Abstract
Our previous study showed that activation of c-jun-N-terminal kinase (JNK) in spinal astrocytes plays an important role in neuropathic pain sensitization. We further investigated how JNK regulates neuropathic pain. In cultured astrocytes, tumor necrosis factor α (TNF-α) transiently activated JNK via TNF receptor-1. Cytokine array indicated that the chemokine CCL2/MCP-1 (monocyte chemoattractant protein-1) was strongly induced by the TNF-α/JNK pathway. MCP-1 upregulation by TNF-α was dose dependently inhibited by the JNK inhibitors SP600125 (anthra[1,9-cd]pyrazol-6(2H)-one) and D-JNKI-1. Spinal injection of TNF-α produced JNK-dependent pain hypersensitivity and MCP-1 upregulation in the spinal cord. Furthermore, spinal nerve ligation (SNL) induced persistent neuropathic pain and MCP-1 upregulation in the spinal cord, and both were suppressed by D-JNKI-1. Remarkably, MCP-1 was primarily induced in spinal cord astrocytes after SNL. Spinal administration of MCP-1 neutralizing antibody attenuated neuropathic pain. Conversely, spinal application of MCP-1 induced heat hyperalgesia and phosphorylation of extracellular signal-regulated kinase in superficial spinal cord dorsal horn neurons, indicative of central sensitization (hyperactivity of dorsal horn neurons). Patch-clamp recordings in lamina II neurons of isolated spinal cord slices showed that MCP-1 not only enhanced spontaneous EPSCs but also potentiated NMDA- and AMPA-induced currents. Finally, the MCP-1 receptor CCR2 was expressed in neurons and some non-neuronal cells in the spinal cord. Together, we have revealed a previously unknown mechanism of MCP-1 induction and action. MCP-1 induction in astrocytes after JNK activation contributes to central sensitization and neuropathic pain facilitation by enhancing excitatory synaptic transmission. Inhibition of the JNK/MCP-1 pathway may provide a new therapy for neuropathic pain management.
Introduction
Neuropathic pain is a devastating disease, and our incomplete understanding of its pathogenesis has hampered effective treatment for this pain. Recently, it has been demonstrated that c-jun N-terminal kinase (JNK), a member of the mitogen-activated protein kinase (MAPK) family, is persistently activated in spinal cord astrocytes after nerve injury (Ma and Quirion, 2002; Zhuang et al., 2006). Spinal infusion of the JNK inhibitor SP600125 (anthra[1,9-cd]pyrazol-6(2H)-one) attenuates neuropathic pain after nerve injury (Obata et al., 2004; Zhuang et al., 2006) and diabetes (Daulhac et al., 2006). In particular, a selective and cell-permeable peptide inhibitor of JNK, D-JNKI-1 (Borsello et al., 2003), is more potent than SP600125 in alleviating neuropathic pain (Zhuang et al., 2006). As a stress-activated protein kinase, JNK is activated by diverse stress-related stimuli and proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and growth factors such as basic fibroblast growth factor (bFGF) (Weston and Davis, 2007; Gao and Ji, 2008). However, the downstream mechanisms by which JNK activation in astrocytes regulates neuropathic pain remain unclear.
Accumulating evidence indicates that the chemokine MCP-1 (monocyte chemoattractant protein-1) (CCL2) plays a critical role in chronic pain facilitation via CCR2 receptors (Abbadie et al., 2003; Tanaka et al., 2004; Menetski et al., 2007; Dansereau et al., 2008). MCP-1 expresses in primary sensory neurons in the dorsal root ganglion (DRG), and this expression increases after nerve injury (Tanaka et al., 2004; White et al., 2005a,b; Zhang and De Koninck, 2006; Jung et al., 2008). So far, two different mechanisms have been proposed for MCP-1 to produce pain hypersensitivity. First, MCP-1 is released from a subset of sensory neurons to stimulate surrounding sensory neurons in the DRG and cause hyperactivity of these neurons (White et al., 2005a,b, 2007). Second, MCP-1 is released from central terminals of DRG neurons in the spinal cord to induce microglia activation, which is regarded as an important mechanism for neuropathic pain development (Tsuda et al., 2005; Zhang et al., 2007; Thacker et al., 2009).
However, MCP-1 is not only expressed in the DRG. Several studies show that activated astrocytes in vitro produce MCP-1 (Croitoru-Lamoury et al., 2003; Meeuwsen et al., 2003; El-Hage et al., 2005; Mojsilovic-Petrovic et al., 2007). MCP-1 is also expressed in brain astrocytes after demyelinating lesions (Van Der Voorn et al., 1999; Tanuma et al., 2006), mechanical injury (Glabinski et al., 1996), entorhinodentate axon transection (Babcock et al., 2003), and focal cerebral ischemia (Yan et al., 2007). In the present study, we found that MCP-1 was upregulated in cultured astrocytes after TNF-α stimulation and in spinal cord astrocytes after nerve injury, and both upregulations required JNK.
Central sensitization manifests as increased sensitivity in spinal cord dorsal horn neurons after tissue and nerve injury and plays an essential role in persistent pain sensitization (Woolf and Salter, 2000). However, how chemokines regulate central sensitization is unclear. Our data showed that, in addition to activating microglia via transcriptional regulation, MCP-1 could produce rapid central sensitization (within minutes) by inducing extracellular signal-regulated kinase (ERK) activation and enhancing excitatory synaptic transmission in dorsal horn neurons via posttranslational regulation.
Materials and Methods
Animals and surgery.
For most experiments, adult CD1 mice (male, 25–35 g), purchased from Charles River Laboratories, were used. For some experiments, TNFR1−− mice (male, 25–35 g), The Jackson Laboratory, and C57BL/6 wild-type control mice (male), were also used. CCR2–green fluorescent protein (GFP) reporter mice were generated by Drs. Jung and Miller (Northwestern University, Chicago, IL) (Jung et al., 2008). Dr. Jung collected spinal cords from these mice and sent us the tissue samples. All animal procedures performed in this study were approved by the Animal Care Committee of Harvard Medical School. To produce a spinal nerve ligation (SNL), animals were anesthetized with isoflurane, and the L6 transverse process was removed to expose the L4 and L5 spinal nerves. The L5 spinal nerve was then isolated and tightly ligated with 6-0 silk thread (Kim and Chung, 1992).
Drugs and administration.
The MAPK inhibitors SP600125, SB203580 [4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole], and U0126 [1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene] were purchased from Calbiochem. MCP-1 was purchased from R & D Systems. D-JNKI-1 was kindly provided by Dr. Isabelle Decosterd (University of Lausanne, Lausanne, Switzerland). For intrathecal injection, spinal cord puncture was made with a 30 gauge needle between the L5 and L6 level to deliver the reagents (10 μl) to the CSF (Hylden and Wilcox, 1980).
Primary astrocyte cultures.
Astrocyte cultures were prepared from cerebral cortexes of neonatal mice [postnatal day 2 (P2)]. The cerebral hemispheres were isolated and transferred to ice-cold Hank's buffer, and the meninges were carefully removed. Tissues were then minced into ∼1 mm pieces, triturated, filtered through a 100 μm nylon screen, and collected by centrifugation at ∼3000 × g for 5 min. The cell pellets were broken with a pipette and resuspended in a medium containing 15% fetal bovine serum (FBS) in low-glucose DMEM. After trituration, the cells were filtered through a 10 μm screen, plated into six-well plates at a density of 2.5 × 105 cells/cm2, and cultured for 10–12 d. The medium was replaced twice a week, first with 15% FBS, then with 10% FBS, and finally with 10% horse serum. Once the cells were grown to 95% confluence (∼10 d), 0.15 mm dibutyryl cAMP (Sigma) was added to induce differentiation. Astrocytes used for immunocytochemistry were cultured onto cover glasses at a density of 2.5 × 104 cells/cm2, and differentiation was induced when cells reached 50% confluence. GFAP immunostaining (see below) was performed to confirm the identity of astrocytes.
ELISA.
Mouse MCP-1 ELISA kit was purchased from R & D Systems. For in vitro experiments, culture medium and cells were collected separately after treatment. For in vivo experiments, animals were transcardially perfused with PBS at 1, 3, 10, and 21 d after SNL surgery or at 1 or 3 h after intrathecal injection of TNF-α. Naive animals and sham surgery animals were used as control for SNL surgery. The lumbar spinal cord segments were dissected. Spinal cord tissues or astrocytes were homogenized in a lysis buffer containing protease and phosphatase inhibitors (Zhuang et al., 2005). Protein concentrations were determined by BCA Protein Assay (Pierce). For each reaction in a 96-well plate, 100 μg of proteins or 100 μl of culture medium were used, and ELISA was performed according to the protocol of the manufacturer. The standard curve was included in each experiment.
