Abstract
The V-ATPase d2 protein constitutes an important subunit of the V-ATPase proton pump, which regulates bone homeostasis; however, currently little is known about its transcriptional regulation. Here, in an attempt to understand regulation of the V-ATPase d2 promoter, we identified the presence of NFATc1, microphthalmia-associated transcription factor (MITF)- and myocyte enhancer factor 2 (MEF2)-binding sites within the V-ATPase d2 promoter using complementary bioinformatic analyses, chromatin immunoprecipitation, and electromobility shift assay. Intriguingly, activation of the V-ATPase d2 promoter by NFATc1 was enhanced by either MEF2 or MITF overexpression. By comparison, coexpression of MITF and MEF2 did not further enhance V-ATPase d2 promoter activity above that of expression of MITF alone. Consistent with a role in transcriptional regulation, both NFATc1 and MITF proteins translocated from the cytosol to the nucleus during RANKL-induced osteoclastogenesis, whereas MEF2 persisted in the nucleus of both osteoclasts and their mononuclear precursors. Targeted mutation of the putative NFATc1-, MITF-, or MEF2-binding sites in the V-ATPase d2 promoter impaired its transcriptional activation. Additionally retroviral overexpression of MITF or MEF2 in RAW264.7 cells potentiated RANKL-induced osteoclastogenesis and V-ATPase d2 gene expression. Based on these data, we propose that MEF2 and MITF function cooperatively with NFATc1 to transactivate the V-ATPase d2 promoter during RANKL-induced osteoclastogenesis.
Bone resorbing osteoclasts are multinucleated giant cells derived from the fusion of mononuclear precursors of hematopoietic lineage (1). Excessive osteoclast activity has been linked to many common bone lytic disorders including osteoporosis, aseptic loosening, nonunion of bone allografts, and tumor-induced bone destruction (2).
The V-ATPase5 complex plays an essential role in osteoclast function and thus represents a candidate target for the treatment of lytic bone disorders (3). Structurally, the V-ATPase complex is composed of two distinct functional domains, a cytoplasmically oriented V1 domain and a membrane-bound V0 domain, and several accessory subunits, including Ac45 and M8-9 (3-5). The functional importance of V-ATPase in osteoclasts is highlighted by the finding that mutations of V-ATPase a3 gene causes infantile malignant osteopetrosis in humans (6), and a3-deficient mice exhibited severe osteopetrosis because of a defect in osteoclast-mediated extracellular acidification (7). The accessory subunit Ac45 is also required for efficient osteoclastic bone resorption (8). Furthermore, targeted disruption of the V-ATPase d2 subunit resulted in a marked increase in bone mass, surprisingly without affecting V-ATPase acidification (9). In addition, the size of osteoclasts derived from V-ATPase d2-/- mice were unexpectedly reduced, both in vivo and in vitro, presumably reflecting impaired fusion capacity of precursor cells (9). Interestingly, V-ATPase d2 gene expression has recently been shown to be regulated by NFATc1 (10), a transcription factor required for the expression of genes associated with the process of osteoclastogenesis (11, 12).
Until this study, the precise loci of the contribution of NFATc1 to V-ATPase d2 gene transactivation and the roles of additional transcription factors were unclear. Herein, we investigate the expression of the V-ATPase d2 subunit during RANKL (receptor activator of NF-κB ligand)-induced osteoclast differentiation. Using complementary EMSA, ChIP, point mutagenesis, and luciferase report assays, we demonstrate that NFATc1, MITF, and MEF2 bind directly within a 1-kb region of V-ATPase d2 promoter. Interestingly, coexpression of NFATc1 with either MITF or MEF2 potentiated whereas mutation of the putative NFATc1, MITF, or MEF2-binding sites impaired transactivation of the V-ATPase d2 promoter. Based on these data, we propose that MITF and MEF2 cooperate with NFATc1 to enhance transactivation of the V-ATPase d2 promoter during osteoclastogenesis.
EXPERIMENTAL PROCEDURES
Reagents and Materials—The primary antibodies used in this study include: a monoclonal anti-NFATc1 (7A6), polyclonal goat anti-MEF-2C (E-17) (Santa Cruz), a monoclonal anti-α-tubulin antibody (Sigma), a mouse anti-actin monoclonal antibody (Clone JLA20) (Calbiochem), a polyclonal rabbit anti-MEF2A (Abcam, 32866), a monoclonal mouse anti-MITF antibody (C5) (ABCAM) or (Neomarkers, Fremont), a polyclonal rabbit anti-VP16 tag antibody (Abcam), an anti-vesicular glutamate transporter 1 antibody (kindly provided by Prof. Reinhard Jahn), and a polyclonal rabbit anti-V-ATPase d2 antibody raised against GST-d2 peptide antigen produced in our laboratory. The secondary antibodies include: Alexa Fluor 546-conjugated goat anti-mouse IgG and Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen). The plasmids used include: pEF6 (Invitrogen), pGL3-Basic and phRL-CMV (Promega), pAd-MEF2 and pAd-MEF2C-R24L (kindly provided by Prof. Leon J. De Windt), pMSCV-caNFATc1 (kindly provided by N. A. Clipstone), and pEF6-MITF (kindly provide by Dr. Alan I. Cassady). Hoechst dye was purchased from Invitrogen. The restriction enzymes and reverse transcriptase were purchased from Promega, and DNA polymerase was from Gene-Works (Adelaide, Australia). GST-rRANKL160-318 recombinant proteins were expressed and affinity-purified in our laboratory as previously described (13).
Cell Culture and Osteoclast Formation—RAW264.7 cells were cultured in α-minimal essential medium and COS-7 cells in DMEM. Both media were supplemented with 10% fetal calf serum, 2 mm l-glutamine, and 100 units/ml penicillin/streptomycin. Medium and fetal calf serum were from Invitrogen. Cell lines were from ATCC (Manassas, VA). RAW264.7 cells were induced to differentiate into osteoclasts by the addition of GST-rRANKL (100 ng/ml) as previously described (13).
Reverse Transcription-PCR—Total RNA was extracted from cells using an RNA extraction kit (Invitrogen), and cDNAs were synthesized using Reverse Transcriptase (Promega), 1 mm dNTPs, 1 μm oligo(dT) primers, and supplied buffer, as previously described (14). PCR amplifications were carried out using the conditions and primer pairs outlined in the supplemental “Experimental Procedures.”
