Abstract
Objective
Vitamin C has been shown to be an effective therapeutic for reducing total serum cholesterol, but epidemiologic studies have determined that low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol are actually better predictive measures of coronary heart disease risk. Therefore, the purpose of this study was to provide a comprehensive meta-analysis of randomized controlled trials to investigate the effect of vitamin C supplementation on LDL and HDL cholesterol as well as triglycerides in patients with hypercholesterolemia.
Methods
Thirteen randomized controlled trials published between 1970 and June 2007 were identified using Medline and a manual search. From the 13 trials, 14 separate group populations with hypercholesterolemia and who were supplemented with at least 500 mg/d of vitamin C for between 3 and 24 weeks were entered into the meta-analysis. This meta-analysis used a random-effects model; and the overall effect sizes were calculated for changes in LDL and HDL cholesterol, as well as triglyceride concentrations.
Results
The pooled estimate of effect for vitamin C supplementation on LDL and HDL cholesterol was −7.9 mg/dL (95% confidence interval [CI], −12.3 to −3.5; P = .000) and 1.1 mg/dL (95% CI, −0.2 to 2.3; not significant), respectively. The pooled estimate of effect for vitamin C supplementation on triglycerides was −20.1 mg/dL (95% CI, −33.3 to −6.8; P < .003).
Conclusion
Supplementation with at least 500 mg/d of vitamin C, for a minimum of 4 weeks, can result in a significant decrease in serum LDL cholesterol and triglyceride concentrations. However, there was a nonsignificant elevation of serum HDL cholesterol.
Key indexing terms: Vitamin C, Ascorbic Acid, Cholesterol, Triglycerides, Meta-analysis, Chiropractic
Introduction
Hypercholesterolemia is a primary risk factor leading to coronary heart disease, which is the leading cause of premature death and disability in the United States. The American Heart Association estimates that approximately 36.6 million American adults have total serum cholesterol levels of greater than 240 mg/dL.1 Over the past couple of decades, a number of nutritional compounds have shown some promise in reducing total serum cholesterol concentrations. One such compound is vitamin C, and a recent meta-analysis found that supplementation with at least 500 mg/d can reduce total serum cholesterol in both borderline-high and high hypercholesterolemic groups by 7.6 and 17.2 mg/dL, respectively.2 A pooled analysis of 9 cohort studies found that those who took at least 700 mg of vitamin C had a 25% reduction in the incidence of coronary heart disease.3 Although total serum cholesterol concentration is a well-known predictor of the incidence of coronary heart disease, a number of epidemiologic studies have determined that low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol are actually better predictive measures of coronary heart disease risk.4-9 The Helsinki Heart Study determined that the LDL/HDL ratio was the best single predictor of cardiac events.10 The role of triglycerides in predicting coronary heart disease remained controversial up until the 8-year follow-up study to the Copenhagen study that found that triglycerides were actually a strong independent risk factor for predicting coronary heart disease.11 This finding was further supported by a meta-analysis of 17 population-based prospective studies that found that plasma triglycerides predict subsequent coronary heart disease.12
It is because of these findings that the National Cholesterol Education Program Expert Panel revised its guidelines and now recommends monitoring triglycerides, LDL cholesterol, and HDL cholesterol in the context of coronary heart disease risk factors.13 Therefore, in light of the fact that vitamin C has been shown to be an effective therapeutic for total serum cholesterol reduction, the purpose of this study was to provide a comprehensive meta-analysis of randomized controlled trials to investigate the effect of vitamin C supplementation on LDL and HDL cholesterol as well as triglycerides in patients with hypercholesterolemia.
Methods
Selection of studies
A comprehensive Medline literature search was performed to locate relevant randomized controlled trials published between 1970 and June 2007. The following headings were combined using the following Boolean operation: (vitamin C OR ascorbic acid OR ascorbate) AND (cholesterol OR triglyceride OR triglycerides). The search was restricted to key terms located in the title/abstract, and language was not an exclusion criteria. Furthermore, only full-length original journal articles were considered; and no attempt was made to include abstracts or unpublished studies. A manual search was also conducted by using reference lists from original research papers and review articles.
To be included in the meta-analysis, a study had to meet the following criteria: (1) the study was conducted using hypercholesterolemic human subjects (total serum cholesterol >200 mg/dL); (2) the study design consisted of at least a single-blind, random allocation of study participants to vitamin C treatment or placebo-controlled groups; (3) vitamin C was given orally with a minimum dose of 500 mg/d; (4) the intervention was greater than 3 weeks and less than 24 weeks; and (5) the study reported the mean LDL cholesterol, HDL cholesterol, and triglyceride concentration changes in both the treatment and control groups. The dose and intervention duration cutoffs were chosen based on the observations that 500 mg/d is the required intake for 95% of the population to achieve a saturated plasma vitamin C concentration14 and that it takes 3 to 4 weeks to reach a plasma steady-state after vitamin C supplementation.15
Only 13 studies of the potential 1363 abstracts met the eligibility criteria and were included in the meta-analysis.16-28 Fourteen separate vitamin C supplementation groups were identified from these 13 studies. Participant and study design characteristics for the 14 groups included in the meta-analysis are presented in Table 1.
Table 1.