Western blot.
Protein samples were prepared in the same way as for ELISA analysis, and 30 μg of proteins were loaded for each lane and separated by SDS-PAGE gel (4–15%; Bio-Rad). After the transfer, the blots were incubated overnight at 4°C with polyclonal antibody against pJNK (1:1000, rabbit; Cell Signaling Technology). For loading control, the blots were probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:20,000, mouse; Millipore Bioscience Research Reagents). These blots were further incubated with HRP-conjugated secondary antibody, developed in ECL solution, and exposed onto Hyperfilm (GE Healthcare) for 1–10 min. Specific bands were evaluated by apparent molecular size. The intensity of the selected bands was analyzed using NIH ImageJ software.
Cytokine array.
The mouse cytokine array kit was purchased from R & D Systems. Protein samples were prepared in the same way as for ELISA analysis. Each reaction was performed according to the protocol of the manufacturer using 150 μg of proteins collected from three samples. In brief, protein samples were incubated with a blot (array) that is precoated with 40 cytokine/chemokine antibodies for 24 h at 4°C. The remaining procedures were same as that of Western blotting.
Immunohistochemistry and immunocytochemistry.
After appropriate survival times, animals were deeply anesthetized with isoflurane and perfused through the ascending aorta with PBS, followed by 4% paraformaldehyde with 1.5% picric acid in 0.16 m phosphate buffer. After the perfusion, the L4–L5 spinal cord segments and DRGs were removed and postfixed in the same fixative overnight. Spinal cord sections (30 μm, free-floating) and DRG sections (14 μm) were cut in a cryostat and processed for immunofluorescence as we described previously (Jin et al., 2003; Kawasaki et al., 2008a). The sections were first blocked with 2% goat or horse serum for 1 h at room temperature. The sections were then incubated overnight at 4°C with the following primary antibodies: GFAP antibody (mouse, 1:5000; Millipore Bioscience Research Reagents), OX-42 antibody (mouse, 1:5000; Serotec), neuronal-specific nuclear protein (NeuN) antibody (mouse, 1:5000; Millipore Bioscience Research Reagents), MCP-1 antibody (rabbit, 1:500; R & D Systems), calcitonin gene-related peptide (CGRP) antibody (chicken, 1:500; Neuromics), and phosphorylated ERK antibody (pERK1/2) (rabbit, 1:500; Cell Signaling Technology). The sections were then incubated for 1 h at room temperature with cyanine 3 (Cy3)- or FITC-conjugated secondary antibodies (1:400; Jackson ImmunoResearch). For double immunofluorescence, sections were incubated with a mixture of polyclonal and monoclonal primary antibodies, followed by a mixture of FITC- and Cy3-congugated secondary antibodies. The stained sections were examined with a Nikon fluorescence microscope, and images were captured with a CCD Spot camera.
For immunocytochemistry, cultured astrocytes, after incubation with TNF-α, were fixed with 4% paraformaldehyde for 20 min and processed for immunofluorescence with MCP-1 (rabbit, 1:500; R & D Systems) and GFAP (mouse, 1:5000; Millipore Bioscience Research Reagents) antibody as shown above. After immunostaining, 4′,6′-diamidino-2-phenylindole (DAPI) (0.1 μg/ml; Sigma) was added for 5 min at room temperature to stain all the nuclei of cells in the cultures.
In situ hybridization.
The animals were perfused with 4% paraformaldehyde, postfixed overnight, and cryoprotected with 15% sucrose. Spinal cord tissues were sectioned at a thickness of 14 μm and mounted on Superfrost plus slides. The vector of mouse CCR2 cDNA was kindly provided by Drs. Flecher White (Loyola University, Chicago, IL) and Richard Miller (Northwestern University, Chicago, IL). The vector and probe were prepared as described previously (Tran et al., 2007). In brief, the CCR2 cDNA fragment was amplified by PCR from mouse cDNA, subcloned into the PCR II-TOPO vector (Invitrogen), and verified by restriction analysis and automated DNA sequencing (PerkinElmer Life and Analytical Sciences). The CCR2 cDNA fragment was 489–1336 of U77349, and the specificity of the probe was tested previously (Tran et al., 2007). Antisense and sense cRNA probes were transcribed in vitro with T7 or SP6 RNA polymerase in the presence of digoxigenin-labeling mix. In situ hybridization was performed as described previously (Chen et al., 2006). Briefly, sections were hybridized with the CCR2 probe overnight at 65°C. After washing, sections were blocked with 10% serum for 1 h at room temperature, followed by incubation with alkaline phosphatase-conjugated anti-digoxigenin antibody (1:2000; Roche Diagnostics) overnight at 4°C. Sections were then washed with alkaline phosphatase buffer and stained with a mixture of nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate in alkaline phosphatase buffer for ∼30 h. The corresponding sense probe was also included as control. Images were captured under bright field with a Leica microscope fitted with a CCD camera.
Reverse transcriptase-PCR.
Total RNA was prepared from spleen, DRG, and spinal cord. cDNA was synthesized with oligo-dT primers, and PCR was performed with CCR2 primers using different reaction cycles (20–35). GAPDH was used as loading control. The sequences of the primers are as follows: CCR2 forward, 5′-GGTCATGATCCCTATGTGG-3′; CCR2 reverse, 5′-CTGGGCACCTGATTTAAAGG-3′; GAPDH forward, 5′-GAAGGGTGGAGCCAAAAGG-3′; GAPDH reverse, 5′-AAGGTGGAAGAGTGGGAGTT- 3′ .
Spinal slice preparation.
As we reported previously (Baba et al., 2003; Kawasaki et al., 2008b), a portion of the lumbar spinal cord (L4–L5) was removed from young mice (3–4 weeks old) under urethane anesthesia (1.5–2.0 g/kg, i.p.) and kept in pre-oxygenated ice-cold Krebs' solution. Some experiments [e.g., recording of spontaneous EPSCs (sEPSCs)] were also repeated in adult mice (8 weeks old). Spinal segment was placed in a shallow groove formed in an agar block and glued to the bottom of the microslicer stage. Transverse slices (600 μm) were cut on a vibrating microslicer. The slices were perfused with Krebs' solution (8 ml/min) saturated with 95% O2 and 5% CO2 at 36 ± 1°C for at least 1–3 h before experiment. The Krebs' solution contained the following (in mm): 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. Some spinal cord slices were used for pERK immunostaining. The slices were stimulated with MCP-1 (100 ng/ml) for 5 min and then fixed with 4% paraformaldehyde for 1 h. Thin spinal cord sections (15 μm) were cut on a cryostat and processed for immunohistochemistry using pERK and NeuN antibodies (see above).
Patch-clamp recordings in spinal slices.
The whole-cell patch-clamp recordings were made from lamina II neurons in voltage-clamp mode (Baba et al., 2003). Under a dissecting microscope with transmitted illumination, the substantia gelatinosa (lamina II) is clearly visible as a relatively translucent band across the dorsal horn. Patch pipettes were fabricated from thin-walled, borosilicate, glass-capillary tubing (1.5 mm outer diameter; World Precision Instruments). After establishing the whole-cell configuration, neurons were held their holding potentials at −70 mV for recording sEPSCs. The resistance of a typical patch pipette is 5–10 MΩ. The internal solution contained the following (in mm): 135 potassium gluconate, 5 KCl, 0.5 CaCl2, 2 MgCl2, 5 EGTA, 5 HEPES, and 5 ATP-Mg. AMPA- or NMDA-induced current was recorded by bath application of AMPA (10 μm; Sigma) or NMDA (50 μm; Sigma) at the holding potential of −70 and −50 mV, respectively. Membrane currents were amplified with an Axopatch 200A amplifier (Molecular Devices) in voltage-clamp mode. Signals were filtered at 2 kHz and digitized at 5 kHz. Data were stored with a personal computer using pClamp 6 software and analyzed with Mini Analysis (Synaptosoft). Those cells that showed >5% changes from the baseline levels were regarded as responding ones (Kawasaki et al., 2008b).
Behavioral analysis.