Western Blot Analysis—Western blotting was performed as previously described (15). In brief, proteins from whole cell extracts were separated by SDS-PAGE and electroblotted onto nitrocellulose membranes (Bio-Rad). The membranes were blocked for 2 h with 5% (w/v) nonfat milk powder in TBST (10 mm Tris, pH 7.5, 150 mm NaCl, 0.1% (v/v) Tween 20) and then probed for 1.5 h with primary antibodies appropriately diluted in the blocking solution. After three washes with TBS, the membranes were incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies diluted 1/5000 in 1% (w/v) nonfat milk powder in TBST. The membranes were washed three times in TBS and then developed using an enhanced chemiluminescence system (Amersham Biosciences).
Promoter Cloning and Site-directed Mutagenesis—A V-ATPase d2 promoter truncation series (-3203, -2109, -1009, and -609 to +1) was prepared by PCR using the following forward primers: 0.6 kb, 5′-AAGCTTGAATTCGGTACCTGTCTCAAAAACTAAGA-3′; 1 kb, 5′-AAGCTTGAATCGGTACCTGCCAGCTGTGCCTTCT-3′; 2 kb, 5′-AAGCTTGAATTCGGTACCACTATGAATGGAGAGGA-3′; 3 kb, 5′-aagcttGAATTCGGTACCAACATGGCAGCAGAACATTTCC-3′; and a common reverse primer, 5′-GAATTCAGATCTGGATCCACTGCTCAGGCTGAAGTGGC-3′. The fragments were subcloned into the pGL3-Basic luciferase reporter vector (Promega). Mutations of the putative NFAT, MITF, or MEF2 sites within the 1-kb V-ATPase d2 promoter luciferase reporter construct were generated by Mutagenex, Inc. (Piscataway, NJ) using the primers outlined in the supplemental “Experimental Procedures.” All of the constructs were confirmed by DNA sequencing.
Transfection and Luciferase Reporter Assays—RAW264.7 cells were transfected with 0.5 μg of the V-ATPase d2 luciferase reporter constructs using DEAE dextran (Sigma) as previously described (16). After transfection cells (0.2 × 106) were seeded in 0.5 ml of complete medium in 24-well plates. The transfected cells were harvested after 72 h, and the luciferase activities were measured using the Promega luciferase assay system according to the manufacturer's instructions. COS-7 cells (4 × 104/well of a 24-well plate) were transfected with 0.01 μg of a Renilla luciferase vector (phRL-CMV), 0.1 μg of 1-kb V-ATPase d2 luciferase reporter construct (wild type or one of its mutants) and 0.2 μg of expression vector(s) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. The cells were lysed 48 h after transfection, and Renilla and firefly luciferase assays performed using the Dual-Glo luciferase assay system (Promega). For each well, the firefly luciferase activity was corrected by Renilla luciferase activity to account for differences in cell numbers and transfection efficiency.
Chromatin Immunoprecipitation—ChIP assays were performed using a kit (Millipore) according to the manufacturer's instructions. In brief, RAW264.7 cells (1 × 106) were treated with RANKL for 0, 1, 3, or 5 days, fixed with 1% formaldehyde for 10 min at 37 °C, washed with phosphate-buffered saline, and then lysed with a 1% SDS, 10 mm EDTA, 50 mm Tris (pH 8.1) containing protease inhibitor mixture (Roche Applied Science). The following lysis samples were sonicated to shear DNA and cell debris removed by centrifugation. Supernatants containing the released cross-linked protein-DNA complexes were subjected to overnight immunoprecipitation at 4 °C with antibodies against NFATc1 (7A6; Santa Cruz), MITF, MEF2A, or control murine IgG (Sigma). Cross-linking between proteins and DNA was reversed by incubation at 65 °C for 4 h, and proteinase-K was added to digest protein. Following phenol/chloroform extraction DNA was precipitated with ethanol and resuspended in distilled water. Purified DNA was subjected to PCR using the following primers: 5′-AAGCTTGAATTCGGTACCTGCCAGCTGTGCCTTCT-3′ (forward) and 5′-GAATTCAGATCTGGATCCACTGCTCAGGCTGAAGTGGC-3′ (reverse), to detect the 1-kb (relative to ATG start codon) V-ATPase d2 promoter sequence.
Immunostaining and Confocal Analysis—For immunofluorescent staining, RAW264.7 cells or osteoclast-like cells cultured on glass coverslips were fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 min at room temperature and washed four times with phosphate-buffered saline. The fixed cells were permeabilized with 0.1% Triton X-100 for 5 min, washed, and then incubated for 1.5 h at room temperature with anti-MEF2 and anti-NFATc1 or anti-MITF at a final dilution of 1:200. Following extensive washing with phosphate-buffered saline containing 2% bovine serum albumin, the cells were incubated for 45 min with either goat anti-mouse Alexa Fluor 546 or both goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 546 (Invitrogen) at a final dilution of 1:500. The cell nuclei were visualized by counterstaining with Hoechst dye (1:10,000, Invitrogen). Fluorescent images were collected on a Bio-Rad MRC 1000/1024 UV Laser Scanning Confocal Microscope as previously described (17).
Transduction of Retroviral Constructs—To generate a pMX-MEF2-VP16 construct, pAd-MEF2-VP16 (18) (kind gift from Prof. Leno de Windt, Hubrecht Institute, Utrecht, The Netherlands) was digested with BglII/XhoI to release the MEF2-VP16 fragment (∼800 bp) which was then inserted into the linearized pMX puro vector (kindly provided by Prof. Kitamura, University of Tokyo) digested with BamHI/XhoI. To generate the pMX-MITF construct, pEF6-MITF (kindly provided by Dr. Alan I. Cassady, University of Queensland) was digested with BamHI/NotI, and the subsequent MITF fragment was subcloned into the pMX puro vector at the BamHI/NotI sites.
PT67 retroviral packaging cells (Clontech) were plated in DMEM (3 × 105 cells/well of a 6-well plate) the day before transfection with pMX-GFP, pMX-MITF, or pMX-MEF2D-VP16 using Lipofectamine 2000 in accordance with the manufacturer's instructions. The medium was replaced 24 h post-transfection, and 48 h later the cells were exposed to puromycin (2 μg/ml). Following four to five passages in puromycin containing medium, the selected cell lines were cultured in 10 ml of DMEM lacking puromycin at 3 × 105 cells/100-mm culture dishes for at least a further 2 days. The conditioned medium containing retroviral particles was collected, centrifuged at 1500 rpm for 5 min, and then filtered through a 0.22-μm filter (Millipore).
RAW264.7 cells (2 × 105) were seeded into 6-well plates in 2 ml of retroviral conditioned medium containing polybrene (8 μg/ml). After 36 h the conditioned medium was replaced with fresh DMEM, and the cells were allowed to recover for 48 h. Transduced cells were then selected in medium containing 4 μg/ml puromycin for 10 days, and resistant colonies were expanded. Successful transductions of GFP, MITF, and MEF2-VP16 into the cells were confirmed by Western blotting using specific antibodies before examining their ability to form osteoclasts.