Participant and study design characteristics of the 14 vitamin C supplementation groups
| Source and Year (Reference) | Sample Size | Mean Age (y) | Male (%) | Study Design | Vitamin C Dose (mg/d) | Duration (wk) | Baseline Lipids (mg/dL) |
||
|---|---|---|---|---|---|---|---|---|---|
| LDL Cholesterol | HDL Cholesterol | Triglyceride | |||||||
| Horsey et al 198116 | 11 | 82 | 55 | PD | 1000 | 6 | 139.4 | 35.9 | |
| Wahlberg and Walldius 198217 | 9 | 55 | 89 | XD | 2000 | 4 | 154.4 | 42.1 | 427.1 |
| Bishop et al 198518 | 25 | 51 | 52 | XD | 500 | 8 | 208.2 | ||
| Bishop et al 198518 | 25 | 60 | 44 | XD | 500 | 8 | 288.0 | ||
| Aro et al 198819 | 27 | 48 | 0 | XD | 2000 | 6 | 44.8 | ||
| Salonen et al 199120 | 39 | 72 | 100 | PD | 600 | 20 | 146.7 | 49.4 | |
| Cerna et al 199221 | 80 | 48 | 42 | PD | 500 | 24 | 203.1 | 53.7 | 196.7 |
| Paolisso et al 199522 | 40 | 72 | 48 | XD | 1000 | 16 | 220.1 | 42.5 | 231.2 |
| Gokce et al 199923 | 21 | 56 | 81 | PD | 500 | 4 | 123 | 39 | 223 |
| Fotherby et al 200024 | 40 | 72 | 50 | XD | 500 | 12 | 135.1 | 60.2 | |
| Singhal et al 200125 | 31 | 55 | 77 | PD | 1000 | 4 | 120.7 | 43.8 | 214.4 |
| Vinson and Jang 200126 | 10 | 53 | 70 | PD | 1000 | 8 | 193.1 | 51.7 | 150.6 |
| Rezaian et al 200227 | 30 | >50 | 50 | PD | 1000 | 10 | 120.8 | 33.3 | 152.8 |
| Shidfar et al 200328 | 17 | 52 | 35 | PD | 500 | 10 | 160.6 | 37.2 | 315 |
PD, Parallel double-blind; XD, crossover double blind.
Data abstraction and statistical analysis
Information on sample size, participant characteristics, study design, vitamin C dosage, duration of intervention, and treatment results with the 3 lipid categories (LDL, HDL, and triglycerides) were abstracted from the 13 studies. In the end, 11 separate LDL cholesterol, 12 HDL cholesterol, and 10 triglyceride group populations were identified from the 13 studies. The pooled demographics for each lipid category are presented in Table 2.
Table 2.
Pooled demographic of subjects included in the meta-analysis for each lipid profile
| Vitamin C Group |
Placebo Group |
|
|---|---|---|
| LDL Cholesterol | ||
| No. of Subjects | 328 | 310 |
| Age (y) | 58.3 | 59.6 |
| % Male | 59.1 | 61.3 |
| Baseline LDL (mg/dL) | 163.7 | 154.6 |
| HDL Cholesterol | ||
| No. of Subjects | 355 | 338 |
| Age (y) | 60.2 | 61.5 |
| % Male | 54.6 | 56.4 |
| Baseline HDL (mg/dL) | 46.9 | 47.2 |
| Triglycerides | ||
| No. of Subjects | 289 | 270 |
| Age (y) | 55.3 | 56.2 |
| % Male | 52.9 | 54.8 |
| Baseline Triglycerides (mg/dL) | 222.2 | 231.5 |
To calculate the overall effect size within each lipid category measurement, studies were weighted by the reciprocal of their variances. The variances for all groups were calculated using the variances at baseline and at the end of follow-up based on the methodology of Follmann et al.29 In this method, a correlation coefficient of 0.5 between the initial and final measures was assumed. Within each trial, equal variance was assumed between the control and intervention groups, as well as between the beginning and end of each trial. For parallel and crossover trials, net changes in measurements were calculated as follows: (measure at end of follow-up in the treatment group − measure at baseline in the treatment group) − (measure at end of follow-up in the control group − measure at baseline in the control group).
Estimates of the mean effect of vitamin C supplementation on each lipid measure and the corresponding 95% confidence intervals (CIs) were calculated using random-effects models. The assumption of heterogeneity implied by the use of the random-effects model was plausible because of differences between trials in such aspects as duration of the trial, dosages used, and sample populations that differed by age and sex. To examine potential publication bias, a funnel plot was constructed where the sample size of each study was plotted against its corresponding effect size. Data analysis was performed using Comprehensive Meta-Analysis software version 2.0 (Biostat, Englewood, NJ).
Results
Characteristics of the studies
The 11 groups making up the LDL cholesterol category consisted of a total of 549 individual subjects (328 participated in the vitamin C supplementation treatment group, and 310 participated in the control group). All of the trials were conducted using adults with an age range of 48 to 82 years and a pooled mean age of 58.9 years. Men made up most of the subjects, with the pooled population consisting of 60% men. Eight trials used a parallel double-blind design, and 3 used a crossover double-blind design. The study duration varied from 4 to 24 weeks, with a median length of 10 weeks. Vitamin C supplementation for 5 of the 11 trials was 500 to 600 mg/d, whereas 5 trials used 1000 mg/d and 1 used 2000 mg/d. The pooled mean baseline LDL cholesterol concentrations for the treatment and control groups were 163.7 and 154.6 mg/dL, respectively. Comparative demographics between the placebo and vitamin C supplementation groups are presented in Table 2.
The 12 groups making up the HDL cholesterol category consisted of a total of 577 individual subjects (355 participated in the vitamin C supplementation treatment group, and 338 participated in the control group). All of the trials were conducted using adults with an age range of 48 to 82 years and a pooled mean age of 60.8 years. Men made up most of the subjects, with the pooled population consisting of 55% men. Eight trials used a parallel double-blind design, and 4 used a crossover double-blind design. The study duration varied from 4 to 24 weeks, with a median length of 10 weeks. Vitamin C supplementation for 5 of the 12 trials was 500 to 600 mg/d, whereas 5 trials used 1000 mg/d and 2 used 2000 mg/d. The pooled mean baseline HDL cholesterol concentrations for the treatment and control groups were 46.9 and 47.2 mg/dL, respectively.
