Abstract
In 1980, the International Agency for Research on Cancer (IARC) determined there was sufficient evidence that inorganic arsenic was a human lung carcinogen based on studies involving exposure through inhalation. In 2004, IARC listed arsenic in drinking water as a cause of lung cancer, making arsenic the first substance established to cause human cancer by two unrelated pathways of exposure. It may initially seem counterintuitive that arsenic in drinking water would cause human lung cancer, and even if it did, one might expect risks to be orders of magnitude lower than those from direct inhalation into the lungs. In this paper we consider lung cancer dose-response relationships for inhalation and ingestion of arsenic by focusing on two key studies, a cohort mortality study in the United States involving Tacoma smelter workers inhaling arsenic, and a lung cancer case-control study involving ingestion of arsenic in drinking water in northern Chile. When exposure was assessed based on absorbed dose identified by concentrations of arsenic in urine, there was very little difference in the dose-response findings for lung cancer relative risks between inhalation and ingestion. The lung cancer mortality rate ratio estimate was 8.0 (95% CI 3.2-16.5, p<0.001) for an average urine concentration of 1179 μg/L following inhalation, and the odds ratio estimate of the lung cancer incidence rate ratio was 7.1 (95% CI 3.4-14.8, p<0.001) for an estimated average urine concentration of 825 μg/L following ingestion. The slopes of the linear dose-response relationships between excess relative risk (RR-1) for lung cancer and urinary arsenic concentration were similar for the two routes of exposure. We conclude that lung cancer risks probably depend on absorbed dose, and not on whether inorganic arsenic is ingested or inhaled.
Keywords: lung cancer, arsenic, inhalation, ingestion
Introduction
Arsenic was one of the first substances recognized to cause cancer in humans. In 1879, high rates of lung cancer in miners in Saxony were attributed by Haerting and Hesse to “irritation caused by inhaled arsenic with other respiratory affections and a bad state of nutrition as predisposing factors,” although later authors thought the increased lung cancer risks were related to radioactive substances in the mining pits (Neubauer, 1947). In 1980, the International Agency for Research on Cancer (IARC) classified inorganic arsenic as a Group 1 carcinogen, with key evidence coming from increased lung cancer risks in workers manufacturing arsenical pesticides and workers inhaling arsenic in copper smelters (IARC, 1980).
At the same time that arsenic inhalation was identified as a cause of lung cancer, ingestion of arsenic in medicines and drinking water was also listed as a Group 1 human carcinogen resulting in skin cancer, but no conclusion was reached about internal cancers (IARC, 1980). The evidence that ingestion of arsenic might increase the risk of lung cancer may seem scientifically implausible. Inhalation is the known pathway of exposure for all other established human lung carcinogens, including cigarette smoking, asbestos, chromium, silica dust, nickel, and radon (Table 1). Yet, the evidence from multiple studies in different countries including Argentina, Chile, Japan and Taiwan, all of which identified associations between ingested arsenic and lung cancer, led IARC to classify arsenic in drinking water as a cause of this cancer in 2004 (IARC, 2004). Even more surprising is the evidence that lung cancer is the main contributor to mortality from arsenic in drinking water, exceeding mortality from other outcomes such as bladder cancer, kidney cancer and cardiovascular disease (Smith, et al., 1992; Yuan, et al., 2007)
Table 1.
Known causes of lung cancer and pathways of exposure.
| Inhalation | |
| Arsenic | Mustard Gas |
| Asbestos | Nickel |
| Beryllium | Radon |
| Bischloromethyl ether | Silica |
| Cadmium | Tobacco smoke |
| Chromium | |
| Ingestion | |
| Arsenic |
The purpose of this paper was to compare lung cancer risks from inhalation and ingestion of arsenic. Given that inhalation results in a more direct exposure to the lung, and given that for all other known lung carcinogens exposure occurs via inhalation, our initial hypothesis was that inhalation risks would be much higher than those resulting from ingestion.
