Abstract
Background
The paraventricular nucleus of the hypothalamus (PVN) has emerged as one of the most important autonomic control centers in the brain, with neurons playing essential roles in controlling stress, metabolism, growth, reproduction, immune, and other more traditional autonomic functions (gastrointestinal, renal and cardiovascular).
Objectives
Traditionally the PVN was viewed very much as a nucleus in which afferent inputs from other regions were faithfully translated into changes in single specific outputs whether those were neuroendocrine or autonomic. Here we will present data which suggest that PVN in fact plays significant and essential roles in integrating multiple sources of afferent input and sculpting an integrated autonomic output by concurrently modifying the excitability of multiple output pathways. In addition we will highlight recent work which suggests that dysfunction of such intranuclear integrative circuitry may contribute to the pathology of conditions such as hypertension and congestive heart failure.
Conclusions
This review highlights data showing that individual afferent inputs (SFO), signaling molecules (orexins, adiponectin), and interneurons (glutamate/GABA), all have the potential to influence (and thus coordinate) multiple PVN output pathways. We also highlight recent studies showing that modifications in this integrated circuitry may play significant roles in the pathology of diseases such as congestive heart failure and hypertension.
1. Introduction
The paraventricular nucleus of the hypothalamus (PVN) has over the past 20 years slowly emerged as one of the most important hypothalamic autonomic control centers, with neurons in this bilateral structure playing essential roles in neuroendocrine/autonomic regulation [1-5]. In the early 1980s anatomical studies had already described the basic architecture of the PVN identifying a complex CNS nucleus comprising cell bodies of neurons which potentially played essential neuroendocrine roles in controlling the hypothalamo-pituitary-adrenal (HPA) axis (corticotropin releasing hormone (CRH) neurons projecting to the median eminence), the thyroid axis (thyrotropin releasing hormone (TRH) neurons projecting to the median eminence), the reproductive axis (dopamine and oxytocin neurons projecting to the median eminence or posterior pituitary), growth and development (somatostatin neurons projecting to the median eminence), the regulation of body fluid balance (vasopressin and oxytocin neurosecretory cells projecting to the posterior pituitary), as well as gastrointestinal and cardiovascular function through traditional autonomic outputs (neurons projecting to caudal medullary and spinal autonomic control centers). Afferent inputs to the PVN from many important integrative centers of the hypothalamus (subfornical organ, medial septum/diagonal band of broca, median preoptic nucleus, arcuate nucleus, suprachiasmatic nucleus), pons (lateral parabrachial nucleus), and medulla (nucleus tractus solitarius, dorsal motor nucleus of the vagus, and the ventrolateral medulla) had also been identified.
In addition the functional unit of the PVN had been suggested to contain a significant proportion of ‘integrative’ interneurons consisting glutamate (within the nucleus) and gama-amino-butyric acid (GABA) (primarily in the halo zone surrounding the nucleus) containing cells. We will first review here recent progress in elucidating the complex intranuclear circuitry through which the PVN exerts control of these seemingly diverse physiological functions and will also consider recent work suggesting that pathological changes in the functioning of this circuitry which has been suggested to contribute to clinical conditions such as congestive heart failure.
2. Functional Circuitry of the Paraventricular Nucleus
As recently as the early 1990s the PVN was regarded as a heterogeneous nucleus composed of magnocellular and parvocellular neurons, the latter subgroup consisting of neurosecretory cells projecting to the median eminence as well as caudally projecting preautonomic cells (neurons projecting to either medullary (nucleus tractus solitarius and rostral ventrolateral medulla) or spinal (intermediolateral cell column column) autonomic control centres). The ability to combinesophisticated immunochemical and anatomical labeling techniques with patch clamp techniques had resulted in a broad characterization of these cells into Type I (putative magnocellular projecting to the posterior pituitary) and Type II (putative parvocellular – projecting to either the median eminence or medullary/spinal autonomic centers) neurons based upon their electrophysiological profiles [6] as illustrated in Figure 1. At the time there was little definitive evidence of interneurons in this nucleus, although speculation of their existence was growing. Although there was an abundant literature describing extensive afferent inputs to the PVN from other regions of the hypothalamus, there was minimal, if any, clear data in support of extensive integration occurring within the nucleus as a consequence of intranuclear circuitry, and thus the PVN was considered largely a throughput center. In this model of the PVN very little cross-integration occurred between systems, other than the summation by output neurons of synaptic inputs from identified afferents with the end result that synaptic input was thought to be channelled in a rather linear fashion from nuclei of origin by PVN system specific efferents. These efferent thus acted as a number of separate the autonomic and neuroendocrine output centers controlling distinct and separate physiological systems. The importance of the PVN therefore lay in the maintenance of fidelity of information and directing signals appropriately.
Figure 1.
