α, a transcriptional co-activator that is also a general regulator of the oxidative stress protection system in endothelial cells.
FIGURE 1.
Foxo3a regulates oxidative stress protection in HUVEC. A, expression of genes involved in oxidative stress protection was determined by qRT-PCR in HUVEC infected with TM-Foxo3a. Expression is shown as the fold induction above the level in cells infected with control virus (GFP). 18 S RNA was used as a loading control. B, Western blot analysis of oxidative stress protection proteins in HUVEC infected with TM-Foxo3a or control (GFP) adenovirus. Right panel, Western blot quantification. C, ChIP analysis of Foxo3a localization in the promoter regions of oxidative stress protection genes in HUVEC infected with TM-Foxo3a adenovirus. β-Actin was used as a negative control. A and C, control samples were assigned the value of 1. D, ROS levels (left panel) and ΔΨm (right panel) were determined by fluorescence-activated cell sorting of DhR6G or TMREM loaded HUVEC infected with TM-Foxo3a or control (GFP) adenovirus. A, B, and D, data are the means ± S.D. *, p < 0.05.
We next tested the impact of Foxo3a on ROS accumulation. For this, HUVEC were infected with TM-Foxo3a or GFP control adenovirus, and ROS production was estimated by two methods. In the first method, the cells were loaded with the redox-active fluorescent probe DhR6G, and ROS were estimated from the fluorescence signal produced by their interaction with this probe. DhR6G fluorescence was significantly lower in cells expressing TM-Foxo3a, indicating a reduction in ROS accumulation and therefore suggesting a more active detoxification system in the presence of TM-Foxo3a and/or reduced ROS production (Fig. 1D, left panel). However, DhR6G is also sensitive to changes in mitochondrial membrane potential (ΔΨm). To rule out that the observed changes were due to differences in ΔΨm, we monitored changes in ΔΨm independently by loading cells with the membrane-potential fluorescent probe tetramethylrhodamine methyl ester. TM-Foxo3a increased the tetramethylrhodamine methyl ester signal, indicating that the reduced DhR6G signal was not a consequence of altered mitochondrial membrane potential (Fig. 1D, right panel). These results were confirmed by the second method, in which cells were loaded with the general ROS probe CM-H2DCFDA (supplemental Fig. S1).
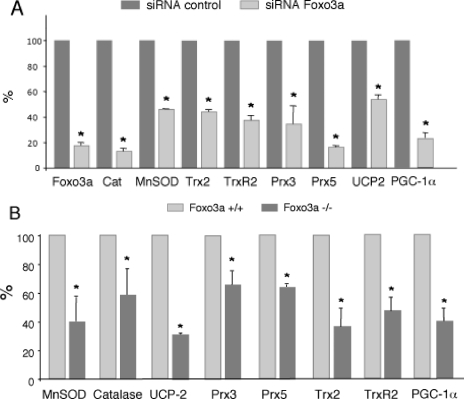
To evaluate the regulation of the mitochondrial detoxification system by endogenous Foxo3a, we first suppressed Foxo3a expression in HUVEC, using Foxo3a-specific small interference RNA. Suppression of endogenous Foxo3a expression resulted in reduced mRNA expression of MnSOD, catalase, UCP-2, Prx-3, Prx5, Trx2, TrxR2, and PGC-1α, indicating that Foxo3a is necessary for the maintenance of the expression of these genes in endothelial cells (Fig. 2A).
FIGURE 2.
Requirement of Foxo3a to maintain oxidative stress protection systems. A, HUVEC were transfected with control or Foxo3a specific small interference RNAs and mRNA expression levels of target genes were determined by qRT-PCR. B, mRNA expression of oxidative stress protection genes in Foxo3a+/+ and Foxo3a-/- MEF. 18 S RNA was used as loading control, and MMP13 was used as negative control. Control samples were assigned the value of 100%. The data are the means ± S.D. *, p < 0.05.
To further investigate the role of endogenous Foxo3a in the regulation of oxidative stress protection genes, we next compared their expression in MEF derived from Foxo3a+/+ and Foxo3a-/- mice. As expected, Foxo3a-/- MEF showed reduced basal expression levels of MnSOD, catalase, UCP-2, Prx3, Prx5, Trx2, TrxR2, and PGC-1α, supporting the notion that Foxo3a is a general regulator of the ROS detoxification system (Fig. 2B).
