Abstract
Dementia can be caused by different diseases including Alzheimer's disease (AD), dementia with Lewy bodies (DLB), or both (AD + DLB). University of Kentucky AD Center pathologically-diagnosed AD and AD + DLB cases were evaluated who had three or more longitudinal antemortem mental status examinations (n = 156). Patients with important concomitant pathology (n = 5) or patients that were profoundly demented at recruitment (intake MMSE < 20; n = 86) were excluded to strengthen our ability to test the association of specific clinical and pathological indices. Patients with pathologically-diagnosed AD + DLB (n = 25) lost cognitive capacity faster than patients with AD alone (n = 40). In both diseases, treatment with acetylcholinesterase inhibitors was associated with a slower rate of cognitive decline.
Keywords: Acetycholinesterase, acetylcholinesterase inhibitors, Alzheimer's disease, cholinesterase, dementia with Lewy bodies, neuropathology
Introduction
For over a decade, acetylcholinesterase inhibitors (AChEIs) have been the most prescribed drugs for dementia. patients [1,2]. Nonetheless, there is controversy about the efficacy of AChEIs, and about the degree to which patients with different types of pathologies respond to treatment. Three underlying diseases account for most dementia cases in Western countries: Alzheimer's disease (AD), dementia with Lewy bodies (DLB), and patients with both (AD + DLB) [3,4]. Neuropathologically, AD is defined by amyloid plaques and neurofibrillary tangles while DLB reflects the presence of cortical Lewy bodies and neurites. Both diagnoses also require a history of clinical dementia. Prior studies have produced differing results about the rates of cognitive decline in AD versus AD + DLB patients [5,6], and about how the different groups respond to AChEIs [7–9].
We sought to evaluate how cognitive decline in these different dementia subytpes is influenced by AChEI treatment. Here we describe a novel strategy to define retrospectively, but quantitatively, the impact of AChEI therapy. The University of Kentucky Alzheimer's Disease Center (UK ADC) follows a clinical group longitudinally with a > 90% autopsy rate [10,11]. In the current study, we employed a nonlinear mixed effects regression model [12] to correlate the rate of premortem cognitive decline with different groups based on definitive neuropathological diagnosis. This approach allows for biologically informative comparisons about the variables associated with faster and slower rates of cognitive decline.
Materials And Methods
The details of UK ADC inclusion criteria, recruitment, and testing have been described [10,13]. The present study focuses on the Mini-Mental State Examination (MMSE) scores as the ‘severity metric’ for premortem cognitive decline. MMSE scores were recorded at each clinic visit. All of the non-demented subjects used in this study were contacted at 6-month intervals, had detailed annual mental status testing, and had neurological and physical examinations at least biannually. Any patient that became demented continued to receive mental status testing and neurological examinations annually.
All of the cases derive from our database of longitudinally-followed patients that have come to autopsy with a UK ADC consensus conference diagnosis of AD or AD + DLB (n = 212) as defined by consensus criteria [4,14]. Patients satisfying the following criteria were included in the study: patients had undergone a minimum of three longitudinal MMSE evaluations (n = 56 excluded); each patient had on first evaluation an MMSE > 20 (additional n = 86 excluded) so that relatively early disease course would be included in the study; and patients were excluded only if there was other confounding subtype(s) of prevalent concomitant neuropathology (cerebrovascular disease, hippocampal sclerosis, etc.) as described previously [10]. More than 60% of patients had concomitant neuropathology as described previously and were excluded from the study [10]. After the application of exclusion criteria, the remaining 65 patients (all of whom were Caucasian) in this study had a total of 430 assessments (average 6.6; range 3–16). By neuropathology, patients were designated as either AD (n = 45) or AD + DLB (n = 20).
