Abstract
The syntheses and characterization of several complexes of Mn(II) with cyclen derivatives having variable numbers of pendant N-acetic acid or N-acetamide arms are reported. X-ray crystallographic results are presented for Mn(DOTAM)Cl2·2H2O (monoclinic C2/c, a=18.5798(15), b=13.6006(11), c=10.5800(8) Å, β=110.490(1)°, Z=4), [Mn(DO3AM)][MnCl4]·EtOH (monoclinic P21/n, a=8.366(8), b=19.483(2), c=16.3627(16) Å, β=99.254(2)°, Z=4), and Mn(H2DOTA) (monoclinic C2/c, a=16.374(3), b=6.6559(13), c=16.750(3) Å, β=98.381(3)°, Z=4), which exhibit 8-, 7-, and 6-coordinate Mn(II), respectively. 1H relaxivity data in water at 20 MHz and 37°C is presented and interpreted in terms of a mechanism involving transient binding of water in an associative intermediate. Relaxivity studies in mixed water/methanol solvents are consistent with this interpretation.
INTRODUCTION
Manganese(II) complexes are attractive as potential MRI contrast agents since they have relatively high electronic spins (5/2), fast water exchange rates, and Mn2+(aq) has a lower intrinsic toxicity than many other paramagnetic metal ions, such as Gd3+.1-5 While recent advances have been made in the search for water soluble manganese-based small molecule contrast agents,6-12 only two Mn2+-containing contrast agents have been approved for clinical use. [Mn(dipyridoxyldiphosphate)]4− is the paramagnetic component of Teslascan®, a liver imaging agent13,14 while LumenHance®, which contains MnCl2, is employed in GI imaging.15
Since Mn2+ is substitutionally labile, chelating ligands are required in order to ensure the thermodynamic and kinetic stability of the complexes. Cyclic polyamine ligands are attractive candidates, but Mn2+ complexes of cyclen (1,4,7,10-tetraazacyclododecane) and related ligands are known to oxidize readily in air to give dimers or insoluble polymers.16-23 This is unfortunate since ligands such as cyclen and its derivatives have a rich aqueous chemistry with other metal ions.24-26 Attaching pendant arms (generally via the nitrogen atoms of the ring) that can also coordinate the metal leads to higher denticity of binding and has proved to be a useful strategy for further stabilizing labile complexes.27,28
Given the potential of Mn2+ complexes as MRI contrast agents, we have developed an interest in more fully understanding the chemical features of these complexes that influence their relaxivities. In this context, it is instructive to explore structure/relaxivity correlations in a series of chemically and structurally related complexes. We report herein on the synthesis, X-ray crystal structures, and solution characterization of several complexes of Mn(II) with the pendant-arm cyclen derivatives shown in Scheme 1. Structural analyses demonstrate that in the solid state the coordination numbers range from 6 to 8. Evidence outlined below suggests that the mechanism of water 1H relaxivity in the 6- and 7-coordinate cases involves transient binding of water to the metal ion in an associative process. This pathway is not available to the 8-coordinate complex [Mn(DOTAM)]2+. The most pronounced correlation appears to be that of higher coordination number with lower relaxivity. The results are also of interest since they suggest that metal complexes without bound water molecules may still dynamically interact with bulk water and exhibit significant relaxivities.
Scheme 1.
EXPERIMENTAL
Manganese salts were obtained from Strem Chemicals, Inc. 1,4,7,10-tetraazacyclododecane (cyclen) and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (H4DOTA) were obtained from Macrocyclics. All other starting materials were obtained from Sigma-Aldrich and used as received. 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetamide (DOTAM),29-31 1,4,7,10-tetraazacyclododecane-1,7-diacetic acid (H2DO2A),32 and 1,4,7,10-tetraazacyclododecane-1-(2-hydroxylcyclohexyl) (cyclen-CyOH)33 were synthesized by published methods. All the complexes are air-stable and no precautions to exclude oxygen were necessary during the syntheses.
Elemental analyses were performed by Atlantic Microlab Inc., Norcross, GA. Mass spectra were obtained using a Waters 2795 HPLC system with ZQ single quadrupole MS and an electrospray ionization source. Samples were introduced using loop injection. IR spectra were obtained as KBr pellets on a Perkin-Elmer Spectrum BX FT-IR System. 1H and 13C NMR spectra were obtained in D2O using a Varian Mercury spectrometer operating at 300 MHz. Cyclic voltammograms were obtained on a CH Instruments (Austin, TX) 650-A Electrochemical System with electronic iR compensation using a glassy carbon working electrode, SCE reference, and Pt wire counter electrode. The aqueous solutions used for electrochemistry contained 0.1 M NaNO3 as supporting electrolyte.
[Mn(DOTAM)]Cl2·3H2O. DOTAM (0.502 mmol) in 30 mL of 80% methanol was heated at reflux and MnCl2·4H2O (0.550 mmol) was added. The solution quickly became pale yellow, after which it was stirred for two hours and allowed to cool. Ethanol (40 mL) was added and the combined solvents were allowed to evaporate. The product was recrystallized from water to give crystals of the dihydrate suitable for X-ray crystallographic analysis. (Yield 0.2909g, 99.9% based on DOTAM) Anal. Calcd for C16H38N8O7MnCl2: C, 33.11; H, 6.60; N, 19.31. Found: C, 32.88; H, 6.20; N, 19.11. Mass spectrum (ESI-MS: m/z = 227.75, [Mn(DOTAM)]2+). IR (KBr pellet) 1655 cm−1, ν(C=O).
[Mn(DO3AM)][MnCl4]·EtOH. Cyclen (5.8 mmol) and NEt3 (6.1 mmol) were heated at reflux in 30 mL ethanol. Over a period of 3 hrs, chloroacetamide (5.8 mmol) in 15 mL ethanol was added dropwise. The mixture was allowed to stir for another hour, then an additional 5.8 mmol of chloroacetamide in ethanol was added dropwise over an additional 3 hrs. MnCl2·4H2O (1.38 mmol) in 10 mL methanol was then added. A white powder precipitated at once and, after cooling, was collected by suction filtration, and washed with 40 mL methanol. The product was recrystallized by diffusion of ethanol into an aqueous solution of the complex, producing crystals suitable for X-ray crystallographic analysis. The overall yield was 0.632 g (17% based on cyclen). Anal. Calcd for C16H35N7O4Mn2Cl4: C, 29.95; H, 5.46; N, 15.29. Found: C, 29.90; H, 5.44; N, 15.33. Mass spectrum (ESI-MS: m/z = 199.27, [Mn(DO3AM)]2+). IR (KBr pellet) 1653 cm−1, ν(C=O).
[Mn(DO3AM)]Cl2·5H2O. [Mn(DO3AM)][MnCl4]·EtOH was dissolved in a minimum of water and acetone was allowed to slowly diffuse into the solution. Pale pink crystals of MnCl2·6H2O developed over a period of about 40 days. After crystal formation ceased, the remaining solution was collected and allowed to evaporate, giving the product as a white powder in approximately 99% yield. Mass spectrum (ESI-MS: m/z = 199.03, [Mn(DO3AM)]2+). Anal. Calcd for C14H35N7O6MnCl2: C, 30.61; H, 5.32; N, 17.85. Found: C, 30.14; H, 5.55; N, 17.03. IR (KBr pellet) 1655 cm−1, ν(C=O).