The 10 groups making up the triglyceride category consisted of a total of 460 individual subjects (289 participated in the vitamin C supplementation treatment group, and 270 participated in the control group). All of the trials were conducted using adults with an age range of 48 to 72 years and a pooled mean age of 55.7 years. Men made up most of the subjects, with the pooled population consisting of 54% men. Six trials used a parallel double-blind design, and 4 used a crossover double-blind design. The study duration varied from 4 to 24 weeks, with a median length of 8 weeks. Vitamin C supplementation for 5 of the 10 trials was 500 mg/d, whereas 4 trials used 1000 mg/d and 1 used 2000 mg/d. The pooled mean baseline triglyceride concentrations for the treatment and control groups were 222.2 and 231.5 mg/dL, respectively.
Serum lipid concentration changes
The mean net changes between the treatment and placebo groups for LDL cholesterol, HDL cholesterol, and triglycerides entered into the meta-analysis are presented in Table 3. In the LDL cholesterol category, 9 of the 11 trials had an intervention-related trend toward a reduction in LDL cholesterol; but only 4 of these trials showed a statistically significant reduction (P < .05) when compared with the control group (Fig 1). For the HDL cholesterol category, a trend toward an intervention-related increase was observed for 6 of the 12 trials; however, only 1 of these trials showed a statistically significant increase when compared with the control group (Fig 2). For the triglyceride category, a trend toward intervention-related reduction was also observed for 5 of the 10 trials; but only 2 of these trials showed a statistically significant reduction when compared with the control group (Fig 3).
Table 3.
Mean net change in LDL cholesterol, HDL cholesterol, and triglycerides after vitamin C supplementation
| Source and Year (Reference) | Sample Size | Net Lipid Changea (95% CI) | P Valueb |
|---|---|---|---|
| LDL Cholesterol | |||
| Horsey et al 198116 | 25 | −13.9 (−32.3, 4.5) | NS |
| Wahlberg and Walldius 198217 | 18 | 1.2 (−31.0, 33.3) | NS |
| Salonen et al 199120 | 78 | 3.9 (−6.3, 14.0) | NS |
| Cerna et al 199221 | 134 | −17.8 (−29.5, −6.0) | .004 |
| Paolisso et al 199522 | 80 | −57.9 (−91.4, −24.4) | .001 |
| Gokce et al 199923 | 46 | −1.0 (−13.2, 11.2) | NS |
| Fotherby et al 200024 | 80 | −3.9 (−14.0, 6.3) | NS |
| Singhal et al 200125 | 63 | −1.9 (−14.0, 10.2) | NS |
| Vinson and Jang 200126 | 18 | −34.0 (−67.3, −0.7) | NS |
| Rezaian et al 200227 | 60 | −26.5 (−44.9, −8.0) | .007 |
| Shidfar et al 200328 | 36 | −21.7 (−40.5, −2.9) | .030 |
| HDL Cholesterol | |||
| Horsey et al 198116 | 25 | 7.3 (−0.7, 15.4) | NS |
| Wahlberg and Walldius 198217 | 18 | −1.5 (−7.8, 4.7) | NS |
| Aro et al 198819 | 54 | −1.5 (−5.0, 1.9) | NS |
| Salonen et al 199120 | 78 | −1.9 (−5.8, 1.9) | NS |
| Cerna et al 199221 | 135 | 2.3 (−1.1, 5.8) | NS |
| Paolisso et al 199522 | 80 | 3.9 (−3.7, 11.4) | NS |
| Gokce et al 199923 | 46 | −4.0 (−8.4, 0.4) | NS |
| Fotherby et al 200024 | 80 | 1.2 (−3.1, 5.4) | NS |
| Singhal et al 200125 | 63 | 0.3 (−3.4, 4.0) | NS |
| Vinson and Jang 200126 | 18 | −0.8 (−8.4, 6.8) | NS |
| Rezaian et al 200227 | 60 | 11.7 (8.0, 15.3) | .000 |
| Shidfar et al 200328 | 36 | −1.4 (−4.9, 2.1) | NS |
| Triglycerides | |||
| Wahlberg and Walldius 198217 | 18 | 59.4 (−62.3, 181.0) | NS |
| Bishop et al 198518 | 50 | −43.4 (−99.9, 13.1) | NS |
| Bishop et al 198518 | 50 | 4.4 (−68.5, 77.4) | NS |
| Cerna et al 199221 | 138 | 0.9 (−44.7, 46.5) | NS |
| Paolisso et al 199522 | 80 | −44.3 (−72.1, −16.5) | .003 |
| Gokce et al 199923 | 46 | 27.0 (−28.5, 82.5) | NS |
| Singhal et al 200125 | 63 | −33.5 (−68.0, 1.0) | NS |
| Vinson and Jang 200126 | 18 | 18.6 (−32.2, 69.4) | NS |
| Rezaian et al 200227 | 60 | −11.1 (−37.9, 15.8) | NS |
| Shidfar et al 200328 | 36 | −52.7 (−103.3, −2.1) | .049 |
NS, Not significant.
For parallel trials, the net change is (intervention final lipid − baseline lipid) − (control final lipid − baseline lipid). For crossover trials, the net change is intervention final lipid − control final lipid.
The P value was calculated by the author.
Fig 1.
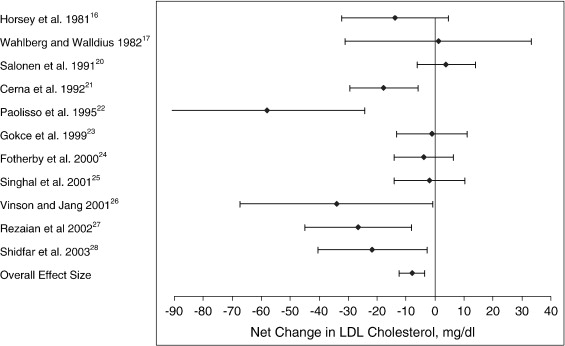
Net change (and 95% CI) in LDL cholesterol concentration associated with vitamin C supplementation. The overall effect size is weighted by the inverse of the total variance of each trial.
Fig 2.