In modeling the post-diagnosis decline in the MMSE scores over time, the following two characteristics of the 430 observations had to be accounted for: (i) between and within patient variability; and (ii) floor and ceiling effects for the scores. The floor score was zero while the ceiling score varied by participant. To account for (i) and (ii) the nonlinear mixed (i.e., fixed and random) effects regression model based on Martins and colleagues [12] was fitted to these data. This model is a three-parameter logistic regression model
where “Yit” represents the MMSE score for the ith participant at time t years post diagnosis. The parameter a is the highest MMSE score, or asymptote, and varies by participant; the parameter b is a scaling effect representing 75% of the highest MMSE score; and the parameter c is the midpoint of the decline curve, or 50% of the highest MMSE score. We assume that the parameters b and c depend only on the fixed effects, while parameter a depends on both fixed and random effects. The fixed effects or covariates of interest are gender, educational level (high school or less versus more than high school), apolipoprotein E (ApoE) genotype (presence or absence of at least one ApoE 4 allele), treatment with a cholinesterase inhibitor (yes or no), and pathology (pure AD versus a mix of AD and DLB). The random effects, within as well as between patient variability, are each assumed to follow an independent normal distribution with mean zero and unknown variance. The purpose of the modeling was to determine how parameters a, b, and c depended on these covariates after accounting for the two sources of variability. All models were fitted using PROC NLMIXED in SAS 9.1.3®.
Patient demographics and pathology subtype information are shown in Table 1. The two groups (AD and AD + DLB) are comparable on baseline age, education level, gender, presence of at least one ApoE 4 allele, family history of first-degree relative with dementia, marital status, and duration of dementia. There was also no significant between-group differences in the number of plaques and tangles counted (data not shown). As the sample comprises only dementia patients, over half of the patients in the study express the ApoE 4 allele (53.8%). Table 2 shows a comparison between the same parameters as Table 1, except comparing treatment (AChEIs) and non-treatment groups. AChEI use was operationalized according to whether or not the patient had ever received the treatment according to our records. Note that none of the demographic variables show significant between-group differences.
Table 1.
Patient demographics by pathology group
| Demographic characteristic | Pure AD (n = 45) |
AD + DLB (n = 20) |
P-value |
|---|---|---|---|
| Baseline Age (mean ± s.d.) | 77.7 (± 7.9) | 74.0 (±9.6) | 0.1 |
| Female | 28 (62.2%) | 12 (60.0%) | 0.87 |
| At least one APOE 4 allele | 24 (53.3%) | 11 (55.5%) | 0.9 |
| Family history | 19 (42.2%) | 8 (40.0%) | 0.87 |
| Married (not widowed or divorced) | 23 (51.1%) | 11 (55.0%) | 0.77 |
| Duration, years (mean ± s.d.) | 4.3 (± 1.9) | 4.9 (± 2.3) | 0.4 |
| Education beyond high school | 31 (68.9%) | 14.0 (70.0%) | 0.93 |
Table 2.
Patient demographics by (AChEIs) treatment group
| Demographic characteristic | Treated (N = 47) |
Untreated (N = 18) |
P-value |
|---|---|---|---|
| Pure AD pathology | 33 (70.2%) | 12 (66.7%) | 0.78 |
| Baseline age (mean ± s.d.) | 76.6 (± 8.0) | 76.6 (±10.2) | 0.98 |
| Female | 28 (59.6%) | 12 (66.7%) | 0.60 |
| Education beyond high school | 32 (68.1%) | 13 (72.2%) | 0.75 |
| At least one APOE 4 allele | 25 (53.2%) | 10 (55.6%) | 0.86 |
| Family history | 20 (42.6%) | 7 (38.9%) | 0.79 |
| Married (not widowed or divorced) | 26 (55.3%) | 8 (44.4%) | 0.43 |
| Duration, years (mean ± s.d.) | 4.4 (± 2.4) | 4.7 (± 1.9) | 0.64 |
The fit of the three parameter logistic model involving only significant covariates is summarized in Table 3. Parameter a varies with gender and pathology, parameter b varies with ApoE 4, treatment, education level, and pathology while parameter c varies with ApoE status, AChEI treatment, gender, and pathology. To motivate and illustrate the model, refer to Fig. 1. In Fig. 1A, the nonlinear decline in MMSE scores is evident in the profile of MMSE scores over time for a typical subject who experienced a decline of approximately 3 points per year. Since the model is fitted to scores obtained post diagnosis of disease, Fig. 1B illustrates this decline in scores for three representative individual patients: one with no treatment and a mixed pathology, one with no treatment and pure AD pathology, and one with treatment and pure AD pathology.