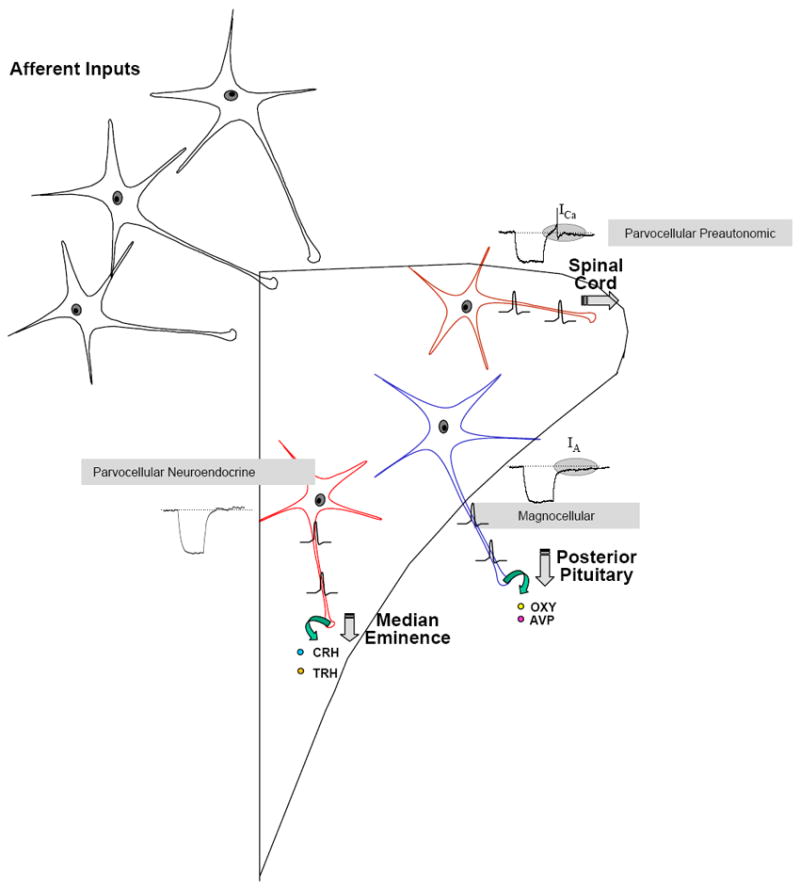
This schematic illustrates the classical view of the organization of the paraventricular nucleus of the hypothalamus. Afferent inputs originating from diverse regions of the CNS make synaptic contacts with one of the three primary functional groups of output neurons of the nucleus. The magnocellular neurons which synthesize and release vasopressin (AVP) or oxytocin (OXY) have been electrophysiologically characterized by the distinctive delayed return to baseline (see highlighted region in inset) following a depolarizing pulse which indicates the presence of a dominant transient potassium conductance known as IA. In contrast the parvocellular preautonomic neurons which project to autonomic control centers of the medulla and spinal cord are characterized by a different electrophysiological fingerprint manifested by a low threshold calcium spike (see highlighted region in inset) following such depolarizing pulses which is the result of activation of a T-type calcium conductance in this subpopulation of neurons. Finally the parvocellular neuroendocrine cells, which express neither of these conductances, synthesize CRH and TRH (as well as other factors), project to the median eminence, where they release these hypophysiotropic factors into the hypophysial portal circulation.
In view of the PVN’s central role in autonomic and neuroendocrine regulation and its extensive connections throughout the hypothalamus and brainstem, studying neurotransmission within this nucleus was becoming increasingly relevant. PVN neurons had been known for some time to be sensitive to both glutamate and GABA and numerous studies had examined the functional roles of these transmitters within the PVN [7;8]. Anatomical studies have now confirmed the presence of glutamate interneurons within the PVN (Figure 2) although it should be emphasized that glutamate inputs to the PVN neurons also originate in other hypothalamic nuclei [9]. In addition anatomical studies have reported extensive distribution of GABA interneurons in the halo zone (including anterior hypothalamic and perifornical regions) surrounding the PVN (Figure 2) as well as occasional GABA neurons within the nucleus [10]. Our inability to routinely record from these subpopulations of neurons had made it difficult to investigate their functional roles, although the recent application of single cell reverse transcriptase polymerase chain reaction (RT-PCR) techniques to the identification of recorded neurons circumvent this difficulty, and should lead to a better understanding of the integrative roles of these neurons in the PVN (see below also). Although they will not be discussed in-depth here it should also be emphasized that recent studies have also highlighted important roles for both dendritic release of peptides produced in the PVN neurons [11;12], and glial cell release of amino acid signaling molecules [13] in shaping the integrated outputs of the PVN.
Figure 2.
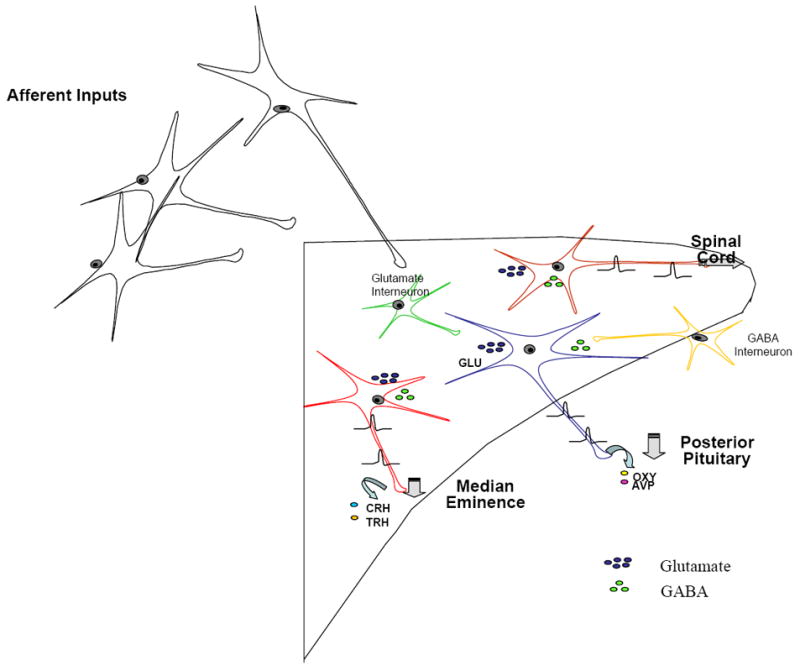
This schematic illustrates modifications to the classical view of the organization of the paraventricular nucleus of the hypothalamus associated with recent work identifying important roles for both glutamate and GABA interneurons in controlling the excitability of magnocellular and parvocellular output neurons.