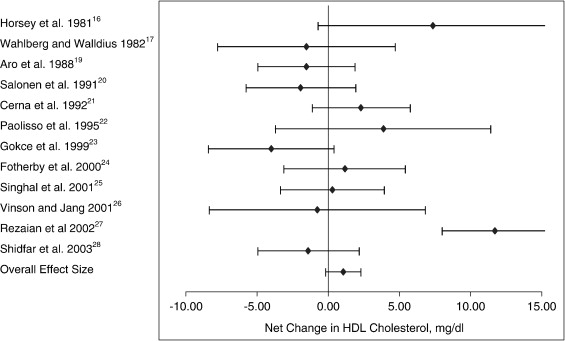
Net change (and 95% CI) in HDL cholesterol concentration associated with vitamin C supplementation. The overall effect size is weighted by the inverse of the total variance of each trial.
Fig 3.
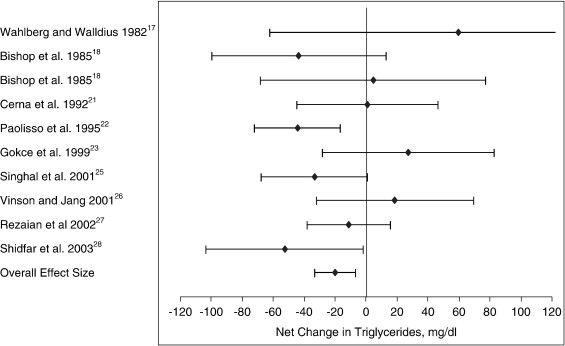
Net change (and 95% CI) in triglyceride concentration associated with vitamin C supplementation. The overall effect size is weighted by the inverse of the total variance of each trial.
The effect size along with the 95% CIs for each trial, as well as the overall effect size for the 3 lipid categories, are presented in Table 4 and Figs 1 through 3. For LDL cholesterol, the overall pooled estimate of the effect of vitamin C supplementation on LDL cholesterol was −7.9 mg/dL (95% CI, −12.3 to −3.5; P = .000). For HDL cholesterol, the overall pooled estimate of the effect of vitamin C supplementation on HDL cholesterol was 1.1 mg/dL (95% CI, −0.2 to 2.3; not significant). For triglycerides, the overall pooled estimate of the effect of vitamin C supplementation on triglycerides was −20.1 mg/dL (95% CI, −33.3 to −6.8; P < .003).
Table 4.
Pooled estimates of treatment effect on the lipid profile
| Variables | No. of Study Groups | Sample Size | Effect Size (95% CI) | Percentage Change | P Value |
|---|---|---|---|---|---|
| Total Cholesterola | 18 | 1119 | −10.67 (−14.0, −7.3) | 4.5 | .000 |
| LDL Cholesterol | 11 | 638 | −7.9 (−12.3, −3.5) | 5.0 | .000 |
| HDL Cholesterol | 12 | 692 | 1.1 (−0.2, 2.3) | 2.3 | .087 |
| Triglycerides | 10 | 555 | −20.1 (−33.3, −6.8) | 8.8 | .003 |
Data for total cholesterol extrapolated from McRae.2
Publication bias
For all 3 lipid profiles, the plot of sample size vs effect size showed a typical “funnel” shape with little variation in effect size for large sample studies and increasing spread of effect size with smaller sample sizes (data not shown). The distribution of effects sizes seen in the individual studies was symmetrically distributed around the pooled mean effect size for the LDL cholesterol and triglyceride category, but asymmetrically distributed for the HDL cholesterol category (Figs 1-3).
Discussion
In this current meta-analysis, vitamin C supplementation provided a significant reduction in both LDL cholesterol (−7.9 mg/dL or 5%) and triglycerides (−20.1 mg/dL or 8.8%), but failed to provide a significant increase in HDL cholesterol (1.1 mg/dL or 2.3%). This last result is surprising because numerous epidemiologic studies have shown that vitamin C intake positively correlates with HDL cholesterol concentrations.30-34 One cross-sectional study found that a 30-mg/dL increase in plasma vitamin C concentration would result in a 3.7% to 5.0% increment in HDL cholesterol.33 However, in interventional studies, the efficacy of vitamin C supplementation has failed to show promising results because only one of the 12 interventional studies included in this meta-analysis showed a significant positive effect. In another 2 studies, not included in this meta-analysis, vitamin C supplementation also failed to provide a positive effect upon HDL cholesterol.35,36 One explanation for the discrepancy between the correlation and intervention studies is that plasma vitamin C levels were not elevated enough in the interventional studies to have a positive effect on HDL cholesterol. However, this does not appear to be the case because the weighted average increase in plasma vitamin C concentration for the trials entered into this meta-analysis was 46 mg/dL.
Although the magnitude of change in LDL cholesterol and triglycerides appeared modest, it can be estimated from the Atherosclerosis Risk in Communities Study37 that an LDL cholesterol change of −7.9 mg/dL could potentially translate to a 6.6% reduction in coronary heart disease and that a change in triglycerides of −20.1 mg/dL could translate to a 2.4% reduction in coronary heart disease risk. Although the change in HDL cholesterol was not statistically significant, the 1.1-mg/dL change in HDL cholesterol could still equate to a 2.1% decrease in coronary heart disease risk. If it were possible to work out that each lipid change was mutually exclusive and played an independent mechanistic role in the future pathogenesis of coronary heart disease, then it could be surmised that vitamin C supplementation should equate to a combined decrease in coronary heart disease risk of 11.1%.
If the changes in the lipid profile established in this meta-analysis are used to determine the change in total serum cholesterol, then it could be calculated that total serum cholesterol decreased by 10.82 mg/dL (where total serum cholesterol = LDL + HDL + [triglycerides/5]). This calculated change in total serum cholesterol also happens to support the finding of a meta-analysis investigating the effects of vitamin C supplementation on total serum cholesterol that found that total serum cholesterol decreased by 10.67 mg/dL.2 However, based upon the Atherosclerosis Risk in Communities Study37 and the above calculated change in total serum cholesterol, this change would only equate to a decrease in coronary heart disease risk of 6.5%, which is 40% less than the coronary heart disease risk calculation of 11.1% determined in the previous paragraph. This suggests that the combined effects of vitamin C on the separate lipid profiles are not mutually exclusive or independent when generating a coronary heart disease risk score. For example, one of the protective mechanisms of HDL cholesterol is to cause inhibition of LDL oxidation.38 Therefore, the benefits of vitamin C supplementation on increasing HDL cholesterol concentrations can indirectly carry over to act as a benefit for LDL cholesterol reduction.