Table 3.
Beta estimates and standard errors for the significant covariates in the three parameter logistic model
| Parameter | Covariate | Beta (standard error) | P-value* |
|---|---|---|---|
| a = asymptote | Intercept | 23.016 (1.384) | < 0.0001 |
| Female gender | 6.398 (1.762) | 0.0006 | |
| Mixed pathology | 7.623 (2.595) | 0.0046 | |
| b = scale | Intercept | 0.424 (0.055) | < 0.0001 |
| APOE4 present | 0.258 (0.040) | < 0.0001 | |
| AChEI treated | −0.114 (0.041) | 0.0073 | |
| High education | −0.094 (0.041) | 0.026 | |
| Mixed pathology | −0.084 (0.042) | 0.053 | |
| c = midpoint | Intercept | 6.825 (0.500) | < 0.0001 |
| APOE 4 present | −1.664 (0.381) | < 0.0001 | |
| AChEI treated | 0.960 (0.263) | 0.0005 | |
| Female gender | −2.747 (0.361) | < 0.0001 | |
| Mixed pathology | −2.301 (0.460) | < 0.0001 | |
| Variability | Between | 11.160 (0.833) | – |
| Within | 19.142 (0.469) | – |
P values based on t-tests with 64 degrees of freedom
Fig. 1.
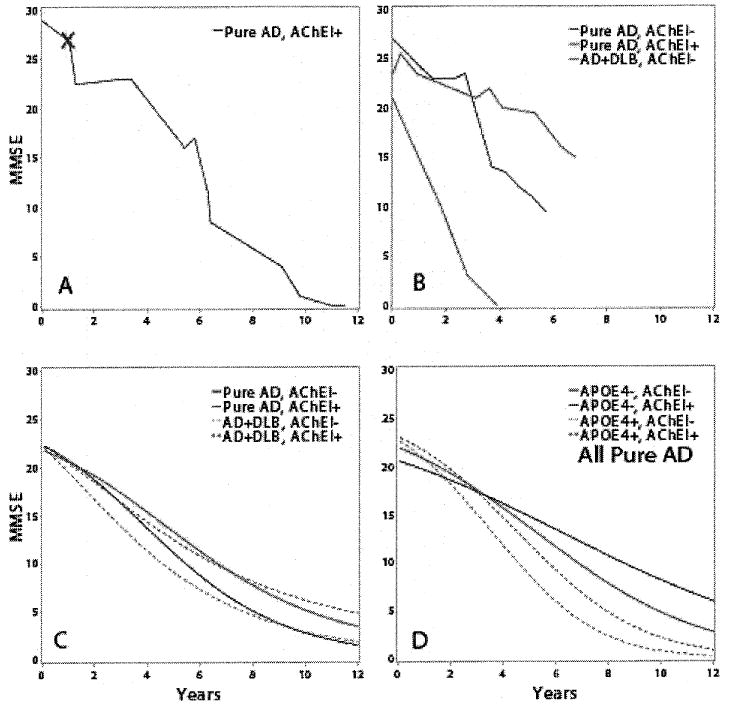
The change of MMSE scores over time is depicted as raw data from dementia patients (A–B), or as statistically rendered group data that allows between-cohort comparisons (C–D). A) MMSE scores from a representative patient (female, no history of AChEI use) with AD pathology at autopsy. Red ‘X’ is time of diagnosis. B) Three traces show representative examples of separate female ApoE-matched individual patients with AD (no AChEI; black); AD (with AChEI; red); and AD + DLB (no AChEI; green). C) Groups of patients with AD (n = 45) and AD + DLB (n = 20) are compared using statistical rendering; the AD + DLB patients decline faster (p < 0.01 for asymptote and midpoint parameters). Rendered curves allow a comparison between the effect of AChEIs on patients with AD pathology versus AD + DLB. AD/AChEl(−): black; AD/AChEI(+): red; AD + DLB/AChEI(−): green; AD + DLB/AChEI(+): blue. D) Within groups, the effect of ApoE4 allele(s) and AchEI therapy are significant. Rendered curves depicting MMSE decline that combine Pure AD patients into four groups. ApoE4(−)/AChEI(−): black; ApoE4(−)/AChEI(+): red; ApoE4(+)/AChEI(−): green; ApoE4(+)/AChEI(+): blue.