1,4,7,10-tetraazacyclododecane-1-(2-hydroxylcyclohexyl)-4,7,10-triacetamide (DO3AM-CyOH). Cyclen-CyOH (3.7 mmol), 2-chloroacetamide (11.3 mmol), and triethylamine (11.3 mmol) were heated at reflux in 35 mL EtOH for three hours. After the reaction mixture cooled, 30 mL of Et2O were added and white precipitate formed. The precipitate was collected by suction filtration and washed twice with 30 mL aliquots of Et2O. The white powder became a slightly yellow oil on standing in air, but was reprecipitated with a 9:1 mixture of acetone and methanol. The powder was collected and washed with acetone to give a 45% yield of DO3AM-CyOH. Anal. Calcd for C20H39N7O4·2.5H2O: C, 49.58.12; H, 9.00; N, 20.16. Found: C, 49.53; H, 8.98; N, 20.13. 1H NMR: 1.1-4.2 (m) -CH2-,-CH. 13C NMR: 22.7, 23.4, 24.0, 24.6, 42.3, 46.5, 47.0 -CH2-; 33.8 -CH; 51.6 -CH(OH)-; 66.5, 67.4 -CH2CONH2; 126.3 -C(O)NH2. Mass spectrum (ESI-MS: m/z = 442.4, [DO3AM-CyOH + H+]). IR (KBr pellet) 1672 cm−1, ν(C=O).
[Mn(DO3AM-CyOH)]Cl2·3H2O. DO3AM-CyOH (0.3366 mmol) in 30 mL of ethanol was heated at reflux and MnCl2·4H2O (0.3366 mmol) in 10 mL methanol was added dropwise. After 30 min, the solvent was evaporated to give a pale yellow solid, which was washed with acetone and dried. (Yield 0.2189g, 99% based on DO3AM-CyOH) Anal. Calcd for C20H49N7O9MnCl2: C, 36.52; H, 7.45; N, 14.90. Found: C, 36.34; H, 7.41; N, 14.84. Mass spectrum (ESI-MS: m/z = 248.20, [Mn(DO3AM-CyOH)]2+). IR (KBr pellet) 1664 cm−1, ν(C=O).
Mn(H2DOTA)·2H2O. MnSO4·H2O (0.15 mmol) was dissolved in approximately 0.5 mL water, H4DOTA (0.15 mmol) was added, and the mixture stirred for 30 min. The water was allowed to evaporate and the product precipitated as a white powder. (Yield 0.0756g, 99% based on MnSO4·H2O) Anal. Calcd for C16H30N4O10Mn: C, 38.99; H, 6.00; N, 11.35. Found: C, 38.48; H, 5.91; N, 11.22. Mass spectrum (ESI-MS: m/z = 458.02, [Mn(H2DOTA) + H+]). IR (KBr pellet) 1736 cm−1, ν(C=O, uncoordinated -COOH); 1579 cm−1, ν(C=O, coordinated -COO−). Pale yellow crystals of the anhydrous salt suitable for X-ray crystallographic analysis were grown by diffusing acetone into an aqueous solution of the complex.
Mn(DO2A)·2H2O. H2DO2A (0.27 mmol) was dissolved in approximately 0.5 mL water and MnCl2·4H2O (0.27 mmol) was added. The mixture was stirred for several minutes and the solvent was allowed to evaporate. The product precipitated as a white powder. (Yield 0.1007 g, 99% based on H2DO2A) Anal. Calcd for C12H26N4O6Mn: C, 38.20; H, 6.89; N, 14.85. Found: C, 38.19; H, 6.86; N, 14.95. Mass spectrum (ESI-MS: m/z = 342.01, [Mn(DO2A) + H+]). IR (KBr pellet) 1612 cm−1, ν(C=O).
For several of the complexes, competitive EDTA binding studies were carried out by preparing 2:1 molar ratios of EDTA and metal complex (∼ 1mM) in water at pH 7.0. The T1 values of the solutions were monitored periodically for several days. In no case was a detectable change of T1 observed.
Relaxivity Measurements
Longitudinal relaxation rates (T1) were obtained using a Bruker Minispec mq20 spectrometer operating at 20 MHz and 37°C using the standard saturation-recovery pulse sequence ([90°-τ-90°] where τ is a variable delay time) provided by Bruker. Twenty scans were averaged for each of twenty saturation delay intervals which were used to define the magnetization curve. Fitting of the magnetization curves in aqueous solutions to a mono-exponential recovery was carried out using routines provided by Bruker. All aqueous magnetization data were successfully fit with mono-exponential curves and there was no evidence for bi-exponential behavior at any concentration. Data in mixed 1:1 mole fraction H2O:MeOH was similarly obtained, but using fifty delay intervals and fit to a bi-exponential expression using a Marquardt-Levenberg algorithm.34 Fits of T1−1 vs concentration were carried out using standard linear least squares fitting approaches and the slopes of the plots are reported as the relaxivities, R1.
X-Ray Crystallography
X-ray Crystallographic analyses were carried out by the X-ray Crystallographic Laboratory at the Department of Chemistry, University of Minnesota. Table 1 contains data collection and solution parameters for all reported structures. Full details are given in the Supporting Material. Suitable single crystals of [Mn(DOTAM)]Cl2·2H2O, [Mn(DO3AM)][MnCl4]·EtOH, and Mn(H2DOTA) were each placed onto the tips of 0.1 mm diameter glass capillaries and mounted on a Bruker CCD area detector diffractometer for data collection at 173 K. The data collection was carried out using MoKα radiation (graphite monochromator) with a frame time of 10 sec for [Mn(DOTAM)]Cl2·3H2O and 60 sec for [Mn(DO3AM)][MnCl4]·EtOH and Mn(H2DOTA) with a detector distance of 4.9 cm in each case. The complete sphere of reciprocal space was surveyed to a resolution of 0.77 Å for [Mn(DOTAM)]Cl2·3H2O and 0.84 Å for Mn(H2DOTA). For [Mn(DO3AM)][MnCl4]·EtOH a randomly oriented region of reciprocal space was surveyed to the extent of 1.5 hemispheres and to a resolution of 0.84 Å. The intensity data were corrected for absorption and decay (SADABS).35 Final cell constants were calculated from the xyz centroids of strong reflections (3505 for [Mn(DOTAM)]Cl2·3H2O, 2813 for [Mn(DO3AM)][McCl4]·EtOH, and 2827 for Mn(H2DOTA)) from the data collection after integration (SAINT 6.01, 1999).36 The structures were solved using SIR9237 and refined using SHELXL-97.38 The final full matrix least squares refinement parameters for each crystal are given in Table 1. The program PLATON was used for verifying the structures.39
Table 1.
Crystal Data and Refinement Parameters for [Mn(DOTAM)]Cl2·H2O, [Mn(DO3AM)][MnCl4]·EtOH, and Mn(H2DOTA).
| [Mn(DOTAM)]Cl2·2H2O | [Mn(DO3AM)][MnCl4]·EtOH | Mn(H2DOTA) | |
|---|---|---|---|
| empirical formula | C16H36Cl2MnN8O6 | C16H35Cl4Mn2N7O4 | C16H26MnN4O8 |
| fw (g mol−1) | 562.37 | 641.19 | 457.35 |
| T (K) | 173(2) | 173(2) | 173(2) |
| Space Group | C2/c | P21/n | C2/c |
| a (Å) | 18.5798(15) | 8.3646(8) | 16.374(3) |
| b (Å) | 13.6006(11) | 19.483(2) | 6.6559(13) |
| c (Å) | 10.5800(8) | 16.3627(16) | 16.750(3) |
| α (°) | 90 | 90 | 90 |
| β (°) | 110.490(1) | 99.254(2) | 98.381(3) |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 2504.4(3) | 2631.9(5) | 1806.0(6) |
| Z | 4 | 4 | 4 |
| Density (calc.) | 1.492 | 1.622 | 1.682 |
| λ (Å) | 0.71073 | 0.71073 | 0.71073 |
|
R indicesa [I/>2σ(I)] |
R1 = 0.0255 wR2 = 0.0629 |
R1 = 0.0536 wR2 = 0.1177 |
R1 = 0.0314 wR2 = 0.0782 |
|
R indicesa (all data) |
R1 = 0.0296 wR2 = 0.0644 |
R1 = 0.0673 wR2 = 0.1230 |
R1 = 0.0392 wR2 = 0.0840 |
R1 = Σ∥Fo|-|Fc∥/Σ|Fo|; wR2 = [Σ[w(Fo2- Fc2)2]/ Σ[w(Fo2)2]1/2.