Foxo3a Regulation of Oxidative Stress Protection Genes Requires PGC-1α—These results presented in Figs. 1 and 2 show that both Foxo3a and PGC-1α regulate a common group of oxidative stress protection genes. Most strikingly, the ChIP analysis (Fig. 1C, Ref. 8, and supplemental Fig. S2) revealed that these factors bind to or associate with the same or overlapping promoter regions. Importantly too, Foxo3a regulates the expression of PGC-1α. Therefore, we aimed to elucidate whether Foxo3a regulation of oxidative stress protection genes requires PGC-1α and, if so, whether PGC-1α is working as a Foxo3a co-activator.
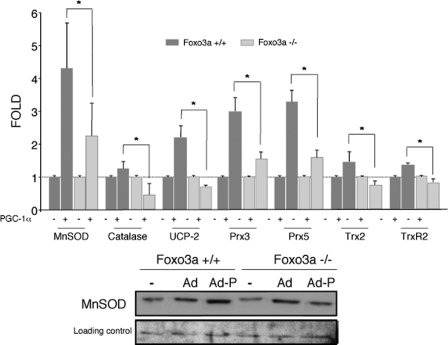
We first tested whether overexpression of PGC-1α could compensate for Foxo3a deficiency in the regulation of oxidative stress protection genes. MEF from Foxo3a+/+ and Foxo3a-/- mice were infected with a PGC-1α adenovirus. The ability of PGC-1α to induce oxidative stress protection genes was severely impaired in Foxo3a-deficient MEF (Fig. 3), suggesting that PGC-1α requires Foxo3a to regulate oxidative stress protection, because PGC-1α overexpression does not compensate for Foxo3a deficiency. Therefore, Foxo3a induction of PGC-1α expression does not seem to be the only mechanism which Foxo3a induces oxidative stress protection genes. These results were supported by the observation that induction of oxidative stress protection genes by PGC-1α was also impaired in MEFs infected with an shFoxo3a adenovirus (supplemental Fig. S3).
FIGURE 3.
PGC-1α induced expression of oxidative stress protection genes is reduced in the absence of Foxo3a. Up-regulation of oxidative stress protection systems by PGC-1α was determined in Foxo3a+/+ and Foxo3a-/- MEF that were infected with PGC-1α (Ad-P) or a control adenovirus (Ad). Upper panel, mRNA expression levels were determined by qRT-PCR. 18 S RNA was used as loading control. Control samples were assigned the value of 1. The data are the means ± S.D. *, p < 0.05. Lower panel, Western blot analysis.
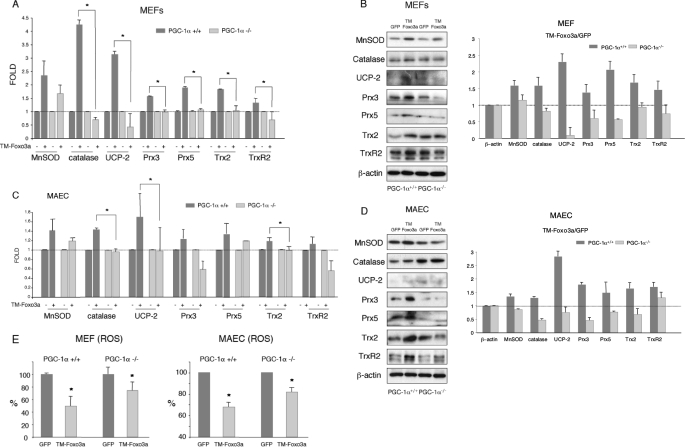
To determine whether Foxo3a-mediated induction of the oxidative protection program was reciprocally dependent on the presence of PGC-1α or could be mediated by a PGC-1α-independent mechanism, we next analyzed the ability of adenovirally expressed TM-Foxo3a to induce oxidative stress protection genes in MEF and MAEC isolated from PGC-1α+/+ and PGC-1α-/- mice. PGC-1α-/- cells have lower expression of several oxidative stress protection genes and increased ROS levels (Ref. 7 and supplemental Fig. S4). Consistently, we found that the ability of Foxo3a to induce mRNA or protein expression of all the genes under investigation was either severely reduced or completely abolished in PGC-1α-/- MEF (Fig. 4, A and B) and MAEC (Fig. 4, C and D), supporting the notion that Foxo3a and PGC-1α cooperate to induce the expression of oxidative stress protection genes both in endothelial and nonendothelial cells. However, not all the Foxo3a targets tested were equally dependent on PGC-1α. Notably, although MnSOD induction seemed to be reduced in PGC-1α-/- MAEC, the difference did not reach statistical significance.
