Abstract
We report here a high-resolution NMR structure of the complete receptor-binding domain of human apolipoprotein E3 (apoE3-NT). Similar to the crystal structure of apoE-NT, the NMR structure displayed an elongated four-helix bundle. However, additional unique structural features were also observed. The segments in the N and C termini, which were missing in the crystal structure, formed α-helices having extensive tertiary contacts with the bundle, which oriented these short helices at specific positions for receptor binding activity. Several buried hydrophilic residues observed in the bundle were located strategically between helices 1 and 2 and between helices 3 and 4, significantly destabilizing these helix-helix interfaces. In addition, these buried hydrophilic residues formed buried H-bonds, which may play a key role in specific lipid-free helix bundle recovery. A short helix, nHelix C, was fully solvent-exposed and nearly perpendicular to the bundle. This short helix likely plays a critical role in initiating protein-lipid interaction, causing a preferred conformational adaptation of the bundle at the weaker helix-helix interfaces. This produces an open conformation with two lobes of helices, helices 1 and 4 and helices 2 and 3, which may be the competent conformation for receptor binding activity. Thus, the NMR structure suggests a unified scheme for the initiation and helix bundle opening of apoE-NT upon lipoprotein-binding and for receptor binding activity.
Human apolipoprotein E (apoE)2 is a 299-residue plasma-exchangeable apolipoprotein with the primary function of transporting lipids from one tissue to another. ApoE performs its functions via interactions with the low-density lipoprotein receptor (LDLR) superfamily (1). The high affinity binding of apoE to the receptors allows apoE-associated lipoprotein particles to be targeted for endocytosis and intracellular degradation. As a subclass of high-density lipoprotein, apoE also influences both cholesterol efflux and influx, thus playing an important role in reverse cholesterol transport (2, 3). Three major isoforms of apoE have been identified: ApoE3 has a cysteine at position 112 and an arginine at position 158, whereas apoE2 has cysteines and apoE4 has arginines at both positions. Although these isoforms differ in only two residues, they show profound functional differences. Recent evidence indicates that apoE is also critical in several other important biological processes, including Alzheimer disease, cognitive functioning, immunoregulation, cell signaling, and infectious diseases (4).
ApoE is a two-domain protein that contains a 22-kDa N-terminal domain (residues 1-191) and a 10-kDa C-terminal domain (residues 216-299) linked by a protease sensitive hinge region. Although the N-terminal domain of apoE (apoE-NT) is primarily responsible for LDL-receptor binding, the C-terminal domain (apoE-CT) binds to lipoprotein with a high affinity (1). The x-ray crystal structure of lipid-free apoE-NT reveals a globular up-and-down four-helix bundle (5). The major receptor-binding region, residues 130-150, is located on the fourth helix. The positively charged residues (Lys and Arg) in this region are critical for interacting with the negatively charged residues in the receptor (1, 6). This structure only contains residues 24-164, whereas the rest of the regions are disordered. However, experimental evidence indicates that regions beyond residues 24-164 are also critical for LDLR binding activity. For example, deletion of residues 167-185 reduces the apoE3 LDLR binding activity to 15%, and a mutation at position Arg-172 reduces the LDLR binding activity to only ∼2% (7). In addition, an E3K mutant of apoE3 enhances the LDLR binding activity by 2-fold (8). Although the x-ray crystal structure of apoE-NT provides a structural explanation of the major receptor-binding domain of apoE, this structure does not explain the above described important experimental data. Thus, our understanding of the structural basis of the receptor binding activity of apoE remains incomplete.
Previous studies using truncation mutants have shown that apoE(1-183) displays nearly 100% LDLR binding activity (9), suggesting that residues beyond position 183 are not important in LDLR binding. We report here a high-resolution NMR structure of the complete LDLR-binding domain of apoE3. Interestingly, our NMR structure shows that the N and C termini form α-helical structures that have extensive contacts with the helix bundle, orienting the two termini at specific positions for potential receptor binding. The NMR structure also displays several novel structural features that may provide the structural basis of a unified scheme for initiation and conformational adaptation of apoE-NT upon lipoprotein binding.
EXPERIMENTAL PROCEDURES
NMR Sample—The NMR sample contains 1 mm 2H (30%)/15N/13C-labeled apoE-NT in 50 mm phosphate buffer containing 0.1 mm NaN3, 10 mm EDTA, and 50 mm dithiothreitol, pH 6.8. Sample preparations followed the published procedure (10, 11).
NMR Spectroscopy—NMR data collected for a complete backbone assignment are reported elsewhere (11). The NMR data collected here were for the side chain assignment and for generation of the NMR restraints, including the NOE, dihedral angle, and H-bond restraints. NMR data were collected using a Varian INOVA 600-MHz NMR spectrometer with a cyrogenic probe. three-dimensional/four-dimensional Nuclear Overhauser effect spectroscopy (NOESY) experiments, including three-dimensional 15N-edited NOESY and four-dimensional 13C/15N-edited NOESY, were collected at 30 °C with a mixing time of 150 ms. In addition, several three-dimensional NMR experiments, including CCC-TOCSY-NNH and HCC-TOCSY-NNH, as well as HCCH-TOCSY and MQ-(H)CCH-TOCSY (12), were collected for side chain assignment. The spectra were processed using NMRPipe (13) and analyzed using NMRView (14) and PIPP software (15). The chemical shift data were used for TALOS (16), PECAN (17), and chemical shift index (CSI) (18) calculations to generate accurate locations of the secondary structure of apoE-NT and dihedral angle restraints.
Structure Calculations—Side chain resonances were assigned based on the NMR experiments collected above. 15N-Edited NOESY and 13C/15N-edited NOESY were used to generate NOE distance restraints. The TALOS program was also used to predict the dihedral angles based on chemical shift information. Structure calculations were performed based on 2980 NOE, 292 dihedral angle, and 218 H-bond restraints with a simulated annealing protocol using CYANA, version 2.1 (19). Structure calculations were carried out in an iterative manner, and each iteration generated 100 NMR structures. The generated structures were analyzed for distance and dihedral angle restraint violations. A new restraint set was generated after these analyses for the next calculation. Fourteen iterations were carried out, and the final iteration generated 30 structures with no NOE violations of >0.2 Å or dihedral angle violations of >3.0°. Twenty best-fit NMR structures were used for further analysis.