RESULTS
The -1 to -1009 Region of the Mouse V-ATPase d2 Promoter Is Responsive to RANKL—To investigate transcriptional regulation of the V-ATPase d2 gene during RANKL-induced osteoclastogenesis, we generated a series of 5′-truncation V-ATPase d2 promoter luciferase reporter constructs (Fig. 1A). RAW264.7 cells, transiently transfected with these constructs, were treated with or without RANKL for 72 h, and luciferase activities were measured. RANKL enhanced transcriptional activity of each of the V-ATPase d2 promoter reporter vectors, with the greatest increase (∼2-fold) occurring in cells transfected with the 1-kb fragment of the V-ATPase d2 promoter (Fig. 1B). The relatively weaker activity of the longer promoter reporters suggests the presence of repressor elements in upstream regions of the V-ATPase d2 promoter.
FIGURE 1.
The V-ATPase d2 promoter is activated in RAW264.7 cells by RANKL. A, schematic depiction of 5′ deletion V-ATPase d2 promoter luciferase reporter constructs. Promoter fragments upstream of the V-ATPase d2 transcriptional start site: -609 to -1 (0.6 kb), -1109 to -1 (1 kb), -2009 to -1 (2.0 kb), and -3009 to -1 (3.0 kb) were subcloned into the pGL3-Basic luciferase reporter vector. B, RAW264.7 cells were transiently transfected, using DEAE dextran, with 0.5 μg of the V-ATPase d2 promoter luciferase reporter constructs, and luciferase activities were measured after 72 h of culture in the absence or presence of 100 ng/ml RANKL. The bars represent fold change in promoter activity following RANKL stimulation. The data are the means ± S.E. of representative results from three independent experiments. C, bioinformatic analysis of the 1-kb V-ATPase d2 promoter. The transcription element search software analysis identified putative NFAT (N1, N2, and N3)-, MITF (M1, M2, and M3)-, and MEF2 (E1 and E2)-binding sites (sequences underlined).
Having established that RANKL transcriptionally activates the V-ATPase d2 promoter, we next employed the transcription element search software to screen for candidate transcriptional binding elements within the 1-kb V-ATPase d2 promoter. Several putative transcription factor-binding sites were identified including three putative NFAT transcription-binding sites (N1, N2, and N3), three putative MITF-binding sites (M1, M2, and M3), and two putative MEF2 sites (E1 and E2) (Fig. 1C).
Expression of V-ATPase d2, NFAT, MITF, and MEF2 during RANKL-induced Osteoclastogenesis—The mRNAs for V-ATPase d2, calcitonin receptor, and cathepsin K (CATH K), markers of osteoclastogenesis, were strongly up-regulated in RAW264.7 cells following RANKL treatment (Fig. 2A). Pro-osteoclastogenic M-CSF and tumor necrosis factor-α also weakly increased V-ATPase d2 mRNA expression as compared with RANKL (10-fold), whereas lipopolysaccharide treatment had no effect (supplemental Fig. S1). NFATc1 mRNA was significantly induced by RANKL but hardly at all by M-CSF and tumor necrosis factor-α treatment (Fig. 2A and supplemental Fig. S1). Interestingly, NFATc2 and NFATc3 were induced by M-CSF, tumor necrosis factor-α, and lipopolysaccharide but not by RANKL stimulation (supplemental Fig. S1). These findings are consistent previous reports indicating that NFATc1 is largely regulated by RANKL during osteoclastogenesis (12, 19). To explore the effect of NFATc1 on V-ATPase d2 gene regulation, we examined the effect of cyclosporine A, an established NFAT inhibitor, on V-ATPase d2 expression in RAW264.7 cells in the presence or absence of RANKL. Cyclosporine A potently suppressed RANKL-induced V-ATPase d2 mRNA expression (supplemental Fig. S1). Furthermore, cyclosporine A also appeared to inhibit the basal expression of the V-ATPase d2 and CATH K genes. Taken together, these data support the view that NFATc1 is an important regulator of V-ATPase d2 transcription. Transcripts for the MITF and MEF2A, C and D genes were present in RAW264.7 cells and did not change during osteoclastogenesis, whereas those of MEF2B were up-regulated by RANKL stimulation, albeit weakly (Fig. 2A).
FIGURE 2.
NFAT, MITF and MEF2 bind to the endogenous V-ATPase d2 promoter and lead to its activation. A, expression profile of the d2, NFATc1, MITF, and MEF2 genes during osteoclast formation. RNA from RAW264.7 cells treated with RANKL for 0 and 4 h and 1, 3, and 5 days were reverse transcribed into cDNA, and 30 cycles of PCR were performed using specific primers for d2, NFATc1, MITF, and MEF2 (isoforms A, B, C, and D), calcitonin receptor (CTR), CATH K, and 36B4. B, upper panel, Western blot analysis of the V-ATPase d2, MITF, and MEF2 proteins during osteoclastogenesis. Vesicular glutamate transporter 1 detection was used as a positive control and α-tubulin as a loading control. Lower panel, phase contrast micrographs of 0 (MOCK) and 5-day RANKL-treated cultures with and without TRAP staining. C, ChIP analysis of the binding of NFATc1, MITF, and MEF2 to the V-ATPase d2 promoter. Following culture in the absence (RAW) or presence (OC) of 100 ng/ml RANKL for up to 5 days, the cells were fixed, and cross-linked protein-chromatin complexes were treated with anti-NFATc1, anti-MITF, anti-MEF2, or isotype matched (IgG) control antibodies. Precipitated DNA was then subjected to PCR using primers directed against the 1-kb V-ATPase d2 promoter region, and products were visualized as described above. D, COS-7 cells were cotransfected with 100 ng of the V-ATPase d2 luciferase reporter construct, 10 ng of the Renilla luciferase reporter construct (phRL-CMV) to correct for transfection efficiency, and 200 ng of constitutively active NFATc1, MITF, or MEF2 overexpression vector. At 72 h post-transfection, firefly and Renilla luciferase activities were assayed. The results are expressed as fold increase over control (firefly luciferase corrected by Renilla luciferase) activity. Each bar is the mean ± S.E. of three independent experiments. **, p < 0.05 compared with control.