Animals were habituated to the testing environment daily for at least 2 d before baseline testing. The room temperature and humidity remained stable for all experiments. For testing mechanical sensitivity, animals were put in boxes on an elevated metal mesh floor and allowed 30 min for habituation before examination. The plantar surface of each hindpaw was stimulated with a series of von Frey hairs with logarithmically incrementing stiffness (0.02–2.56 g; Stoelting), presented perpendicular to the plantar surface (3–5 s for each hair). The 50% paw-withdrawal threshold was determined using Dixon's up-down method (Chaplan et al., 1994). For testing heat sensitivity, animals were put in plastic boxes and allowed 30 min for habituation before examination. Heat sensitivity was tested by radiant heat using Hargreaves apparatus (IITC Life Science) and expressed as paw-withdrawal latency (PWL). The radiant heat intensity was adjusted so that basal PWL was between 9 and 12 s, with a cutoff of 18 s to prevent tissue damage.
Quantification and statistics.
The density of specific bands from Western blotting and specific dots from cytokine array was measured with a computer-assisted imaging analysis system (NIH ImageJ). The number of pERK-immunoreactive cells in the superficial dorsal horn (laminae I–III) was quantified in five spinal cord sections randomly selected from all sections collected from a spinal cord segment of each mouse. All data were expressed as mean ± SEM. Differences between groups were compared using Student's t test or ANOVA, followed by Newman–Keuls test. The criterion for statistical significance was p < 0.05.
Results
TNF-α induces JNK activation and chemokine upregulation in cultured astrocytes
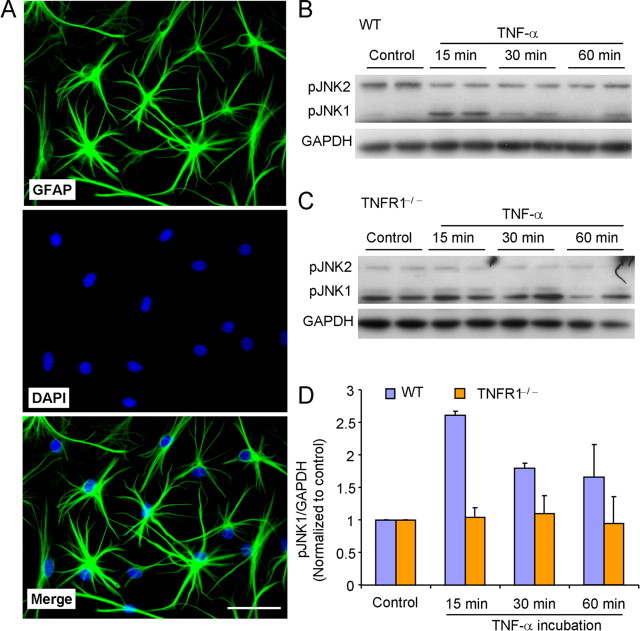
To determine the role of JNK in astrocyte signaling, we prepared primary astrocyte cultures from cerebral cortexes of neonatal mice (P2). To mimic mature astrocytes that dominate in the adult spinal cord, we differentiated astrocytes with 0.15 mm dibutyryl cAMP. Figure 1A shows the morphology of GFAP-expressing astrocytes, with fully extended processes. Majority of cultured cells (DAPI+) were astrocytes (GFAP+) (Fig. 1A). Because astrocytes are known to be activated by inflammatory mediators after nerve injury, we tried to mimic astrocyte activation in vitro with TNF-α, a critical trigger for inflammatory responses. The phosphorJNK1 (pJNK1) level was very low in nonstimulated cultures. After TNF-α (10 ng/ml, 0.59 nm) exposure, there was a rapid but transient activation (phosphorylation) of JNK1 (Fig. 1B). pJNK1 induction reached to a peak within 15 min and recovered at 60 min. In contrast, pJNK2 level did not increase after TNF-α stimulation (Fig. 1B).
Figure 1.
TNF-α induces JNK1 activation via TNF receptor type 1 in astrocytes. A, Double staining of GFAP (astrocyte marker, green) and DAPI (nuclear marker, blue) shows that all DAPI+ cells also express GFAP-IR. Scale bar, 50 μm. B, C, Western blot shows that TNF-α induces transient activation of JNK1 in astrocytes from wild-type (WT) mice (B) but not in astrocytes from TNFR1 null mice (C). D, Density of pJNK1 bands, which is normalized to GAPDH loading control and expressed as ratio of nontreated control.
TNF receptor type 1 (TNFR1) is known to mediate most of biological effects produced by TNF-α (Smith et al., 1994). We prepared astrocytes from mice lacking TNFR1 (TNFR1−−). TNF-α failed to activate JNK1 in these TNFR1-deficient astrocytes, supporting an essential role of TNFR1 for JNK1 activation by TNF-α in astrocytes (Fig. 1C,D).
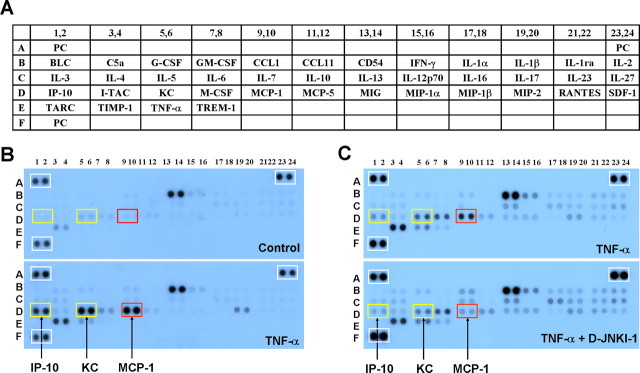
To determine whether the TNF-α/JNK pathway would regulate the expression of cytokines and chemokines, we performed a brief screening using a cytokine array (blot) that contains 40 different cytokine and chemokine antibodies (Fig. 2A). We incubated the blots with cell lysates from control or TNF-α-treated (10 ng/ml, 0.59 nm, 1 h) astrocytes simultaneously. TNF-α produced a marked increase in the levels of several chemokines, such as IP-10 (IFN-γ-inducible protein 10/CXCL10, 3.2-fold), KC (keratinocyte-derived chemokine/CXCL1, 3.2-fold), and MCP-1 (3.6-fold) (Fig. 2B). Pretreatment of astrocyte cultures with the JNK inhibitor D-JNKI-1 (50 μm) suppressed the TNF-α-induced increase of IP-10, KC, and MCP-1 by 40, 30, and 70%, respectively (Fig. 2C). Notably, the expression of positive controls was not affected by TNF-α or D-JNKI-1 treatment (Fig. 2B,C). After this initial screening, we chose MCP-1 for additional characterization, because (1) MCP-1 is the most regulated protein by the TNF-α/JNK pathway in astrocytes, and (2) the role of MCP-1 in pain sensitization is well documented (see Introduction).
Figure 2.
Cytokine array reveals a JNK-dependent upregulation of chemokines in astrocytes after TNF-α stimulation. A, Illustration of the cytokine array that contains 40 different antibodies with duplicates. The array also contains three positive control (PC) proteins. For more details, see the instructions of the manufacturer (R & D Systems). B, TNF-α treatment (10 ng/ml) for 1 h markedly increases the expression of the chemokines IP-10 (IFN-γ-inducible protein 10/CXCL10), KC (keratinocyte-derived chemokine/CXCL1), and MCP-1 in astrocytes. Note that the PC protein levels in the white boxes of the two arrays do not change. C, Pretreatment of the JNK inhibitor D-JNKI-1 (50 μm) reduces TNF-α (10 ng/ml, 1 h)-induced upregulation of the chemokines. Note that the PC protein levels in the white boxes of the two arrays are comparable. The two arrays (blots) in B and C were processed under the same conditions after stimulation. This experiment was repeated two to three times.