Structural Analysis—The 20 final best-fit NMR structures of apoE-NT were analyzed using VADAR (20) and PROCHECK (21). VADAR analyzed the structural coordinates, gave secondary structure locations, and also provided the fractional solvent-accessible surface area (ASA) of the side chain of each residue, as well as H-bonding information. From the ASA, we identified buried/partially buried hydrophilic residues. A program was written to summarize the VADAR results of the 20 best-fit NMR structures and list those buried hydrophilic residues and buried H-bonds that existed in more than 10 best-fit structures (shown in Tables 1 and 2). In fact, we found that the majority of buried hydrophilic residues and buried H-bonds existed in more than 18 best-fit NMR structures, indicating high resolution of these NMR structures. The threshold used was an ASA < 0.15 for buried and 0.15 < ASA < 0.30 for partially buried hydrophilic residues.
TABLE 1.
Buried/partially buried hydrophilic residues in apoE-NT NMR structure
Buried hydrophilic residues are highlighted in bold. Partially buried residues are in plain text. Buried hydrophilic residues that are between helices N/C and the bundle are underlined.
| Helix N | Helix 1 | Helix 1′ | Helix 2 | Loop2 | Helix 3 | Helix 4 | Helix C | |
|---|---|---|---|---|---|---|---|---|
| Helix N | Gln-163 | |||||||
| Helix 1 | Glu-50 | Glu-59, Glu-70 | Arg-145, Gln-156 | |||||
| Helix 2 | Glu-50 | Cys-112, Arg-119 | Arg-180 | |||||
| Helix 3 | Thr-57 | Thr-83 | Asp-151, Arg-158, Tyr-162 | Glu-179 | ||||
| Helix 4 | Thr-18, Ser-22 | Thr-83 | Asp-107, Arg-114, Tyr-118 | |||||
| Helix C | Lys-75, Glu-79 | Arg-90, Ser-94, Gln-101 |
TABLE 2.
Interhelical hydrogen bonds in the apoE N-terminal domain NMR structure
Residues highlighted in bold are completely buried residues, and residues in plain text are partially buried residues. Buried H-bonds that are between helices N/C and the bundle are underlined. “s” stands for side chain atom, and “b” stands for the backbone atom that forms the H-bonds.
| Helix N | Helix 1 | Helix 1′ | Helix 2 | Loop 2 | Helix 3 | Helix 4 | |
|---|---|---|---|---|---|---|---|
| Helix 2 | Trp-26b—Glu-70s | Glu-50s—Gln-55sa | |||||
| Arg-25b—Glu-70s | Glu-50b—Val-56ba | ||||||
| Helix 3 | Lys-1b—Arg-92sb | ||||||
| Helix 4 | Gln-21s—Gln-163b | Leu-37b—Arg-145s | Glu-45s—Arg-134sb | Tyr-74s—Leu-159bc | T83s-V161ba | Asp-107s—Leu-144b | |
| Ser-22s—Gln-163s | Glu-27s—Gln-156s | Asp-107s—Arg-147s | |||||
| Asp-107s—Leu-148b | |||||||
| Helix C | Glu-79s—Pro-183b | Ser-94s—Glu-179s |
The buried H-bonds between helices 1′ and 2 and between loop 2 and helix 4 favor helix bundle recovery via the preferred helix bundle opening.
These are exposed H-bonds.
This buried H-bond favors helix bundle recovery via both preferred and non-preferred bundle openings.
RESULTS
Structural Description—Fig. 1 depicts 20 best-fit NMR structures and a ribbon representation of the average structure of lipid-free apoE-NT. The structure consists of an up-and-down helix bundle of four long amphipathic α-helices. Short loops link the helices, except between helices 1 and 2, which are connected by a short helix (helix 1′). The helix bundle structure between the NMR and crystal structures is very similar, with a root-mean-square deviation of 1.22 Å for the backbone atoms. Supplemental Fig. S1 summarizes all of the NMR parameters and displays the helical locations. Supplemental Table S1 shows the structural statistics and root-mean-square deviations for the ensemble of 20 structures. The root-mean-square deviation of backbone atoms of the helix bundle is 0.42 ± 0.05 Å, indicating a high-resolution NMR structure. The precision of this NMR structure allowed us to accurately analyze the buried hydrophilic residues, buried H-bonds, and tertiary contacts. PROCHECK analysis indicated that in addition to 10 Gly and 4 Pro residues, 140 residues (83.3%) were located in the most favored regions, 22 residues (13.1%) in additional allowed regions, and 4 residues (2.4%) in the generously allowed region of the Ramachandran plot. Only two residues (Val-2 and Gln-24) are in the disallowed region. Val-2 is in the N terminus, and Gln-24 is between helix N and helix 1 of the bundle.
FIGURE 1.
NMR structure of apoE-NT. A, 20 best-fit NMR structures superimposed based on well defined helices, with backbone in white and side chain heavy atoms in green. B, 20 best-fit NMR structures superimposed based on well defined helices, with only backbone atoms displayed. The helices are colored as follows: helix N, dark blue; helix 1, light blue; helix 1′, light green; helix 2, green; helix 3, yellow; helix 4, orange; helix C, red. C, ribbon representation of the average structure of apoE-NT, with each helix colored same as in B.
A striking difference is the helix formation of the two regions that are missing in the crystal structure. Residues 12-22 form a flexible short helix (Fig. 1, dark blue helix) that is nearly perpendicular to the bundle. We termed this helix N. Residues 168-180 also form a flexible helix (red helix), which is termed helix C. This helix initially was found perpendicular to the bundle at residues 168-172 (termed nHelix C), which is on the top of one end of the bundle opposite Helix 1′. It breaks at residue Gly-173, which makes a sharp, almost 90° turn, resulting in the remaining helix (termed cHelix C) adopting a parallel orientation to the bundle. CD data indicated 74-78% of α-helix content for apoE-(1-183) in solution, which is consistent with the 81% helix content observed in this NMR structure. Extensive tertiary contacts were observed between helix N/helix C and the bundle. Fig. 2 shows several NOE cross-peaks between helix N(right panel) and helix C (left panel) of the bundle in a four-dimensional 13C/15N-edited NOESY spectrum, demonstrating these tertiary contacts.