RESULTS AND DISCUSSION
Synthesis and Stability of Complexes
The complexes whose syntheses are reported in the experimental section have not been previously isolated. Mn(H2DOTA)40-42 and Mn(DO2A)42 have been previously reported as components of solutions containing the free ligands with manganese salts, but were not previously obtained as solids. The syntheses of the manganese complexes proceed by relatively straightforward direct ligand substitution reactions. Of particular note, however, is that [Mn(DO3AM)]Cl2 was prepared without isolating the free ligand. Although the reaction mixture was not extensively characterized, the major expected side products of the reaction of cyclen with chloroacetamide are unreacted cyclen and the monosubstituted DO1AM (1,4,7,10-tetraazacyclododecane-1-monoacetamide). Treatment of this mixture with a substoichiometric quantity of MnCl2 yields the [Mn(DO3AM)]2+ product, presumably due to the high denticity of the ligand and consequent thermodynamic stability. Other manganese-containing side products that may form remain in solution under these conditions and [Mn(DO3AM)]2+ is the only cationic constituent of the isolated precipitate. The DO3AM free ligand has been previously synthesized independently,43,44 but the in situ approach we report represents a novel strategy for the synthesis of a tri-substituted cyclen complex. The recrystallized product of the reaction mixture is [Mn(DO3AM)][MnCl4]·EtOH. This salt has been structurally characterized by X-ray crystallography as described below. Since the [MnCl4]2− ion is paramagnetic and would interfere with relaxivity measurements, the ion was removed by exploiting the differential solubilites of the desired complex and MnCl2 in a water/acetone mixture. MnCl2 is significantly less soluble and precipitates, leaving [Mn(DO3AM)]2+ and chloride ion in solution. Attempts to grow crystals of this salt suitable for X-ray studies were unsuccessful and there remains some ambiguity regarding the solid state structure. Either the salt contains the seven-coordinate [Mn(DO3AM)]2+ cation (as found in the tetrachloromanganate salt) with two chloride counterions, or one of the chloride ions binds to form an eight coordinate [Mn(DO3AM)Cl]+ cation with one additional chloride as the counterion. Chloride binding to [Mn(DO3AM)]2+ should, however, be very weak. The structurally confirmed isolation of [Mn(DO3AM)][MnCl4]·EtOH indicates that Cl− preferentially binds to “[MnCl3]−” rather than [Mn(DO3AM)]2+. Chloride binding to Mn2+ is known to be very weak45-47 and the formation constant for [MnCl4]2− in aqueous solution has never been determined. Thus chloride is expected to be even more weakly bound to the metal ion of [Mn(DO3AM)]2+ in solution and should be fully dissociated. Furthermore, the mass spectrum of solutions of [Mn(DO3AM)]Cl2 is dominated by peaks at m/z = 199.03 (corresponding to [Mn(DO3AM)]2+) and at m/z = 397.00 (corresponding to [Mn(DO3AM)]2+ − H+). Only very small peaks are apparent 433.00 and 435.00 (corresponding to [Mn(DO3AM)]2+ + Cl−).
The susceptibility of polyazamacrocyclic complexes of Mn2+ to air oxidation is well known and has limited the development of their coordination chemistry.16-23 Mn(H2DOTA), Mn(DO2A), [Mn(DOTAM)]2+, [Mn(DO3AM)]2+, and [Mn(HOCy-DO3AM)]2+, however, are all air stable in aqueous solution for at least several days. These results are further supported by electrochemical studies detailed in the Supporting Information. All the complexes exhibit irreversible Mn(II/III) oxidations at potentials greater than +0.8V vs SCE. These potentials are consistent with the relatively high oxidation potentials observed in related complexes with nitrogen and oxygen donor ligands.48 The conclusion is that cyclic polytetraamine ligands with two or more π-accepting pendant arms are capable of stabilizing Mn2+ to air oxidation.
Stability constants for the Mn(II) complexes of DOTA (log K = 19.89)40,41 and DO2A (log K = 14.54)40 have been previously reported. For [Mn(DOTAM]2+ and [Mn(DO3AM)]2+, thermodynamic binding studies have not been carried out. Incubation of solutions of [Mn(DOTAM]2+ or [Mn(DO3AM)]2+ with a two-fold excess of EDTA, however, results in no changes in the relaxivities even after several days. EDTA is known49 to bind Mn2+ with a dissociation constant of logK = 13.9 and the reported R1 of [Mn(EDTA)]2− is 2.20 mM−1s−1.50 These results indicate that regardless of the thermodynamic stability of the complexes, they are kinetically inert to ligand substitution on the timescale of the relaxivity experiments.
X-ray Crystallography
[Mn(DOTAM)]Cl2·2H2O represents a relatively rare example of a structurally characterized 8-coordinate Mn(II) complex.51-63 The molecular structure is illustrated in Figure 1 and some of the key structural parameters are summarized in Table 2. The four oxygen atoms define an approximate square plane, as do the four nitrogen ring atoms. The crystallographically imposed symmetry of the cation is C2, but the complex has essentially S4 point group symmetry. The overall coordination geometry may be described as a slightly compressed square prism in which the basal faces are rotated about the pseudo-fourfold axis by 23° with respect to each other. The Mn-O and Mn-N bond lengths fall within the range of previously reported Mn-O and Mn-N bond lengths in 8-coordinate species. 51-63 Weak hydrogen-bonding interactions between the amide hydrogens and the oxygen of water are also apparent.
Figure 1.
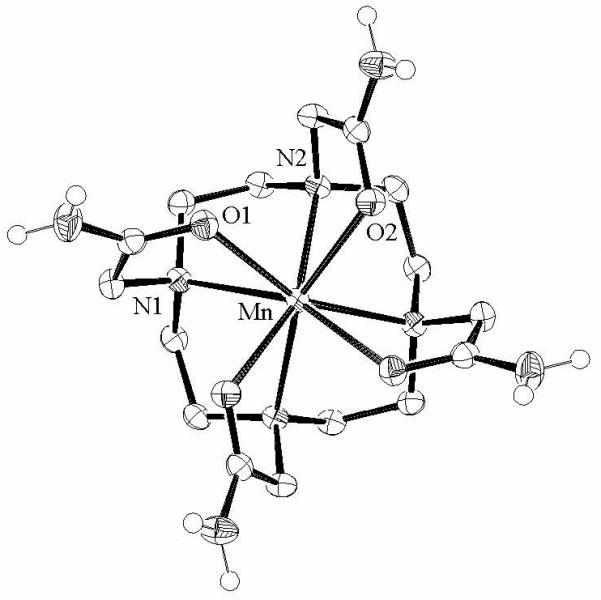
Molecular structure of [Mn(DOTAM)]2+ viewed approximately along the C2 axis. For clarity, only the amide hydrogens are shown. Selected bond lengths [Å] and angles [°]: Mn-N1, 2.4351(11); Mn-N2, 2.4218(11); Mn-O1, 2.3128(10); Mn-O2, 2.3080(9); N1-Mn-N2, 73.73(4); N2-Mn-N1′, 73.79(4); O1-Mn-N1, 68.96(4); O1-Mn-O2, 73.83(3); O2-Mn-N2, 68.82(4).