2.1 Peptidergic Regulation of PVN Output Neurons
In addition to this work demonstrating a complex intranuclear circuitry within the PVN many studies were beginning to identify potentially important roles for a number of non-classical peptidergic neurotransmitters in controlling the excitability of magnocellular and parvocellular neurons within the PVN. A complete description of all such peptidergic input systems is beyond the scope of this paper. We will use three characterized peptidergic input systems to highlight the potential roles of neuropeptides in regulating the excitability of the PVN magnocellular and parvocellular neurons.
2.1.1 Angiotensin II
Angiotensin II (ANG) was first suggested to be a neurotransmitter utilized by subfornical organ neurons projecting to the PVN following anatomical studies which identified this projection as ‘angiotensinergic’ using double labeling anatomical techniques [14]. Studies reporting effects of exogenous ANG administered into the PVN on hormone secretion [15;16], and cardiovascular regulation [17;18], added further credibility to this suggestion. Functional electrophysiological studies demonstrated that activation of this pathway resulted in increases in the excitability of both magnocellular [19] and parvocellular [19-22] neurons in the PVN. Later studies using non-peptidergic angiotensin type 1 receptor (AT1) antagonists showing that treatment with such pharmacological tools blocked these excitatory effects [23;24] confirmed the role of angiotensin II as a neurotransmitter used by this projection. Additional work focusing on the mechanisms through which angiotensin II modulated the excitability of PVN neurons examined the effects of this peptide on ion channels expressed in PVN neurons. Interestingly, these studies showed that while ANG specifically inhibited the transient potassium conductance IA (while there were not effects on the delayed rectifier IK) in magnocellular neurons, the depolarizing effects of ANG on these cells were the result of simultaneous actions on a glutamate interneuron as these effects were abolished by glutamate antagonists and blockers of synaptic transmission [25]. In contrast the effects of ANG on parvocellular neuroendocrine cells (putative CRH and TRH) appear to result from combined inhibition of IK and activation of a non selective cationic conductance (NSCC) [26]. These multiple direct effects of ANG on different subpopulations of neurons in the PVN are summarized schematically in Figure 3. Additional indirect effects of ANG in regulating the excitability of PVN neurons have been reported and will be discussed later when we consider effects mediated by glutamate and GABA interneurons (Figure 3).
Figure 3.
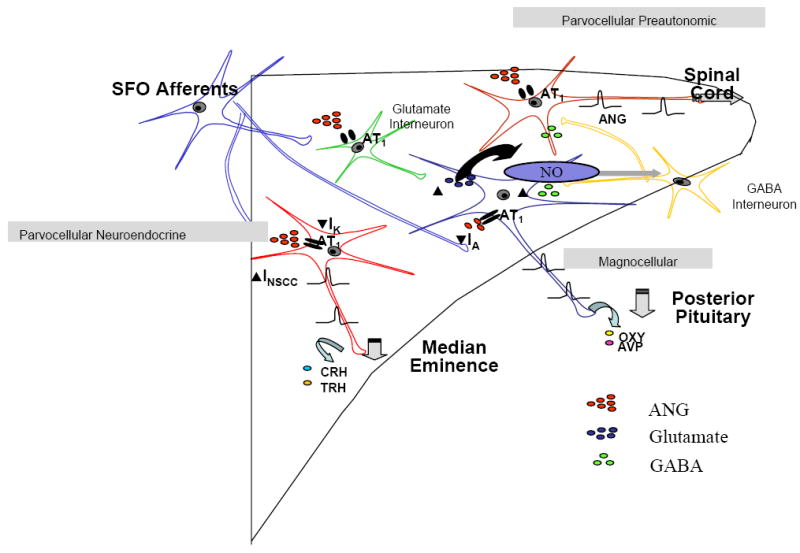
This schematic illustrates our current understanding of the multiple mechanisms through which ANG influences the excitability of magnocellular, parvocellular neuroendocrine and parvocellular preautonomic neurons in the paraventricular nucleus of the hypothalamus. In magnocellular neurons ANG has direct effects to inhibit a transient potassium conductance IA, and indirect depolarizing effects resulting from the activation of glutamate interneurons. In addition the ANG mediated activation of these neurons appears to be held in check by the subsequent production of nitric oxide which in turn activates a short loop inhibitory feedback pathway as a consequence of nitric oxide actions on GABA interneurons in the halo zone surrounding the nucleus. In contrast ANG appears to depolarize parvocellular neuroendocrine cells as a result of direct modulation of both a non selective cationic conductance and a sustained potassium conductance (likely IK), again with such activation likely activating NO/GABA feedback circuits. Finally, although there is no definitive evidence identifying ion channels regulated by ANG in PVN preautonomic neurons, effects have been shown to be AT1 receptor mediated and again appear to activate NO/GABA feedback loops, dysfunction of which have been implicated in congestive heart failure and hypertension.