FIGURE 2.
Two planes of the four-dimensional 13C/15N-edited NOESY spectrum of apoE-NT in 50 mm phosphate buffer containing 0.1 mm NaN3, 10 mm EDTA and 50 mm dithiothreitol, pH 6.8. The experiment was conducted using a mixing time of 150 ms. Left panel, a region of the NOESY plane at chemical shift for 15N, 122.89 ppm, and 1HN, 8.14 ppm. Right panel, a region of the plane at chemical shift for 15N, 121.39 ppm, and 1HN, 7.91 ppm. The spectral assignments are based on the chemical shift assignment for apoE-NT deposited in the Biological Magnetic Resonance Bank data base with accession number 6524. The cross-peaks that are highlighted in gray are long range NOEs from either helix N (left) or helix C (right) to the helix bundle of apoE-NT. The cross-peaks labeled by an asterisk are those with stronger intensity in the adjacent planes and have been assigned in a different plane.
Structural Features of the Helix Bundle—Analysis of the four-helix bundle of apoE-NT revealed several key structural features that are potentially critical for apoE functions, including receptor binding and lipoprotein binding. The NMR structure maintains all of the structural features, as revealed by the crystal structure, indicating that the helix bundle is organized in such a way that the hydrophobic faces orient toward each other forming a hydrophobic core, whereas the hydrophilic surfaces are exposed to the solvent. The hydrophobic residues are clustered together, providing the main driving force for bundle formation. The major receptor-binding region, residues 130-150, is located on the fourth helix. The positively charged residues (Lys and Arg) in this region are fully solvent-exposed (except Arg-145) and form a massive positively charged surface available for receptor binding (5). However, other novel structural features were also observed. Through VADAR analysis (20) we found that numerous polar (Gln, Asn, Ser, and Thr) and ionizable residues (Asp, Glu, His, Arg, and Lys) are located in the otherwise hydrophobic interior of the bundle, potentially destabilizing the bundle structure. More importantly, they are clustered specifically between helices 1 and 2 and helices 3 and 4. Fig. 3 shows a helix wheel diagram based on the NMR structure, highlighting in dark gray the locations and orientations of these buried hydrophilic residues. Supplemental Fig. S2B shows the structure of apoE-NT, highlighting the cluster of the buried hydrophilic residues between helices 1 and 2 and helices 3 and 4. In contrast, only a few hydrophilic residues were observed between helices 1 and 4 and helices 2 and 3 (supplemental Fig. S2A). Table 1 lists a total of 15 buried hydrophilic residues within the bundle. Two buried hydrophilic residues (Glu-59, Glu-70) are between helices 1 and 2, and six buried hydrophilic residues (Asp-107, Arg-114, Tyr-118, Asp-151, Arg-158, and Tyr-162) are between helices 3 and 4. In addition, two buried hydrophilic residues, Glu-50 on helix 1′ and Thr-83 on loop 2, are located between helices 1 and 2 and helices 3 and 4, respectively. In contrast, only two buried hydrophilic residues (Arg-145, Gln-156) are located between helices 1 and 4, and three buried hydrophilic residues (Cys-112, Arg-119, Thr-57) are located between helices 2 and 3. VADAR analysis of the x-ray crystal structure showed the same result. Three more buried hydrophilic residues, Thr-67, Tyr-74, and Gln-81, point to the center of the bundle. Because buried hydrophilic residues destabilize helix-helix interfaces, these data indicate weaker helix-helix interfaces between helices 1 and 2 and helices 3 and 4 and stronger interfaces between helices 1 and 4 and helices 2 and 3.
FIGURE 3.
A helix wheel diagram of apoE-NT based on NMR structure. Buried hydrophobic residues are shown as black circles and exposed residues as open circles. Buried hydrophilic residues that are inside the helix bundle are in gray with the residue name (one-letter code) and number in white. Buried hydrophilic residues and buried hydrophobic residues, which are between helices N/C and the bundle, are also in gray but with the residue name (one-letter code) and number in black. The letter under each residue represents the accessible surface area of the side chain of this residue. b, completely buried; p, partially buried; e, exposed.
The second novel feature is the buried H-bonds. VADAR analysis indicated that the buried hydrophilic residues form buried H-bonds, which are also unevenly distributed inside the bundle (Fig. 4). Table 2 lists six buried and five partially buried interhelical H-bonds inside the bundle. In addition, one exposed H-bond was also observed between residues Glu-45 and Arg-134 (helices 1′ and 4). Three buried H-bonds and two partially buried H-bonds were observed between helices 1 and 2 and helices 3 and 4, whereas only one buried H-bond and one partially buried H-bond were found between helices 1 and 4. No buried/partially buried H-bond was observed between helices 2 and 3. Two buried/partially buried H-bonds were observed between helices 1′ and 2. In addition, one partially buried H-bond is between Tyr-74s and Leu-159b (helices 2 and 4) and another buried H-bond is between Thr-83s and Val-161b (loop 2 and helix 4). We believe that these buried H-bonds may play an important role in regulating the biological functions of apoE.
FIGURE 4.
Ribbon representation of the NMR structure of apoE-NT highlighting the buried H-bonds between different helix-helix interfaces. The side chain heavy atoms of the buried H-bonds are displayed as sticks. The H-bonds are represented by blue dotted lines. The residues in the front helices are colored in green, and the residues in the back helices are in red. The helices are labeled and colored as follows: helices 1 and 4, wheat; helices 2 and 3, light blue; helix 1′, violet; helix N, salmon; helix C, marine blue. A, the buried H-bonds located in the interfaces between helices 1 and 4 and between helices 1′ and 4. No buried H-bonds are found between helices 2 and 3. B, the buried H-bonds located in the interfaces between helices 1 and 2 and between helices 3 and 4. Also shown are the buried H-bonds between helix N/C and the helix bundle.