To examine whether protein levels reflected the observed mRNA levels during RANKL induced differentiation of RAW264.7 cells, we performed Western blot analysis on whole cell extracts. An affinity-purified rabbit polyclonal antibody specific for the V-ATPase d2 isoform revealed an immunoreactive polypeptide, corresponding to the predicted size (∼38 kDa) of V-ATPase d2, following 3 and 5 days of RANKL stimulation (Fig. 2B). Similar up-regulation of the RANKL-regulated osteoclast marker, vesicular glutamate transporter 1 protein (20) was observed, whereas α-tubulin levels (the loading control) remained constant. The expression of NFATc1 protein peaked at day 3 and decreased after 5 days of RANKL treatment, whereas levels of MITF protein remained constant. Interestingly, the expression of MEF2A and MEF2C proteins appeared to fluctuate during osteoclast differentiation (Fig. 2B).
NFATc1, MEF2, and MITF Bind to the V-ATPase d2 Promoter, and Their Overexpression Leads to Its Activation—To determine whether NFATc1, MITF, and MEF2 were capable of binding to the -1 to -1009 region of the V-ATPase d2 promoter during osteoclastogenesis, ChIP assays were performed. There was a dramatic increase in NFATc1 and MITF binding to the 1-kb region of the V-ATPase d2 promoter region following 5 days of RANKL-induced differentiation of RAW264.7 cells (Fig. 2C, left and middle panels). However, the strong constitutive binding of MEF2 to the 1-kb V-ATPase d2 promoter region was unaltered by up to 5 days of RANKL treatment (Fig. 2C, right panel). No PCR products were observed from DNA precipitated by any of the isotype matched control antibodies.
To confirm that NFATc1, MITF, and MEF2 were capable of activating the V-ATPase d2 promoter, each of these putative transcriptional activators was transfected into COS-7 cells together with the 1-kb V-ATPase d2 promoter luciferase reporter and a CMV-driven Renilla luciferase reporter. NFATc1, MITF, and MEF2 each increased (4-, 14-, and 2-fold, respectively) transcription from the 1-kb V-ATPase d2 promoter construct (Fig. 2D).
MEF2 and MITF Cooperate with NFATc1 to Transactivate the 1-kb V-ATPase d2 Promoter—To determine whether NFATc1, MEF2, and MITF were able to cooperate in the activation of the 1-kb V-ATPase d2 promoter, we compared the effect of overexpressing each transcription factor alone with overexpression of various combinations of transcription factors. Cotransfection of MITF and MEF2 together with the 1-kb V-ATPase d2 luciferase reporter construct into COS-7 cells did not induce any greater transactivation of the 1-kb V-ATPase d2 promoter than seen with MITF alone (Fig. 3A, second and fourth bars). A dominant negative form of MEF2 (MEF2 R24L), which cannot bind to DNA but retains its dimerization ability (21), reversed MEF2 transactivation capacity (Fig. 3A, third and fifth bars). However, MEF2 R24L did not affect MITF transactivating potential, implying that MEF2 and MITF did not exhibit a coactivation effect.
FIGURE 3.
MEF2 and MITF cooperate with NFATc1 to activate the 1-kb V-ATPase d2 promoter. COS-7 cells were cotransfected with the 1-kb V-ATPase d2 luciferase reporter construct (100 ng) and combinations of NFATc1, MITF, MEF2 wild type, or the dominant negative MEF2 R2L expression vectors as displayed in each panel. Expression vectors were transfected at 200 ng/vector, and the total amount of DNA transfected was kept constant at 500 ng with empty expression vector DNA. The bars are means ± S.E. of at least three independent experiments. A, MEF2 does not cooperate with MITF to activate the 1-kb V-ATPase d2 promoter. B, MITF cooperates with NFATc1 to activate the 1-kb V-ATPase d2 promoter. C, MEF2 cooperates with NFATc1 to activate the 1-kb V-ATPase d2 promoter. ca, constitutively active.
Remarkably, cotransfection of NFATc1 and MITF resulted in enhanced transcriptional induction of the V-ATPase d2 promoter compared with the overexpression of NFATc1 (from ∼6- to 26-fold; Fig. 3B, second and fourth bars) or MITF (from ∼14- to 26-fold; Fig. 3B, third and fourth bars) alone. Intriguingly, cotransfection of NFATc1 and MEF2 also resulted in enhanced transcriptional induction of the V-ATPase d2 promoter (from ∼6- to 11-fold; Fig. 3C, second and fourth bars) compared with the overexpression of NFATc1 alone. The dominant negative form of MEF2 (MEF2 R24L) failed to enhance V-ATPase d2 promoter activity alone (Fig. 3C, first and fifth bars) or cooperate with NFATc1 on the V-ATPase d2 promoter (Fig. 3C, second and sixth bars).
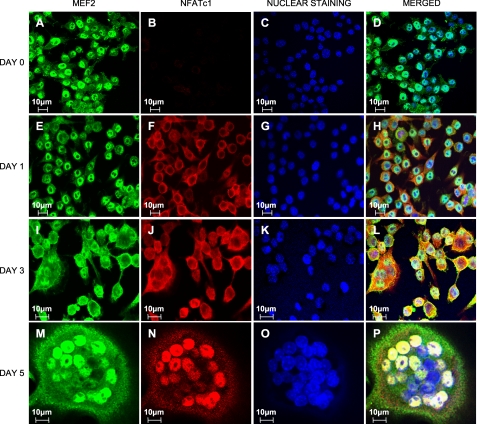
Colocalization of NFATc1 and MEF2 and Translocation of MITF to the Nucleus Following RANKL-induced Osteoclastogenesis—Given our novel observations that NFATc1 and MEF2 can cooperate to activate the V-ATPase d2 promoter, we next examined the intracellular localization of MEF2 with NFATc1 during osteoclastogenesis. For this purpose, RAW264.7 cells were treated with RANKL for 0, 1, 3, and 5 days, then fixed, and incubated with antibodies against MEF2 and NFATc1. Confocal microscopy revealed that at all time points studied MEF2 was largely localized in the nuclear compartment (Fig. 4, A, E, I, and M). By comparison, during the course of RANKL stimulation, NFATc1 progressively translocated from the cytosol to the nucleus with nearly complete nuclear localization after 5 days (Fig. 4, D, H, L, and P). The colocalization of nuclear NFATc1 and MEF2 immunostaining is consistent with the notion that MEF2 and NFATc1 cooperatively regulate V-ATPase d2 transcription during osteoclast differentiation.
FIGURE 4.
NFATc1 and MEF2 colocalize during RANKL-induced osteoclast formation. RAW264.7 cells seeded on glass slips were stimulated with RANKL for 0, 1, 3, and 5 days. The cells were fixed and exposed to anti-MEF2 (green) and anti-NFATc1 (red) antibodies before being subjected to fluorescent-conjugated secondary antibodies. The cell nuclei were stained with Hoechst 33258 (blue). The images were recorded using a confocal laser scanning microscope (MRC-1000; Bio-Rad). The cells exposed to secondary antibody only served as negative controls and demonstrated no fluorescent signals.