Methods
We first identified epidemiological studies regarding lung cancer resulting from arsenic exposure via inhalation, and that resulting from ingestion of arsenic in drinking water, focusing on studies with dose-response data. Urinary arsenic concentrations are the most widely used measure of absorbed dose as about 70% of absorbed arsenic is excreted in the urine (Biggs, Kalman, Moore, Hopenhayn-Rich, Smith and Smith, 1997). We therefore searched for studies that either included urinary arsenic concentrations, or could be linked to measurements of urine arsenic concentrations in the exposed populations.
We plotted dose-response relationships using lung cancer relative risk estimates as the measure of effect, and urinary arsenic concentrations during exposure as the biological marker of the absorbed dose rate for both inhalation and ingestion of arsenic. The slopes of the dose-response lines were then compared to see which pathway of exposure resulted in the greatest increase in lung cancer risks.
Urine arsenic concentration gives a measure of dose rate of arsenic, rather than cumulative dose. There is a widespread misconception that cumulative dose is the best measure of exposure for epidemiological studies, perhaps based on evidence that absolute risks increase with cumulative dose. However relative risk is a ratio estimate involving both additional cases owing to a particular exposure, and the background cases already occurring from other causes. If both the background cases from other causes and the additional cases from the exposure of interest increase in a similar cumulative manner, one would expect that the relative risk might remain constant once there had been a sufficient latency from the commencement of the added exposure of interest. As evidence that dose rate might be a better measure than cumulative dose to use in studies estimating relative risks, we present data on smoking and lung cancer from the American Cancer Society cohort study, which is the largest cohort study ever conducted (Halpern, Gillespie and Warner, 1993). The data show that the relative risk for lung cancer remains constant with age (see Results), and therefore, by implication, with cumulative dose of cigarettes smoked, suggesting that cumulative dose is not a good metric for exposure when the outcome measure is relative risk. This fits with the general principle that when relative risk is the measure of effect, then in steady state (i.e. with long-term exposure and after appropriate latency) relative risk will be determined by dose rate, rather than cumulative dose. As the populations of interest in this paper both had received long-term steady-state exposures, we have used dose rate in steady state as reflected in urine arsenic concentrations, rather than cumulative dose, in our dose-response analyses.
Results
Identification of inhalation studies
The two largest occupational cohort studies involving inhalation of arsenic are the Tacoma smelter study (Enterline, Henderson and Marsh, 1987) and the Anaconda smelter study (Welch, Higgins, Oh and Burchfiel, 1982) in the United States. Both studies showed marked increases in lung cancer mortality and dose-response relationships based on air concentrations of arsenic. However, the Tacoma smelter study also had measurements of urinary arsenic concentrations for workers in different jobs (Enterline, Henderson and Marsh, 1987). They were therefore able to estimate lung cancer risks based on absorbed dose of arsenic (Enterline, Henderson and Marsh, 1987). Table 2 presents data from this study, with the mean urinary arsenic concentration in workers grouped by exposure, and the corresponding lung cancer standardized mortality ratios (SMRs), which increase to 8.0 (95% CI 3.2-16.5, p<0.001) in the highest exposure category of 1179 μg/L.
Table 2.
Urinary arsenic concentrations resulting from inhalation of arsenic and standardized mortality ratios (SMR) for respiratory cancer in copper smelter workers in Tacoma, WA
| Mean Arsenic Concentration in Urine (μg/Liter) | Adjusted Mean Arsenic Concentration in Urine (μg/Liter)a | Observed Number of Respiratory Cancer Deaths | SMR | 95% CI | |
|---|---|---|---|---|---|
| 15.0 | 0 | 1 | |||
| 114.4 | 99.4 | 6 | 1.8 | 0.7 | 3.9 |
| 355.6 | 340.6 | 2 | 2.6 | 0.3 | 9.5 |
| 605.8 | 590.8 | 4 | 4.9 | 1.3 | 12.7 |
| 1178.5 | 1163.5 | 7 | 8.0 | 3.2 | 16.5 |
Adjusted by subtracting the baseline concentration of 15 μg/L.