Table 2.
Selected Bond Distances and Angles for [Mn(DOTAM)] 2+, [Mn(DO3AM)]2+, and Mn(H2DOTA).
| [Mn(DOTAM)]2+ | [Mn(DO3AM)]2+ | Mn(H2DOTA) | ||
|---|---|---|---|---|
| Bond Distances(Å) | ||||
| Mn-N | 2.4351(11) | 2.423(4) | 2.3803(18) | |
| 2.4218(11) | 2.351(4) | 2.3481(19) | ||
| 2.348(4) | ||||
| 2.312(5) | ||||
| Mn-O | 2.3128(10) | 2.297(4) | 2.1601(16) | |
| 2.3080(9) | 2.238(3) | |||
| 2.162(4) | ||||
| Bond Angles(°) | ||||
| N-Mn-N | 73.79(4) | 77.12(13)) | 76.99(6) | |
| 73.73(4) | 76.16(15) | 74.72(6) | ||
| 75.71(14) | ||||
| 74.40(14) | ||||
| O-Mn-N | 73.83(3) | 74.66(13) | 73.81(6) | |
| 68.96(4) | 72.22(12) | |||
| 71.24(13) | ||||
| O-Mn-O | 68.82(4) | 91.73(14) | 101.03(9) | |
| 84.38(13) | ||||
| 76.28(13) | ||||
Among the 8-coordinate Mn(II) complexes that have been reported,51-63 [Mn(DOTAM]2+ most resembles other pendant-arm macrocycles such as [Mn(2-pyridylmethyl)4cyclen]2+, which has 2-pyridyl groups in place of the amide groups of DOTAM,62 and [Mn(pyrazol-1-ylmethyl)4cyclen]2+ in which imadozole nitrogens also coordinate the metal.57 In each case the fundamental geometry is square prismatic with one of the square planes rotated about a pseudo-fourfold axis.
While DOTAM typically exhibits octadentate coordination to lanthanides,29,64-67 structural studies have shown that its binding to transition metal or main group cations is more diverse.30 The Cd2+ and Zn2+ complexes are 6-coordinate, while the Ca2+ complex is a more regular 8-coordinate geometry similar to that of [Mn(DOTAM)]2+. The differences in coordination have been ascribed to steric interactions between the coordinated oxygens which lead to lower coordination numbers for the smaller ions.30 The Mn2+ ion, however, has an effective ionic radius slightly smaller than that of Cd2+,68 suggesting that there are other contributions that play a role in determining the observed solid state structure.
Crystals of [Mn(DO3AM)][MnCl4]·EtOH give a disordered structure. The most successful model for the structure of the [Mn(DO3AM)]2+ ion is shown in Figure 2 and selected structural parameters are given in Table 2. The structure shows a 7-coordinate Mn(II) coordinated by the four amine nitrogens of the ring as well as by three oxygen atoms of the pendant amide groups. There is, however, residual difference electron density on the order of 0.9 eÅ−3 in the area where a fourth pendant arm would be located. This is also the general vicinity of the ethanol solvate molecule. The most successful model was based on 88.5% occupation of the site by ethanol and 11.5% occupation by an amide pendant arm resulting from a 90° rotation of the complex about an axis perpendicular to the plane of the ring nitrogens.
Figure 2.
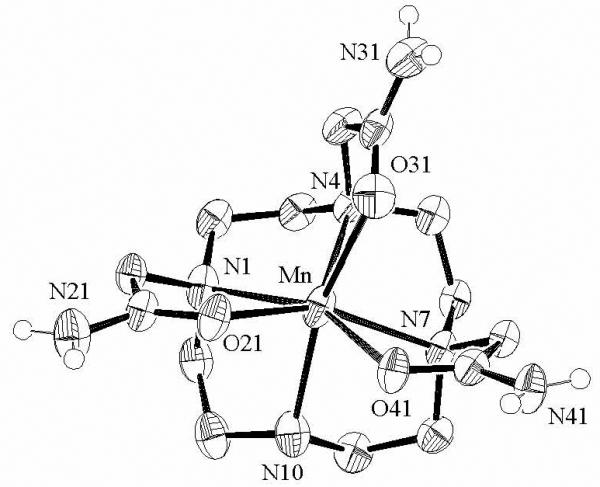
Molecular structure of [Mn(DO3AM)]2+. For clarity, only the amide hydrogens are shown. Selected bond lengths [Å] and angles [°]: Mn-N1, 2.423(4); Mn-N4, 2.342(4); Mn-N7, 2.351(4); Mn-N10, 2.312(5);Mn-O21, 2.162(4); Mn-O31, 2.297(4); Mn-O41, 2.238(3);N1-Mn-N4, 74.40(14); N4-Mn-N7, 77.12(13); N7-Mn-N10, 75.71(15); N1-Mn-N10, 76.16(15); O21-Mn-N1, 74.66(13); O31-Mn-N4, 71.24(13); O41-Mn-N7, 72.22(12); O21-Mn-O31, 91.73(14) ; O31-Mn-O41, 76.28(13) ; O21-Mn-O41, 84.38(13).
The complex can be described as a distorted capped trigonal prism in which N10 is the capping ligand. Several other structurally characterized 7-coordinate Mn(II) complexes are based on capped trigonal prismatic geometries69-71 although pentagonal bipyramidal structures have also been observed.72-74 A comparison with the structure of [Mn(DOTAM)]2+ shows that the bond lengths decrease and the bond angles increase somewhat for the lower coordination number. For example, the average Mn-N bond length for [Mn(DO3AM)]2+ is 2.374Å compared to 2.428Å for [Mn(DOTAM)]2+. Since the DO3AM ligand has somewhat reduced steric constraints on binding, such differences are not unexpected. A number of hydrogen-bonding interactions between amide hydrogens and chlorines of the anion are apparent in the solid state structure.
The structure of Mn(H2DOTA) is given in Figure 3 with some of the key structural parameters summarized in Table 2. The manganese ion is located on a crystallographic C2 axis and displays a distorted octahedral geometry at the metal with four amino nitrogen donors and two carboxylate oxygen donors occupying cis coordination positions. The bound carboxylates are from the 1 and 7 positions of the macrocyclic ring. The other two carboxylates are protonated and not directly coordinated to the metal ion. The closest distance between an oxygen of an uncoordinated acetate group and the metal ion is 3.01 Å. The hydrogen atoms of the –COOH groups have weak hydrogen-bonding interactions with the water molecules in the lattice.
Figure 3.