We also examined the localization of MITF in osteoclasts and their precursor cells. RAW264.7 cells were treated with RANKL for 5 days or left untreated, fixed, and incubated with an antibody to MITF. Confocal analysis revealed that MITF was predominantly localized in the cytoplasm of untreated cells (supplemental Fig. S2) but translocated into the nucleus in osteoclast-like cells (supplemental Fig. S2). Studies on the colocalization of MITF and NFATc1 could not be conducted because only murine monoclonal MITF and NFATc1 antibodies were available.
Mapping the NFATc1, MITF, and MEF2-binding Sites within the V-ATPase d2 Promoter—To identify the specific NFATc1-binding sites responsible for transcriptional regulation of the V-ATPase d2 promoter, we employed EMSA. Nuclear extracts from control and RANKL-treated RAW264.7 cells were incubated with 32P-labeled double-stranded DNA fragments overlapping the putative NFAT-binding sites (N1, N2, or N3; Fig. 1C) and then run on a gel. Retarded complexes (two) could be seen with the N2 and N3 but not the N1 probes. Complexes bound more strongly to the N2 than the N3 probe and were more prominent from RANKL-treated than control nuclear extracts (supplemental Fig. S3). Excess (×100) unlabeled N2 probe out competed binding to labeled N2 probe for each of the two complexes (supplemental Fig. S3). An antibody to NFATc1 significantly diminished the intensity of each of the retarded bands, producing a faint supershifted complex irrespective of whether binding was conducted at 4 or 37 °C (supplemental Fig. S3). These data demonstrate the existence of a strong NFATc1-binding site (N2) at -555 to -561 and a weaker (N3) NFATc1-binding site at -319 to -324 within the first 1 kb of the V-ATPase d2 promoter region.
To determine the impact of the N2 and N3 sites on NFATc1-induced V-ATPase d2 transactivation, we generated 1-kb V-ATPase d2 luciferase reporter constructs with mutations in the N2 (M(N2)), N3 (M(N3)), or N1-N3 (M(N1-3)) sites (Fig. 5A, upper panel). COS-7 cells were then cotransfected with either a wild type, M(N2), M(N3), or M(N1-N3) 1-kb V-ATPase d2 luciferase reporter and a constitutively active form of NFATc1. Overexpression of NFATc1 increased transcription 4-fold from the wild type 1-kb V-ATPase d2 promoter, whereas transcription from the mutated promoters (M(N2), M(N3), and M(N1-3)) was at a similar level of the control unstimulated promoter (Fig. 5A, lower panel). Interestingly, no significant difference was evident between reporter constructs with mutations at N2, N3, and N1-3, suggesting that both the N2 and N3 NFATc1-binding sites are required to transactivate the V-ATPase d2 promoter.
FIGURE 5.
Mapping the NFATc1-, MITF-, and MEF2-binding sites within the V-ATPase d 2 promoter. A, mutation of the putative NFAT-binding sites within the 1-kb V-ATPase d2 promoter suppresses NFATc1-induced transcriptional activity. Upper panel, schematic representation of the mutations M(N2), M(N3), and M(N1-3) of the putative NFAT-binding sites within the 1-kb V-ATPase d2 promoter luciferase reporter construct. Lower panel, COS-7 cells were cotransfected with the 1-kb V-ATPase d2 luciferase reporter constructs (100 ng), CMV driven Renilla reporter construct (10 ng), and 200 ng of NFATc1 expression vector or empty overexpression vector (CTRL). The total amount of DNA transfected was kept constant at 500 ng with empty expression vector DNA. At 72 h post-transfection, firefly luciferase activities were measured. The activities are expressed as fold increases in activity after correcting by Renilla reporter activity. Each bar is the mean ± S.E. of three independent experiments. B, mutation of the putative MITF-binding sites within the 1-kb V-ATPase d2 promoter diminishes MITF-induced transcriptional activity. Upper panel, schematic representation of the mutations M(M1), M(M2), M(M3), and M(M1-3) of the putative MITF-binding sites within the 1-kb V-ATPase d2 promoter luciferase reporter construct. Lower panel, as in A except that the MITF overexpression vector was used. C, mutation of putative MEF2-binding sites within the 1-kb V-ATPase d2 promoter diminishes ability of MEF2 to enhance NFATc1 transcriptional activity. Upper panel, schematic representation of the 1-kb V-ATPase d2 promoter luciferase construct without (WT) or with mutations of the putative MEF2-binding sites M(E1), M(E2), or M(E1-2). Lower panel, COS-7 cells were cotransfected with the 1-kb V-ATPase d2 luciferase constructs (100 ng) and either NFATc1 (200 ng), MEF2 (200 ng), or NFATc1 with MEF2 expression vectors together. The total amount of DNA transfected was kept constant at 500 ng with empty expression vector DNA. Luciferase activities were measure 72 h post-transfection. The bars represent the means ± S.E. of at least three independent experiments, and statistical analysis was carried out using analysis of variance (#, p < 0.05 compared with control. Response of M(E1), M(E2), and M(E1-2) did not differ significantly from controls. **, p < 0.01 and ***, p < 0.001 compared with wild type cotransfected with CaNFATc1 and MEF2).
In addition, RAW264.7 cells were transfected with the 1-kb V-ATPase d2 reporter constructs (either wild type, M(N2), M(N3), or M(N1-3)) and treated with RANKL for 3 days. RANKL strongly transactivated the wild type 1-kb V-ATPase d2 promoter, whereas it poorly transactivated the M(N2), M(N3), and M(N1-3) 1-kb promoters (supplemental Fig. S4). Collectively, these data suggest that the N2 and N3 sites are functional and are likely to be responsible for up-regulation of transcription of the V-ATPase d2 gene by NFAT during RANKL-induced osteoclastogenesis.
Next, we used similar approaches to map the MITF-binding site in the d2 promoter. Probes spanning the three putative MITF sites (M1, M2, and M3; Fig. 1C) each formed retarded complexes in EMSA with nuclear extracts from control and RANKL-treated RAW264.7 cells. Only complexes binding to the M2 probe increased in intensity with RANKL treatment (data not shown). Excess unlabeled probe competed for binding factors in these nuclear extracts, whereas probes with mutations in the MITF-like binding region failed to compete. However, we were unable to obtain successful supershifts of the retarded complexes because of the lack of suitable anti-MITF antibodies for the EMSA assay (data not shown).