Structural Features of Helix N and Helix C—Helix N and helix C also display important structural features. First, the locations and orientation of these two short helices are very interesting. Helix N is located at the same end of the bundle as helix C, which is at the opposite end from helix 1′. Residue 23 is a Gly, causing a helix break and reorientation of helix N nearly perpendicular to the bundle. This short helix has extensive tertiary contacts with helix 4, stabilizing the short helix and orienting it to a strategic location that ensures the proper position of the N-terminal loop (residues 1-11). NOEs are observed from the N-terminal end of this loop to helix 3, indicating tertiary contacts with the bundle. For helix C, the striking feature is a surprising, nearly 90° orientation change at different portions of this 12-residue short helix. nHelix C is located on top of and perpendicular to the bundle and is fully solvent-exposed. This helix breaks at residue Gly-173 and then extends to cHelix C with a nearly 90° orientation change that make it almost parallel to the helix bundle. Extensive long range NOEs were observed between the first five-residue helix fragment to loop 2 that links helices 2 and 3, as well as the second seven-residue helix fragment to either helix 2 or helix 3. These tertiary contacts stabilize helix C and also drive it to make a near 90° sharp turn in the second half of helix C.
Another striking observation was that of several buried hydrophilic residues in both helices C and N (Table 1): Thr-18 and Ser-22 in helix N and Glu-179 and Arg-180 in helix C. In addition, several buried hydrophilic residues were also observed in the helix bundle with orientations toward helices N and C, including Gln-163 on helix 4 (with helix N), Lys-75 and Glu-79 on helix 2, and Arg-90, Ser-94, and Gln-101 on helix 3 (with helix C). Interestingly, no buried hydrophilic residues were found for nHelix C, as this helix segment is completely solvent-exposed. Accordingly, several buried and partially buried H-bonds were also observed between helices N and C with the bundle (Table 2), including Gln-21s—Gln-163b, Ser-22s—Gln-163s (between helices N and 4), Glu-79s—Pro-183b (between helices 2 and C), and Ser-94s—Glu-179s (between helices 3 and C). Once again, no buried H-bonds were observed between nHelix C and loop 2. Interestingly, one exposed H-bond between Lys-1 and Arg-92 was observed, indicating that the N-terminal loop between residues 1-11 indeed adopts a defined location that is close to the N-terminal end of helix 3.
DISCUSSION
Although important progress has been made in apoE structural studies, significant challenges remain (22). Currently, the crystal structure of apoE-NT is still the only high-resolution structure available for this important protein. The crystal structure of human apoE-NT spans only residues 24-164, with significant portions of the molecule in both the N and C termini missing, even though they are important for apoE functions. The crystal structure of mouse apoE-NT shows a helix in the N terminus and in the C terminus (23), which is similar to our NMR structure. In addition, it is found that lipid interaction induces an extension of helix 4 in human apoE beyond the boundary defining its lipid-free helix bundle (24). Table 3 shows the helical locations of different apoE-NT structures, indicating similar helical locations between mouse and human apoE. However, significant orientation differences are observed for helices N and C between the two apoE structures. Whereas helix N in mouse apoE is part of helix 1, with only an ∼15° orientation change, the same helix in human apoE breaks at Gly-23 and changes its orientation by ∼75°, making helix N nearly perpendicular to the bundle. Mouse apoE has an Asn-23 and lacks the first eight amino acids of human apoE. These differences may account for the orientation difference of helix N. Helix C also displays a major orientation difference. Although a nearly identical sequence, except for residue 174 (Val-174 in mouse apoE; Leu-174 in human apoE), is observed between mouse and human apoE, helix C in mouse apoE points away from the bundle and is nearly perpendicular to the bundle. In addition, helix C in mouse apoE shows no tertiary interactions with the bundle. In contrast, helix C in human apoE displays extensive tertiary contacts with the bundle (Fig. 2), which places this helix in a strategic position relative to the bundle for possible interaction with the receptor. Interestingly, helix C of human apoE changes its orientation from perpendicular for nHelix C to parallel to the bundle for cHelix C. Once again, Gly-173 breaks helix C and makes this dramatic 90° orientation change possible. Nevertheless, a common structural feature is the flexibility of this short helix. Indeed, the loop (residues 166-172) linking the bundle and helix C is missing in mouse apoE, demonstrating the dynamic nature of this region.
TABLE 3.
A comparison of helix locations in human and mouse apoE N-terminal domain
Mouse apoE is eight residues shorter than human apoE. The helix locations shown here are based on the sequence comparison with human apoE. h, human; m, mouse; NA, not applicable.
The NMR structure of apoE-NT retains the important structural features of the helix bundle as revealed by the x-ray crystal structure (5), including an unusually elongated four-helix bundle, a tightly packed hydrophobic core, and a cluster of positively charged surface residues on helix 4 that is important for LDL receptor binding. We have further demonstrated additional structural features in apoE-NT, including buried hydrophilic residues and buried H-bonds inside the bundle that are placed at specific positions. The NMR structure also reveals two additional helices, helices N and C. Both helices N and C contain extensive tertiary contacts with the bundle, placing several key residues, including E3K mutation and Arg-172, in critical positions for receptor binding. Furthermore, nHelix C provides the only fully solvent-exposed short helix that is located at one end and perpendicular to the bundle, potentially serving as a recognition helix to the lipoprotein surface to initiate apoE-lipoprotein interaction.