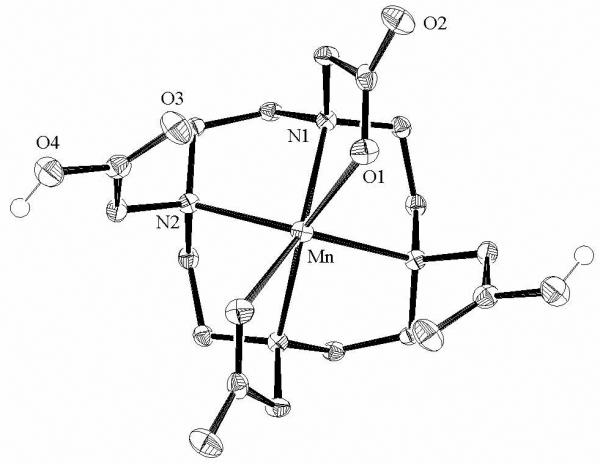
Molecular structure of Mn(H2DOTA) viewed approximately along the C2 axis. All hydrogens except –COOH have been omitted for clarity. Selected bond lengths [Å] and angles [°]: Mn-N1, 2.3481(19); Mn-N2, 2.3803(18); Mn-O1, 2.1601(16); N1-Mn-N2, 74.72(6); N2-Mn-N1′, 76.99(6); O1-Mn-N1, 73.81(6); O1-Mn-O1′, 101.03(9).
Several metal complexes of H2DOTA2− have been previously characterized and the structure of Mn(H2DOTA) is very similar to those of Cu(H2DOTA) and Ni(H2DOTA).75 The Mn-N bond lengths are in the expected range, while the Mn-O bond lengths are somewhat shorter than those in [Mn(DOTAM)]2+ or [Mn(DO3AM)]2+. This observation may be rationalized by noting that both the increased electrostatic attraction of the negatively charged carboxylate and the decreased steric demands in the 6-coordinate complex favor shorter Mn-O bond lengths relative to the amide complexes.
1H Relaxivities
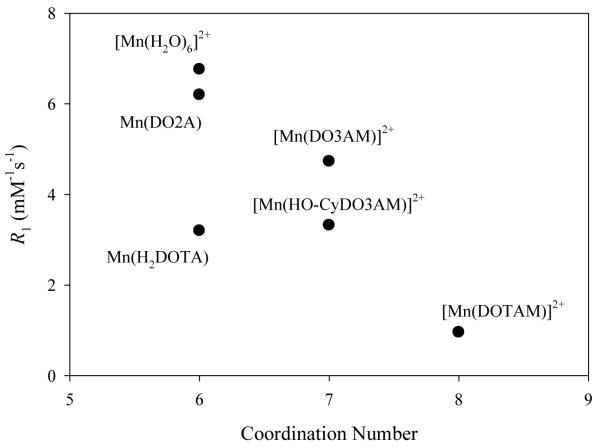
The 1H relaxivities of the complexes at in water 20 MHz and 37° C are reported in Table 3. The data are also displayed graphically in Figure 4 as a plot of the relaxivity vs the crystallographically defined coordination number of the metal ion. The relaxivity of the 6-coordinate complex Mn(DO2A) is nearly as high as that of Mn2+(aq) in acidic media. As the coordination number of the complexes increases to seven the relaxivity decreases somewhat and for a coordination number of eight, a larger decrease is apparent. The relaxivity of [Mn(DOTAM)]2+ is low relative to most previously reported Mn(II) complexes.76,1,7,8,40,77,78 The complex Mn(H2DOTA) is an apparent exception to these trends (however, see below), with a relaxivity significantly lower than those of the other 6-coordinate species.
Table 3.
Relaxivities of the complexes in water at 20 MHz and 37°C.
| Complex | R1 (mM−1s−1) | Coordination No. |
|---|---|---|
| [Mn(H2O)6](ClO4)2 | 6.76a | 6 |
| Mn(DO2A) | 6.20 | 6 |
| Mn(H2DOTA) | 3.20 | 6 |
| [Mn(DO3AM)]Cl2 | 4.73 | 7 |
| [Mn(DO3AM-CyOH)]Cl2 | 3.32 | 7 |
| [Mn(DOTAM)]Cl2 | 0.96 | 8 |
in 0.1M HClO4.
Figure 4.
Graph of longitudinal relaxivities at 20 MHz and 37°C vs crystallographically defined coordination number for the complexes in Table 2.
Manganese(II) is a substitutionally labile species and the inner sphere contributions to the relaxivities of Mn2+(aq) and its small molecular complexes has most often been interpreted in terms of exchange mechanisms involving coordinated water.7,8,40,77 There are, however, relatively few published studies of the correlations between the relaxivities, coordination numbers, and water exchange mechanisms for Mn2+(aq) and its complexes.7,40,74,78,79 Most 7-coordinate complexes investigated so far exhibit positive volumes of activation and/or entropies of activation for water exchange and these parameters have been interpreted in terms of a dissociative interchange (Id) mechanism for water exchange.7,74 While some 6-coordinate complexes also appear to exchange by Id mechanisms,74 for both [Mn(H2O)6]2+ and [Mn2(ENOTA)(H2O)],80 a associative interchange (Ia) mechanism for exchange of bound water has been implicated.40,74
The polyazamacrocyclic complexes in Table 3, however, do not have water directly bound to the metal ion in the solid state, although an inner sphere exchange of water for the 6-and 7-coordinate complexes would best account for their relatively high relaxivities. Two limiting mechanistic possibilities are suggested within this interpretive framework. The crystallographic results indicate that in this type of complex, Mn2+ reaches coordinative saturation at a maximum coordination number of 8. Thus for Mn(DOTAM)2+ an associative addition of water into the primary coordination sphere to form a transient 9-coordinate species is not possible and the observed relaxivity reflects only outer sphere contributions. For the seven-coordinate complexes, Mn(DO3AM)2+ and Mn(DO3AM-CyOH)2+, it is possible to expand the coordination sphere to form an 8-coordinate associative intermediate. This site provides a pathway for transient inner sphere coordination of water and would be expected to provide a significant contribution to the relaxivity. For complexes such as Mn(DO2A) an associative mechanism through a seven-coordinate intermediate is possible in an analogous fashion and has been experimentally demonstrated for Mn2+(aq).79 Such exchanges are expected to be faster that those proceeding through eight-coordinate intermediates due to relaxed steric constraints. The relative rates for complexes with the same overall coordination number are more likely to differ due to specific steric effects than to electronic effects at the Mn2+ center.
The second possibility is that of a dissociative interchange mechanism involving release of one bound pendant arm to open a site for a water molecule to bind transiently. This alternative seems less chemically reasonable since it would require a very coordinatively unsaturated 5-coordinate intermediate for Mn(DO2A). Additionally, a dissociative mechanism suggests that inner sphere pathways should still contribute to the relaxivity of Mn(DOTAM)2+ via a 7-coordinate intermediate, but experiments in mixed protic solvents detailed below are inconsistent with this interpretation.
In 1:1 mixtures of MeOH and H2O, there are two populations of relaxable protons.81-83 The hydroxyl protons are highly exchangeable and thus relax primarily by inner sphere mechanisms. The methyl protons are not exchangeable and are relaxed only through an outer sphere mechanism. For a complex for which water exchange is important the magnetization vs time curves in the mixed solvent clearly exhibit bi-exponential behavior with the slow component corresponding to outer sphere relaxation (primarily CH3) and the faster component to inner-sphere hydroxyl proton exchange. The best fit for Mn2+(aq) at 20 MHz and 37°C is shown in Figure 5 (top). The parameters of the fit are: T1fast = 9.13 ± 0.11 ms (rel. contrib. = 49.5%); T1slow = 58.82 ± 0.69 ms (rel. contrib. = 50.5%); R2 = 0.9999, entirely consistent with an inner sphere water exchange mechanism for relaxation.
Figure 5.

Magnetization vs time curves in 1:1 MeOH:H2O for Mn2+(aq) fit to bi-exponential kinetics (top) and [Mn(DOTAM)]2+ fit to mono-exponential kinetics(bottom).