To further examine a potential role for the M1, M2, and M3 sites in MITF-induced V-ATPase d2 transactivation, reporter constructs with mutations in the M1 (M(M1)), M2 (M(M2)), M3 (M(M3)), or all three sites (M(M1-3)) sites were made (Fig. 5B, upper panel). COS-7 cells were then cotransfected with either the wild type 1-kb V-ATPase d2 luciferase reporter or one of the MITF mutant reporters and MITF. Overexpression of MITF strongly transactivated the wild type 1-kb V-ATPase d2 promoter, whereas it poorly transactivate the M(M1), M(M2), M(M3), and M(M1-3) 1-kb promoters (Fig. 5B, lower panel). The similar degree of suppression that each of the MITF site mutations had on MITF-induced enhancement of the 1-kb V-ATPase d2 promoter suggests that each of the three MITF sites are required for transactivation of the V-ATPase d2 promoter.
To define the MEF2-binding sites within the 1-kb V-ATPase d2 promoter region, EMSA assays were performed with probes spanning the putative MEF2 E1 (-336 to -346) and E2 (-563 to -573) sites (supplemental Fig. S5). Both probes bound to factors in nuclear extracts from unstimulated and RANKL-treated RAW264.7 cells, with the E1 probe, adjacent to the -555 to -561 NFATc1 site, demonstrating the stronger binding (supplemental Fig. S5). There was no difference in the amount of complexes formed on either of the MEF2 probes with nuclear extracts from control or RANKL-treated RAW264.7 cells. Complexes bound to the MEF2 E1 or E2 probes were diminished by competition with unlabeled probe but were less affected by competition with unlabeled probes that had their MEF2 site mutated (supplemental Fig. S5). Supershift experiments were carried out with anti-MEF2A, which also binds MEF2C, and a weak supershifted band was evident for complexes bound to either the E1 or E2 probe (supplemental Fig. S5). In contrast, an isotype-matched control antibody did not show any supershifted band (supplemental Fig. S5).
To demonstrate the functionality of the MEF2 sites, 1-kb V-ATPase d2 luciferase reporter vectors with mutations of the E1, E2, or both sites were constructed (Fig. 5C). Mutation of either or both MEF2 sites blocked the induction of transcription from the 1-kb V-ATPase d2 promoter in COS-7 cells overexpressing MEF2 (Fig. 5C). Furthermore, mutations of the E1, E2, or both MEF2 sites impaired the coactivation of the V-ATPase d2 promoter by MEF2 and NFATc1 (Fig. 5C). Taken together, we have provided fine mapping and functionality of the NFATc1-, MITF-, and MEF2-binding sites within the V-ATPase d2 promoter.
Overexpression of MITF and MEF2 Induces the Expression of the V-ATPase d2 Gene and Potentiates Osteoclastogenesis—Given that the role of NFAT in V-ATPase d2 gene expression and osteoclastogenesis has been recently documented (10), we next focused on examining the effect of overexpressing MITF or MEF2 on V-ATPase d2 gene expression and osteoclastogenesis. To this end, we generated retroviral expression constructs pMX-MITF and pMX-MEF2-VP16, a constitutively active form of MEF2. Following transduction into RAW264.7 cells and selection with puromycin, the overexpression of MITF and MEF2-VP16 was demonstrated by Western blot analysis (Fig. 6A). Notably, RAW264.7 cells transduced with either MITF or pMX-MEF2-VP16 and then treated with RANKL demonstrated enhanced osteoclastogenic potential with an increase in the number of multinucleated TRAP-positive cells (Fig. 6B). In addition, reverse transcription-PCR analysis revealed that RAW264.7 cells transduced with MITF or pMX-MEF2-VP16 expressed higher levels of mRNA for V-ATPase d2, TRAP, and CATH K compared with cells transduced with GFP, both in the absence (basal level) and/or presence of RANKL (Fig. 6C). Collectively, these data provide evidence that MEF2 and MITF play an important role in V-ATPase d2 gene expression and osteoclastogenesis.
FIGURE 6.
Overexpression of MITF or MEF2 promotes osteoclastogenesis and up-regulates expression of the d2, TRAP, and cathepsin K genes. A, Western blot analysis showing the overexpression of pMX-GFP, pMX-MITF, and pMX-MEF2-VP16 (lanes 1, 2, and 3, respectively) in RAW264.7 cells. B, MITF or MEF2 overexpression enhances osteoclast formation. RAW264.7 cells stably transduced with pMX-GFP, pMX-MITF, or pMX-MEF2-VP16 were cultured in the presence of 100 ng/ml RANKL for 5 days to form multinucleated osteoclast-like cells. The bars represent the number of TRAP-positive cells with greater than three nuclei (means ± S.E. from randomly selected fields). C, MITF or MEF2 overexpression induces enhanced expression of the V-ATPase d2, TRAP, and CATH K genes. Upper panel, RNA was extracted from RAW264.7 cells treated as in B and reverse transcribed into cDNA. Following 30 cycles of PCR using specific primers for V-ATPase d2, TRAP, cathepsin K, and 36B4 products were separated on agarose gels, stained with ethidium bromide, and photographed. Lower panels, PCR products were quantified by densitometry and normalized to 36B4 expression. #, p < 0.05 compared with basal level of GFP control. **, p < 0.05 compared with RANKL-treated GFP control.
DISCUSSION
Genetic studies have recently uncovered the crucial importance of the V-ATPase d2 subunit in maintaining physiological bone homeostasis. Mice lacking the d2 gene exhibiting a phenotype of enhanced bone mass with defects in osteoclast fusion but not V-ATPase activity (9). Although the precise molecular mechanisms underlying this phenomenon are currently unclear, this finding implies an unconventional role for the V-ATPase d2 subunit in preosteoclastic fusion during osteoclastogenesis (9). Our study provides novel insights into the molecular machinery involved in the activation of V-ATPase d2 gene expression via the collaboration of MEF2 and MITF with NFATc1 during RANKL-induced osteoclastogenesis.
During osteoclastogenesis, RANKL activates signaling of several transcription factors including NF-κB, c-Fos, MITF, PU-1, and NFATc1, each indispensable for osteoclast development (1, 22). For example, embryonic stem cells lacking NFATc1 fail to differentiate into osteoclasts (12). On the other hand, ectopic expression of a constitutively active form of NFATc1 is able to induce osteoclast formation in the absence of RANKL (11, 12). Recent studies by Yamashita et al. (23) hinted that NFATc1 induction occurred downstream of NF-κB p50 and p52 activation following RANKL-induced signaling in osteoclasts because overexpression of NFATc1 alone was sufficient to rescue osteoclastogenesis from p50/p52-deficient cells (23). Based on these findings, NFATc1 has been suggested to be a master regulator of RANKL-induced osteoclast differentiation (12).