FIGURE 4.
Foxo3a-dependent expression of oxidative stress protection genes is reduced in the absence of PGC-1α. Induction of oxidative stress protection genes by Foxo3a was determined in PGC-1α+/+ and PGC-1α-/- MEF (A and B) and MAEC (C and D) that were infected with TM-Foxo3a or a control adenovirus. A and C, mRNA expression levels were determined by qRT-PCR. 18 S RNA was used as loading control. B and D, Western blot analysis. Right panels, Western blot quantification. E, PGC-1α+/+ and PGC-1α-/- MAEC or MEF were infected with TM-Foxo3a or a control adenovirus, and ROS levels were determined by fluorescence-activated cell sorting of CM-H2DCFDA loaded cells. Control samples were assigned the value of 1. The data are the means ± S.D. *, p < 0.05.
Overall MAEC responded more poorly to TM-Foxo3a than HUVEC or MEF. Although that may be due to an inherent characteristic of these cells, we think it is more likely to be a consequence of the extended time that these cells have to be in culture before use, because we have consistently observed relatively poor responses of MAEC to both PGC-1α overexpression (8) and nitric oxide donor treatment (26). It is also worth noting that genes whose mRNAs were only slightly induced in MEF and MAEC by TM-Foxo3a, such as Trx2 and TrxR2, showed markedly elevated protein levels. Also of interest, in PGC-1α-/- cells some proteins were down-regulated by TM-Foxo3a. Finally, we found that the capacity of TM-Foxo3a to lower ROS was reduced in the absence of PGC-1α (Fig. 4E).
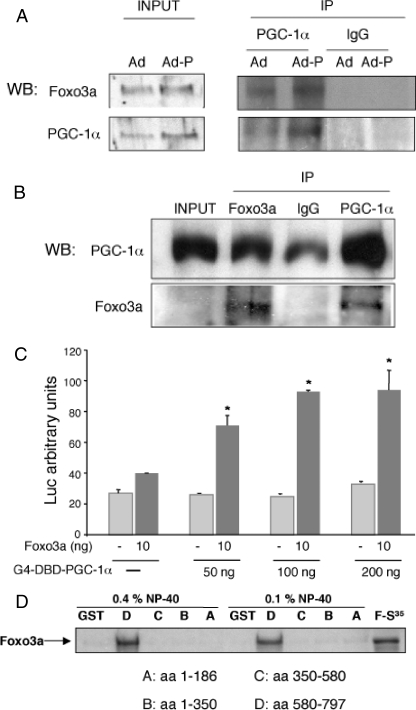
Foxo3a and PGC-1α Can Interact Directly—To investigate whether Foxo3a and PGC-1α form part of the same transcriptional complex in endothelial cells, we infected HUVEC with an adenovirus expressing PGC-1α. Foxo3a was detected in PGC-1α immunoprecipitates, and the co-immunoprecipitation was significantly enhanced by PGC-1α overexpression (Fig. 5A). Consistently, PGC-1α was detected in Foxo3a immunoprecipitates (Fig. 5B).
FIGURE 5.
Foxo3a interacts with PGC-1α. A, HUVEC were infected with PGC-1α (Ad-P) or control adenovirus (Ad). PGC-1α was immunoprecipitated (IP), and Foxo3a was detected in the immunoprecipitated material by Western blotting (WB) but not in a control immunoprecipitation. Input, Western blot of whole cell lysate. B, HUVEC were infected with a PGC-1α adenovirus (Ad-P). Foxo3a was immunoprecipitated and PGC-1α was detected in the immunoprecipitated material by Western blot. C, mammalian two-hybrid assay. HUVEC were co-transfected with the Gal4 luciferase reporter plasmid 5×UAS-Luc, the indicated amounts of G4-DBD-PGC-1α (encoding PGC-1α fused to the DNA-binding domain of Gal4 to provide a DNA tethering domain), and a TM-Foxo3a expression vector, or the corresponding control vectors. Luciferase activity was determined 24 h post-transfection. D, GST pulldown assay mapping the interaction between Foxo3a and PGC-1α. GST-fusions with the indicated PGC-1α fragments (fragments A–D) were immobilized on glutathione beads and incubated with 35S-labeled Foxo3a. After extensive washing, the samples were separated by SDS/PAGE, and bound 35S-labeled Foxo3a protein was detected by autoradiography.