It was surprising to note that the magnitude of change in triglycerides was more than doubled when compared with the change in LDL cholesterol (−20.1 vs −7.9 mg/dL, respectively). This observation is of particular interest because the literature on vitamin C mechanism and actions focuses primarily on changes in LDL and HDL cholesterol. Even reviews by Trout,39 Simon,40 Howard and Meyers,41 and Lynch et al42 had little to say about vitamin C's actions and efficacy in reducing triglycerides. However, in a review paper by Hemila on vitamin C and plasma cholesterol, he did extensively remark upon vitamin C's ability to decrease triglyceride levels.43 By extrapolating the data from 27 groups of subjects examined in Hemila's review,43 the weighted decrease in triglycerides was approximately 8% when compared with the control group. This finding corroborates the percentage change observed in this meta-analysis, which was 8.8%.
It has been observed that the LDL/HDL ratio is a good predictor of coronary heart disease10; and in this analysis, the weighted average decrease in the LDL/HDL ratio was −0.60. When compared with the control group, this equates to a 16.2% reduction from baseline. From this, it can be surmised that such a drop can translate to a 10.2% reduction in coronary heart disease risk,44 which is in keeping with our first predicted estimate.
The triglyceride to HDL ratio has also been shown to be a good predictor of coronary heart disease45; and in this analysis, the weighted average decrease in the triglyceride/HDL ratio was −0.47. When compared with the control group, this equates to a 9.4% reduction from baseline. Although the magnitude of change in the triglyceride to HDL ratio was smaller than that observed in the LDL/HDL ratio, this could still account for a similar change in coronary heart risk because it has been shown that the LDL/HDL ratio may underestimate coronary heart disease risk when compared with the estimation achieved with the triglyceride to HDL ratio.46
In regard to mechanism of action, it has been shown that vitamin C is able to intercept reactive oxygen species in the aqueous phase of plasma, thereby significantly reducing plasma lipid peroxide levels and thus inhibiting oxidative modification of LDLs.47-49 This protection preserves the ability of LDL to be recognized by LDL receptors in the liver and therefore expedite its removal from the blood by LDL cholesterol catabolic pathways.50 Vitamin C may also have a protective effect on these LDL receptors that were shown to decrease in number by approximately 25% in guinea pigs fed suboptimal vitamin C intakes.51 This same guinea pig study also found that suboptimal vitamin C intake caused an increase in the activity of 2 cholesterol-regulating enzymes, acyl–coenzyme A:cholesterol acyltransferase and cholesterol ester transfer protein, by 20% and 30%, respectively. Increased activity of acyl–coenzyme A:cholesterol acyltransferase may result in elevated serum LDL cholesterol concentrations,52 where an increase in cholesterol ester transfer protein activity may cause a reduction in HDL cholesterol.53
Vitamin C has also been shown to protect HDL cholesterol from lipid oxidation, therefore allowing it to be involved in a process known as reverse cholesterol transport.54 Reverse cholesterol transport involves the removal of unesterified cholesterol from extrahepatic cell membranes to where it is esterified via lecithin:cholesterol acyltransferase. The cholesterol esters inside the HDL lipoproteins are then finally transferred back to the liver for further processing and excretion via the bile. It is known that HDL oxidation modifies apolipoprotein A-I structure, which alters the ability of the HDL lipoproteins to activate lecithin:cholesterol acyltransferase, therefore inhibiting the esterification and removal of extrahepatic cholesterol.55,56 Most important then is the finding that vitamin C supplementation has been shown to significantly increase apolipoprotein A-I concentrations and therefore preserve the reverse cholesterol transport process.57
As was noted earlier, HDL lipoproteins also inhibit LDL oxidation; and this free radical scavenging effect occurs via an antioxidant enzyme called HDL-associated paraoxonase.58 Vitamin C shows a capacity to prevent the loss of paraoxonase activity during oxidant stress, therefore attenuating the oxidative modification of LDL cholesterol.59
Vitamin C's effectiveness in reducing triglyceride concentrations was first documented in guinea pig models where a chronic borderline vitamin C deficiency led to hypertriglyceridemia.60 It was later stated that the hypertriglyceridemia was caused by a slow uptake and removal of very low-density lipoprotein triglycerides from the plasma.61 Vitamin C's antioxidant protection of very low-density lipoprotein may therefore facilitate its uptake by the liver and hence promote its removal from the plasma.62,63 It has also been shown that vitamin C stimulates fatty acid utilization in hepatocytes by enhancing carnitine synthesis.64 Carnitine is synthesized from the amino acids lysine and methionine, and vitamin C is required as a cofactor in 2 hydroxylation reactions in the pathway of carnitine biosynthesis. If increased hepatic carnitine concentration results in further hepatic fatty acid β-oxidation, then as a result, there will be a reduction in the plasma triglyceride concentration.65,66
In regard to the limitations of this meta-analysis, first and foremost was the pooling of clinical trials that include a considerable amount of heterogeneity in design and population characteristics. Average subject age varied between 48 and 82 years; and it is known that vitamin C concentration in serum decreases with aging, whereas a concomitant increase in total serum concentration occurs.67 Differences in age and dietary characteristics may result in unevenly matched baseline plasma vitamin C concentrations. In the 9 groups where baseline plasma vitamin C concentrations were observed, the range varied between 28 and 75 μmol. This may confound both the starting baseline total serum cholesterol levels as well as the absorbability of vitamin C supplementation that is dependent upon initial preabsorption plasma concentrations.15 Furthermore, not having evenly matched baseline cholesterol and triglyceride concentrations could confound the results because populations with higher concentrations could possibly exhibit a greater posttreatment effect with vitamin C supplementation. Confounders also included differences between studies in vitamin C supplementation dose (range, 500-2000 mg/d) and study duration (range, 4-24 weeks).