2.1.2 Orexins
The discovery of the orexins (also known as hypocretins) identified a population of orexin producing neurons in the lateral hypothalamic/perifornical region of the hypothalamus involved in autonomic regulation which projected to diverse regions of the brain including the PVN [27;28]. Although these early studies identified specific roles for the orexins in the regulation of food intake and metabolism the later demonstration of a narcoleptic phenotype in orexin knockout mice [29] refocused the study of these peptides on the regulation of sleep/wakefulness and arousal. Orexinergic projections to the PVN have been shown to have clear effects on both autonomic regulation [30;31], and the control of the excitability of PVN magnocellular and parvocellular neurons [32;33]. Using whole cell patch clamp techniques we have reported both indirect effects on magnocellular neurons (see section of interneurons below), and direct (maintained in synaptic isolation) depolarizing effects of orexin A on PVN parvocellular neurons [33;34]. Using voltage clamp techniques we have shown that the latter direct action of orexins results from the modulation of a NSCC [33], thus suggesting a common point for co-regulation of the excitability of these neurons by both ANG and the orexins. This may explain the similar actions of ANG and orexin to not only stimulate sympathetic function but also arousal and the release of stress hormones.
2.1.3 Adiponectin
In addition to the peptides such as ANG and orexin which are believed to be released from angiotensinergic and orexinergic nerve terminals in the PVN respectively to elicit their physiological effects, a number of chemical messengers including steroids, cytokines and adipokines [35-38] have been shown to influence the excitability of PVN neurons presumably as a consequence of hormonal or paracrine delivery to their sites of action. One such regulator is the adipokine adiponectin which acts as an insulin sensitizing hormone [39;40], the circulating concentrations of which are inversely correlated with adipose tissue mass. ADP concentrations are also reduced in obesity-related diseases such as insulin resistance and metabolic syndrome. Treatment with ADP lowers hepatic gluconeogenesis, serum glucose and ameliorates insulin resistance in mice [40-43]. Recent studies demonstrating that microinjection of ADP into the cerebral ventricles of mice also caused changes in glucose, lipid handling, and body weight have identified the brain and the PVN in particular as a potentially important target for ADP actions [44].Two different adiponectin receptors have been identified, adipoR1 and adipoR2 [40], and we have confirmed expression of adiponectin receptors in the PVN and also have identified direct hyperpolarizing effects of adiponectin on magnocellular oxytocin secreting neurons in the PVN, cells which also express mRNA for both adiponectin receptors (shown using single cell RTPCR) [45]. In contrast, we have also obtained preliminary data showing depolarizing effects of adiponectin on parvocellular CRH and TRH neurons in hypothalamic slices (again identified using single cell RTPCR) and have correlated these observations with data from conscious animals showing that intracerebroventricular adiponectin also increases plasma ACTH concentrations (Hoyda et al. – unpublished). Adiponectin thus clearly has the ability to modulate different functional subpopulations of neurons in the PVN, effects which we would speculate contribute to and integrated physiological response to changes in this adipokine. Future studies will be needed to clarify not only the source of centrally active adiponectin (peripheral or CNS) but also the cellular mechanisms through which this adipokine influences the excitability of PVN neurons.
2.2 Roles for Interneurons in the regulation of PVN output Neurons
Noradrenergic effects on magnocellular PVN neurons were demonstrated to be the result of catecholamine actions on glutamate interneurons rather than direct effects on the magnocellular neuron itself [46]. Subsequently, a number of studies which have suggested important roles for glutamate interneurons in mediating the excitatory effects of ANG [25], orexin [33], and prokineticin 2 [47] on magnocellular neurons, capsaicin on preautonomic neurons [48], and on the response of neuroendocrine CRH neurons to restraint stress [49]. Work from our lab added further to the complexity by demonstrating that excitation of magnocellular neurons activated a nitric oxide/GABA mediated negative feedback system within the PVN [50;51] which effectively provided ultra-short loop regulation of the activity of PVN neurons, an intriguing system within a nucleus functionally focused of homeostatic regulation. In addition, these observations suggested important roles for GABA interneurons, the majority of which are localized in the halo zone surrounding the PVN [10], as an additional gatekeeper/integrator in controlling the excitability of PVN outputs. Important roles for these GABA interneurons have now been suggested in mediating inhibitory effects of adrenomedullin [52] on magnocellular, excitatory effects (presumably through disinhibition) of ANG on preautonomic [53], and IL-1β [37] on parvocellular neurons.
3. Pathophysiological Consequences of PVN Dysfunction
Along with our developing understanding of the complex neuronal circuitry within the PVN, and the multiple chemical messengers which together continuously modulate the excitability of PVN neurons, has come the realization that pathological changes in the function of this integrated circuitry may contribute to the development of certain disease processes.