To confirm the specificity of this interaction, we next carried out a mammalian two-hybrid assay. BAEC were transiently transfected with increasing doses of a construct encoding the Gal4-DBD-PGC-1α fusion protein, in which PGC-1α is fused to the DNA-binding domain of Gal4 to provide a DNA tethering domain, together with the luciferase reporter plasmid 5×UAS-Luc, which contains five copies of the Gal4-binding site (5×UAS) in the promoter region. Co-transfection with a TM-Foxo3a expression vector significantly increased the transcriptional activity of the promoter (Fig. 5C), supporting the notion that PGC-1α and Foxo3a can form a functional complex in endothelial cells.
To determine whether PGC-1α and Foxo3a interact directly and to identify the PGC-1α domain involved in the interaction, we generated affinity-purified GST fusion proteins of four PGC-1α domains (domains A–D) and tested their interaction in vitro with 35S-radiolabeled Foxo3a (from a reticulocyte translation system). In the pulldown assays Foxo3a was associated only with the D domain of PGC-1α (Fig. 5D), corresponding to the C-terminal region (amino acids 580–797). We therefore conclude that the association of PGC-1α with Foxo3a in endothelial cells is likely to be dependent on the direct interaction of these two proteins.
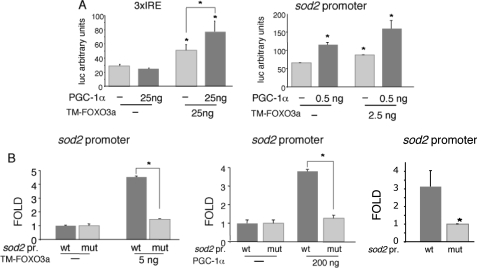
PGC-1α-mediated Induction of sod2 Requires a Functional FoxO Site—Taking into account that Foxo3a and PGC-1α require each other to regulate oxidative stress protection genes and that these factors seem to be able to interact in cells, we analyzed whether Foxo3a and PGC-1α could form a functional complex on their common target genes. We first tested whether PGC-1α can work as a co-activator of Foxo3a on FoxO sites by carrying out transient transfection experiments in BAEC with a luciferase reporter construct driven by a promoter harboring three copies of a canonical FoxO binding site (3×IRE-luc). We observed that PGC-1α and Foxo3a could activate this construct cooperatively (Fig. 6A, left panel), suggesting that PGC-1α is in fact a Foxo3a co-activator.
FIGURE 6.
PGC-1α is a co-activator of Foxo3a. A, BAEC were transfected with the FoxO activity luciferase reporter construct 3×IRE-luc (left panel) or with the 2 kb sod2 promoter-luciferase reporter vector (right panel) and with the indicated amounts of Foxo3a and PGC-1α expression vectors or the corresponding controls. B, BAEC were transfected with the wild type 2-kb sod2 promoter (wt) or version containing a point mutation in the functional FoxO site (mut) and with the indicated amounts of expression vectors encoding Foxo3a (left panel) or PGC-1α (right panel) or the corresponding controls. Luciferase activity was determined 24 h post-transfection. Control samples were assigned the value of 1. The data are the means ± S.D. *, p < 0.05.
To determine whether PGC-1α co-activates Foxo3a on natural promoters, we tested Foxo3a and PGC-1α co-regulation on the sod2 promoter, a well characterized target of both factors. We cloned a 2-kb fragment of the sod2 (MnSOD-encoding gene) promoter, which contains a well characterized functional FoxO site, into a luciferase reporter vector. When we co-transfected PGC-1α and TM-Foxo3a expression vectors together with the wild type sod2 promoter construct, PGC-1α and Foxo3a were able to cooperate to activate this promoter, suggesting that PGC-1α is a Foxo3a co-activator (Fig. 6A, right panel).