Figure 1C uses the model in Table 2 to illustrate the effect of pathology taking into account treatment status. Figure 1D illustrates a larger effect for treatment after accounting for the same ApoE 4 status. It also illustrates that the largest effect is due to ApoE 4 taking into account the treatment status. Untreated AD patients lose half their maximum score approximately 1.4 or 1.7 years earlier when compared to treated AD patients with and without at least one ApoE 4 allele. AD patients harboring the ApoE4 allele lose half their scores 2.4 and 2.7 years earlier when compared to untreated and treated patients who do not express a 4 allele. ApoE status has a larger effect on decline than treatment status. A similar relationship holds for patients with mixed (AD + DLB) pathology.
Discussion
Using the methods outlined above, we studied the rate of MMSE decline associated with the administration of AChEIs stratified by neuropathological findings of AD or AD + DLB. To allow comparisons between groups, we combined the data from multiple longitudinal tests on patients from the UK ADC autopsy cohort and then applied a nonlinear mixed effects regression model. Patients with AD + DLB declined faster in cognition than ‘pure’ AD patients. More importantly, AChEIs appear to slow dementia progression irrespective of whether the postmortem diagnosis was AD or AD + DLB.
Several factors constituted limitations or potential confounds to the current study. One limitation to this study was the use of the MMSE exam as our sole metric for the severity of cognitive decline. This is an important caveat and the limitations of this metric need to be kept in mind. However, in many research centers and in different contexts, MMSE has been found to be a robust indicator of dementia severity [15–17]. Our clinical cohort is relatively homogenous (white, higher educated, and aged), and we find no evidence for demographic bias in AChEI prescriptions (Table 2). The number of cases evaluated in the study was relatively small at 65; however, this number allowed for statistical significance to be achieved. Further, there may be selection bias with subjects and families perceiving benefit who are likely to remain in therapy. Finally, we did not stratify our results relative to the type of AChEI or in regard to any additional therapies.
Among the strengths of our study is the cross-comparison referent to cognitive decline of groups defined by well-characterized neuropathological outcomes. We assessed the change in MMSE scores over time using a statistical model based on the algorithm of Martins et al. [12]. We were able to find differences between the evaluated groups that were statistically significant and relevant in clinical and biological terms. We also excluded patients with prevalent confounding pathologies because they are a source of systematic biases [10].
Some prior studies reported that patients with pure AD decline cognitively at the same rate as patients with AD + DLB pathology (reviewed in [5]). By contrast, we find that patients with AD + DLB decline more rapidly. This finding agrees with reports by Olichney and coworkers [18] and Kraybill and coworkers [19] indicating that patients with AD + DLB decline faster than patients with pure AD. These data underscore the need for autopsy confirmation in neurodegenerative disease cases. Indeed, prospective studies of drug efficacy will face confounds if randomization results in disparate numbers of patients with DLB pathology in the study arms.
We also found a significant effect on rate of cognitive decline associated with the administration of AChEIs. Specifically, patients with dementia taking AChEI show MMSE scores that decline at a slower rate than patients not taking the drugs. Some reports suggest a different effect of AChEIs on patients with AD versus AD + DLB [20]. The present results suggest a beneficial effect regardless of the underlying presence of mixed pathology.
Acknowledgments
We are profoundly grateful to all of the participants in our longitudinal aging study and to the patients with neurodegenerative diseases in our Alzheimer's Disease Center's research clinic. We thank Ela Patel, Ann Tudor, Dr. Huaichen Liu, Paula Thomason, and Sonya Anderson, for technical support, and Nancy Stiles, MD and Allison Caban-Holt, PhD, for clinical evaluations.
This study was supported by grant 5-P30-AG028383 and K08 NS050110 from the National Institutes of Health, Bethesda, MD, and a grant from the Healy Family Foundation.