One such example can be found in the critical role of PVN CRH neurons in controlling the hypothalamic-pituitary-adrenal (HPA) stress axis. Multiple neural (including inputs from limbic structures) and hormonal signals (glucocorticoids, vasoactive peptides, cytokines) converge on the final output pathway, the CRH neurons of the PVN. Intriguingly, PVN AT1 receptor expression is increased during repeated restraint stress and after 24 h of isolation stress [54;55]. Stimulation of these receptors is essential for HPA axis activation and in fact can be blocked by ANG antagonists but the ionic mechanisms which mediate these phenomena are not known. These data suggest that AT1 receptor blockade may have a place in the prevention and treatment of stress-related disorders. However, the role of ANG in modulating these complex inputs remains poorly understood and future studies examining how such inputs regulate the excitability of CRH neurons may throw important new light on understanding the roles of the areas which play such critical roles in the regulation of stress in the human population.
The conventional view of the pathophysiology of most cardiovascular diseases has until recently focused on pathological changes within the heart and/or vasculature itself as the most likely explanation for the onset and progression of these complex disorders. Innovative new theories however are emerging which hypothesize that the CNS likely plays a critical role in congestive heart failure (CHF) and hypertension. Intriguingly, recent work has suggested that dysfunctional hypothalamic feedback loops (the short loop NO feedback we have described above) play a critical in the development of CHF and hypertension [17].
Neurohumoral responses in CHF include increased thirst and sodium appetite, increased AVP release, increased ACTH and cortisol release, and increased sympathetic renal nerve activity [56]. Unfortunately, the mechanisms underlying autonomic/CNS dysfunction in CHF are not well established but clearly involve the hypothalamus and in particular the PVN. Perhaps the first study demonstrating that the PVN was involved in the pathogenesis of CHF was performed by Patel et al. who showed that metabolic activity with the parvocellular and magnocellular regions of the PVN was increased in ischemia-induced CHF models [57]. In fact, a number of neuroactive peptides have been shown to be elevated in CHF including ANG, adrenomedullin, atrial natriuretic peptide, aldosterone and pro-inflammatory cytokines all of which have documented actions within the PVN [58-63]. ANG levels known to be elevated in CHF act within the PVN to increase renal sympathetic nerve activity (RSNA), increased renin release, and ultimately increased peripheral ANG release [64]. Normally, the PVN is under potent inhibitory influence of GABA and NO but these feedback loops are thought to be dysfunctional in CHF resulting in exaggerated responses to glutamate, ANG and potentially all other excitatory inputs to these neurons. The consequences of such changes have been clearly demonstrated by studies showing that the mild pressor effects which normally occur in response to electrical stimulation of the PVN are dramatically increased when this GABA/NO feedback loop is blocked by injection of GABA antagonists or NOS inhibitors into the PVN [65;66].
The decreased expression of NOS in ischemia-induced models of CHF suggests that NO may also contribute to the increased sympathoexcitation and vasopressin levels associated with CHF [67]. We have shown that inhibition of NOS results in a decrease in inhibitory feedback in magnocellular neurons concomitant with an augmented excitatory response to ANG [68]. Whether or not a disruption of this pathway exists in CHF remains to be investigated but is a plausible explanation for the increase in AVP seen in these models. The observation of increased number of c-fos expressing magnocellular PVN neurons of CHF compared to sham operated rats further suggests enhanced activation of these neurosecretory cells [69]. Li et al. demonstrated that AT1 receptors within the PVN mediate an excitatory effect on renal sympathetic nerve discharge, arterial blood pressure, and heart rate [64]. Increases in RSNA result in elevated circulating levels of renin, ANG, and aldosterone, each of which are partly responsible for in the increase in intravascular volume observed in CHF. The expansion of blood volume seen in CHF has been shown to increase heart rate reflexively via volume receptors at the venous-atrial junctions of the heart [70]. This reflex response to an increase in plasma volume consists of a distinctive unique pattern of sympathetic activity in which the PVN is intimately involved. Neurons in the PVN show early gene activation on stimulation of atrial stretch receptors [70]. A similar pattern of cardiac sympathetic excitation and renal inhibition can be evoked by electrical stimulation of PVN neurons [70]. In CHF the atrial reflex is blunted and evidence suggests that this is a result of downregulation of NOS and NO production and reduced GABA activity in the PVN [71]. A complete review of the literature describing potential changes in PVN circuitry congestive heart failure is beyond the scope of this article although the reader is referred to a review by Patel [72].
The PVN is also known to play an important role in the regulation of blood pressure. Not only do PVN neurons synthesize, store, and secrete AVP from the posterior pituitary but they also send axonal projections to the brainstem (NTS, AP) and spinal cord (IML), which play significant roles in controlling autonomic output [1]. Indeed, certain forms of hypertension (eg.1 kidney 1 clip) are abolished in those animals, which undergo electrolytic lesion of the PVN [73]. ANG has also been suggested to play a central role in cardiovascular disease with elevated central concentrations of ANG in hypothalamus and expression of the AT1 receptor in the PVN of spontaneously hypertensive rats (SHR) [74-76].