To investigate whether PGC-1α activity on sod2 required functional FoxO sites, a mutant sod2 promoter construct was generated by introducing a point mutation into the functional FoxO site. The mutation reduced the basal promoter activity about 3-fold (Fig. 6B). In transient co-transfection experiments in BAEC, TM-Foxo3a was able to transactivate the wild type but not the mutant promoter, as previously described (15). Importantly, PGC-1α was also able to transactivate wild type sod2 but not the mutant promoter construct. This finding indicates that the functional FoxO site is required for PGC-1α-mediated induction of the sod2 promoter (Fig. 6B).
DISCUSSION
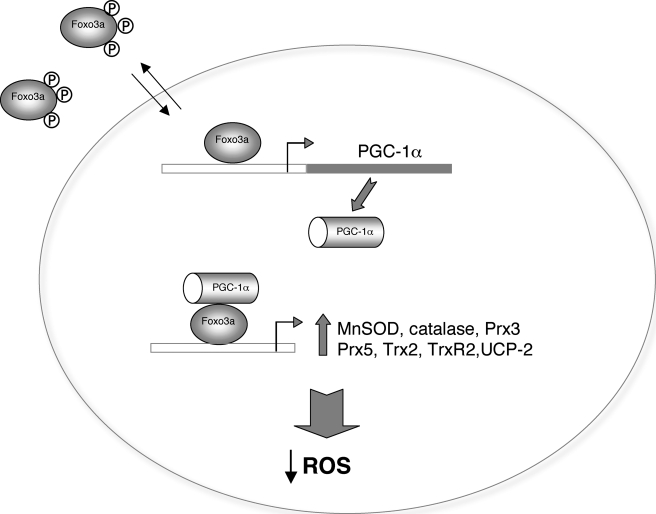
Oxidative stress in the endothelium is a key event in the development of vascular disease. In this study we show that Foxo3a regulates a group of oxidative stress protection genes in vascular endothelial cells and provide strong evidence that the transcriptional co-activator PGC-1α is a necessary partner of Foxo3 in the regulation of these genes and that its expression is directly regulated by Foxo3a (Fig. 7).
FIGURE 7.
Proposed model of Foxo3a regulation of oxidative stress protection genes. Foxo3a induces PGC-1α expression and interacts with PGC-1α to regulate the expression of oxidative stress protection genes.
Foxo3a Regulates ROS Homeostasis in the Vascular Endothelium—Although other FoxO factors besides Foxo3a have been implicated in ROS homeostasis, our loss-of-function studies show that Foxo3a is necessary for the maintenance of normal levels of detoxification enzymes, both in primary endothelial cells and MEF. Foxo3a has been previously shown to be able to directly regulate MnSOD, catalase, and Prx3 (15, 17, 18). Here we provide evidence that this regulation also takes place in the endothelium, and we further show that Foxo3a is also directly involved in the regulation of Prx5, UCP-2, Trx2 (probably also TrxR2), and PGC-1α. Consistent with the increased levels of ROS detoxification proteins, cells that express TM-Foxo3a have reduced levels of ROS.
Previous studies showed that FoxO factors control endothelial proliferation and differentiation processes (31, 32). We show here that Foxo3a is also required for protection against oxidative stress in the endothelium. Understanding how FoxO controls seemingly disparate pathways such as oxidative stress protection and induction of a pro-apoptotic program will be crucial to our understanding of Foxo3a function in the endothelium and other systems (33). Therefore, further studies to identify proteins that modulate FoxO target specificity are warranted.
Foxo3a and PGC-1α Are Partners in the Regulation of ROS Detoxification Genes—PGC-1α can coordinately regulate the mitochondrial oxidative stress protection system in various cell types including endothelial cells (8). Because PGC-1α cannot bind directly to the promoter sequences of its target genes, it needs a transcription factor to mediate its protective role. The data presented here suggest that Foxo3a can be this mediator.
Our results demonstrate that in the absence of PGC-1α, the capacity of Foxo3a to regulate ROS protection genes is drastically curtailed, an indication that PGC-1α is a necessary partner of Foxo3a in this regulation. Supporting this, PGC1–1α-dependent induction of oxidative stress protection genes is impaired in the absence of Foxo3a. We propose that this functional partnership is dependent on the direct interaction between these two proteins. This hypothesis is supported by the observations that Foxo3a and PGC-1α can interact in vitro, that they form part of the same complex, and that PGC-1α can co-activate Foxo3a. The notion that this interaction is important for the co-regulation of oxidative stress protection genes is supported by the observation that both proteins can locate on the same promoter regions of the co-regulated genes and by the finding that a functional FoxO site is required for activation of the sod2 promoter by PGC-1α. It is important to note that we cannot extend our observations to all Foxo3a targets. Indeed, PGC-1α activity has been reported to inhibit Foxo3a activation of the atrogin-1 gene (34).