Identification of ingestion studies
There have been several ecological studies showing increased lung cancer mortality with arsenic in drinking water (IARC, 2004). Only two published lung cancer studies have individual data on exposure, a cohort study conducted in Taiwan (Chen, et al., 2004) and a case-control study conducted in Chile (Ferreccio, Gonzalez, Milosavjlevic, Marshall, Sancha and Smith, 2000). The cohort study in Taiwan used measurements of well water arsenic concentrations (Chen, et al., 2004). Although there were wide ranges of well water concentrations in some villages, the investigators took the median for each village as a measure of individual exposure. The lung cancer rate ratio estimates obtained increased up to 3.3 (95% CI 1.6-6.8) for average arsenic water concentrations over 700 μg/L. However, in view of the wide range of exposures within many villages, the use of the village medians likely caused this rate ratio estimate to be biased. One source of bias is that within any village, those who get lung cancer as result of arsenic in the water are more likely to have obtained drinking water from the high arsenic concentration tube wells rather than the low concentration tubewells. Hence, their exposure would be underestimated by the median arsenic concentration tube wells. Another source of bias in this study is that arsenic water concentrations were averaged over lifetimes. As lung cancer relative risks would be dependent on exposures within an appropriate latency time window, averaging arsenic concentrations from periods of exposure that are not likely relevant to cancer causation could also bias relative risk estimates.
The lung cancer case-control study in Chile was a little larger than the Taiwan study in terms of numbers of lung cancer cases (159 versus 139) (Chen, et al., 2004; Ferreccio, Gonzalez, Milosavjlevic, Marshall, Sancha and Smith, 2000). A major advantage of this study is the unique nature of the exposure scenario in northern Chile, where the study was conducted. There is very little rainfall in the area and very few private wells. Each city and town receives water from a municipal source, and arsenic has been measured in these sources since the 1950s (Ferreccio, Gonzalez, Milosavjlevic, Marshall, Sancha and Smith, 2000). Thus, merely identifying the town or city that participants lived in can serve to determine the drinking water arsenic concentration for each person in the study. Relative risk estimates were calculated in this study for arsenic concentrations during the high arsenic exposure periods, as well as for lifetime averages. The arsenic water concentrations for the high exposure periods and odds ratio estimates for the peak exposures are given in Table 3. The odds ratios reached 7.1 (95% CI 3.4-14.8, p<0.001) at the highest arsenic water concentrations in the range above 700 μg/L, corresponding to an equivalent urinary arsenic concentration of 825 μg/L. The urinary arsenic concentrations were obtained by multiplying each water concentration given in Table 3 by a factor of 0.97. The conversion factor was derived from data collected in the town of San Pedro in northern Chile (Biggs, Kalman, Moore, Hopenhayn-Rich, Smith and Smith, 1997) by dividing the mean excreted arsenic concentration measured in 123 individuals (582.4 μg/L urine) by the mean arsenic concentration in the town's drinking water (600 μg/L). The key reason why this study was selected is the unique situation in northern Chile, where each town has a single water source as observed earlier. In the absence of alternate water sources, the arsenic concentration in the municipal supply is an accurate measure of arsenic intake from water. We would therefore anticipate the conversion factor of 0.97 to be a good approximation to estimate urinary arsenic concentrations throughout the northern Chile region, where the single-source scenario applies.
Table 3.
Equivalent urinary arsenic concentrations resulting from ingestion of arsenic and lung cancer odds ratios (OR) from a case-control study in Chile.
| Arsenic Concentration in Drinking Water (μg/Liter)a | Cases | Controls | Mean Arsenic Concentration in Drinking Water μg/Liter) | Mean Arsenic Concentration in Urine (μg/Liter)b | Adjusted Mean Arsenic Concentration in Urine (μg/Liter)c | ORd | 95% | CI |
|---|---|---|---|---|---|---|---|---|
| 0-9 | 11 | 92 | 5 | 4.9 | 0 | 1 | ||
| 10-59 | 7 | 81 | 35 | 34.0 | 29.1 | 0.7 | 0.3 | 1.7 |
| 60-199 | 35 | 87 | 130 | 126.1 | 121.3 | 3.4 | 1.8 | 6.5 |
| 200-399 | 23 | 44 | 300 | 291.0 | 286.2 | 4.7 | 2.0 | 11.0 |
| 400-699 | 11 | 12 | 550 | 533.5 | 528.7 | 5.7 | 1.9 | 16.9 |
| 700-999 | 64 | 103 | 850 | 824.5 | 819.7 | 7.1 | 3.4 | 14.8 |
Average concentration during the period of peak arsenic exposure from 1958 to 1970.