Nitric oxide in the PVN has been shown to have an inhibitory effect on RSNA [77;78], while microinjection injection of NOS inhibitors into the PVN increases RSNA and elevates blood pressure dramatically in response to ANG or glutamate. This sympathoinhibitory effect of NO is eliminated by bicuculline supporting the role of GABA interneurons in mediating this action [65]. Interestingly, GABA receptor binding sites measured using quantitative autoradiography and GAD levels were significantly lower in the PVN of spontaneously hypertensive rats when compared with normotensive controls [79;80]. The basal firing rate of preautonomic PVN neurons also has been reported to be significantly decreased in normotensive versus SHR and both the frequency and amplitude of GABAergic spontaneously inhibitory postsynaptic currents were reduced in SHR [81]. These data suggest that the elevated levels of ANG associated with hypertension may stimulate neurons in the PVN either through exaggerated presynaptic disinhibition or a dysfunctional GABAergic feedback system.
4. Expert Opinion
Over the last decade we have witnessed significant advances in our understanding of the functional integration by the PVN of multiple diverse afferent signals. Clearly, we can no longer consider the PVN as a simple input/output relay station maintaining the fidelity of multiple unrelated signals which then control the output of separate neuroendocrine and autonomic pathways. Rather it would seem that the PVN represents a unique CNS site at which multiple input signals can be assessed and integrated such that a complex multifactorial autonomic output can be generated. Thus, the role of the PVN in generation of the integrated physiological response to stress would first involve the collection of sensory input from cortical, thalamic, hypothalamic, medullary, spinal and circulating signals followed by the integration of these signals. Ten years ago we would have attributed this integration to each separate collection of neuroendocrine and preautonomic output neurons (CRH, TRH, oxytocin, vasopressin) performing such functions in isolation, a conclusion which makes little physiological sense when we know the importance of multiple sets of these output neurons to normal physiological functions. Here presumably there may be vital roles for both glutamate and GABA interneurons in the PVN (including the so-called halo zone), in performing such integrated functions. Experiments directed toward understanding the integrative functional roles of these neurons will provide important new information and will hopefully be greatly facilitated by the recent development of single cell RT-PCR tools which allow focused functional analysis of these neuronal subtypes. Also important to emphasize is the fact that within such an integrated system these interneurons have the potential to take such integrated signals and control outputs of the integrated functional output of the nucleus.
How these data impact on our understanding of the pathology of a variety of different autonomic diseases remains to be determined, although the above discussion does at least suggest that elevated levels of ANG in the PVN of both CHF and hypertensive animal models contribute to the pathogenesis of these disorders. Whether dysfunctional feedback or inappropriate feedforward loops are responsible for these phenomena remains to be determined. What is clear however is that these observations are of particular importance in the clinical setting where ACE inhibitors (e.g., enalapril), angiotensin receptor blockers (e.g., losartan), and aldosterone antagonists (e.g., spironolactone) have become first-line therapy in managing heart failure, hypertension and post myocardial infarction. Traditionally these drugs have been viewed as afterload and intravascular volume reducers with concomitant changes in ventricular and arterial remodeling, rather that regulators of CNS autonomic control centers.
References
- 1.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 2.Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- 3.Coote JH. Cardiovascular function of the paraventricular nucleus of the hypothalamus. Biol Signals. 1995;4:142–149. doi: 10.1159/000109434. [DOI] [PubMed] [Google Scholar]
- 4.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 5.Williams G, Harrold JA, Cutler DJ. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc. 2000;59:385–396. doi: 10.1017/s0029665100000434. [DOI] [PubMed] [Google Scholar]
- 6.Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol (Lond) 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469.. *This paper highlighted the different electrophysiological properties of magnocellular and parvocellular neurons of the PVN
- 7.van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993;13:2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decavel C, van den Pol AN. GABA: A dominant neurotransmitter in the hypothalamus. The Journal of Comparative Neurology. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 9.Csaki A, Kocsis K, Halasz B, Kiss J. Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]D-aspartate autoradiography. Neuroscience. 2000;101:637–655. doi: 10.1016/s0306-4522(00)00411-5.. **Definitive anatomical demonstration of glutamate interneurons within the PVN
- 10.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. The Journal of Comparative Neurology. 1993;332:123–143. doi: 10.1002/cne.903320109.. **This study described the anatomical location of GABA interneurons in and around the PVN.