Although it is currently unknown what signals trigger the association between Foxo3a and PGC-1α, it is interesting that both Foxo3a and PGC-1α have been shown to be induced or activated by elevated cell levels of ROS and to be negatively modulated by two major sensors of metabolic status, the PI3K/Akt pathway (13, 35) and the deacetylase SirT1 (36).
Recently, it has been suggested that the orphan nuclear receptor estrogen-related receptor α plays a role in the regulation of oxidative stress protection genes by PGC-1α (37). It will thus be interesting to see whether estrogen-related receptor α plays a role in the formation of a Foxo3a·PGC-1α complex or works through a Foxo3a-independent mechanism.
An understanding of how the response to oxidative stress is orchestrated requires knowledge not only of the transcription factors involved but also of how their levels and activities are modulated in response to oxidative stress. It has been reported that Foxo3a nuclear localization is increased under oxidative stress conditions (38) and that PGC-1α expression is induced by H2O2 via activation of cAMP-responsive element-binding protein (7). Our finding that Foxo3a is also a direct transcriptional regulator of PGC-1α suggests that activation of PGC-1α under stress conditions could be also elicited via Foxo3a.
Metabolic disorders usually affect the physiology of the vascular endothelium and result in oxidative stress (39). Here we show that both PGC-1α and Foxo3a are likely to be key players in the interplay between metabolic control and oxidative stress protection in the vascular endothelium. Understanding the key signals and components of this regulatory pathway may identify new targets for pharmacological intervention in cardiovascular diseases associated with metabolic disorders.
Supplementary Material
Acknowledgments
We thank Dr. B. Spiegelman for providing the PGC-1α-/- mice and Dr. K. Arden for the Foxo3a-/- MEF. The FoxO reporter pGL3 construct (3×IRE-luc) was a gift from Dr. L. del Peso. Foxo-TM expression vector and adenovirus were a gift from Dr. K. Walsh. Editorial support was provided by Dr. S. Bartlett. We thank Dr. Stavit Drori, Dr. Ana Aranda, Dr. Kenneth McCreath, and Dr. Eva Cano for careful reading of the manuscript and Cristina Sanchez and Andrea Caneva for technical support.
This work was supported in part by Grant SAF2006-01619 from the Plan Nacional de I+D+I of the Ministerio de Educación y Ciencia (to M. M.), Grant CSD 2007-00020 from the Ministerio de Educación y Ciencia (to M. M. and S. L.), and Grant SAF2006-02410 from the Ministerio de Educación y Ciencia and a grant-in-aid from the Spanish Society of Nephrology (to S. L.). The Centro Nacional de Investigaciones Cardiovasculares is supported by the Spanish Ministry of Health and Consumer Affairs and the Pro-Centro Nacional de Investigaciones Cardiovasculares Foundation.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Footnotes
The abbreviations used are: ROS, reactive oxygen species; UCP, uncoupling proteins; MnSOD, manganese superoxide dismutase; Prx, peroxiredoxin; Trx2, thioredoxin 2; TrxR2, thioredoxin reductase 2; PGC-1α, peroxisome proliferator-activated receptor γ co-activator 1-α; HUVEC, human umbilical vein endothelial cells; BAEC, bovine aortic endothelial cells; MAEC, mouse aortic endothelial cells; MEF, mouse embryonic fibroblasts; TM-Foxo3a, constitutively active form of Foxo3a; qRT-PCR, real time PCR of retro transcribed cDNA; ChIP, chromatin immunoprecipitation; CM-H2DCFDA, 5-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester; DhR6G, dihydrorhodamine 6G; GST, glutathione S-transferase; GFP, green fluorescent protein.