In contrast, the magnetization decay curve for [Mn(DOTAM)]2+ in the mixed solvent was successfully fit by a single exponential expression with T1 = 72.96 ± 0.22 ms (R2 = 0.9999), also shown in Figure 5 (bottom). Attempts to fit the data to a bi-exponential expression gave only small (<5%) contributions from the second, much longer decay term with larger standard errors on the parameters (see Supporting Information). These results are consistent with solvent relaxation by [Mn(DOTAM)]2+ proceeding through an outer sphere mechanism only. In this case both the CH3 and OH protons have essentially identical relaxation times due to similar through space dipole-dipole interactions with the Mn2+ ion. The apparent T1 the [Mn(DOTAM)]2+ data is somewhat longer than that for the outer-sphere component of Mn2+(aq) and is consistent with the increased size of the complex relative to Mn2+(aq). Outer sphere relaxivities generally increase inversely with both the distance of closest approach for water molecules and the diffusion coefficient of the ion.1,84-88 Both of these quantities are expected to be larger for [Mn(DOTAM)]2+ than for Mn2+(aq), and thus consistent with the observed T1 values.
It is also of note that the complexes have different charges, which might affect the relaxivities. For series of structurally related Gd3+ complexes, it has been generally found that the relaxivity decreases as the charge increases,89,90 although exceptions have also been noted.91 This effect has been rationalized in terms of a water exchange mechanism and enhanced electrostatic interactions between positive charges and the oxygen atom of the water molecule, which lead to slower water exchange rates.89,90 All of the amide-based complexes in Table 3 have 2+ charges while the carboxylates are neutral and Mn2+(aq) carries a large positive charge. If the charge of the complex were dominant, all the amide-based complexes would have relaxivities similar to that of Mn2+(aq) and they should be among the lowest relaxivities in the series. It is notable that Mn(DO2A) has the relaxivity most similar to Mn2+(aq), even though there is a large difference in charge. Thus it appears that the differences in coordination numbers are far more important overall determinants of relaxivities.
In terms of the data presented in Table 3 and Figure 4, Mn(H2DOTA) appears to be an exception. Although the crystal structure clearly indicates a six-coordinate Mn2+ ion, the relaxivity of an aqueous solution of the complex is considerably lower than that of Mn(DO2A), which is also 6-coordinate. NMRD data on solutions containing Mn2+ and excess H2DOTA2− at pH values of 6.2-6.5 exhibit relaxivity profiles characteristic of an outer sphere mechanism.40 It should be noted, however, that the uncoordinated –COOH groups have reported pKa values of 2.99 and 4.26,41,42 and are expected to ionize to a significant extent in aqueous solution. In fact, a 1 mM solution of Mn(H2DOTA) has a pH of 3.7, indicating significant ionization. The relaxivity vs pH curve for Mn(H2DOTA) shows a prominent inflection with an effective pKa of 2.79 and limiting R1 values of 7.2 mM−1 s−1 at low pH (<2) and 2.7 mM−1 s−1 at high pH (>4).92 In contrast, for all the other complexes in Table 3, the relaxivities vary less than 10% in the pH 2-6 range. This observation is consistent with the fact that of all the complexes, only Mn(H2DOTA) has functional groups that are protonatable or deprotonatable over this pH range. Thus for all the complexes in Table 3, except Mn(H2DOTA), there is only one significant species present in solution.
For Mn(H2DOTA), however, the predominant solution structure may be considerably different from the crystal structure and the solution contains a mixture of species in a variety of protonation states. For example, deprotonation of an uncoordinated pendant acetate arm may lead to binding of the arm and a net increase in the coordination number of the metal. If such processes occur, it is predicted that the relaxivity should be relatively low at higher pH values and should increase as inner sphere relaxation pathways contribute at lower pH values. Such a pH dependence has been observed for Mn(H2DOTA) 92 and will be the addressed in a subsequent publication.93
An important additional conclusion to be drawn from the results described above is that complexes with easily expandable coordination spheres may be potential candidates for MRI contrast agents, even if there is no water directly coordinated in the solid form of the complex. In such complexes, water may be able bind transiently to form higher coordinate intermediates that are long-lived enough to provide an efficient relaxation pathway for water protons.
Manganese complexes can exhibit relaxivities that rival that of the active ingredient of the most common clinically used gadolinium-based contrast agent, Magnevist® ([Gd(diethylenetriamine pentaacetic acid)]2−, R1 = 4.34 mM−1 s−1 at 20 MHz and 39°C).94 In fact, both Mn(DO2A) and [Mn(DO3AM)]2+ exhibit relaxivities in water that exceed that of Magnevist under similar conditions. The implications for contrast development are that Mn2+, lacking crystal field stabilization effects, can assume a variety of total coordination numbers with relatively small energetic differences. For complexes of relatively low coordination number, an associative mechanism for water interaction with the metal ion is allowed thus providing an inner sphere pathway for solvent relaxation. These results suggest the possibility of many new Mn2+-based candidates for clinically useful contrast agents once a more complete understanding of their dynamics of interaction with water has been developed.
Supplementary Material
Acknowledgement
Drs. Neil R. Brooks and Victor G. Young, Jr. of the X-Ray Crystallographic Laboratory at the University of Minnesota are acknowledged for the crystallographic analyses. Financial support from the National Institutes of Health (R15 GM061724) and Wesleyan University is also gratefully acknowledged.