Calculated by multiplying arsenic concentration in drinking water by conversion factor of 0.97.
Adjusted by subtracting the baseline concentration of 4.9 μg/Liter.
Adjusted for age, sex, smoking status, employment in copper smelting, and socioeconomic status.
Dose-response analyses
To explore the dose-response relationship between lung cancer and inhaled arsenic, we performed a linear regression of lung cancer SMRs versus the mean urinary arsenic concentration observed in four exposure categories of Tacoma smelter workers (Table 2) (Enterline, Henderson and Marsh, 1987). The baseline concentration of 15 μg/L, for which the authors reported an SMR of 1.0, was subtracted from the mean urinary arsenic concentration in each exposure category prior to performing the regression. To assess the impact of ingested arsenic, we carried out a similar regression for lung cancer odds ratio estimates observed in eight exposure categories in the Chile study (Ferreccio, Gonzalez, Milosavjlevic, Marshall, Sancha and Smith, 2000). Because of small numbers, we pooled the 10-29 μg/L stratum with the 30-59 μg/L stratum, and the 60-89 μg/L stratum with the 90-199 μg/L stratum, resulting in a total of 6 strata (Table 3). The mean urinary arsenic concentration for each stratum was obtained by multiplying the drinking water arsenic concentration by the conversion factor of 0.97 derived above. The background value of 4.9 μg/L, which was the equivalent urinary arsenic concentration for the exposure category yielding an odds ratio of 1.0, was subtracted from the calculated urinary arsenic concentrations.
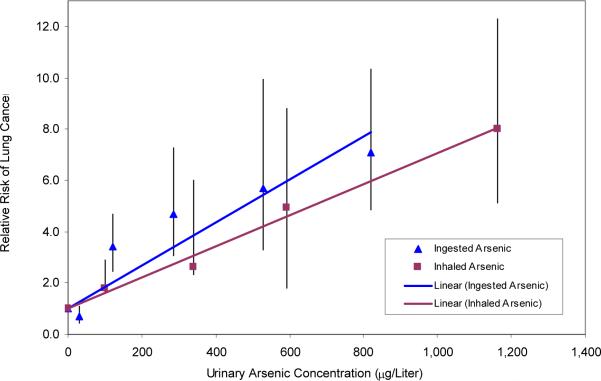
The regressions showed that, for each 100 μg/L increase in urinary arsenic concentration, the increase in lung cancer relative risk was 0.61 (95% CI 0.49-0.73) for inhalation and 0.84 (95% CI 0.43-1.25) for ingestion. The two-tailed t-test comparing the slopes from the regressions yielded a p-value of 0.16.
We used the urinary arsenic concentrations as our exposure metric even though cumulative dose is often used as the measure of exposure in cancer studies. We believe that the dose rate (in our case, measured by urinary arsenic concentrations), in an appropriate time period as far as latency is concerned, is generally the best measure of exposure when the effect measure is relative risk.
As evidence for using dose rate in our analyses rather than trying to estimate cumulative dose, we analyzed data from the American Cancer Society cohort study on smoking and lung cancer (Halpern, Gillespie and Warner, 1993). A cumulative exposure measure, such as pack-years of smoking, consists of two components: years exposed (exposure duration) and intensity of exposure (dose rate). The relationship between cumulative exposure and lung cancer risks could be due to either one or both of these components. One way to assess which one is the more important factor in determining lung cancer relative risk estimates is to compare relative risks across different groups of age. Age is strongly associated with exposure duration (years smoked), but not necessarily with dose rate (cigarettes smoked per day). Owing to the strong association between age and exposure duration, if exposure duration were a truly important determinant of lung cancer relative risk, then the relative risk should increase as age increases among smokers who continue to smoke.