References
- 1.Finkel, T. (2000) FEBS Lett. 476 52-54 [DOI] [PubMed] [Google Scholar]
- 2.Maulik, N. (2002) Antioxid. Redox. Signal. 4 805-815 [DOI] [PubMed] [Google Scholar]
- 3.Monsalve, M., Borniquel, S., Valle, I., and Lamas, S. (2007) Front. Biosci. 12 1131-1153 [DOI] [PubMed] [Google Scholar]
- 4.Giannotti, G., and Landmesser, U. (2007) Herz 32 568-572 [DOI] [PubMed] [Google Scholar]
- 5.Balakumar, P., Kaur, T., and Singh, M. (2008) Toxicology 245 49-64 [DOI] [PubMed] [Google Scholar]
- 6.Liang, H., and Ward, W. F. (2006) Adv. Physiol. Educ. 30 145-151 [DOI] [PubMed] [Google Scholar]
- 7.St-Pierre, J., Drori, S., Uldry, M., Silvaggi, J. M., Rhee, J., Jager, S., Handschin, C., Zheng, K., Lin, J., Yang, W., Simon, D. K., Bachoo, R., and Spiegelman, B. M. (2006) Cell 127 397-408 [DOI] [PubMed] [Google Scholar]
- 8.Valle, I., Alvarez-Barrientos, A., Arza, E., Lamas, S., and Monsalve, M. (2005) Cardiovasc. Res. 66 562-573 [DOI] [PubMed] [Google Scholar]
- 9.Mootha, V. K., Lindgren, C. M., Eriksson, K. F., Subramanian, A., Sihag, S., Lehar, J., Puigserver, P., Carlsson, E., Ridderstrale, M., Laurila, E., Houstis, N., Daly, M. J., Patterson, N., Mesirov, J. P., Golub, T. R., Tamayo, P., Spiegelman, B., Lander, E. S., Hirschhorn, J. N., Altshuler, D., and Groop, L. C. (2003) Nat. Genet. 34 267-273 [DOI] [PubMed] [Google Scholar]
- 10.Sano, M., and Schneider, M. D. (2005) Cell Metab. 1 216-218 [DOI] [PubMed] [Google Scholar]
- 11.Weydt, P., Pineda, V. V., Torrence, A. E., Libby, R. T., Satterfield, T. F., Lazarowski, E. R., Gilbert, M. L., Morton, G. J., Bammler, T. K., Strand, A. D., Cui, L., Beyer, R. P., Easley, C. N., Smith, A. C., Krainc, D., Luquet, S., Sweet, I. R., Schwartz, M. W., and La Spada, A. R. (2006) Cell Metab. 4 349-362 [DOI] [PubMed] [Google Scholar]
- 12.Paik, J. H., Kollipara, R., Chu, G., Ji, H., Xiao, Y., Ding, Z., Miao, L., Tothova, Z., Horner, J. W., Carrasco, D. R., Jiang, S., Gilliland, D. G., Chin, L., Wong, W. H., Castrillon, D. H., and DePinho, R. A. (2007) Cell 128 309-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, H., and Tindall, D. J. (2007) J. Cell Sci. 120 2479-2487 [DOI] [PubMed] [Google Scholar]
- 14.Pierrou, S., Hellqvist, M., Samuelsson, L., Enerback, S., and Carlsson, P. (1994) EMBO J. 13 5002-5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kops, G. J., Dansen, T. B., Polderman, P. E., Saarloos, I., Wirtz, K. W., Coffer, P. J., Huang, T. T., Bos, J. L., Medema, R. H., and Burgering, B. M. (2002) Nature 419 316-321 [DOI] [PubMed] [Google Scholar]
- 16.Essers, M. A., Weijzen, S., de Vries-Smits, A. M., Saarloos, I., de Ruiter, N. D., Bos, J. L., and Burgering, B. M. (2004) EMBO J. 23 4802-4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiribau, C. B., Cheng, L., Cucoranu, I. C., Yu, Y. S., Clempus, R. E., and Sorescu, D. (2008) J. Biol. Chem. 283 8211-8217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemoto, S., and Finkel, T. (2002) Science 295 2450-2452 [DOI] [PubMed] [Google Scholar]
- 19.Potente, M., Urbich, C., Sasaki, K., Hofmann, W. K., Heeschen, C., Aicher, A., Kollipara, R., DePinho, R. A., Zeiher, A. M., and Dimmeler, S. (2005) J. Clin. Investig. 115 2382-2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz, C., Castellanos, M. C., Alfranca, A., Vara, A., Esteban, M. A., Redondo, J. M., and de Landazuri, M. O. (1996) J. Immunol. 157 3587-3597 [PubMed] [Google Scholar]