- 11.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 13.Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046.. **This was the first paper to show the existence of angiotensinergic neurons in the CNS
- 15.Shoji M, Share L, Crofton JT. Effect on vasopressin release of microinjection of angiotensin II into the paraventricular nucleus of concious rats. Neuroendocrinology. 1989;50:327–333. doi: 10.1159/000125241. [DOI] [PubMed] [Google Scholar]
- 16.Tsushima H, Mori M, Matsuda T. Microinjections of angiotensin II into the supraoptic and paraventricular nuclei produce potent antidiureses by vasopressin release mediated through adrenergic and angiotensin receptors. Jpn J Pharmacol. 1994;66:241–246. doi: 10.1254/jjp.66.241. [DOI] [PubMed] [Google Scholar]
- 17.Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol. 2002;282:H2039–H2045. doi: 10.1152/ajpheart.00854.2001. [DOI] [PubMed] [Google Scholar]
- 18.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for a neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p.. **This study provided functional data in support of the conclusion that angiotensin II was in fact used as a neurotransmitter by SFO neurons which projected to PVN
- 19.Ferguson AV, Day TA, Renaud LP. Subfornical organ efferents influence the excitability of neurohypophysial and tuberoinfundibular paraventricular nucleus neurons in the rat. Neuroendocrinology. 1984;39:423–428. doi: 10.1159/000124015. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson AV, Day TA, Renaud LP. Influence of subfornical organ stimulation on the excitability of hypothalamic paraventricular neurons projecting to the dorsal medulla. Am J Physiol. 1984;247:R1088–R1092. doi: 10.1152/ajpregu.1984.247.6.R1088. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson AV, Day TA, Renaud LP. Subfornical organ stimulation excites paraventricular neurons projecting to the dorsal medulla. Am J Physiol. 1984;247:R1088–R1092. doi: 10.1152/ajpregu.1984.247.6.R1088. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson AV, Day TA, Renaud LP. Subfornical organ stimulation excites paraventricular neurons projecting to dorsal medulla. Am J Physiol. 1984;247:R1088–R1092. doi: 10.1152/ajpregu.1984.247.6.R1088. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Bains JS, Ferguson AV. Functional evidence that the angiotensin antagonist losartan crosses the blood-brain barrier in the rat. Brain Res Bull. 1993;30:33–39. doi: 10.1016/0361-9230(93)90036-b. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Ferguson AV. Subfornical organ efferents to the paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol. 1993;265:R302–R309. doi: 10.1152/ajpregu.1993.265.2.R302. [DOI] [PubMed] [Google Scholar]
- 25.Latchford KJ, Ferguson AV. ANG II-induced excitation of paraventricular nucleus magnocellular neurons: a role for glutamate interneurons. Am J Physiol Regul Integr Comp Physiol. 2004;286:R894–R902. doi: 10.1152/ajpregu.00603.2003.. *This paper describes roles for glutamate interneurons in mediating angiotensin II actions on PVN neurons.
- 26.Latchford KJ, Ferguson AV. Angiotensin depolarizes parvocellular neurons in paraventricular nucleus through modulation of putative nonselective cationic and potassium conductances. Am J Physiol Regulatory Integrative Comp Physiol. 2005;289:R52–R58. doi: 10.1152/ajpregu.00549.2004. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. see comments. [DOI] [PubMed] [Google Scholar]
- 28.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 30.Samson W, Resch ZT. The hypocretin/orexin story. TRENDS ENDOCRINOL METAB. 2000;11:257–262. doi: 10.1016/s1043-2760(00)00273-3. [DOI] [PubMed] [Google Scholar]
- 31.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 32.Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2001;281:R1114–R1118. doi: 10.1152/ajpregu.2001.281.4.R1114. [DOI] [PubMed] [Google Scholar]
- 33.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545:855–867. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samson WK, Taylor MM, Follwell MJ, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- 35.Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing Crosstalk between Leptin and Glucocorticoids Rapidly Modulates Synaptic Excitation via Endocannabinoid Release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powis JE, Bains JS, Ferguson AV. Leptin depolarizes rat hypothalamic paraventricular neurons. Am J Physiol. 1997;274:R14682–R1472. doi: 10.1152/ajpregu.1998.274.5.R1468. [DOI] [PubMed] [Google Scholar]
- 37.Ferri CC, Ferguson AV. Interleukin-1 beta depolarizes paraventricular nucleus parvocellular neurones. J Neuroendocrinol. 2003;15:126–133. doi: 10.1046/j.1365-2826.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 38.Ferri CC, Yuill EA, Ferguson AV. Interleukin-1beta depolarizes magnocellular neurons in the paraventricular nucleus of the hypothalamus through prostaglandin-mediated activation of a non selective cationic conductance. Regul Pept. 2005;129:63–71. doi: 10.1016/j.regpep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 40.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 41.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 42.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 44.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029.. **The first paper to show effects of adiponectin in the brain, as well as idnetifying the PVN as a potential target.
- 45.Hoyda TD, Fry M, Ahima RS, Ferguson AV. Adiponectin Selectively Inhibits Oxytocin Neurons of the Paraventricular Nucleus of the Hypothalamus. J Physiol. 2007 doi: 10.1113/jphysiol.2007.144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daftary SS, Boudaba C, Szabo K, Tasker JG. Noradrenergic excitation of magnocellular neurons in the rat hypothalamic paraventricular nucleus via intranuclear glutamatergic circuits. J Neurosci. 1998;18:10619–10628. doi: 10.1523/JNEUROSCI.18-24-10619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuill EA, Hoyda TD, Ferri CC, Zhou QY, Ferguson AV. Prokineticin 2 depolarizes paraventricular nucleus magnocellular and parvocellular neurons. Eur J Neurosci. 2007;25:425–434. doi: 10.1111/j.1460-9568.2006.05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol. 2004;92:1807–1816. doi: 10.1152/jn.00171.2004. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler DR, Herman JP. Local integration of glutamate signaling in the hypothalamic paraventricular region: regulation of glucocorticoid stress responses [In Process Citation] Endocrinology. 2000;141:4801–4804. doi: 10.1210/endo.141.12.7949. [DOI] [PubMed] [Google Scholar]
- 50.Bains JS, Ferguson AV. Nitric oxide regulates NMDA driven GABAergic inputs to type I neurons of the rat paraventricular nucleus. J Physiol (Lond) 1997;499.3:733–746. doi: 10.1113/jphysiol.1997.sp021965.. *This paper identified the circuitry in PVN through which activation of output neurons could activate a nitric oxide drive GABA mediated feedback circuit.