Conclusion
This meta-analysis of 13 randomized controlled trials indicates that supplementation with at least 500 mg/d of vitamin C, for a minimum of 4 weeks, can result in a significant decrease in serum LDL cholesterol and triglyceride concentrations. However, there was a nonsignificant elevation of serum HDL cholesterol. Although these changes are modest, any small change can have beneficial effects on the incidence of coronary heart disease, especially in light of the low cost and absence of toxicity when supplementing vitamin C within the ranges of 500 to 1000 mg/d.68
References
- 1.Rosamond W., Flegal K., Friday G. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.McRae M.P. Vitamin C supplementation for treating hypercholesterolemia: a meta-analysis of 16 randiomized controlled trials. J Am Nutraceut Ass. 2007;10(2):21–28. [Google Scholar]
- 3.Knekt P., Ritz J., Pereira M.A., O'Reilly E.J., Augustsson K., Fraser G.E. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80(6):1508–1520. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 4.Abbott R.D., Wilson P.W., Kannel W.B., Castelli W.P. High density lipoprotein cholesterol, total cholesterol screening, and myocardial infarction. The Framingham Study. Arteriosclerosis. 1988;8(3):207–211. doi: 10.1161/01.atv.8.3.207. [DOI] [PubMed] [Google Scholar]
- 5.Castelli W.P., Anderson K., Wilson P.W., Levy D. Lipids and risk of coronary heart disease. The Framingham Study. Ann Epidemiol. 1992;2(1-2):23–28. doi: 10.1016/1047-2797(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 6.Corti M.C., Guralnik J.M., Salive M.E., Harris T., Field T.S., Wallace R.B. HDL cholesterol predicts coronary heart disease mortality in older persons. JAMA. 1995;274(7):539–544. [PubMed] [Google Scholar]
- 7.Assmann G., Schulte H., von Eckardstein A., Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):S11–S20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 8.Després J.P., Lemieux I., Dagenais G.R., Cantin B., Lamarche B. HDL-cholesterol as a marker of coronary heart disease risk: the Québec cardiovascular study. Atherosclerosis. 2000;153(2):263–272. doi: 10.1016/s0021-9150(00)00603-1. [DOI] [PubMed] [Google Scholar]
- 9.Franceschini G. Epidemiologic evidence for high-density lipoprotein cholesterol as a risk factor for coronary artery disease. Am J Cardiol. 2001;88(12A):9N–13N. doi: 10.1016/s0002-9149(01)02146-4. [DOI] [PubMed] [Google Scholar]
- 10.Manninen V., Tenkanen L., Koskinen P. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85(1):37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Jeppesen J., Hein H.O., Suadicani P., Gyntelberg F. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation. 1998;97(11):1029–1036. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- 12.Austin M.A., Hokanson J.E., Edwards K.L. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 13.Tulenko T.N., Sumner A.E. The physiology of lipoproteins. J Nucl Cardiol. 2002;9(6):638–649. doi: 10.1067/mnc.2002.128959. [DOI] [PubMed] [Google Scholar]
- 14.Brubacher D., Moser U., Jordan P. Vitamin C concentrations in plasma as a function of intake: a meta-analysis. Int J Vitam Nutr Res. 2000;70(5):226–237. doi: 10.1024/0300-9831.70.5.226. [DOI] [PubMed] [Google Scholar]
- 15.Levine M., Conry-Cantilena C., Wang Y. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci. 1996;93(8):3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsey J., Livesley B., Dickerson J.W. Ischaemic heart disease and aged patients: effects of ascorbic acid on lipoproteins. J Hum Nutr. 1981;35(1):53–58. doi: 10.3109/09637488109143486. [DOI] [PubMed] [Google Scholar]
- 17.Wahlberg G., Walldius G. Lack of effect of ascorbic acid on serum lipoprotein concentrations in patients with hypertriglyceridaemia. Atherosclerosis. 1982;43(2-3):283–288. doi: 10.1016/0021-9150(82)90029-6. [DOI] [PubMed] [Google Scholar]
- 18.Bishop N., Schorah C.J., Wales J.K. The effect of vitamin C supplementation on diabetic hyperlipidaemia: a double blind, crossover study. Diabet Med. 1985;2(2):121–124. doi: 10.1111/j.1464-5491.1985.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 19.Aro A., Kyllastinen M., Kostiainen E., Gref C.G., Elfving S., Uusitalo U. No effect on serum lipids by moderate and high doses of vitamin C in elderly subjects with low plasma ascorbic acid levels. Ann Nutr Metab. 1988;32(3):133–137. doi: 10.1159/000177426. [DOI] [PubMed] [Google Scholar]
- 20.Salonen J.T., Salonen R., Seppanen K. Effects of antioxidant supplementation on platelet function: a randomized pair-matched, placebo-controlled, double-blind trial in men with low antioxidant status. Am J Clin Nutr. 1991;53(5):1222–1229. doi: 10.1093/ajcn/53.5.1222. [DOI] [PubMed] [Google Scholar]
- 21.Cerna O., Ramacsay L., Ginter E. Plasma lipids, lipoproteins and atherogenic index in men and women administered vitamin C. Cor Vasa. 1992;34(3):246–254. [PubMed] [Google Scholar]