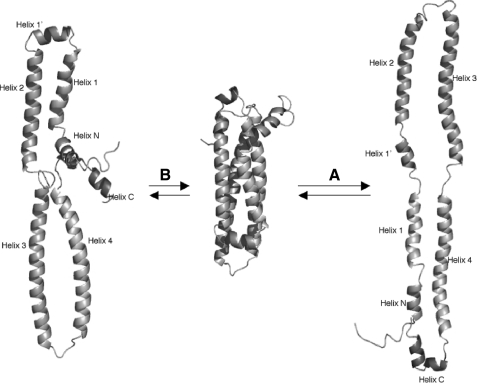
Reversible Lipoprotein Binding and Helix Bundle Opening and Recovery—The reversible lipoprotein binding activity represents the functional characteristics of apoE, because the full biological activity of apoE requires its association with a lipoprotein particle. Protein-lipid interaction is hydrophobic, and the helix bundle of apoE-NT is unable to bind lipid surfaces in the absence of a conformational change because its hydrophobic surfaces are sequestered inside the bundle. A conformational adaptation hypothesis has been proposed (1, 25), suggesting that interaction with a lipid surface induces the helix bundle to open at the hinges of one end of the bundle, resulting in large, exposed, continuous, hydrophobic surfaces capable of lipid binding. This hypothesis is supported by considerable experimental evidence (26, 27). In addition, a 10 Å structure of apoE bound to dipalmitoylphosphatidylcholine derived from a crystal structural study shows that the apoE molecule is folded into a helical hairpin with the binding regions for the LDL receptor at its apex (28), which provides direct experimental evidence to support this open conformation model.
A critical question remains unanswered: What is the structural feature in the lipid-free helix bundle of apoE-NT that dictates its specific opening and recovery? Using disulfide bond engineering, it has been suggested that helices 1 and 2 and helices 3 and 4 preferentially remain paired in the open conformation (27). We showed here that different helix-helix interfaces display different stability in the apoLp-III helix bundle. This is because the buried hydrophilic residues are located only in specific helix-helix interfaces, thus destabilizing these interfaces. Upon lipid binding, the helix bundle will be preferentially opened at the weaker helix-helix interfaces (29, 30). The NMR structure of apoE-NT shows the same patterns of buried hydrophilic residues. Table 1 indicates weaker helix-helix interfaces between helices 1 and 2 and helices 3 and 4 (10 buried hydrophilic residues, including Glu-50 and Thr-83), suggesting that the lipid-free apoE-NT helix bundle may open using the hinge at helix 1′ end, so that the preferred open conformation contains two lobes formed by helices 1 and 4 and helices 2 and 3 (Fig. 5A). This preferred helix bundle opening is consistent with the published data indicating that helices 1 and 3 move further apart upon lipid binding (31). The helix bundle can also possibly open using the hinge at nHelix C end, resulting in two lobes consisting of helices 1 and 2 and helices 3 and 4 (five buried hydrophilic residues) (Fig. 5B). We cannot exclude this alternative bundle opening, even though it may not be the preferred one. Indeed, once helices 1 and 2/helices 3 and 4 are tethered by disulfide bridges, this alternative opening is observed (27). Only when all of the helices of the bundle are tethered, the lipid binding activity is completely diminished (27).
FIGURE 5.
Proposed conformational adaptation of apoE-NT upon lipid binding-helix bundle opening. A, proposed preferred helix bundle opening at the hinges formed by helix 1′ and loop 3 (between helices 3 and 4). nHelix C may initiate protein-lipid interaction, inducing the helix bundle opening around the hinges to form two lobes (helices 1 and 4 and helices 2 and 3). The observed patterns of the buried hydrophilic residues and buried H-bonds in apoE-NT support this suggestion. B, proposed non-preferred helix bundle opening.
Given the increased stability of lipid-associated apoE-NT, the open conformation will be retained until metabolic processes eliminate the binding sites, resulting in apoE release from lipoprotein surface and recovery of the lipid-free helix bundle. Re-establishment of hydrophobic interactions between helix-helix interfaces initially drives the bundle recovery. However, these hydrophobic interactions are rather nonspecific and usually result in molten globules (32). The observed buried H-bonds serve as the specific interaction that locks the protein into the well defined helix bundle (32). For recovery of the preferred bundle opening, both lobes (helices 1 and 4 and helices 2 and 3) in the open conformation will be repositioned initially by the nonspecific hydrophobic interactions around the hinge to establish helix-helix interactions between helices 1 and 2 and helices 3 and 4. The observed buried interhelical H-bonds are located predominantly at the interfaces between the two lobes (eight buried H-bonds), whereas only two buried H-bonds are found within one lobe (between helices 1 and 4) (Table 2). Such a spatial arrangement strongly suggests that the interlobe H-bonds are critical to guiding the two lobes to specifically recover into the lipid-free helix bundle, supporting the preferred helix bundle opening.
The preferred helix bundle opening (Fig. 5A) would require the initial dissociation of helices N and C from the bundle. Several buried hydrophilic residues were observed specifically between helix N and helix 4 (Thr-18, Ser-22, Gln-163) and between helix C and helices 2 and 3 (Lys-75, Glu-79, Arg-90, Ser-94, Gln-101, Glu-179, Arg-180) (Table 1). These residues are critical in adjusting the stability of helix-helix interfaces between the bundle and helices N and C, making their dissociation possible, which leads to the bundle opening. In addition, two buried H-bonds are observed between helices N and 4, and two buried H-bonds are between helices C and 2/3 (Table 2). These buried H-bonds are most likely critical in locating the accurate positions of helices N and C relative to the bundle during lipid-free helix bundle recovery.
This NMR structure does not contain the apoE C-terminal domain. However, the C-terminal domain of apoE is the major lipoprotein-binding domain. It has been proposed that lipid binding of the C-terminal domain may induce a conformational change in the N-terminal domain for a competent conformation of LDL receptor binding (2, 22). In addition, a domain-domain interaction in apoE was suggested to regulate specific functional difference between different apoE isoforms (2, 4). Based on the chemical shift difference between apoE-(1-183) and full-length apoE, our NMR data indicated that in addition to Arg-61, several other residues, such as Glu-45 and Arg-134, also are potentially involved in this domain-domain interaction (33). We further demonstrated that the flexible hinge domain of apoE that connects the N- and C-terminal domains is important in mediating this domain-domain interaction (34). The NMR structure of the apoE N-terminal domain supports the hypothesis that lipid binding to the C-terminal domain induces the specific helix bundle opening of the N-terminal domain, as the N-terminal domain contains all of the structural features that permit such a specific bundle opening. We further suggest that the location of helix C may properly position the flexible hinge domain for its role in mediating domain-domain interaction. However, a detailed structural understanding of how lipid binding at the C-terminal domain induces the N-terminal helix bundle opening requires the structure of full-length apoE; we are currently working on generating the NMR structure of full-length apoE.