TNF-α induces MCP-1 upregulation in cultured astrocytes via activation of the JNK pathway
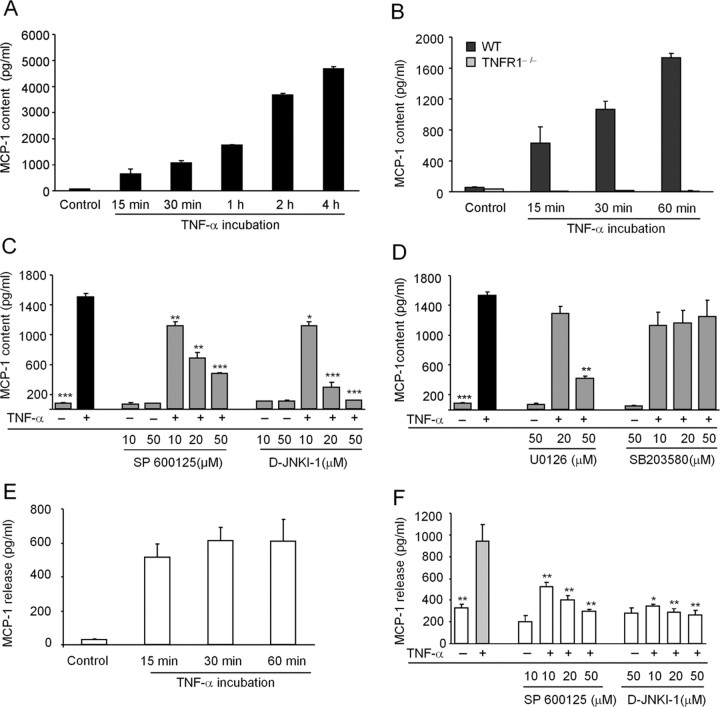
Next, we performed MCP-1 ELISA to determine MCP-1 production in astrocyte lysates and MCP-1 release in culture medium. In the nonstimulated conditions, astrocytes expressed low levels of MCP-1 (55 pg/ml). Exposure to TNF-α (10 ng/ml, 0.59 nm) induced rapid and time-dependent increase of MCP-1 expression. This increase was visible within 15 min and continued after 4 h. At 1 and 4 h after TNF-α stimulation, there were 31.3- and 84.1-fold increases in MCP-1 expression, respectively, compared with the control (Fig. 3A). TNF-α treatment also produced a profound increase in the intensity of MCP-1 immunofluorescence in astrocytes (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). TNF-α-induced MCP-1 upregulation was abrogated in astrocytes lacking TNFR1−− (Fig. 3B), supporting a critical role of TNFR1 in TNF-α-induced MCP-1 increase in astrocytes.
Figure 3.
ELISA demonstrates a JNK-dependent MCP-1 upregulation in astrocytes after TNF-α stimulation. A, TNF-α (10 ng/ml) induces a time-dependent upregulation of MCP-1 in astrocytes. B, TNF-α fails to increase MCP-1 expression in astrocytes prepared from TNFR1 null mice. C, JNK inhibitors SP600125 and D-JNKI-1 dose dependently inhibit TNF-α (10 ng/ml, 1 h)-induced MCP-1 upregulation. D, MEK inhibitor U0126, but not p38 inhibitor SB203580, reduces TNF-α-induced MCP-1 upregulation. E, F, TNF-α (10 ng/ml, 1 h) also induces MCP-1 release in the astrocyte culture medium (E), which is inhibited by the JNK inhibitors SP600125 and D-JNKI-1 (F). *p < 0.05, **p < 0.01, ***p < 0.001 versus TNF-α treatment (positive control); n = 3.
To further define the role of the JNK pathway in TNF-α-induced MCP-1 expression, we examined dose-dependent effects of D-JNKI-1 on MCP-1 expression using ELISA. Pretreatment of D-JNKI-1, 30 min before TNF-α application, dose dependently inhibited MCP-1 expression by 25.7, 80.4, and 92.2% at the doses of 10, 20, and 50 μm, respectively (Fig. 3C). Another JNK inhibitor, SP600125, also reduced MCP-1 expression in a dose-dependent manner after TNF-α stimulation: SP600125 inhibited TNF-α-induced MCP-1 expression by 16.2, 54.9, and 68.3% at the doses of 10, 20, and 50 μm, respectively (Fig. 3C). D-JNKI-1 was more potent than SP600125 in inhibiting MCP-1 expression (Fig. 3C). However, D-JNKI-1 or SP600125 alone (10 or 50 μm) had no effect on basal MCP-1 expression (Fig. 3C).
To determine whether other MAPK pathways are also involved in the MCP-1 expression, we blocked the p38 pathway with the p38 inhibitor SB203580 and the ERK pathway with the MAPK kinase (MEK) inhibitor U0126. U0126 at a high concentration (50 μm) but not a low concentration (20 μm), suppressed TNF-α-induced MCP-1 upregulation. In contrast, SB203580 had no effects at all the concentrations (Fig. 3D). These data suggest an involvement of the ERK but not the p38 pathway in MCP-1 upregulation by TNF-α.
We also tested MCP-1 release in the culture medium. TNF-α not only increased MCP-1 expression but also increased MCP-1 release. The increase in MCP-1 release started at 15 min but remained constant at 1 h, suggesting that MCP-1 release is very rapid (Fig. 3E). The JNK inhibitor SP600125 or D-JNKI-1 also inhibited TNF-α-induced increase in MCP-1 release (Fig. 3F).
Spinal injection of TNF-α induces pain hypersensitivity and MCP-1 upregulation via activation of the JNK pathway
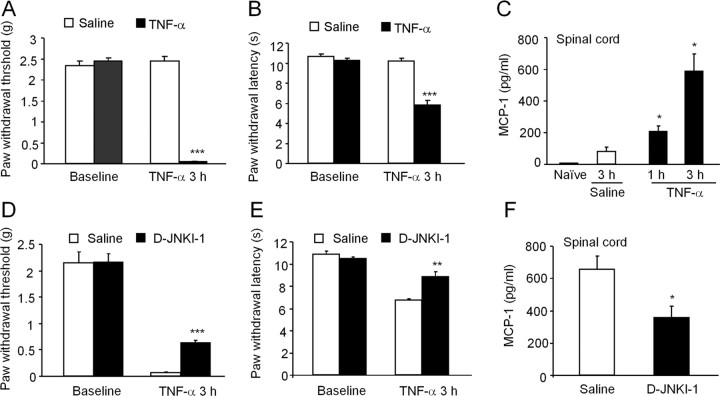
To determine whether the JNK/MCP-1 pathway observed in vitro would also be active in the spinal cord in vivo, we applied TNF-α (20 ng, 1.18 pmol) to mouse spinal cord via lumbar puncture. TNF-α injection decreased paw-withdrawal threshold to von Frey hair stimulation and paw-withdrawal latency to radiant heat, indicating the development of mechanical allodynia (Fig. 4A) and heat hyperalgesia (Fig. 4B), in support of a previous study in mice (Narita et al., 2008). However, Reeve et al. (2000) did not observe changes in pain behavior after intrathecal injection of TNF-α in rats. This discrepancy may result from different species, drug doses, and behavior testing times. A dose–response study indicated that TNF-α at 20 ng produced the most distinct heat hyperalgesia compared with lower doses (0.2 and 2 ng) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Figure 4.
Spinal administration of TNF-α induces JNK-dependent pain hypersensitivity and MCP-1 upregulation in the spinal cord. A–C, Spinal injection of TNF-α (20 ng) induces mechanical allodynia (A), heat hyperalgesia (B), and MCP-1 increase in spinal cord (C). D–F, Pretreatment of the JNK inhibitor D-JNKI-1 (50 nmol, i.p.), starting 1 h before TNF-α injection, reduces TNF-α-induced mechanical allodynia (D), heat hyperalgesia (E), and MCP-1 upregulation (F). *p < 0.05, **p < 0.01, ***p < 0.001 versus corresponding saline control, n = 5.
MCP-1 levels in the spinal cord were also significantly increased after TNF-α application (Fig. 4C). Systemic administration of the JNK inhibitor D-JNKI-1 (50 nmol, i.p.) partially prevented TNF-α-induced mechanical allodynia (Fig. 4D), heat hyperalgesia (Fig. 4E), and MCP-1 upregulation in the spinal cord (Fig. 4F), suggesting that the TNF-α/JNK/MCP-1 pathway is functional both in vitro and in vivo. Furthermore, TNF-α-induced mechanical allodynia, heat hyperalgesia, and MCP-1 upregulation in the spinal cord were all reduced in mice lacking TNFR1 (supplemental Fig. 3A–C, available at www.jneurosci.org as supplemental material).