Footnotes
Supporting Information Available: Details of the crystallographic analyses (including data collection and refinement details, atomic coordinates and isotropic displacement parameters, bond lengths and angles, anisotropic displacement parameters, hydrogen coordinates and isotropic displacement parameters, and analysis of hydrogen bonding), fits of relaxation data in mixed MeOH:H2O solvent, and summary of electrochemical data (28 pgs). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Lauffer RB. Chem. Rev. 1987;87:901–927. [Google Scholar]
- 2.Lauterbur PC, Mendonca-Dias MH, Rudin AM. Frontiers of Biological Energetics. Academic Press; New York: 1978. [Google Scholar]
- 3.Wagnon BK, Jackels SC. Inorg. Chem. 1989;28:1923–1927. [Google Scholar]
- 4.Bradshaw JE, Gillogly KA, Wilson LJ, Kumar K, Wan X, Tweedle MF, Hernandez G, Bryant RG. Inorg. Chim. Acta. 1998;275-276:106–116. [Google Scholar]
- 5.Merbach AE, Toth E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. John Wiley & Sons, Ltd.; New York: 2001. [Google Scholar]
- 6.Schwert DD, Davies JA, Richardson N. Topics in Curr. Chem. 2002;221:165–199. [Google Scholar]
- 7.Aime S, Anelli PL, Botta M, Brocchetta M, Canton S, Fedeli F, Gianolio E, Terreno E. J. Biol. Inorg. Chem. 2002;7:58–67. doi: 10.1007/s007750100265. [DOI] [PubMed] [Google Scholar]
- 8.Troughton JS, Greenfield MT, Greenwood JC, Dumas S, Wiethoff AJ, Wang J, Spiller M, McMurry TJ, Caravan P. Inorg. Chem. 2004;43:6313–6323. doi: 10.1021/ic049559g. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Koretsky AP. Curr. Pharm. Biotech. 2004;5:529–537. doi: 10.2174/1389201043376607. [DOI] [PubMed] [Google Scholar]
- 10.Bock NA, Silva AC. Future Neurology. 2007;2:297–305. [Google Scholar]
- 11.Li Z, Li W, Li X, Pei F, Wang X, Lei H. J. Inorg. Biochem. 2007;101:1036–1042. doi: 10.1016/j.jinorgbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Lovejoy KS, Jasanoff A, Lippard SJ. Proc. Natl. Acad. of Sci. U.S.A. 2007;104:10780–10785. doi: 10.1073/pnas.0702393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocklage SM, Cacheris WP, Quay SC, Hahn FE, Raymond KN. Inorg. Chem. 1989;28:477–485. [Google Scholar]
- 14.Lim KO, Stark DD, Leese PT, Pfefferbaum A, Rocklage SM, Quay SC. Radiology. 1991;178:79–82. doi: 10.1148/radiology.178.1.1898539. [DOI] [PubMed] [Google Scholar]
- 15.Small WC, DeSimone-Macchi D, Parker JR, Sukerkar A, Hahn PF, Rubin DL, Zelch JV, Kuhlman JE, Outwater EK, Weinreb JC, Brown JJ, de Lange EE, Woodward PJ, Arildsen R, Foster GS, Runge VM, Aisen AM, Muroff LR, Thoeni RF, Parisky YR, Tanenbaum LN, Totterman S, Herfkens RJ, Knudsen J, Laster RE, Jr., Duerinckx A, Stillman AE, Spritzer CE, Saini S, Rofsky NM, Bernardino ME. J. of Magn. Reson. Imaging. 1999;10:15–24. doi: 10.1002/(sici)1522-2586(199907)10:1<15::aid-jmri3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Chiswell B, McKenzie ED, Lindoy LF. Manganese. In: Wilkinson G, editor. Comprehensive Coordination Chemistry. Vol. 4. Pergamon Press; Oxford: 1987. p. 76. [Google Scholar]
- 17.Goodson PA, Hodgson DJ, Glerup J, Michelsen K, Weihe H. Inorg. Chim. Acta. 1992;197:141–147. [Google Scholar]
- 18.Brewer KJ, Calvin M, Lumpkin RS, Otvos JW, Spreer LO. Inorg. Chem. 1989;28:4446–4451. [Google Scholar]
- 19.Goodson PA, Hodgson DJ, Michelsen K. Inorg. Chim. Acta. 1990;172:49–57. [Google Scholar]
- 20.Létumier F, Broeker G, Barbe J-M, Guilard R, Lucas D, Dahaoui-Gindrey V, Lecomte C, Thouin L, Amatore C. J. Chem. Soc., Dalton Trans. 1998:2233–2239. [Google Scholar]
- 21.Weighardt K, Bossek U, Gebert W. Angew. Chem., Int. Ed. Engl. 1983;22:328–329. [Google Scholar]
- 22.Weighardt K, Bossek U, Nuber B, Weiss J, Bonvoison J, Corbella M, Vitolis SE, Girerd J-J. J. Amer. Chem. Soc. 1988;110:7398–7411. [Google Scholar]
- 23.Hubin TJ, McCormick JA, Collinson SR, Alcock NW, Clase HJ, Busch DH. Inorg. Chim. Acta. 2003;346:76–86. [Google Scholar]
- 24.Lindoy LF, Busch DH. Prep. Inorg. Reactions. 1971;6:1–61. [Google Scholar]
- 25.Melson GA, editor. Coordination Chemistry of Macrocyclic Compounds. Plenum; New York: 1979. [Google Scholar]
- 26.Connors H, McAuliffe CA, Tames J. Rev. Inorg. Chem. 1981;3:199–257. [Google Scholar]
- 27.Hancock RD, Martell AE. Chem .Rev. 1989;89:1875–1914. [Google Scholar]
- 28.Martell AE, Hancock RD. Metal Complexes in Aqueous Solution. Plenum Press; New York: 1996. pp. 127–133. [Google Scholar]
- 29.Amin S, Morrow JR, Lake CH, Churchill MR. Angew. Chem., Int. Ed. Engl. 1994;33:773–775. [Google Scholar]
- 30.Maumela H, Hancock RD, Carlton L, Reibenspies JH, Wainwright KP. J. Amer. Chem. Soc. 1995;117:6698–6707. [Google Scholar]
- 31.Note that by extending the reported reaction time yields of up to 90% may be obtained.
- 32.Van Westrenen J, Sherry AD. Bioconjugate Chem. 1992;3:524–532. doi: 10.1021/bc00018a011. [DOI] [PubMed] [Google Scholar]
- 33.de Sousa AS, Hancock RD, Reibenspies JH. J. Chem. Soc., Dalton Trans. 1997:939–944. [Google Scholar]
- 34.a) Marquardt DW. J. Soc. Ind. Appl. Math. 1963;11:431–441. [Google Scholar]; b) Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes. Cambridge University Press; Cambridge: 1986. [Google Scholar]
- 35.Blessing R. Acta Cryst. A51. 1995:33–38. doi: 10.1107/s0108767394005726. [DOI] [PubMed] [Google Scholar]
- 36.SAINT V6.1. Bruker Analytical X-Ray Systems; Madison, WI: [Google Scholar]
- 37.Altomare A, Cascarano G, Giacovazzo C, Gualardi A. J. Appl. Cryst. 1993;26:343–350. [Google Scholar]
- 38.SHELXTL-Plus V5.10. Bruker Analytical X-Ray Systems; Madison, WI: [Google Scholar]
- 39.Spek AL. J.Appl.Cryst. 2003;36:7–13. [Google Scholar]
- 40.Geraldes CFGC, Sherry AD, Brown RD, III, Koenig SH. Magn. Reson. Med. 1986;3:242–250. doi: 10.1002/mrm.1910030207. [DOI] [PubMed] [Google Scholar]
- 41.Chaves S, Delgado R, Da Silva JJRF. Talanta. 1992;39:249–254. doi: 10.1016/0039-9140(92)80028-c. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi A, Calabi L, Giorgi C, Losi P, Mariani P, Palano D, Paoli P, Rossi P, Valtancoli B. J. Chem. Soc., Dalton Trans. 2001:917–922. [Google Scholar]