Recognition Short Helix—Another important question remains unanswered: Which structural element initiates the helix bundle opening of apoE3-NT upon lipoprotein binding? Previously, by a comparison of available structures of lipid-free helix bundles of exchangeable apolipoproteins, we proposed helix 1′ as the recognition short helix to lipoprotein surfaces (29, 30). An alternative proposal suggested that the 80's loop, located in the opposite end of helix 1′, initiates lipid binding and bundle opening (35). It has been suggested that the negatively charged side chains of glutamate residues may be attracted to the quaternary amino group of the phosphatidylcholine moiety at the lipid surface, whereas the flexibility of the 80's loop facilitates the required conformational change (35). A more recent study, in which helix 1′ was replaced by a β-turn, concluded that helix 1′ is not essential for recognition or initiation of lipid binding and helix bundle opening (36).
We notice a short helix in the NMR structure of apoE-NT, the nHelix C (168EGAER172), which is located in the same end as the 80's loop and nearly perpendicular to the bundle. VADAR analysis indicates that this short helix is the most solvent-exposed helix in apoE-NT. In contrast, the 80's loop is somewhat buried because of the tertiary interactions with this short helix. Indeed, the sequence of this short helix contains a Gly and an Ala, making it very flexible. In addition, this short helix contains two negatively charged side chains of Glu. Thus, we suggest that nHelix C may serve as the recognition helix that initiates apoE-NT-lipoprotein interaction and helix bundle opening.
This short helix is missing in the x-ray crystal structures of human apoE-NT. Even in mouse apoE-NT, helix C starts at residue 173, whereas residues 164-172 are missing in the electron density map (23). However, structural comparisons of the NMR structure of apoE-NT with the published structures of apoLp-III demonstrate that nHelix C displays identical structural features for recognition helices in apoLp-IIIs (29, 30) including that it: (i) is fully solvent-exposed; (ii) is located at one end and perpendicular to the bundle; (iii) is very flexible; (iv) is rich in negatively charged residues; and (v) favors the preferred lipid-free helix bundle opening. Obviously, these structural features are critical for nHelix C to serve as the recognition helix. Indeed, nHelix C was located in the critical turn connecting both the N- and C-terminal domains in the helical hairpin model structure of lipid-bound apoE, further suggesting that nHelix C may serve as a recognition short helix for both apoE domains to initiate their interaction with phospholipids (28). Helix 1′ does not contain these structural features. Indeed, nHelix C may initiate the preferred apoE-NT bundle opening upon lipoprotein binding at the “hinges” formed by helix 1′ and the loop connecting helices 3 and 4, so that the lobe containing helices 1 and 4 moves away from the other lobe containing helices 2 and 3 (Fig. 5A). Therefore, we have provided a unified scheme for the initiation and helix bundle opening of apoE-NT upon lipoprotein-binding.
Possible Binding Mode for the VLDL Receptor—Lipoprotein association is required for full LDLR binding activity (1). However, lipid-free apoE binds to very low-density lipoprotein receptor (VLDLR) with a nanomolar binding constant, and VLDLR also mediates the internalization of different lipid-poor apoE isoforms (37). Based on the x-ray crystal structure of LDLR at endosomal pH (38) and sequence homology between LDLR and VLDLR, we built a model structure of the ligand-binding domain of VLDLR. We then modeled the interfaces between lipid-free apoE-NT and the ligand-binding domain of VLDLR. ApoE contains several regions that are critical for receptor binding, including the major receptor-binding region (residues 136-150) (1) and the region around Arg-172 (7). Residues Lys-143 and Lys-146 in apoE are the critical binding residues for the LDLR, by forming stable salt bridges with the acidic residues in the LDLR type-A (LA) modules, analogous to the binding mode between the receptor-associated protein and LDLR (39). Interestingly, VLDLR recognizes the same region in apoE that binds to LDLR, and the LA3-LA6 of VLDLR gives 100% binding activity to lipid-free apoE (37). With this information, we built a docking model structure of lipid-free apoE and the LA3-LA5 of VLDLR. Fig. 6, upper panel, shows a sequence alignment of LA3-LA6 between LDLR and VLDLR, highlighting our rationale for using LA3-LA5.
FIGURE 6.
Upper panel, sequence alignment of LA3-LA6 of VLDLR with LA2-LA5 of LDLR. The possible apoE-binding residues in each LA module are shaded yellow for Asp/Glu/Asn/Gln and green for Trp/Phe. The Ca2+-binding residues are shaded light blue and underlined. Residues that use backbone carbonyl to chelate Ca2+ are labeled with italic letters for the one-letter code of the amino acid of these residues. Residues that bind to both apoE-NT and Ca2+ are underlined and shaded based on apoE binding. LA3 and LA5 of VLDLR contain a typical consensus sequence for ligand binding (WXCDX(D/E)XDC), whereas LA4 has the sequence FXCNXQXDC. (Boldface letters are residues involved in the interaction with the ligand.) Lower panels, docking model for apoE-NT binding to LA3-LA5 of VLDLR. Lower left, overview of this docking model. Both apoE-NT and LA3-LA5 of VLDLR are rendered as ribbons, with the side chain of the binding residues (Lys/Arg in apoE-NT and Asp/Glu/Asn/Gln/Trp/Phe in LA3-LA5 of VLDLR) shown as sticks. The helix bundle of apoE-NT is colored purple, and the major receptor-binding region (residues 136-150) and helices N and C are colored green. For LA3-LA5 of VLDLR: LA3, light blue; LA4, light orange; LA5, gray. Insets A, B, and C is the lower left panel show the interaction regions of apoE and the receptor. The insets are zoomed and displayed in the right panels. Lower right, A, binding interface between Lys-143/Lys-146 of apoE-NT and Asp-135/Glu-137/Asp-139/Trp-132/Gly-136 of LA3 of VLDLR. B, possible binding interface between Lys-1/Lys-3 of apoE-NT and Asn-174/Gln-176/Glu-178/Phe-171 of LA4 of VLDLR. C, binding interface between Arg-172/Arg-178 of apoE-NT and Asp-213/Asp-215/Asp-217/Trp-210 of LA5. The side chain carbons in apoE-NT, brown sticks; side chain carbon in LA modules, pink sticks; side chain nitrogen, blue stick; oxygen, red stick; side chain hydrogen, green stick. The salt bridges are indicated by dashed blue lines, and H-bonds are shown by dashed red lines.