JNK inhibition reduces SNL-induced neuropathic pain and MCP-1 increase
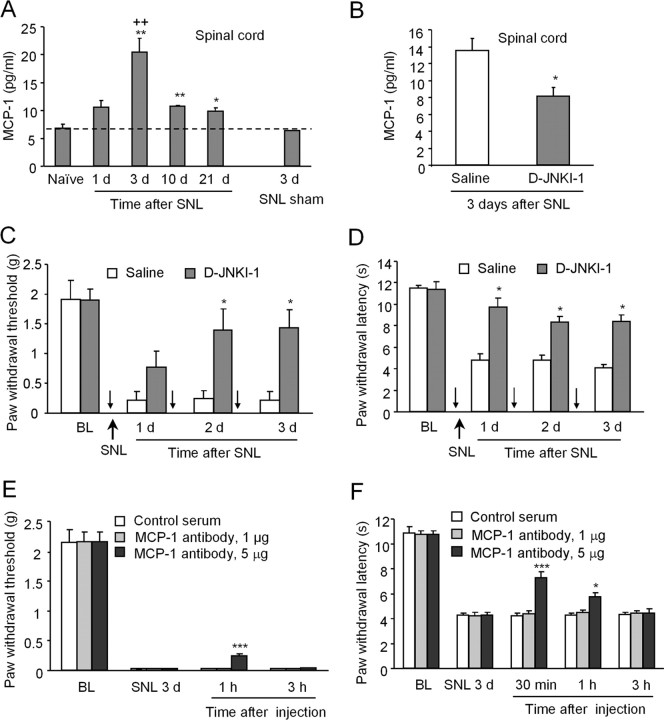
To investigate the role of the JNK/MCP-1 pathway in neuropathic pain, we used the SNL model to induce neuropathic pain. ELISA assay showed that SNL induced a persistent MCP-1 increase in the spinal cord, starting at day 1, peaking at day 3, and maintaining at day 21 (Fig. 5A). To test the effect of D-JNKI-1 on SNL-induced pain hypersensitivity and MCP-1 increase, we administrated D-JNKI-1 daily for 3 d (50 nmol, i.p.), starting 1 h before SNL, and tested neuropathic pain behavior 24 h after each injection. D-JNKI-1 not only reduced SNL-induced MCP-1 upregulation in the spinal cord (Fig. 5B) but also attenuated SNL-induced mechanical allodynia (Fig. 5C) and heat hyperalgesia (Fig. 5D) for 3 d. Intrathecal injection of D-JNKI-1 also attenuated the development of SNL-induced heat hyperalgesia (supplemental Fig. 4, available at www.jneurosci.org as supplemental material), suggesting a possible involvement of spinal mechanism. To define the role of MCP-1 in neuropathic pain sensitization, we blocked MCP-1 signaling by spinal injection of an MCP-1 neutralizing antibody on SNL day 3. The neutralizing antibody but not the control serum partly reversed SNL-induced mechanical allodynia (Fig. 5E) and heat hyperalgesia (Fig. 5F), in a dose-dependent manner, suggesting that MCP-1 is essential for neuropathic pain sensitization.
Figure 5.
SNL induces neuropathic pain and MCP-1 upregulation in the spinal cord, in a JNK-dependent manner. A, SNL induces persistent MCP-1 increase in the L5 spinal cord. *p < 0.05, **p < 0.01 versus naive control; ++p < 0.01 versus 3 d sham control; n = 3. B–D, Systemic application of D-JNKI-1 (50 nmol, i.p., once a day for 3 d, indicated by small arrows) attenuates SNL-induced MCP-1 increase (B), mechanical allodynia (C), and heat hyperalgesia (D). *p < 0.05 versus corresponding saline control; n = 5. E, F, Intrathecal application of MCP-1 neutralizing antibody, 3 d after SNL, partially reverses SNL-induced mechanical allodynia (E) and heat hyperalgesia (F). *p < 0.05, ***p < 0.001 versus control serum; n = 5. BL, Baseline.
MCP-1 is upregulated in spinal cord astrocytes after nerve injury
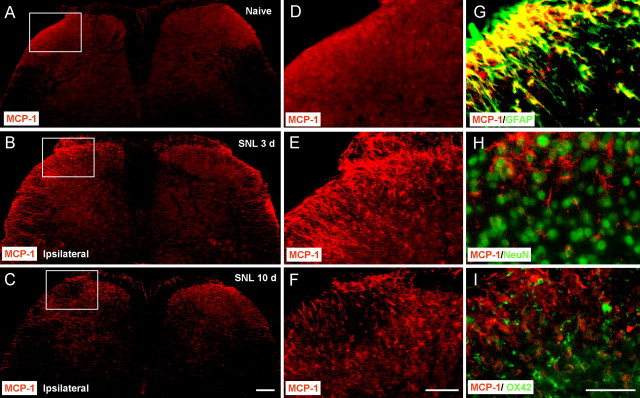
Immunofluorescence showed that SNL induced a marked increase of MCP-1-IR in the ipsilateral side of the lumbar spinal cord at days 3 and 10 after SNL (Fig. 6A–F). The MCP-1 expression on the contralateral side also showed mild increase after SNL (Fig. 6A–C). MCP-1-immunoreactive cells were mainly found in the superficial layers (laminae I–III) of the dorsal horn (Fig. 6E,F). To define the cellular distribution of MCP-1, we performed double staining of MCP-1 with different cell markers. MCP-1-IR was primarily colocalized with the astrocytic marker GFAP (Fig. 6G) but not with neuronal marker NeuN (Fig. 6H) or microglia marker OX-42 (Fig. 6I). In support of previous studies (Zhang and De Koninck, 2006; Dansereau et al., 2008; Jung et al., 2008), we observed similar MCP-1 expression patterns in DRG neurons (supplemental Fig. 5A, available at www.jneurosci.org as supplemental material). MCP-1 was also found in some central terminals of primary afferents that express CGRP-IR in the superficial spinal cord (laminae I–II) (supplemental Fig. 5B, available at www.jneurosci.org as supplemental material). Collectively, these results suggest that, in the spinal cord, MCP-1 is primarily induced in astrocytes, although it also expressed in some primary afferents.
Figure 6.
SNL induces MCP-1 upregulation in spinal cord astrocytes. A–C, MCP-1 expression in the spinal cord of naive animals (A) and SNL animals at 3 d (B) and 10 d (C). Scale bar, 200 μm. D–F, High-magnification images of A–C, indicated in the white boxes of A–C, show the dorsal horn of the ipsilateral spinal cord. Scale bar, 100 μm. G–I, Double staining shows that MCP-1 is colocalized with GFAP, a marker for astrocytes (G), but not with NeuN, a marker for neurons (H) or OX-42, a marker for microglia (I). Scale bar, 50 μm.
Spinal injection of MCP-1 induces heat hyperalgesia and ERK activation in spinal cord neurons
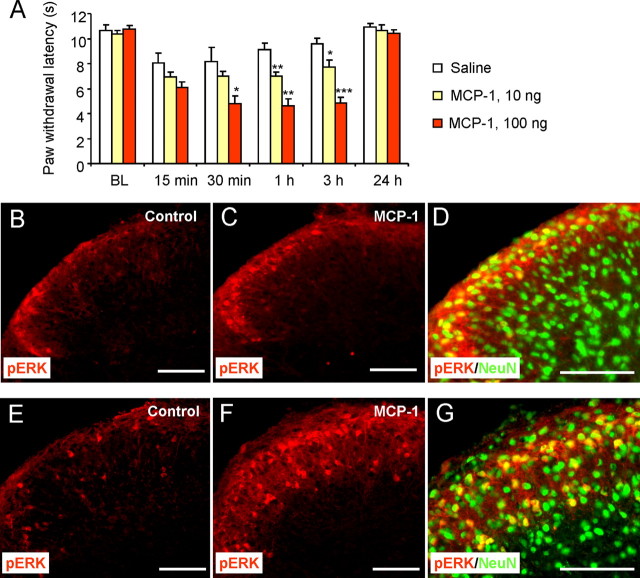
To investigate whether MCP-1 is sufficient to produce pain, we injected MCP-1 (10 or 100 ng) into spinal CSF and tested pain behavior at different times after the injection. MCP-1 produced heat hyperalgesia in a dose-dependent manner. This hyperalgesia began at 15 min, peaked at 30 min, maintained at 3 h, and completely recovered after 24 h (Fig. 7A).
Figure 7.
Spinal injection of MCP-1 induces heat hyperalgesia and ERK activation in superficial dorsal horn neurons. A, Spinal injection of MCP-1 (10 or 100 ng) induces a dose-dependent heat hyperalgesia. *p < 0.05, **p < 0.01, ***p < 0.001 versus saline (A); n = 5. BL, baseline. B, C, Spinal injection of MCP-1 (100 ng) induces pERK at 30 min after the injection in superficial dorsal horn. D, Majority of pERK-immunoreactive cells in intact spinal cord (in vivo) express the neuronal marker NeuN. E, F, Incubation of isolated spinal cord slices with MCP-1 (100 ng/ml, 5 min) induces substantial ERK activation in the superficial dorsal horn. G, Majority of pERK-immunoreactive cells in spinal cord slices (ex vivo) express NeuN. Scale bars, 100 μm. BL, Baseline.
Activation of ERK in superficial spinal cord dorsal horn neurons after noxious stimulation or inflammation is not only robust and specific but also essential for the induction of central sensitization (Ji et al., 1999; Karim et al., 2001). Thus, activation (phosphorylation) of ERK in dorsal horn neurons could serve as a marker for central sensitization. Spinal injection of MCP-1 produced a robust pERK induction in the superficial layers (laminae I–II) of the spinal cord at 30 min after the injection (Fig. 7B,C). The number of pERK-immunoreactive neurons per spinal cord section increased from 2.0 ± 0.5 before stimulation to 9.5 ± 0.4 after stimulation (p < 0.01; n = 4). The majority of pERK-immunoreactive cells also expressed the neuronal marker NeuN (Fig. 7D), suggesting that ERK is primarily activated in dorsal horn neurons by MCP-1.