- 22.Paolisso G., Balbi V., Volpe C. Metabolic benefits deriving from chronic vitamin C supplementation in aged non–insulin dependent diabetics. J Am Coll Nutr. 1995;14(4):387–392. doi: 10.1080/07315724.1995.10718526. [DOI] [PubMed] [Google Scholar]
- 23.Gokce N., Keaney J.F., Frei B. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- 24.Fotherby M.D., Williams J.C., Forster L.A., Craner P., Ferns G.A. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons. J Hypertens. 2000;18(4):411–415. doi: 10.1097/00004872-200018040-00009. [DOI] [PubMed] [Google Scholar]
- 25.Singhal S., Gupta R., Goyle A. Comparison of antioxidant efficacy of vitamin E, vitamin C, vitamin A and fruits in coronary heart disease: a controlled trial. J Assoc Phys India. 2001;49:327–331. [PubMed] [Google Scholar]
- 26.Vinson J.A., Jang J. In vitro and in vivo lipoprotein antioxidant effect of a citrus extract and ascorbic acid on normal and hypercholesterolemic human subjects. J Med Food. 2001;4(4):187–192. doi: 10.1089/10966200152744454. [DOI] [PubMed] [Google Scholar]
- 27.Rezaian G.R., Taheri M., Mozaffari B.E., Mosleh A.A., Ghalambor M.A. The salutary effects of antioxidant vitamins on the plasma lipids of healthy middle aged-to-elderly individuals: a randomized, double-blind, placebo-controlled study. J Med Liban. 2002;50(1-2):10–13. [PubMed] [Google Scholar]
- 28.Shidfar F., Keshavarz A., Jallali M., Miri R., Eshraghian M. Comparison of the effects of simultaneous administration of vitamin C and omega-3 fatty acids on lipoproteins, apo A-I, apo B, and malondialdehyde in hyperlipidemic patients. Int J Vitam Nutr Res. 2003;73(3):163–170. doi: 10.1024/0300-9831.73.3.163. [DOI] [PubMed] [Google Scholar]
- 29.Follmann D., Elliott P., Suh I., Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 30.Jacques P.F., Hartz S.C., McGandy R.B., Jacob R.A., Russell R.M. Ascorbic acid, HDL, and total plasma cholesterol in the elderly. J Am Coll Nutr. 1987;6(2):169–174. doi: 10.1080/07315724.1987.10720177. [DOI] [PubMed] [Google Scholar]
- 31.Dallal G.E., Choi E., Jacques P., Schaefer E.J., Jacob R.A. Ascorbic acid, HDL cholesterol, and apolipoprotein A-I in an elderly Chinese population in Boston. J Am Coll Nutr. 1989;8(1):69–74. doi: 10.1080/07315724.1989.10720279. [DOI] [PubMed] [Google Scholar]
- 32.Itoh R., Yamada K., Oka J., Echizen H., Suyama Y., Murakami K. Serum ascorbic acid and HDL cholesterol in a healthy elderly Japanese population. Int J Vitam Nutr Res. 1990;60(4):360–365. [PubMed] [Google Scholar]
- 33.Jacques P.F. Effects of vitamin C on high-density lipoprotein cholesterol and blood pressure. J Am Coll Nutr. 1992;11(2):139–144. [PubMed] [Google Scholar]
- 34.Hallfrisch J., Singh V.N., Muller D.C., Baldwin H., Bannon M.E., Andres R. High plasma vitamin C associated with high plasma HDL- and HDL2 cholesterol. Am J Clin Nutr. 1994;60(1):100–105. doi: 10.1093/ajcn/60.1.100. [DOI] [PubMed] [Google Scholar]
- 35.Joshi V.D., Joshi L.N., Gokhale L.V. Effect of ascorbic acid on total and high density lipoprotein cholesterol of plasma in normal human subjects. Indian J Physiol Pharmacol. 1981;25(4):348–350. [PubMed] [Google Scholar]
- 36.Johnson G.E., Obenshain S.S. Nonresponsiveness of serum high-density lipoprotein–cholesterol to high dose ascorbic acid administration in normal men. Am J Clin Nutr. 1981;34(10):2088–2091. doi: 10.1093/ajcn/34.10.2088. [DOI] [PubMed] [Google Scholar]
- 37.Sharrett A.R., Ballantyne C.M., Coady S.A. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 38.Parthasarathy S., Barnett J., Fong L.G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044(2):275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 39.Trout D.L. Vitamin C and cardiovascular risk factors. Am J Clin Nutr. 1991;53(1 Suppl):322S–325S. doi: 10.1093/ajcn/53.1.322S. [DOI] [PubMed] [Google Scholar]
- 40.Simon J.A. Vitamin C and cardiovascular disease: a review. J Am Coll Nutr. 1992;11(2):107–125. [PubMed] [Google Scholar]
- 41.Howard P.A., Meyers D.G. Effect of vitamin C on plasma lipids. Ann Pharmacother. 1995;29(11):1129–1136. doi: 10.1177/106002809502901112. [DOI] [PubMed] [Google Scholar]
- 42.Lynch S.M., Gaziano J.M., Frei B. Ascorbic acid and atherosclerotic cardiovascular disease. Subcell Biochem. 1996;25:331–367. doi: 10.1007/978-1-4613-0325-1_17. [DOI] [PubMed] [Google Scholar]
- 43.Hemilä H. Vitamin C and plasma cholesterol. Crit Rev Food Sci Nutr. 1992;32(1):33–57. doi: 10.1080/10408399209527579. [DOI] [PubMed] [Google Scholar]
- 44.Panagiotakos D.B., Pitsavos C., Skoumas J. Importance of LDL/HDL cholesterol ratio as a predictor for coronary heart disease events in patients with heterozygous familial hypercholesterolaemia: a 15-year follow-up (1987-2002) Curr Med Res Opin. 2003;19(2):89–94. [PubMed] [Google Scholar]
- 45.Jeppesen J., Hein H.O., Suadicani P., Gyntelberg F. Relation of high TG-low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen Male Study. Arterioscler Thromb Vasc Biol. 1997;17(6):1114–1120. doi: 10.1161/01.atv.17.6.1114. [DOI] [PubMed] [Google Scholar]