- 43.Woods M, Kiefer GE, Bott S, Castillo-Munquiz A, Eshelbrenner C, Michaudet L, McMillan K, Mudigunda SDK, Ogrin D, Tircsó G, Zhang S, Zhao P, Sherry AD. J. Amer. Soc. Soc. 2004;126:9248–9256. doi: 10.1021/ja048299z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nwe K, Richard JP, Morrow JR. J. Chem. Soc., Dalton Trans. 2007:5171–5178. doi: 10.1039/b710072h. [DOI] [PubMed] [Google Scholar]
- 45.Morris DFC, Short EL. J. Chem. Soc. 1961:5148–5153. [Google Scholar]
- 46.Tribalat S, Caldero J-M. Comp. Rend. 1962;255:925–927. [Google Scholar]
- 47.Gammons CH, Seward TM. Geochim. Cosmochim. Acta. 1996;60:4295–4311. [Google Scholar]
- 48.Schultz FA, Duncan CT, Rigsby MA. Electrochemistry of the Group 7 Elements. In: Scholz F, Pickett CJ, editors. Inorganic Electrochemistry. 7a. Wiley-VCH; Weinheim: 2006. pp. 401–404. and references therein. [Google Scholar]
- 49.Byegård J, Skarnemark G, Skålberg M. J. Radioanal. Nucl. Chem. 1999;241:281–290. [Google Scholar]
- 50.Tweedle MF, Gaughan GT, Hagen J, Wedeking PW, Sibley P, Wilson LJ, Lee DW. Nucl. Med. Biol. 1988;15:31–36. doi: 10.1016/0883-2897(88)90157-2. [DOI] [PubMed] [Google Scholar]
- 51.Neupert-Laves K, Dobler M. Helv. Chim. Acta. 1977;60:1861–1871. doi: 10.1002/hlca.19770600604. [DOI] [PubMed] [Google Scholar]
- 52.Hughes BB, Haltiwanger RC, Pierpont CG, Hampton M, Blackmer GL. Inorg. Chem. 1980;19:1801–1803. [Google Scholar]
- 53.Neupert-Laves K, Dobler M. J. Crystallogr. Spectrosc. Res. 1982;12:271–286. [Google Scholar]
- 54.Vardosanidze TO, Sokol VI, Sobolev AN, Shvelashvili AE, Porai-Koshits MA. Zh. Neorg. Khim. 1985;30:1745–1751. [Google Scholar]
- 55.Zhorzholiani NB, Shvelashvili AE, Sobolev AN, Vardosanidze IO. Soobshch. Akad. Nauk Gruzi. SSR. 1986;123:541–544. [Google Scholar]
- 56.Hagen KS. Angew. Chem., Int. Ed. Engl. 1992;31:764–766. [Google Scholar]
- 57.Di Vaira M, Mani F, Stoppioni P. J. Chem. Soc., Dalton Trans. 1992:1127–1130. [Google Scholar]
- 58.Gultneh Y, Farooq A, Karlin KD, Liu S, Zubieta J. Inorg. Chim. Acta. 1993;211:171–175. [Google Scholar]
- 59.Brychcy K, Jens KJ, Tilset M, Behrens U. Chem. Ber. 1994;127:991–995. [Google Scholar]
- 60.Brooker S, McKee V, Metcalfe T. Inorg. Chim. Acta. 1996;246:171–179. [Google Scholar]
- 61.Reid HON, Kahwa IA, White AJP, Williams DJ. Inorg. Chem. 1998;37:3868–3873. doi: 10.1021/ic980035o. [DOI] [PubMed] [Google Scholar]
- 62.Bu X-H, Chen W, Mu L-J, Zhang Z-H, Zhang R-H, Clifford T. Polyhedron. 2000;19:2095–2100. [Google Scholar]
- 63.Junk PC, Smith MK, Steed JW. Polyhedron. 2001;20:2979–2988. [Google Scholar]
- 64.Amin S, Voss DA, Jr., Horrocks WD, Jr., Lake CH, Churchill MR, Morrow JR. Inorg. Chem. 1995;34:3294–3300. [Google Scholar]
- 65.Aime S, Barge A, Bruce JI, Botta M, Howard JAK, Moloney JM, Parker D, de Sousa AS, Woods M. J. Am. Chem. Soc. 1999;121:5762–5771. [Google Scholar]
- 66.Hancock RD, Reibenspies JH, Maumela H. Inorg. Chem. 2004;43:2981–2987. doi: 10.1021/ic030277a. [DOI] [PubMed] [Google Scholar]
- 67.Bombieri G, Marchini N, Ciattini S, Mortillaro A, Aime S. Inorg. Chim. Acta. 2006;359:3405–3411. [Google Scholar]
- 68.a) Shannon RD, Prewitt CT. Acta Crystallogr. 1969;B25:925–946. [Google Scholar]; b) Shannon RD, Prewitt CT. Acta Crystallogr. 1970;B26:1046–1048. [Google Scholar]
- 69.Inoue MB, Navarro RE, Inoue M, Fernando Q. Inorg. Chem. 1995;34:6074–6079. [Google Scholar]
- 70.Mikuriya M, Hatano Y, Asato E. Chem. Lett. 1996:849–850. [Google Scholar]
- 71.Hureau CH, Blanchard S, Nierlich M, Blain G, Rivière E, Girerd J-J, Anxolabéhère-Mallart E, Blondin G. Inorg. Chem. 2004;43:4415–4426. doi: 10.1021/ic035332u. [DOI] [PubMed] [Google Scholar]
- 72.Riley DP, Weiss RH. J. Am. Chem. Soc. 1994;116:387–388. [Google Scholar]
- 73.Liu Y-C, Ma S-L, Guo Q-L, Zhang J, Xu M-Q, Zhu W-X. Inorg. Chem. Commun. 2005;8:574–577. [Google Scholar]
- 74.Dees A, Zahl A, Puchta R, van Eikema Hommes NJR, Heinemann FW, Ivanović-Burmazović I. Inorg. Chem. 2007;46:2459–2470. doi: 10.1021/ic061852o. [DOI] [PubMed] [Google Scholar]
- 75.Riesen A, Zehnder M, Kaden TA. Helv. Chim. Acta. 1986;69:2067–2073. [Google Scholar]
- 76.Relaxivities for small molecule complexes of Mn2+ under similar conditions are typically in the range of 1.5-6 mM−1s−1.
- 77.Jackels SC, Durham MM, Newton JE, Henniger TC. Inorg. Chem. 1992;31:234–239. [Google Scholar]
- 78.Balogh E, He Z, Hseih W, Liu S, Tóth E. Inorg. Chem. 2007;46:238–250. doi: 10.1021/ic0616582. [DOI] [PubMed] [Google Scholar]
- 79.Ducommun Y, Newman KE, Merbach AE. Inorg. Chem. 1980;19:3696–3703. [Google Scholar]
- 80.ENOTA is 1,2-bis[1-(1,4,7-triazacyclononyl-1,4-diacetic acid)]ethane. See reference 23.
- 81.Luz Z, Meiboom S. J. Chem. Phys. 1964;40:1058–1066. [Google Scholar]
- 82.Luz Z, Meiboom S. J. Chem. Phys. 1964;40:1066–1068. [Google Scholar]
- 83.Gossuin Y, Roch A, Muller RN, Gillis P. J. Magn. Reson. 2002;158:36–42. doi: 10.1016/s1090-7807(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 84.Freed JH. J. Chem. Phys. 1978;68:4034–4037. [Google Scholar]
- 85.Tóth E, Helm L, Merbach AE. Topics in Curr. Chem. 2002;221:61–101. [Google Scholar]
- 86.Abragam A. Principles of Nuclear Magnetism. Oxford University Press; Oxford: 1961. [Google Scholar]
- 87.Fries PH, Belorizky E. J. Phys. (Paris) 1978;39:1263–1282. [Google Scholar]
- 88.Albrand JP, Taieb MC, Fries PH, Belorizky E. J. Chem. Phys. 1983;78:5809–5815. [Google Scholar]
- 89.Gilbert J, Kassab D, Pawlow JH, Santora BP, Fiel RJ, Joshi VN, Kozik M. Inorg. Chem. 1995;34:924–927. [Google Scholar]
- 90.Burai L, Tóth E, Bazin H, Benmelouka M, Jászberényi Z, Helm L, Merbach AE. J. Chem. Soc., Dalton Trans. 2006:629–634. doi: 10.1039/b506700f. [DOI] [PubMed] [Google Scholar]
- 91.Jocher CJ, Botta M, Avedano S, Moore EG, Xu J, Aime S, Raymond KN. Inorg. Chem. 2007;46:4796–4798. doi: 10.1021/ic700399p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siddiqui SA, Chipendo PI, Westmoreland TD. Abstracts of Papers; 235th National Meeting of the American Chemical Society; New Orleans, LA. Apr 6-10, 2008; Washington, D.C.: American Chemical Society; 2008. INOR 176. [Google Scholar]
- 93.Siddiqui SA, Chipendo PI, Westmoreland TD. manuscript in preparation. [Google Scholar]
- 94.Xie D, Kennan RP, Gore JC. Proc. Intl. Soc. Mag. Reson. Med. 2001;9:890. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.