There is now accumulating evidence that NFATc1 cooperates with other transcription factors such as MITF, PU.1, and p38 to regulate osteoclast genes including TRAP, CATH K, calcitonin receptor, DC-STAMP, OSCAR, and human integrin β3 (19, 24-28). A recent study by Kim et al. (10) has also revealed that NFATc1 regulates the 5-kb promoter region of V-ATPase d2. Our study further supports and extends the importance of NFATc1 in the regulation of induction of the V-ATPase d2 gene. Moreover, using a series of complementary molecular, biochemical, and morphological assays, we have now identified and mapped the precise NFATc1-binding sites within the V-ATPase d2 promoter.
The NFATc1 and V-ATPase d2 proteins were up-regulated during the first 3 days of RANKL treatment; however, beyond this time point the expression of the former protein diminished, whereas that of the latter was sustained. The exact reason for the differing expression profiles is unclear, but it suggests that factors other than NFATc1 contribute to the transcriptional regulation of the V-ATPase d2 gene. Indeed our bioinformatic analyses, for the first time, identify the existence of putative MEF2- and MITF-binding sites within the 1-kb V-ATPase promoter. We demonstrate that NFATc1-induced activation of the V-ATPase d2 promoter is enhanced in the presence of MITF, which has been shown to regulate the promoters of CATH K (29) and CLC-7 (27). Unfortunately, at this stage we have been unable to conclusively demonstrate the binding of MITF to putative binding sites within the V-ATPase d2 promoter by EMSA because of a lack of suitable reagents. Despite this, our CHIP and V-ATPase d2 promoter luciferase reporter assays clearly demonstrate functional binding, thus supporting the view that MITF cooperates with NFATc1 to regulate V-ATPase d2 promoter activity.
We also report the novel finding that NFATc1-induced activation of the V-ATPase d2 promoter is enhanced by overexpression of MEF. Although the MEF2 family was originally identified and named as a result of their role in muscle differentiation (30), they are not restricted to that tissue. It has been known for more than a decade that MEF2 members are abundantly expressed in neurons, where their contributions to the development and function of the nervous system are currently being elucidated (31). More recently, MEF2C has been described as an early regulator of chondrocyte hypertrophy and subsequent growth plate maturation (32).
Calcineurin has been shown to dephosphorylate MEF2 directly (33, 34), thereby regulating its activity. It has also been suggested that calcineurin may regulate the association of MEF2 with specific enhancer elements, as well as with transcriptional coactivators and corepressors to generate transcriptional outcomes tuned to specific patterns of calcium flux (30, 31). Moreover, MEF2 and NFAT3 have been shown to cooperatively activate several genes by binding adjacent sites, raising the possibility that they converge on common downstream target genes in pathways leading to muscle hypertrophy (33). Our data extend observations on the cooperative action of MEF2 and NFAT proteins (namely NFATc1) to osteoclasts and in particular to RANKL-regulated genes such as the d2 subunit of the V-ATPase proton pump.
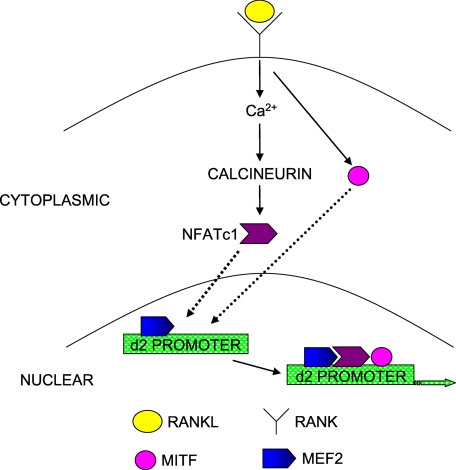
In conclusion, our data support an important role for NFATc1 in RANKL-induced activation of V-ATPase d2 gene transcription. Additionally, we propose a model of cooperation between MITF and NFATc1 or MEF2 and NFATc1 in the transactivation of the V-ATPase d2 promoter (Fig. 7). Deci-phering the biological mechanisms regulating V-ATPase d2 expression will provide important new insights into their enigmatic roles in osteoclast differentiation, potentially uncovering novel therapeutic targets for the treatment and alleviation of osteoclast-related bone diseases such as osteoporosis.
FIGURE 7.
Hypothetical model of the cooperation between MITF, MEF2, and NFATc1 in driving d2 gene expression following RANKL stimulation.
Supplementary Material
Acknowledgments
We thank Prof. Leon J. De Windt, Dr. Alan I. Cassady, Dr. N. A. Clipstone, and Prof. Reinhard Jahn for generously providing reagents.
This work was supported by the National Health and Medical Research Council of Australia.
The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. S1-S5.
Footnotes
The abbreviations used are: V-ATPase, vacuolar H+-adenosine triphosphatase; NFAT, nuclear factor of activated T cells; CATH K, cathepsin K; TRAP, tartrate-resistant acid phosphatase; GST, glutathione S-transferase; M-CSF, macrophage-colony stimulating factor; MEF2, myocyte enhancer factor 2; MITF, microphthalmia-associated transcription factor; EMSA, electromobility shift assay; ChIP, chromatin immunoprecipitation; DMEM, Dulbecco's modified Eagle's medium; CMV, cytomegalovirus; GFP, green fluorescent protein.