We further investigated ERK activation in isolated spinal cord slices, because in this ex vivo condition, drug application can be accurately controlled. There was moderate pERK expression in the nonstimulated mouse spinal slices (Fig. 7E) attributable to tissue injury during spinal slice preparation. However, incubation of the spinal slices with 100 ng/ml MCP-1 for 5 min induced a substantial ERK activation in the superficial spinal cord, predominantly in the laminae I–II (Fig. 7E,F). The number of pERK-immunoreactive neurons per spinal cord section increased from 11.6 ± 0.2 before stimulation to 40.4 ± 0.8 after stimulation (p < 0.001; n = 4). Again, pERK was primarily induced in NeuN-positive neurons (Fig. 7G). Notably, the activation of the ERK in dorsal horn neurons was very rapid (<5 min) (Fig. 7G), indicating that MCP-1 may directly act on neurons.
MCP-1 enhances excitatory synaptic transmission in spinal cord neurons
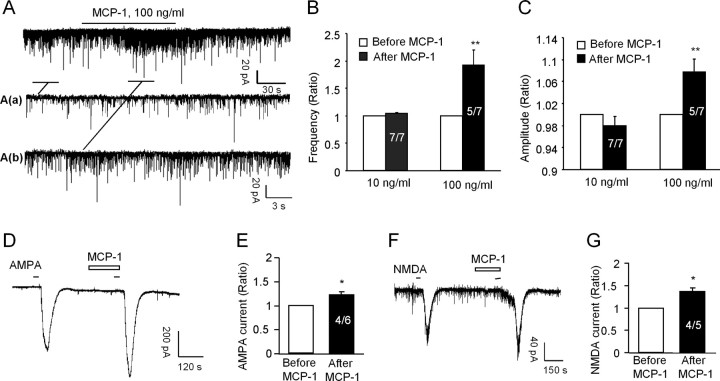
To further determine the role of MCP-1 in central sensitization, we prepared spinal cord slices from young mice (3–4 weeks old) and performed patch-clamp recordings in lamina II neurons in which many nociceptive neurons are localized (Baba et al., 2003; Kawasaki et al., 2008b). We first recorded sEPSCs. Superfusion of MCP-1 at a concentration (100 ng/ml) that caused substantial ERK activation (Fig. 7E,F) resulted in significant increases (p < 0.01) in both the frequencies and amplitudes of sEPSCs (Fig. 8A–C), although the increase in frequencies (93 ± 28%) was much more dramatic than that in amplitudes (8 ± 2%). The increases in both the frequencies and amplitudes of sEPSC suggest possible involvement of both presynaptic mechanism of MCP-1 to enhance glutamate releases (Baba et al., 2003; Kohno et al., 2005) and postsynaptic mechanism of MCP-1 to enhance glutamate receptor function (Kohno et al., 2005). However, superfusion of a lower concentration of MCP-1 (10 ng/ml) for 2 min had no effect on the frequencies (104 ± 2% of the pretreated control; p > 0.05) and amplitudes (98 ± 1% of the pretreated control; p > 0.05) (Fig. 8B,C), indicating dose-dependent effects of MCP-1. We also prepared spinal cord slices from adult mice (8 weeks old) and found a similar increase in the frequency and amplitudes in sEPSCs after MCP-1 application (supplemental Fig. 6, available at www.jneurosci.org as supplemental material).
Figure 8.
Bath application of MCP-1 increases excitatory synaptic transmission and AMPA- and NMDA-induced currents. A, Patch-clamp recording of sEPSCs shows increase in the frequency and amplitude of sEPSC after perfusion of MCP-1 (100 ng/ml, 2 min). Aa and Ab are enlarged recording before and after MCP-1 treatment, respectively. B, C, Ratio of the frequency and amplitude of sEPSCs after MCP-1 treatment. **p < 0.01 versus pretreatment baseline. D–G, Patch-clamp recording demonstrates increase in AMPA-induced currents (D, E) and NMDA-induced currents (F, G) after MCP-1 treatment (100 ng/ml, 2 min). The amplitude of AMPA- and NMDA-induced currents is shown in E and G, respectively. *p < 0.05 versus pretreatment baseline. Inside each column, the number of total recorded neurons and number of responding neurons are indicated (B, C, E, G).
Because excitatory synaptic transmission is mainly mediated by AMPA and NMDA receptors, we further examined the effects of the MCP-1 on inward currents induced by AMPA (10 μm, 30 s) and NMDA (50 μm, 30 s) when holding the voltage at −70 and −50 mV, respectively. MCP-1 (100 ng/ml) significantly enhanced the inward currents elicited by both AMPA (121 ± 5% of pretreated control; p < 0.05) (Fig. 8D,E) and NMDA (136 ± 8% of pretreated control; p < 0.05) (Fig. 8F,G). The increase in the amplitude of NMDA currents appeared to be larger than that of AMPA currents after bath application of MCP-1 (Fig. 8D–G).
CCR2 expression in the spinal cord
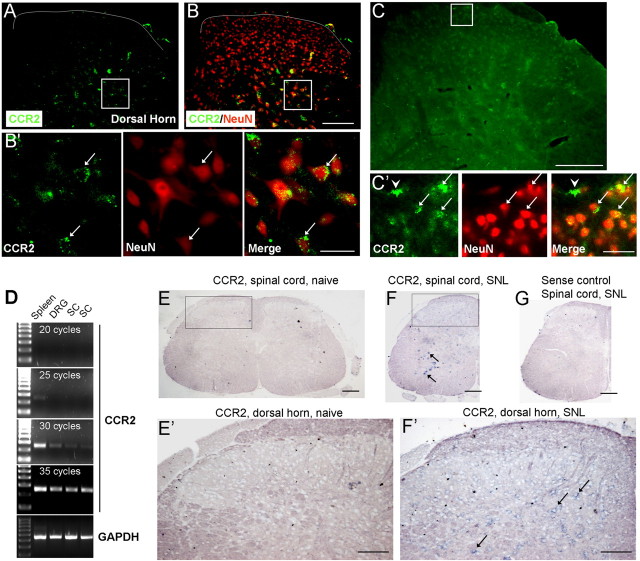
To investigate whether an increase in AMPA/NMDA currents by MCP-1 in spinal cord neurons results from a direct effect of MCP-1 on these neurons, we checked the expression of CCR2, a major type of receptor for MCP-1 (Abbadie et al., 2003; Abbadie, 2005; White et al., 2005a,b), in the spinal cord. Because we had concerns about the specificity of commercially available CCR2 antibodies, we used three different approaches to check CCR2 expression.
First, we collected spinal sections from CCR2–GFP reporter mice (Jung et al., 2008). Although CCR2 signal was weak in the normal spinal cord, we observed some CCR2-positive neurons (NeuN+) in the deep dorsal horn (Fig. 9A,B). After we increased the gain during image capture, we also found some weak staining of CCR2 in superficial dorsal horn neurons (Fig. 9C). Interestingly, the staining was present in punctuate structures close to the membrane of neurons (Fig. 9B′,9C′). Notably, CCR2 was also expressed in some non-neuronal cells in normal conditions (Fig. 9C′).
Figure 9.
Expression of CCR2 in the spinal cord. A–C, Spinal cord from CCR2–GFP-report mice shows CCR2 expression in NeuN-positive neurons in the deep (A, B, B′) and superficial (C, C′) dorsal horn. B is a double staining of CCR2–GFP and NeuN. B′ shows high-magnification images of A and B (inset squares). Arrows indicate double-labeled cells. Scale bars: B, C, 100 μm; B′, C′, 25 μm. D, RT-PCR shows CCR2 mRNA expression in the spleen, DRG, and spinal cord (SC) of naive mice after 20–35 cycles of amplification. E–G, In situ hybridization shows CCR2 mRNA expression in spinal cord in normal and nerve injury conditions using a CCR2 antisense probe. CCR2 mRNA-positive cells are not detectable in naive animals (E). Three days after SNL, CCR2 mRNA-positive neurons are seen in deep dorsal horn and ventral horn (F). E′ and F′ are high-magnification images of E and F (inset squares). Hybridization using a control sense probe shows no signal in the spinal cord of SNL animals (G). Scale bars: E–G, 200 μm; E′, F′, 100 μm.