- 46.Lemieux I., Lamarche B., Couillard C. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161(22):2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 47.Polidori M.C., Mecocci P., Levine M., Frei B. Short-term and long-term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation. Arch Biochem Biophys. 2004;423(1):109–115. doi: 10.1016/j.abb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Balkan J., Dogru-Abbasoglu S., Aykac-Toker G., Uysal M. Serum pro-oxidant–antioxidant balance and low-density lipoprotein oxidation in healthy subjects with different cholesterol levels. Clin Exp Med. 2004;3(4):237–242. doi: 10.1007/s10238-004-0031-6. [DOI] [PubMed] [Google Scholar]
- 49.Jialal I., Vega G.L., Grundy S.M. Physiologic levels of ascorbate inhibit the oxidative modification of low density lipoprotein. Atherosclerosis. 1990;82:185–191. doi: 10.1016/0021-9150(90)90039-l. [DOI] [PubMed] [Google Scholar]
- 50.Sakuma N., Yoshikawa M., Hibino A. Ascorbic acid protects against peroxidative modification of low-density lipoprotein, maintaining its recognition by LDL receptors. J Nutr Sci Vitaminol. 2001;47(1):28–31. doi: 10.3177/jnsv.47.28. [DOI] [PubMed] [Google Scholar]
- 51.Montano C.E., Fernandez M.L., McNamara D.J. Regulation of apolipoprotein B–containing lipoproteins by vitamin C level and dietary fat saturation in guinea pigs. Metabolism. 1998;47(7):883–891. doi: 10.1016/s0026-0495(98)90131-7. [DOI] [PubMed] [Google Scholar]
- 52.Carr T.P., Parks J.S., Rudel L.L. Hepatic ACAT activity in African green monkeys is highly correlated to plasma LDL cholesteryl ester enrichment and coronary artery atherosclerosis. Arterioscler Thromb. 1992;12(11):1274–1283. doi: 10.1161/01.atv.12.11.1274. [DOI] [PubMed] [Google Scholar]
- 53.Agellon L.B., Walsh A., Hayek T. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991;266(17):10796–10801. [PubMed] [Google Scholar]
- 54.Hillstrom R.J., Yacapin-Ammons A.K., Lynch S.M. Vitamin C inhibits lipid oxidation in human HDL. J Nutr. 2003;133(10):3047–3051. doi: 10.1093/jn/133.10.3047. [DOI] [PubMed] [Google Scholar]
- 55.Anantharamaiah G.M., Hughes T.A., Iqbal M. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988;29(3):309–318. [PubMed] [Google Scholar]
- 56.Nagano Y., Arai H., Kita T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Natl Acad Sci. 1991;88(15):6457–6461. doi: 10.1073/pnas.88.15.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacques P.F., Sulsky S.I., Perrone G.E., Jenner J., Schaefer E.J. Effect of vitamin C supplementation on lipoprotein cholesterol, apolipoprotein, and triglyceride concentrations. Ann Epidemiol. 1995;5(1):52–59. doi: 10.1016/1047-2797(94)00041-q. [DOI] [PubMed] [Google Scholar]
- 58.Nofer J.R., Kehrel B., Fobker M., Levkau B., Assmann G., von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161(1):1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 59.Calla M.S., Lynch S.M. Vitamin C preserves the cardio-protective paraoxonase activity of high-density lipoprotein during oxidant stress. Arch Biochem Biophys. 2006;452(2):129–137. doi: 10.1016/j.abb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Bobek P., Ginter E., Ozdín L., Mikus L. The effect of chronic marginal vitamin C deficiency on the rate of secretion and the removal of plasma triglycerides in guinea-pigs. Physiol Bohemoslov. 1980;29(4):337–343. [PubMed] [Google Scholar]
- 61.Bobek P., Ginter E., Ozdin L., Poledne R., Potucek J. Effect of long-term marginal vitamin C deficiency on plasma triglyceride kinetics in guinea-pigs. Biomed Biochim Acta. 1983;42(4):413–416. [PubMed] [Google Scholar]
- 62.Gugliucci A., Menini T., Stahl A.J. Susceptibility to copper-enhanced autoxidation of VLDL+LDL fractions from diabetic patients. Biochem Mol Biol Int. 1994;32(1):139–147. [PubMed] [Google Scholar]
- 63.Hasegawa N., Niimi N., Odani F. Vitamin C is one of the lipolytic substances in green tea. Phytother Res. 2002;16(Suppl 1):S91–S92. doi: 10.1002/ptr.843. [DOI] [PubMed] [Google Scholar]
- 64.Ha T.Y., Otsuka M., Arakawa N. Ascorbate indirectly stimulates fatty acid utilization in primary cultured guinea pig hepatocytes by enhancing carnitine synthesis. J Nutr. 1994;124(5):732–737. doi: 10.1093/jn/124.5.732. [DOI] [PubMed] [Google Scholar]
- 65.Ha T.Y., Otsuka M., Arakawa N. The effect of graded doses of ascorbic acid on the tissue carnitine and plasma lipid concentrations. J Nutr Sci Vitaminol. 1990;36(3):227–234. doi: 10.3177/jnsv.36.227. [DOI] [PubMed] [Google Scholar]
- 66.Otsuka M., Matsuzawa M., Ha T.Y., Arakawa N. Contribution of a high dose of l-ascorbic acid to carnitine synthesis in guinea pigs fed high-fat diets. J Nutr Sci Vitaminol. 1999;45(2):163–171. doi: 10.3177/jnsv.45.163. [DOI] [PubMed] [Google Scholar]
- 67.Kothari L.K., Pramod J., Sharma P., Chaturvedi S.K. Influence of age and vitamin C status on serum cholesterol. Int J Epidemiol. 1988;17(4):929–930. doi: 10.1093/ije/17.4.929-a. [DOI] [PubMed] [Google Scholar]
- 68.Rivers J.M. Safety of high-level vitamin C ingestion. Int J Vitam Nutr Res Suppl. 1989;30:95–102. [PubMed] [Google Scholar]