References
- 1.Teitelbaum, S. L. (2000) Science 289 1504-1508 [DOI] [PubMed] [Google Scholar]
- 2.Phan, T. C., Xu, J., and Zheng, M. H. (2004) Histol. Histopathol. 19 1325-1344 [DOI] [PubMed] [Google Scholar]
- 3.Xu, J., Cheng, T., Feng, H. T., Pavlos, N. J., and Zheng, M. H. (2007) Histol. Histopathol. 22 443-454 [DOI] [PubMed] [Google Scholar]
- 4.Forgac, M. (1999) J. Bioenerg. Biomembr. 31 57-65 [DOI] [PubMed] [Google Scholar]
- 5.Stevens, T. H., and Forgac, M. (1997) Annu. Rev. Cell Dev. Biol. 13 779-808 [DOI] [PubMed] [Google Scholar]
- 6.Kornak, U., Schulz, A., Friedrich, W., Uhlhaas, S., Kremens, B., Voit, T., Hasan, C., Bode, U., Jentsch, T. J., and Kubisch, C. (2000) Hum. Mol. Genet. 9 2059-2063 [DOI] [PubMed] [Google Scholar]
- 7.Li, Y. P., Chen, W., Liang, Y., Li, E., and Stashenko, P. (1999) Nat. Genet. 23 447-451 [DOI] [PubMed] [Google Scholar]
- 8.Feng, H., Cheng, T., Pavlos, N. J., Yip, K. H., Carrello, A., Seeber, R., Eidne, K., Zheng, M. H., and Xu, J. (2008) J. Biol. Chem. 283 13194-13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, S. H., Rho, J., Jeong, D., Sul, J. Y., Kim, T., Kim, N., Kang, J. S., Miyamoto, T., Suda, T., Lee, S. K., Pignolo, R. J., Koczon-Jaremko, B., Lorenzo, J., and Choi, Y. (2006) Nat. Med. 12 1403-1409 [DOI] [PubMed] [Google Scholar]
- 10.Kim, K., Lee, S. H., Ha Kim, J., Choi, Y., and Kim, N. (2008) Mol. Endocrinol. 22 176-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirotani, H., Tuohy, N. A., Woo, J. T., Stern, P. H., and Clipstone, N. A. (2004) J. Biol. Chem. 279 13984-13992 [DOI] [PubMed] [Google Scholar]
- 12.Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., Saiura, A., Isobe, M., Yokochi, T., Inoue, J., Wagner, E. F., Mak, T. W., Kodama, T., and Taniguchi, T. (2002) Dev. Cell 3 889-901 [DOI] [PubMed] [Google Scholar]
- 13.Xu, J., Tan, J. W., Huang, L., Gao, X. H., Laird, R., Liu, D., Wysocki, S., and Zheng, M. H. (2000) J. Bone Miner. Res. 15 2178-2186 [DOI] [PubMed] [Google Scholar]
- 14.Wang, C., Steer, J. H., Joyce, D. A., Yip, K. H., Zheng, M. H., and Xu, J. (2003) J. Bone Miner. Res. 18 2159-2168 [DOI] [PubMed] [Google Scholar]
- 15.Yip, K. H., Zheng, M. H., Steer, J. H., Giardina, T. M., Han, R., Lo, S. Z., Bakker, A. J., Cassady, A. I., Joyce, D. A., and Xu, J. (2005) J. Bone Miner. Res. 20 1462-1471 [DOI] [PubMed] [Google Scholar]
- 16.Yip, K. H., Zheng, M. H., Feng, H. T., Steer, J. H., Joyce, D. A., and Xu, J. (2004) J. Bone Miner. Res. 19 1905-1916 [DOI] [PubMed] [Google Scholar]
- 17.Yip, K. H., Feng, H., Pavlos, N. J., Zheng, M. H., and Xu, J. (2006) Am. J. Pathol. 169 503-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Oort, R. J., van Rooij, E., Bourajjaj, M., Schimmel, J., Jansen, M. A., van der Nagel, R., Doevendans, P. A., Schneider, M. D., van Echteld, C. J., and De Windt, L. J. (2006) Circulation 114 298-308 [DOI] [PubMed] [Google Scholar]
- 19.Kim, K., Kim, J. H., Lee, J., Jin, H. M., Lee, S. H., Fisher, D. E., Kook, H., Kim, K. K., Choi, Y., and Kim, N. (2005) J. Biol. Chem. 280 35209-35216 [DOI] [PubMed] [Google Scholar]
- 20.Morimoto, R., Uehara, S., Yatsushiro, S., Juge, N., Hua, Z., Senoh, S., Echigo, N., Hayashi, M., Mizoguchi, T., Ninomiya, T., Udagawa, N., Omote, H., Yamamoto, A., Edwards, R. H., and Moriyama, Y. (2006) EMBO J. 25 4175-4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molkentin, J. D., Black, B. L., Martin, J. F., and Olson, E. N. (1996) Mol. Cell. Biol. 16 2627-2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003) Nature 423 337-342 [DOI] [PubMed] [Google Scholar]
- 23.Yamashita, T., Yao, Z., Li, F., Zhang, Q., Badell, I. R., Schwarz, E. M., Takeshita, S., Wagner, E. F., Noda, M., Matsuo, K., Xing, L., and Boyce, B. F. (2007) J. Biol. Chem. 282 18245-18253 [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto, M., Kogawa, M., Wada, S., Takayanagi, H., Tsujimoto, M., Katayama, S., Hisatake, K., and Nogi, Y. (2004) J. Biol. Chem. 279 45969-45979 [DOI] [PubMed] [Google Scholar]
- 25.Crotti, T. N., Flannery, M., Walsh, N. C., Fleming, J. D., Goldring, S. R., and McHugh, K. P. (2006) Gene (Amst.) 372 92-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi, M., Ninomiya, K., Fujita, N., Suzuki, T., Iwasaki, R., Morita, K., Hosogane, N., Matsuo, K., Toyama, Y., Suda, T., and Miyamoto, T. (2007) J. Bone Miner. Res. 22 992-1001 [DOI] [PubMed] [Google Scholar]
- 27.Meadows, N. A., Sharma, S. M., Faulkner, G. J., Ostrowski, M. C., Hume, D. A., and Cassady, A. I. (2007) J. Biol. Chem. 282 1891-1904 [DOI] [PubMed] [Google Scholar]
- 28.Beranger, G. E., Momier, D., Guigonis, J. M., Samson, M., Carle, G. F., and Scimeca, J. C. (2007) J. Bone Miner. Res. 22 975-983 [DOI] [PubMed] [Google Scholar]
- 29.Motyckova, G., Weilbaecher, K. N., Horstmann, M., Rieman, D. J., Fisher, D. Z., and Fisher, D. E. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 5798-5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black, B. L., and Olson, E. N. (1998) Annu. Rev. Cell Dev. Biol. 14 167-196 [DOI] [PubMed] [Google Scholar]
- 31.Heidenreich, K. A., and Linseman, D. A. (2004) Mol. Neurobiol. 29 155-166 [DOI] [PubMed] [Google Scholar]
- 32.Arnold, M. A., Kim, Y., Czubryt, M. P., Phan, D., McAnally, J., Qi, X., Shelton, J. M., Richardson, J. A., Bassel-Duby, R., and Olson, E. N. (2007) Dev. Cell 12 377-389 [DOI] [PubMed] [Google Scholar]
- 33.Passier, R., Zeng, H., Frey, N., Naya, F. J., Nicol, R. L., McKinsey, T. A., Overbeek, P., Richardson, J. A., Grant, S. R., and Olson, E. N. (2000) J. Clin. Investig. 105 1395-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, H., Rothermel, B., Kanatous, S., Rosenberg, P., Naya, F. J., Shelton, J. M., Hutcheson, K. A., DiMaio, J. M., Olson, E. N., Bassel-Duby, R., and Williams, R. S. (2001) EMBO J. 20 6414-6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.