Table 4 presents lung cancer relative risk estimates calculated from data from the American Cancer Society for smokers in different age groups (Halpern, Gillespie and Warner, 1993). The table shows that the relative risk estimate increases for the first 10 years shown, but then stabilizes at about 22 for continuing smokers compared to never smokers. Thus, in those age groups that allow for an appropriate time frame for latency from first exposure (ages 50 and over, which is about 20-30 years after most people first start smoking), relative risk estimates remain constant with increasing age, and are therefore not dependent on duration of smoking. This constant relative risk by age provides evidence that the intensity of exposure is the key determinant of relative risk. With this in mind, we have used urine concentrations, which reflect the intensity of exposure to arsenic, in our dose-response analysis.
Table 4.
Relative Risk of Lung Cancer Mortality for Smokers and Non-Smokers
| Age Group | Never Smokers | Current Smokers | RR* | ||
|---|---|---|---|---|---|
| Person Years | Deaths | Person Years | Deaths | ||
| 40-43 | 82335 | 0 | 46626 | 5 | |
| 44-48 | 248278 | 9 | 135527 | 62 | 12.62 |
| 49-53 | 426334 | 20 | 237120 | 195 | 17.53 |
| 54-58 | 475964 | 33 | 253832 | 398 | 22.62 |
| 59-63 | 466829 | 61 | 217673 | 592 | 20.81 |
| 64-68 | 394931 | 75 | 144344 | 622 | 22.69 |
| 69-73 | 291341 | 91 | 80558 | 518 | 20.59 |
| 74-80 | 235547 | 93 | 38664 | 332 | 21.75 |
The data from which the relative risk estimates were calculated come from Table 4 of Halpern M et al.
Discussion
Inorganic arsenic is unique in that it has been established to cause lung cancer with exposure through both ingestion and inhalation. However, if ingestion were to increase the risks of lung cancer, one might expect that the risks from inhalation with direct exposure to lung cells would be much greater than the risks from ingestion, perhaps orders of magnitude greater. There are several uncertainties in the analysis we have conducted, but it is evident that the risks are not markedly different when based on absorbed dose through inhalation or through ingestion. Uncertainties in our comparisons include those related to age distributions, ethnic differences between the study populations, potential differences in genetic susceptibility, differences in study design and exposure assessment, and differences in interacting factors such as smoking. For instance, it should be noted that the Tacoma study used mortality as the outcome measure, whereas the Chile case-control study focused on incident lung cancer cases during the study period. However, as the large majority of lung cancer cases are fatal within five years, relative risks for incidence and mortality are approximately the same. Moreover, the Tacoma smelter study presented SMRs rather than mortality rate ratios. However, in any one age stratum, the SMR estimate obtained using observed divided by expected deaths is effectively a mortality rate ratio estimate. The overall summing of expected and observed deaths over age strata is only likely to result in minor differences from different age group weighting, therefore, one can reasonably think of the SMR as estimating the underlying lung cancer mortality rate ratio. The Chile lung cancer case-control study, on the other hand, estimated odds ratios. As it was a population-based case-control study identifying incident cases, the odds ratio provides an estimate of the rate ratio of lung cancer incidence in the source population.
Despite the uncertainties mentioned, we believe it is evident that the risks are not orders of magnitude different between inhalation and ingestion. In fact, the dose-response relationships are surprisingly close. These findings led us to re-think the idea that risks should be much higher with exposure via inhalation. The exact reason why exposure via inhalation and via ingestion seem to cause the same level of risk for internal dose is unknown. One possible explanation could be related to arsenic metabolism. The primary route of metabolism of internally absorbed inorganic arsenic is methylation, first to monomethylarsonic acid (MMA) then to dimethylarsenic acid (DMA). Methylation of inorganic arsenic to MMA and then DMA were once thought to be detoxification steps (Hopenhayn-Rich, Smith and Goeden, 1993). However in the last few years, evidence has been mounting that the fist step to MMA may be one of activation, as monomethylarsonous acid (MMA3) has been found to be more toxic than arsenite (As3), which was earlier thought to be the most toxic form of arsenic (Cullen, McBride, Manji, Pickett and Reglinski, 1989). If the first step of methylation is indeed an activation step then one potential reason for the absorbed dose of arsenic determining lung cancer risks is that the inhaled inorganic arsenic may first need to be methylated, and this step might not occur in lung cells themselves. In other words, inhaled arsenic may need to enter the circulation, get methylated to MMA in some other part of the body, and then return to the lung where it may then exert a carcinogenic effect.