Fig. 6, lower panel, shows the docking structure of the complex. The lower left panel shows the overall view, indicating that the helix bundle of apoE-NT remains unchanged. Helices N and C are strategically located in positions that are very close to the LA modules of VLDLR due to the arc shape of the receptor. Because different modules do not interact with each other (38), we made a few changes in the dihedral angles of the linker residues between different LA modules in the receptor to best fit the interaction between apoE-NT and VLDLR. Insets A and C (Fig. 6, lower left) show the zoom-in view of detailed interactions between the LA5 and the Arg-172 region (inset C) and between LA3 and the major receptor-binding region (inset A). In particular, Arg-172 fits well into the negatively charged pocket formed by Asp-213, Asp-215, and Asp-217 of LA5, and Lys-143 of apoE is encircled by a similar negatively charged pocket formed by Asp-135, Glu-137, and Asp-139 of LA3. Side chain carboxylate oxygen atoms of three Asp/Glu residues in each LA module form a tripartite salt bridge with the positively charged side chain of Lys/Arg of apoE. A Trp residue (Trp-132 of LA3 and Trp-210 of LA5) packs its side chains against the aliphatic portion of the Lys/Arg in the binding pocket. The second Lys/Arg (Lys-146 and Arg-178) also forms a salt bridge with one of the Asp of the LA module (Glu-137 in LA3 and Asp-213 in LA5). This binding interaction belongs to the typical binding interaction between ligand and LDLR, as revealed by the x-ray crystal structure of the LDLR·RAP (receptor-associated protein) complex (39). In addition, a possible H-bond may also be formed between this second Lys/Arg and a backbone carbonyl of LA modules (Gly-136 in LA3 and Asp-213 in LA5).
During the docking process, we noticed that the N terminus of apoE-NT was in a close proximity of LA4, suggesting a possible interaction between the N terminus of apoE and LA4. Because the E3K mutation enhances the LDLR binding activity of apoE by 2-fold (8), we suggest that this mutation may also be involved in VLDLR binding. In the NMR structure, an H-bond was observed between Lys-1 and Arg-92, strategically placing Lys-1 in a favorable position for a possible interaction with LA4. We suggest that the E3K mutation may allow the N terminus of apoE to bind to LA4, using Lys-1 and Lys-3 (Fig. 6, lower right, panel B). LA4 contains the FXCNXQXDC binding pocket, which is different from the consensus binding sequence of LA3/5 (WXCDX(D/E)XDC). A salt bridge may be formed between Lys-1 and Asp-178. Three H-bonds are possible between the amino protons of the ε-amino group of Lys-1 and the carbonyl oxygen of the side chain of Asn-174 and Gln-176 plus the backbone carbonyl oxygen of Asn-174. Similarly, Phe-171 is also tightly packed with the aliphatic side chain of Lys-1. Similar to Arg-178 and Lys-146, the Lys-3 side chain may form a salt bridge with the Asp-178 side chain. In addition, an H-bond may also be possible between the side chain amino proton of Lys-3 and the side chain carbonyl oxygen of Gln-176. Thus, lys-1/Lys-3 may display a binding mode similar to Lys-143/Lys-146 and Arg-172/Arg-178 for LA4 binding, enhancing the binding interaction between apoE and the receptor.
Supplementary Material
Acknowledgments
We thank Victoria Murray and Dr. Chris McCullough for critical reading of the manuscript. We are also grateful to Drs. Karl Weisgraber (Gladstone Institute of Neurological Disease) and Robert Ryan (Children's Hospital Oakland Research Institute) for critical reading of the manuscript and for insightful suggestions.
The atomic coordinates and structure factors (code 2kc3) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 HL074365 (to J. W.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
Footnotes
The abbreviations used are: apoE, human apolipoprotein E; apoE-NT, apoE N-terminal domain; apoE-CT, apoE C-terminal domain; apoLp-III, apolipophorin III; LDL, low-density lipoprotein; LDLR, LDL receptor; VADAR, volume, area, dihedral angle reporter; PROCHECK, protein structure checks; LA, LDLR type A; VLDLR, very low-density lipoprotein receptor; NOE, nuclear Overhauser effect; NOESY, Nuclear Overhauser effect spectroscopy; ASA, accessible surface area.