Second, we performed reverse transcriptase (RT)-PCR and compared CCR2 mRNA expression in the spleen, DRG, and spinal cord in naive animals. CCR2 was highly expressed in the spleen, but the expression was quite lower in the DRG and spinal cord (Fig. 9D).
Finally, we performed in situ hybridization using an antisense probe of CCR2. In agreement with a previous study (Eltayeb et al., 2007), we did not detect CCR2 mRNA expression in the spinal cord of naive animals. This negative observation may result from lower sensitivity of in situ hybridization with digoxigenin labeling system compared with GFP reporter system. However, 3 d after spinal nerve ligation, we found CCR2 mRNA signal in motor neurons and also in deep dorsal horn neurons (Fig. 9F,F′), indicating that CCR2 is upregulated after nerve injury. In contrast, the control sense probe did not produce any signal (Fig. 9F′).
Discussion
Accumulating evidence supports an important role of spinal microglia in neuropathic pain development (for review, see Tsuda et al., 2005), but recent evidence also indicates a role of spinal astrocytes in neuropathic pain sensitization (for review, see Ji et al., 2006). Compared with microglia, astrocytes appear to be more important for the maintenance of neuropathic pain (Raghavendra et al., 2003; Zhuang et al., 2005, 2006; Kawasaki et al., 2008a; Wei et al., 2008). In this study, we have shown how astrocytes can regulate central sensitization via neural–glial interaction in the spinal cord. We have made the following findings. First, TNF-α induced a substantial upregulation of MCP-1 in both cultured astrocytes and intact spinal cord, and this upregulation required the activation of the JNK pathway. Second, nerve injury by SNL induced persistent and JNK-dependent MCP-1 increase predominantly in astrocytes of the spinal cord. Third, spinal inhibition of JNK or MCP-1 alleviated neuropathic pain after SNL. Fourth, spinal application of MCP-1 induced heat hyperalgesia and ERK activation in dorsal horn neurons. Finally, MCP-1 increased excitatory synaptic transmission and enhanced NMDA and AMPA currents in the lamina II neurons.
TNF-α upregulates MCP-1 via JNK in both astrocyte cultures and the spinal cord
The proinflammatory cytokine TNF-α is an essential trigger for an inflammatory cascade that underlies the development of neuropathic pain after nerve injury (Sommer et al., 2001; Winkelstein et al., 2001; Schäfers et al., 2003; Lee et al., 2004; Xu et al., 2006; Mata et al., 2008). We showed that spinal administration of TNF-α is sufficient to induce neuropathic pain symptoms such as heat hyperalgesia and mechanical allodynia (Kawasaki et al., 2008b) (Fig. 4). Previous studies showed that inhibition of TNF-α could prevent but not reverse neuropathic pain, suggesting a unique role of TNF-α in the induction of neuropathic pain (Schäfers et al., 2003; Xu et al., 2006) (but see Hao et al., 2007). TNF-α in the spinal cord can enhance pain via several different mechanisms. First, TNF-α can directly and rapidly (within minutes) enhance excitatory synaptic transmission in dorsal horn neurons (Kawasaki et al., 2008b). Second, TNF-α can activate p38 MAPK in spinal microglia (Svensson et al., 2005; Boyle et al., 2006; Hao et al., 2007), which is essential for neuropathic pain sensitization (Jin et al., 2003; Schäfers et al., 2003; Tsuda et al., 2004). Third, the present study has shown that TNF-α can further activate JNK in spinal astrocytes, leading to neuropathic pain hypersensitivity (Fig. 4).
TNF-α binds its receptors TNFR1 and TNFR2 to activate TNF-α associated factor, protein kinases, and transcription factors (Baud and Karin, 2001). Our results demonstrated that TNFR1 was essential for the TNF-α-induced JNK1 activation in astrocytes, as well as spinal TNF-α-induced heat hyperalgesia and mechanical allodynia. Interestingly, in neuropathic pain rats, TNF-α was shown to induce JNK-dependent long-term potentiation in the spinal cord (Liu et al., 2007), suggesting a role of the TNF-α/JNK pathway in regulating spinal long-term potentiation, a particular form of central sensitization (Sandkuhler, 2000; Ji et al., 2003).
After activation, JNK can phosphorylate several transcriptional factors such as c-Jun and induces gene transcription (Bogoyevitch and Kobe, 2006). Our cytokine array data showed that JNK activation by TNF-α in cultured astrocytes resulted in an upregulation of several chemokines, including MCP-1, IP-10, and KC. Particularly, the upregulation of MCP-1 after TNF-α stimulation was rapid (<15 min) and persistent (>4 h), in support of a previous study (Croitoru-Lamoury et al., 2003). The persistent upregulation of MCP-1 is in sharp contrast to transient JNK activation (<1 h) by TNF-α. This discrepancy may result from the following possibilities. First, TNF-α may activate other signaling pathways such as the ERK pathway. This pathway in astrocytes is also required for MCP-1 upregulation (Fig. 3D) and further activated after nerve injury (Zhuang et al., 2005). Second, the chemokines (e.g., MCP-1, KC, and IP-10) released from astrocytes may further activate astrocytes to produce more MCP-1 via a positive feedback loop (Luo et al., 2000; Croitoru-Lamoury et al., 2003). Whereas TNF-α produces transient JNK activation, the growth factor bFGF, which is upregulated in primary sensory neurons (Ji et al., 1995) and spinal cord astrocytes (Madiai et al., 2003) after nerve injury and which contributes to neuropathic pain sensitization (Madiai et al., 2005; Ji et al., 2006), induces persistent JNK activation in astrocytes (Ji et al., 2006).
The TNF-α/JNK/MCP-1 pathway acts not only in vitro but also in vivo. Thus, TNF-α-induced hyperalgesia, allodynia, and spinal MCP-1 upregulation were also suppressed by D-JNKI-1 (Fig. 4). In particular, the JNK/MCP-1 pathway is activated in spinal cord astrocytes in a neuropathic pain condition after SNL (see below).
MCP-1 upregulation in astrocytes and neuropathic pain
Our data showed that SNL induced a persistent MCP-1 increase in the spinal cord that was JNK dependent. Furthermore, SNL-induced neuropathic pain was attenuated by D-JNKI-1 and the MCP-1 neutralizing antibody, in support of previous studies (Zhuang et al., 2006; Thacker et al., 2009). These data suggest that the JNK/MCP-1 pathway is indispensable for neuropathic pain. It is generally believed that MCP-1 mediates pain via CCR2 receptors. Thus, mice lacking CCR2 fail to develop of tactile allodynia after partial sciatic nerve ligation (Abbadie et al., 2003; Zhang et al., 2007), and CCR2 receptor antagonist attenuates neuropathic pain after focal nerve demyelination (Bhangoo et al., 2007).
Previous studies focused on MCP-1 expression in DRG neurons, because this expression is upregulated after nerve injury (Tanaka et al., 2004; White et al., 2005a,b). In the spinal cord, MCP-1 is expressed in CGRP-immunoreactive primary afferents (Dansereau et al., 2008; Jung et al., 2008) (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). Remarkably, we found that MCP-1 was predominantly induced in spinal astrocytes after SNL, which is in parallel with the following reports. First, JNK is persistently activated in spinal astrocytes (Zhuang et al., 2006) and induces MCP-1 in astrocytes. Second, propentofylline, an anti-allodynic agent, was shown to dampen MCP-1 release from astrocytes (Tawfik et al., 2006). Third, MCP-1 is also expressed in brain astrocytes under disease conditions (Van Der Voorn et al., 1999; Tanuma et al., 2006; Yan et al., 2007). Finally, mice overexpressing MCP-1 in astrocytes display enhanced nociceptive responses (Menetski et al., 2007).
MCP-1 and central sensitization
MCP-1 has been shown to modulate pain by different mechanisms. In particular, the peripheral mechanisms of MCP-1 (peripheral sensitization) are well documented. For example, MCP-1 released from DRG neurons could activate neighboring CCR2 bearing nociceptive neurons and enhance excitability of these neurons by activation of TRPV1 channels (Jung et al., 2008) and inhibition of voltage-dependent K+ channels (Sun et al., 2006). The central mechanisms of MCP-1 have also begun to be revealed. Spinal injection of MCP-1 activates spinal microglia in wild-type but not CCR2 knock-out mice (Zhang et al., 2007; Thacker et al., 2009). Nerve injury-induced activation of spinal microglia is also attenuated by a MCP-1 neutralizing antibody (Thacker et al., 2009). Furthermore, nerve injury-induced p38 activation in spinal microglia is attenuated in CCR2 knock-out mice (Abbadie et al., 2003). Because microglia activation is critical for neuropathic pain generation (Watkins et al., 2001; Jin et al., 2003; Raghavendra et al., 2003; Coull et al., 2005; Tsuda et al., 2005; Bradbury and McMahon, 2006), MCP-1 may enhance neuropathic pain via this mechanism.