- 51.Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol. 1994;266:R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306.. **This was the first study showing direct effects of nitric oxide in PVN
- 52.Follwell MJ, Ferguson AV. Adrenomedullin influences magnocellular and parvocellular neurons of paraventricular nucleus via separate mechanisms. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1293–R1302. doi: 10.1152/ajpregu.00191.2002. [DOI] [PubMed] [Google Scholar]
- 53.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress. 2007;10:185–193. doi: 10.1080/10253890701350735. [DOI] [PubMed] [Google Scholar]
- 55.Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res. 2007;1142:92–99. doi: 10.1016/j.brainres.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2003;284:R259–R276. doi: 10.1152/ajpregu.00317.2002. [DOI] [PubMed] [Google Scholar]
- 57.Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol. 1993;265:R923–R928. doi: 10.1152/ajpregu.1993.265.4.R923. [DOI] [PubMed] [Google Scholar]
- 58.Francis GS. Neurohumoral activation and progression of heart failure: hypothetical and clinical considerations. J Cardiovasc Pharmacol. 1998;32(Suppl 1):S16–S21. doi: 10.1097/00005344-199800003-00004. [DOI] [PubMed] [Google Scholar]
- 59.Jougasaki M, Wei C, McKinley LJ, Burnett JC. Elevation of circulating and ventricular adrenomedullin in congestive heart failure. Circulation. 1995;92:286–289. doi: 10.1161/01.cir.92.3.286. [DOI] [PubMed] [Google Scholar]
- 60.Jougasaki M, Burnett JC., Jr Adrenomedullin: potential in physiology and pathophysiology. Life Sci. 2000;66:855–872. doi: 10.1016/s0024-3205(99)00358-6. [DOI] [PubMed] [Google Scholar]
- 61.Nagaya N, Nishikimi T, Horio T, Yoshihara F, Kanazawa A, Matsuo H, Kangawa K. Cardiovascular and renal effects of adrenomedullin in rats with heart failure. Am J Physiol. 1999;276:R213–R218. doi: 10.1152/ajpregu.1999.276.1.R213. [DOI] [PubMed] [Google Scholar]
- 62.Riegger AJ. Interaction between atrial natriuretic peptide, renin system and vasopressin in heart failure. European Heart Journal. 1990;11:79–83. doi: 10.1093/eurheartj/11.suppl_b.79. [DOI] [PubMed] [Google Scholar]
- 63.Tang J, Song DL, Suen MZ, Xie CW, Chang D, Chang JK. Alpha-human atrial natriuretic polypeptide (Alpha-hANP) in normal volunteers and patients with heart failure or hypertension. Peptides. 1986;7:33–37. doi: 10.1016/0196-9781(86)90057-4. [DOI] [PubMed] [Google Scholar]
- 64.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 65.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728.. *This study identified roles of nitric oxide in PVN in controlling sympathetic nerve discharge.
- 66.Zhang H, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol Renal,Fluid ELectrolyte Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- 67.Patel KP, Zhang H. Neurohumoral activation in heart failure: role of paraventricular nucleus. Clin Exp Pharmacol Physiol. 1996;23:722–726. doi: 10.1111/j.1440-1681.1996.tb01765.x.. **This study identified roles for PVN in heart failure.
- 68.Bains JS, Ferguson AV. Angiotensin II neurotransmitter actions in paraventricular nucleus are potentiated by a nitric oxide synthase inhibitor. Regul Pept. 1994;50:53–59. doi: 10.1016/0167-0115(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 69.Vahid-Ansari F, Leenan FHH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol. 1998;275:H2140–H2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 70.Coote JH. Homeostasis and stress. Clin Auton Res. 2005;15:247–248. doi: 10.1007/s10286-005-0297-0. [DOI] [PubMed] [Google Scholar]
- 71.Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Res. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2.. *This study identified roles of nitric oxide in PVN in heart failure.
- 72.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 73.Earle ML, Pittman QJ. Involvement of the PVN and BST in 1K1C hypertension in the rat. Brain Res. 1995;669:41–47. doi: 10.1016/0006-8993(94)01222-4. [DOI] [PubMed] [Google Scholar]
- 74.Meyer JM, Felten DL, Weyhenmeyer JA. Measurement of immunoreactive angiotensin II levels in microdissected brain nuclei from developing spontaneously hypertensive and Wistar Kyoto rats. Exp Neurol. 1990;107:164–169. doi: 10.1016/0014-4886(90)90154-k. [DOI] [PubMed] [Google Scholar]
- 75.Phillips MI, Kimura B. Brain angiotensin in the developing spontaneously hypertensive rat. J Hypertens. 1988;6:607–612. doi: 10.1097/00004872-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J Hypertens. 1988;6:79–84. doi: 10.1097/00004872-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 77.Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol. 2002;88:2664–2674. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- 78.Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- 79.Horn EM, Shonis CA, Holzwarth MA, Waldrop TG. Decrease in glutamic acid decarboxylase level in the hypothalamus of spontaneously hypertensive rats. J Hypertens. 1998;16:625–633. doi: 10.1097/00004872-199816050-00010. [DOI] [PubMed] [Google Scholar]
- 80.Kunkler PE, Hwang BH. Lower GABAA receptor binding in the amygdala and hypothalamus of spontaneously hypertensive rats. Brain Res Bull. 1995;36:57–61. doi: 10.1016/0361-9230(94)00164-v. [DOI] [PubMed] [Google Scholar]
- 81.Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]