References
- 1.Weisgraber, K. H. (1994) Adv. Protein Chem. 45 249-302 [DOI] [PubMed] [Google Scholar]
- 2.Mahley, R. W., Huang, Y., and Weisgraber, K. H. (2006) J. Clin. Invest. 116 1226-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang, Y., von Eckardstein, A., Wu, S., Maeda, N., and Assmann, G. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 1834-1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahley, R. W., and Rall, S. C., Jr. (2000) Annu. Rev. Genomics Hum. Genet. 1 507-537 [DOI] [PubMed] [Google Scholar]
- 5.Wilson, C., Wardell, M. R., Weisgraber, K. H., Mahley, R. W., and Agard, D. A. (1991) Science 252 1817-1822 [DOI] [PubMed] [Google Scholar]
- 6.Rudenko, G., and Deisenhofer, J. (2003) Curr. Opin. Struct. Biol. 13 683-689 [DOI] [PubMed] [Google Scholar]
- 7.Morrow, J. A., Arnold, K. S., Dong, J., Balestra, M. E., Innerarity, T. L., and Weisgraber, K. H. (2000) J. Biol. Chem. 275 2576-2580 [DOI] [PubMed] [Google Scholar]
- 8.Wardell, M. R., Rall, S. C., Jr., Schaefer, E. J., Kane, J. P., and Weisgraber, K. H. (1991) J. Lipid Res. 32 521-528 [PubMed] [Google Scholar]
- 9.Lalazar, A., and Mahley, R. W. (1989) J. Biol. Chem. 264 8447-8450 [PubMed] [Google Scholar]
- 10.Fisher, C. A., Wang, J., Francis, G. A., Sykes, B. D., Kay, C. M., and Ryan, R. O. (1997) Biochem. Cell Biol. 75 45-53 [PubMed] [Google Scholar]
- 11.Xu, C., Sivashanmugam, A., Hoyt, D., and Wang, J. (2005) J. Biomol. NMR 32 177. [DOI] [PubMed] [Google Scholar]
- 12.Zheng, Y., Giovannelli, J. L., Ho, N. T., Ho, C., and Yang, D. (2004) J. Biomol. NMR 30 423-429 [DOI] [PubMed] [Google Scholar]
- 13.Delaglio, F., Grzesiek, S., Vuister, G. W., Zhu, G., Pfeifer, J., and Bax, A. (1995) J. Biomol. NMR 6 277-293 [DOI] [PubMed] [Google Scholar]
- 14.Johnson, B. A., and Blevins, R. A. (1994) J. Biomol. NMR 4 603-614 [DOI] [PubMed] [Google Scholar]
- 15.Garrett, D. S., Powers, R., Gronenborn, A. M., and Clore, G. M. (1991) J. Magn. Reson. 95 214-220 [DOI] [PubMed] [Google Scholar]
- 16.Cornilescu, G., Delaglio, F., and Bax, A. (1999) J. Biomol. NMR 13 289-302 [DOI] [PubMed] [Google Scholar]
- 17.Eghbalnia, H. R., Wang, L., Bahrami, A., Assadi, A., and Markley, J. L. (2005) J. Biomol. NMR 32 71-81 [DOI] [PubMed] [Google Scholar]
- 18.Wishart, D. S., Sykes, B. D., and Richards, F. M. (1992) Biochemistry 31 1647-1651 [DOI] [PubMed] [Google Scholar]
- 19.Guntert, P. (2004) Methods Mol. Biol. 278 353-378 [DOI] [PubMed] [Google Scholar]
- 20.Willard, L., Ranjan, A., Zhang, H., Monzavi, H., Boyko, R. F., Sykes, B. D., and Wishart, D. S. (2003) Nucleic Acids Res. 31 3316-3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laskowski, R. A., MacArthur, M. W., Moss, D. S., and Thornton, J. M. (1993) J. Appl. Crystallogr. 26 283-291 [Google Scholar]
- 22.Hatters, D. M., Peters-Libeu, C. A., and Weisgraber, K. H. (2006) Trends Biochem. Sci. 31 445-454 [DOI] [PubMed] [Google Scholar]
- 23.Hatters, D. M., Peters-Libeu, C. A., and Weisgraber, K. H. (2005) J. Biol. Chem. 280 26477-26482 [DOI] [PubMed] [Google Scholar]
- 24.Gupta, V., Narayanaswami, V., Budamagunta, M. S., Yamamato, T., Voss, J. C., and Ryan, R. O. (2006) J. Biol. Chem. 281 39294-39299 [DOI] [PubMed] [Google Scholar]
- 25.Breiter, D. R., Kanost, M. R., Benning, M. M., Wesenberg, G., Law, J. H., Wells, M. A., Rayment, I., and Holden, H. M. (1991) Biochemistry 30 603-608 [DOI] [PubMed] [Google Scholar]
- 26.Fisher, C. A., Narayanaswami, V., and Ryan, R. O. (2000) J. Biol. Chem. 275 33601-33606 [DOI] [PubMed] [Google Scholar]
- 27.Lu, B., Morrow, J. A., and Weisgraber, K. H. (2000) J. Biol. Chem. 275 20775-20781 [DOI] [PubMed] [Google Scholar]
- 28.Peters-Libeu, C. A., Newhouse, Y., Hatters, D. M., and Weisgraber, K. H. (2006) J. Biol. Chem. 281 1073-1079 [DOI] [PubMed] [Google Scholar]
- 29.Fan, D., Zheng, Y., Yang, D., and Wang, J. (2003) J. Biol. Chem. 278 21212-21220 [DOI] [PubMed] [Google Scholar]
- 30.Wang, J., Sykes, B. D., and Ryan, R. O. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 1188-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher, C. A., and Ryan, R. O. (1999) J. Lipid Res. 40 93-99 [PubMed] [Google Scholar]
- 32.Bryson, J. W., Betz, S. F., Lu, H. S., Suich, D. J., Zhou, H. X., O'Neil, K. T., and DeGrado, W. F. (1995) Science 270 935-941 [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Y., Chen, J., and Wang, J. (2008) Biomol. NMR Assign. 2 207-210 [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Y., Vasudevan, S., Sojitrawala, R., Zhao, W., Cui, C., Xu, C., Fan, D., Newhouse, Y., Balestra, R., Jerome, W. G., Weisgraber, K., Li, Q., and Wang, J. (2007) Biochemistry 46 10722-10732 [DOI] [PubMed] [Google Scholar]
- 35.Segelke, B. W., Forstner, M., Knapp, M., Trakhanov, S. D., Parkin, S., Newhouse, Y. M., Bellamy, H. D., Weisgraber, K. H., and Rupp, B. (2000) Protein Sci. 9 886-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redmond, K. A., Murphy, C., Narayanaswami, V., Kiss, R. S., Hauser, P., Guigard, E., Kay, C. M., and Ryan, R. O. (2006) FEBS J. 273 558-567 [DOI] [PubMed] [Google Scholar]
- 37.Ruiz, J., Kouiavskaia, D., Migliorini, M., Robinson, S., Saenko, E. L., Gorlatova, N., Li, D., Lawrence, D., Hyman, B. T., Weisgraber, K. H., and Strickland, D. K. (2005) J. Lipid Res. 46 1721-1731 [DOI] [PubMed] [Google Scholar]
- 38.Rudenko, G., Henry, L., Henderson, K., Ichtchenko, K., Brown, M. S., Goldstein, J. L., and Deisenhofer, J. (2002) Science 298 2353-2358 [DOI] [PubMed] [Google Scholar]
- 39.Fisher, C., Beglova, N., and Blacklow, S. C. (2006) Mol. Cell 22 277-283 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.