MCP-1 appears to induce microglia activation (e.g., IBA-1 upregulation) via transcriptional regulation, which takes hours to manifest. However, spinal application of MCP-1 potentiates synaptic transmission and activates ERK in dorsal horn neurons within minutes (Figs. 7, 8) and induces heat hyperalgesia within tens of minutes (Fig. 7). Thus, our data support a direct action of MCP-1 on dorsal horn neurons to generate central sensitization and pain sensitization. Consistently, the MCP-1 receptor CCR2 was found in spinal cord neurons in the superficial and deep dorsal horn (Fig. 9) (Gosselin et al., 2005) (but see Abbadie et al., 2003). In cultured spinal neurons, MCP-1 was shown to inhibit GABAergic transmission (Gosselin et al., 2005), further supporting a direct effect of MCP-1 on spinal neurons. This finding also indicates that MCP-1 may regulate neuropathic pain via disinhibition (Coull et al., 2005). However, we should not exclude presynaptic mechanisms of MCP-1 for pain regulation in the spinal cord, because (1) MCP-1 induced an increase in the frequency of sEPSC that is caused by an increase in presynaptic glutamate release (Baba et al., 2003; Kawasaki et al., 2008b), and (2) CCR2 is expressed in central terminals of primary afferents (White et al., 2005b; Knerlich-Lukoschus et al., 2008).
Finally, we should point out that MCP-1 remarkably changes markers of central sensitization. MCP-1 application to the spinal cord produced substantial activation of ERK in dorsal horn neurons both in vivo and ex vivo (Fig. 7). ERK activation in dorsal horn neurons contributes importantly to the induction of central sensitization (Ji et al., 1999; Karim et al., 2001). Furthermore, perfusion of spinal slices with MCP-1 rapidly (within 2 min) increased the activity of NMDA receptors (Fig. 8). Hyperactivity of NMDA receptors is the best known mechanism for the induction of central sensitization (Woolf and Salter, 2000; Ji et al., 2003).
Conclusions
In the present study, we have demonstrated that activation of the JNK/MCP-1 pathway in astrocytes plays an important role in promoting neuropathic pain. We have not only identified MCP-1 as a critical downstream target of the JNK signaling pathway but also revealed a new mechanism of MCP-1 for central sensitization, by which MCP-1 enhances excitatory synaptic transmission and increases NMDA receptor activity in dorsal horn neurons. Thus, MCP-1 serves as a critical signaling molecule for neural–glial interaction; it not only signals from DRG neurons to microglia as demonstrated previously but also signals from astrocytes to dorsal horn neurons as currently demonstrated. Targeting the JNK/MCP-1 pathway may provide a novel therapeutic approach for the treatment of devastating neuropathic pain.
Footnotes
This work was supported by National Institutes of Health Grants DE17794, NS54932, and TW7180 (R.-R.J), National Natural Science Foundation of China (NSFC) Grant 30500153 and Natural Science Research Program of Jiangsu Province Grant 05KJB180100 (Y.-J.G.), and Swiss National Science Foundation Grant PBLAB118504 (M.R.S.). L.Z. was supported by a fellowship from East China Normal University and NSFC Grant 30600171. We thank Dr. Fletcher A. White (Loyola University, Chicago, IL) for his helpful comments on CCR2 expression and Drs. Hosung Jung, Dongjun Ren, and Richard Miller (Northwestern University, Chicago, IL) for providing the vector of CCR2 cDNA and spinal cord samples from CCR2–GFP reporter mice. We thank Drs. Isabelle Decosterd and Christophe Bonny (University of Lausanne, Lausanne, Switzerland) for providing D-JNKI-1 peptide.
References
- Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, Sorkin L, Firestein GS. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3:e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Croitoru-Lamoury J, Guillemin GJ, Boussin FD, Mognetti B, Gigout LI, Chéret A, Vaslin B, Le Grand R, Brew BJ, Dormont D. Expression of chemokines and their receptors in human and simian astrocytes: evidence for a central role of TNF alpha and IFN gamma in CXCR4 and CCR5 modulation. Glia. 2003;41:354–370. doi: 10.1002/glia.10181. [DOI] [PubMed] [Google Scholar]
- Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem. 2008;106:757–769. doi: 10.1111/j.1471-4159.2008.05429.x. [DOI] [PubMed] [Google Scholar]
- Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-d-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltayeb S, Berg AL, Lassmann H, Wallström E, Nilsson M, Olsson T, Ericsson-Dahlstrand A, Sunnemark D. Temporal expression and cellular origin of CC chemokine receptors CCR1, CCR2 and CCR5 in the central nervous system: insight into mechanisms of MOG-induced EAE. J Neuroinflammation. 2007;4:14. doi: 10.1186/1742-2094-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci Lett. 2008;437:180–183. doi: 10.1016/j.neulet.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95:1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14:1010–1016. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Zhang X, Piehl F, Reilly T, Pettersson RF, Hökfelt T. Prominent expression of bFGF in dorsal root ganglia after axotom. Eur J Neurosci. 1995;7:2458–2468. doi: 10.1111/j.1460-9568.1995.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RW., 4th Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008a;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008b;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, Juraschek M, Blömer U, Lucius R, Mehdorn HM, Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J Neurotrauma. 2008;25:427–448. doi: 10.1089/neu.2007.0431. [DOI] [PubMed] [Google Scholar]
- Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Lee HL, Lee KM, Son SJ, Hwang SH, Cho HJ. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport. 2004;15:2807–2811. [PubMed] [Google Scholar]
- Liu YL, Zhou LJ, Hu NW, Xu JT, Wu CY, Zhang T, Li YY, Liu XG. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52:708–715. doi: 10.1016/j.neuropharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Luo Y, Fischer FR, Hancock WW, Dorf ME. Macrophage inflammatory protein-2 and KC induce chemokine production by mouse astrocytes. J Immunol. 2000;165:4015–4023. doi: 10.4049/jimmunol.165.7.4015. [DOI] [PubMed] [Google Scholar]
- Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002;99:175–184. doi: 10.1016/s0304-3959(02)00097-0. [DOI] [PubMed] [Google Scholar]
- Madiai F, Hussain SR, Goettl VM, Burry RW, Stephens RL, Jr, Hackshaw KV. Upregulation of FGF-2 in reactive spinal cord astrocytes following unilateral lumbar spinal nerve ligation. Exp Brain Res. 2003;148:366–376. doi: 10.1007/s00221-002-1286-3. [DOI] [PubMed] [Google Scholar]
- Madiai F, Goettl VM, Hussain SR, Clairmont AR, Stephens RL, Jr, Hackshaw KV. Anti-fibroblast growth factor-2 antibodies attenuate mechanical allodynia in a rat model of neuropathic pain. J Mol Neurosci. 2005;27:315–324. doi: 10.1385/JMN:27:3:315. [DOI] [PubMed] [Google Scholar]
- Mata M, Hao S, Fink DJ. Gene therapy directed at the neuroimmune component of chronic pain with particular attention to the role of TNF alpha. Neurosci Lett. 2008;437:209–213. doi: 10.1016/j.neulet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Shimamura M, Imai S, Kubota C, Yajima Y, Takagi T, Shiokawa M, Inoue T, Suzuki M, Suzuki T. Role of interleukin-1beta and tumor necrosis factor-alpha-dependent expression of cyclooxygenase-2 mRNA in thermal hyperalgesia induced by chronic inflammation in mice. Neuroscience. 2008;152:477–486. doi: 10.1016/j.neuroscience.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Sandkühler J. Learning and memory in pain pathways. Pain. 2000;88:113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Tran HM, Soo VW, McQuiston SA, Tartaglia LA, Goeddel DV, Epstein LB. Enhanced synthesis of tumor necrosis factor-inducible proteins, plasminogen activator inhibitor-2, manganese superoxide dismutase, and protein 28/5.6, is selectively triggered by the 55-kDa tumor necrosis factor receptor in human melanoma cells. J Biol Chem. 1994;269:9898–9905. [PubMed] [Google Scholar]
- Sommer C, Lindenlaub T, Teuteberg P, Schäfers M, Hartung T, Toyka KV. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913:86–89. doi: 10.1016/s0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Schäfers M, Jones TL, Powell H, Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;379:209–213. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-McMenemy N, Harris BT, Deleo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia. 2006;54:193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005a;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005b;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–139. [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]