There are other possible mechanisms that may explain why risks are similar with either pathway of exposure. The idea of direct contact of carcinogens with lung cells after inhalation may be simplistic. In fact, each cell has its own steady-state relationship with nutrition from the circulatory system, and with the lymphatic system. Each of the populations studied, both the smelter workers and those drinking arsenic-contaminated water in Chile, has steady-state long-term exposures. The concentration of inorganic arsenic in lung cells, the target site, may therefore be no higher when exposure is through inhalation compared to exposure through ingestion. Inhalation of arsenic in the workplace may be more or less continuous during each workday, and the pulmonary cell concentration of arsenic arriving through inhalation would reach steady state. The same would be true for ingestion of arsenic in drinking water throughout each day. Thus, the intracellular concentration would relate to absorbed dose, which is reflected in urinary arsenic concentrations, and lung cancer risks in turn would relate to the intracellular concentration of arsenic, and only indirectly to the concentration of arsenic in inhaled air.
In conclusion, inorganic arsenic causes lung cancer whether inhaled or ingested. The evidence we have presented from these two major studies of lung cancer, one involving inhalation and the other involving ingestion, suggests that the lung cancer risks relate to absorbed dose and are not dependent on the particular pathway of exposure. This finding is pertinent to the consideration of biological mechanisms for arsenic-induced lung cancer, and also to the assessment of population risks, which appear to be independent of the pathway of exposure.
Figure 1.
Relative Risk of Lung Cancer from Ingestion and Inhalation of Arsenic as Function of Urinary Arsenic Concentration
Note: The error bars indicate one standard deviation from the relative risk point estimate.
Acknowledgments
This research was supported by National Institutes of Health grants R01-HL081520-01, P42-ES04705 and R01-ES014032-01A2. Thanks to Chiron Alston for assisting in the preparation of this manuscript. Conflict of interest: none declared.
References
- Biggs ML, Kalman DA, Moore LE, Hopenhayn-Rich C, Smith MT, Smith AH. Relationship of urinary arsenic to intake estimates and a biomarker of effect, bladder cell micronuclei. Mutat Res. 1997;386(3):185–195. doi: 10.1016/s1383-5742(97)00012-4. [DOI] [PubMed] [Google Scholar]
- Chen CL, Hsu LI, Chiou HY, Hsueh YM, Chen SY, Wu MM, Chen CJ. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. Jama. 2004;292(24):2984–2990. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- Cullen WR, McBride BC, Manji H, Pickett AW, Reglinski J. The metabolism of methylarsine oxide and sulfide. Applied Organometallic Chemistry. 1989;3(1):71–78. [Google Scholar]
- Enterline PE, Henderson VL, Marsh GM. Exposure to arsenic and respiratory cancer. A reanalysis. Am J Epidemiol. 1987;125(6):929–938. doi: 10.1093/oxfordjournals.aje.a114631. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11(6):673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst. 1993;85(6):457–464. doi: 10.1093/jnci/85.6.457. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Smith AH, Goeden HM. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environ Res. 1993;60(2):161–177. doi: 10.1006/enrs.1993.1024. [DOI] [PubMed] [Google Scholar]
- IARC Some metals and metallic compounds (IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, Vol. 23); International Agency for Research on Cancer; Lyon, France: 1980. [PubMed] [Google Scholar]
- IARC . Some metals and metallic compounds (IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, Vol. 84) International Agency of for Research on Cancer; Lyon, France: 2004. [Google Scholar]
- Neubauer O. Arsenical Cancer : A Review. Br J Cancer. 1947;1:192–251. doi: 10.1038/bjc.1947.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KB, Higgins I, Oh M, Burchfiel C. Arsenic Exposure, Smoking, and Respiratory Cancer in Copper Smelter Workers. Arch Environ Health. 1982;37(6):325–335. doi: 10.1080/00039896.1982.10667586. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, Bates MN, Smith AH. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;166(12):1381–1391. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]