Dr. Greg Jicha is a member of the speakers' bureau for Pfizer, Inc.
Footnotes
Copyright Compliance Notice: The copyright law of the United States (Title 17, U. S. Code) governs the making of photocopies or other reproductions of copyrighted materials. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use,” that user may be liable for copyright infringement.
This institution reserves the right to refuse to accept a copying order, if, in its judgment, fulfillment of the order would involve violation of copyright law.
References
- 1.Gruber-Baldini AL, Stuart B, Zuckerman IH, Simoni-Wastila L, Miller R. Treatment of dementia in community-dwelling and institutionalized medicare beneficiaries. J Am Geriatr Soc. 2007;55:1508–1516. doi: 10.1111/j.1532-5415.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- 2.Grutzendler J, Morris JC. Cholinesterase inhibitors for Alzheimer's disease. Drugs. 2001;61:41–52. doi: 10.2165/00003495-200161010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Heidebrink JL. Is dementia with Lewy bodies the second most common cause of dementia? J Geriatr Psychiatry Neurol. 2002;15:182–187. doi: 10.1177/089198870201500402. [DOI] [PubMed] [Google Scholar]
- 4.Rahkonen T, Eloniemi-Sulkava U, Rissanen S, Vatanen A, Viramo P, Sulkava R. Dementia with Lewy bodies according to the consensus criteria in a general population aged 75 years or older. J Neurol Neurosurg Psychiatry. 2003;74:720–724. doi: 10.1136/jnnp.74.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern Y, Jacobs D, Goldman J, Gomez-Tortosa E, Hyman BT, Liu Y, Troncoso J, Marder K, Tang MX, Brandt J, Albert M. An investigation of clinical correlates of Lewy bodies in autopsy-proven Alzheimer disease. Arch Neurol. 2001;58:460–465. doi: 10.1001/archneur.58.3.460. [DOI] [PubMed] [Google Scholar]
- 6.Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67:1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]
- 7.Bhasin M, Rowan E, Edwards K, McKeith I. Cholinesterase inhibitors in dementia with Lewy bodies: a comparative analysis. Int J Geriatr Psychiatry. 2007;22:890–895. doi: 10.1002/gps.1759. [DOI] [PubMed] [Google Scholar]
- 8.Pakrasi S, Thomas A, Mosimann UP, Cousins DA, Lett D, Burn DJ, O'Brien JT, McKeith IG. Cholinesterase inhibitors in advanced Dementia with Lewy bodies: increase or stop? Int J Geriatr Psychiatry. 2006;21:719–721. doi: 10.1002/gps.1547. [DOI] [PubMed] [Google Scholar]
- 9.Shea C, MacKnight C, Rockwood K. Donepezil for treatment of dementia with Lewy bodies: a case series of nine patients. Int Psychogeriatr. 1998;10:229–238. doi: 10.1017/s1041610298005341. [DOI] [PubMed] [Google Scholar]
- 10.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal aging: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. Gerontologist. 2001;41:716–722. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- 12.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65:1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- 13.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 14.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91–94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 15.Cherbuin N, Anstey KJ, Lipnicki DM. Screening for dementia: a review of self- and informant-assessment instruments. Int Psychogeriatr. 2008;20:431–458. doi: 10.1017/S104161020800673X. [DOI] [PubMed] [Google Scholar]
- 16.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 17.Mendiondo MS, Ashford JW, Kryscio RJ, Schmitt FA. Modelling mini mental state examination changes in Alzheimer's disease. Stat Med. 2000;19:1607–1616. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1607::aid-sim449>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology. 1998;51:351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- 19.Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, Kukull WA, Leverenz JB, Cherrier MM. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel W, Caligiuri M, Galasko D, Lacro J, Marini M, McClure FS, Warren K, Jeste DV. Better cognitive and psychopathologic response to donepezil in patients prospectively diagnosed as dementia with Lewy bodies: a preliminary study. Int J Geriatr Psychiatry. 2000;15:794–802. doi: 10.1002/1099-1166(200009)15:9<794::aid-gps178>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]