- 21.Suh, S. H., Vennekens, R., Manolopoulos, V. G., Freichel, M., Schweig, U., Prenen, J., Flockerzi, V., Droogmans, G., and Nilius, B. (1999) Pfluegers Arch. Eur. J. Physiol. 438 612-620 [DOI] [PubMed] [Google Scholar]
- 22.Oblander, S. A., Zhou, Z., Galvez, B. G., Starcher, B., Shannon, J. M., Durbeej, M., Arroyo, A. G., Tryggvason, K., and Apte, S. S. (2005) Dev. Biol. 277 255-269 [DOI] [PubMed] [Google Scholar]
- 23.Jacks, T., Remington, L., Williams, B. O., Schmitt, E. M., Halachmi, S., Bronson, R. T., and Weinberg, R. A. (1994) Curr. Biol. 4 1-7 [DOI] [PubMed] [Google Scholar]
- 24.Ueki, K., Yamamoto-Honda, R., Kaburagi, Y., Yamauchi, T., Tobe, K., Burgering, B. M., Coffer, P. J., Komuro, I., Akanuma, Y., Yazaki, Y., and Kadowaki, T. (1998) J. Biol. Chem. 273 5315-5322 [DOI] [PubMed] [Google Scholar]
- 25.Monsalve, M., Wu, Z., Adelmant, G., Puigserver, P., Fan, M., and Spiegelman, B. M. (2000) Mol. Cell 6 307-316 [DOI] [PubMed] [Google Scholar]
- 26.Borniquel, S., Valle, I., Cadenas, S., Lamas, S., and Monsalve, M. (2006) FASEB J. 20 1889-1891 [DOI] [PubMed] [Google Scholar]
- 27.Wersto, R. P., Rosenthal, E. R., Crystal, R. G., and Spring, K. R. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 1167-1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Peso, L., Gonzalez, V. M., Hernandez, R., Barr, F. G., and Nunez, G. (1999) Oncogene 18 7328-7333 [DOI] [PubMed] [Google Scholar]
- 29.Puigserver, P., Adelmant, G., Wu, Z., Fan, M., Xu, J., O'Malley, B., and Spiegelman, B. M. (1999) Science 286 1368-1371 [DOI] [PubMed] [Google Scholar]
- 30.Anderson, M. J., Viars, C. S., Czekay, S., Cavenee, W. K., and Arden, K. C. (1998) Genomics 47 187-199 [DOI] [PubMed] [Google Scholar]
- 31.Tran, H., Brunet, A., Grenier, J. M., Datta, S. R., Fornace, A. J., Jr., DiStefano, P. S., Chiang, L. W., and Greenberg, M. E. (2002) Science 296 530-534 [DOI] [PubMed] [Google Scholar]
- 32.Dijkers, P. F., Medema, R. H., Lammers, J. W., Koenderman, L., and Coffer, P. J. (2000) Curr. Biol. 10 1201-1204 [DOI] [PubMed] [Google Scholar]
- 33.Liu, J. W., Chandra, D., Rudd, M. D., Butler, A. P., Pallotta, V., Brown, D., Coffer, P. J., and Tang, D. G. (2005) Oncogene 24 2020-2031 [DOI] [PubMed] [Google Scholar]
- 34.Sandri, M., Lin, J., Handschin, C., Yang, W., Arany, Z. P., Lecker, S. H., Goldberg, A. L., and Spiegelman, B. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 16260-16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, X., Monks, B., Ge, Q., and Birnbaum, M. J. (2007) Nature 447 1012-1016 [DOI] [PubMed] [Google Scholar]
- 36.Nemoto, S., Fergusson, M. M., and Finkel, T. (2005) J. Biol. Chem. 280 16456-16460 [DOI] [PubMed] [Google Scholar]
- 37.Rangwala, S. M., Li, X., Lindsley, L., Wang, X., Shaughnessy, S., Daniels, T. G., Szustakowski, J., Nirmala, N. R., Wu, Z., and Stevenson, S. C. (2007) Biochem. Biophys. Res. Commun. 357 231-236 [DOI] [PubMed] [Google Scholar]
- 38.Wang, F., Nguyen, M., Qin, F. X., and Tong, Q. (2007) Aging Cell 6 505-514 [DOI] [PubMed] [Google Scholar]
- 39.Van Gaal, L. F., Mertens, I. L., and De Block, C. E. (2006) Nature 444 875-880 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.