Abstract
Bacterial vaginosis (BV) is a highly prevalent vaginal infection that is associated with adverse pregnancy outcomes. Vitamin D exerts an influence on the immune system and may play a role in BV. The objective of this study was to examine the association between maternal vitamin D status and the prevalence of BV in early pregnancy. Women (n = 469) enrolled in a pregnancy cohort study at <16 wk underwent a pelvic examination and provided a blood sample for determination of serum 25-hydroxyvitamin D [25(OH)D]. BV was diagnosed using Gram-stained vaginal smears interpreted using the method of Nugent. Approximately 41% of women had BV (Nugent score 7–10) and 52% had a serum 25(OH)D concentration <37.5 nmol/L. The mean unadjusted serum 25(OH)D concentration was lower among BV cases (29.5 nmol/L; 95% CI: 27.1, 32.0) compared with women with normal vaginal flora (40.1 nmol/L; 95% CI: 37.0, 43.5; P < 0.001). BV prevalence decreased as vitamin D status improved (P < 0.001). Approximately 57% of the women with a serum 25(OH)D concentration <20 nmol/L had BV compared with 23% of women with a serum 25(OH)D concentration >80 nmol/L. There was a dose-response association between 25(OH)D and the prevalence of BV. The prevalence declined as 25(OH)D increased to 80 nmol/L, then reached a plateau. Compared with a serum 25(OH)D concentration of 75 nmol/L, there were 1.65-fold (95% CI: 1.01, 2.69) and 1.26-fold (1.01, 1.57) increases in the prevalence of BV associated with a serum 25(OH)D concentration of 20 and 50 nmol/L, respectively, after adjustment for race and sexually transmitted diseases. Vitamin D deficiency is associated with BV and may contribute to the strong racial disparity in the prevalence of BV.
Introduction
Bacterial vaginosis (BV),6 a vaginal infection that affects nearly 1 in 3 reproductive-aged women (1), is a syndrome characterized by the loss of normal vaginal flora, predominantly hydrogen peroxide-producing Lactobacillus species, and an increased prevalence of anaerobic bacteria (2). BV is a serious problem, because it is associated with a number of gynecologic conditions and adverse pregnancy outcomes (3). The relation between BV and the risk of preterm birth is one of the strongest and most consistent associations (4,5). Randomized trials have shown a reduction in preterm birth with eradication of BV among high-risk women, although this relation in the general population is more controversial (6). Clearly, prevention of BV remains a public health priority.
Although sociodemographic characteristics and reproductive history are important predictors of BV infections, maternal black race is one of the strongest known risk factors. Black women are 3 times as likely as white women to have BV (1). The racial disparity is not entirely explained by black women's tendency to have a lower socioeconomic status or more frequent douching, coitus, or use of vaginal products (1,7).
Vitamin D may contribute to the racial disparity in BV. In all stages of a woman's life, vitamin D deficiency is far more common in black females than in their white counterparts (8,9). We found that 83% of black pregnant women residing in Pittsburgh, PA had mid-gestation circulating 25-hydroxyvitamin D [25(OH)D] levels indicative of vitamin D insufficiency compared with 50% of their white counterparts (10). Black women carry the burden of vitamin D deficiency, because their dark skin pigmentation prevents adequate cutaneous synthesis of cholecalciferol from casual exposure to sunlight (11). Moreover, black women's dietary and supplemental vitamin D intakes fail to meet national recommendations (12).
Vitamin D may be important for BV, because it influences a number of aspects of the immune system (13). Vitamin D deficiency has been associated with a wide range of immune disorders and chronic infections such as those due to mycobacteria (14–16), but to our knowledge, it has not been studied in relation to BV, one of the most important lower genital tract infections. Our objective was to examine the association between maternal vitamin D status and the prevalence of BV in early pregnancy.
Materials and Methods
Data came from an ongoing prospective cohort study of pregnant women seeking care at Magee-Womens Hospital antepartum clinics in Pittsburgh, PA. The antepartum clinics serve a predominantly uninsured, low-income population that is ∼55% black and 44% white. Eligible women self-reported their race as black or white, had singleton pregnancies, and had no known preexisting conditions, vaginal bleeding, fetal anomalies, or current or planned cervical cerclage. From June 2003 to June 2007, 552 women agreed to participate in the study (75% response rate). Enrollment took place at <16 wk of gestation [9.5 ± 3.2 wk (mean ± SD)] after women provided informed, written consent. The University of Pittsburgh Institutional Review Board approved the study.
At enrollment, women completed an interviewer-administered questionnaire to collect data on sociodemographic characteristics; medical, reproductive, and sexual history; and maternal behaviors. Also at enrollment, women provided a nonfasting blood sample and underwent a standard pelvic examination. Of the 552 women who enrolled in the study, 526 had complete data available to diagnose BV. From this cohort, we excluded 57 women due to missing first-trimester serum (n = 43) or not completing the baseline interview (n = 14). The final analytical sample was n = 469.
Quantitation of 25(OH)D.
Maternal serum samples were stored in aliquots at −80°C until they were analyzed for 25(OH)D [25(OH)-erocalciferol + 25(OH)-cholecalciferol]. Quantitation of serum 25(OH)D was performed using a DiaSorin RIA, which detects 100% of 25(OH)-ergocalciferol and 100% of 25(OH)-cholecalciferol. The interassay CV was 9.5%. The RIA could detect 25(OH)D in the range of 3.75 to 250 nmol/L. No sample in our analysis fell outside this detectable range.
We classified women into 1 of 5 groups [25(OH)D <20, 20 to <37.5, 37.5 to <50, 50–80, or >80 nmol/L]. Levels above 80 are typically viewed as optimal and the remaining cut-points are gradations of vitamin D insufficiency (17,18). These categories were chosen to illustrate the range of poor vitamin D status in this cohort.
Determination of BV.
In accordance with a standardized protocol, a pelvic examination was performed using a clean, nonlubricated speculum. Two Dacron swabs were placed in the cervix and left there for 10 s to achieve saturation. These swabs were placed in a plastic tube containing 400 mL of purified bovine serum (final dilution of 1:5) and stored at −80°C until assay. Two vaginal swabs were also collected for culture and identification of vaginal flora. BV was diagnosed by a vaginal pH ≥ 4.7 and a score of 7–10 from a Gram-stained vaginal smear interpreted using the method of Nugent et al. (19). Intermediate flora was defined as having a Nugent score of 4–6 and normal flora was defined as having a Nugent score of 0–3.
Covariates.
Women were classified as having a sexually transmitted disease (Trichomonas vaginalis, Chlamydia trachomatis, or Neisseria gonorrheae) based on specimens obtained from the enrollment pelvic exam, as described previously (20). Women self-reported their race/ethnicity as white or black. The interviewer-administered questionnaire also solicited information on smoking status since becoming pregnant, maternal education, marital status, parity, household yearly income, and job status. Prepregnancy BMI [weight (kg)/height (m)2] was based on maternal self-reported height and pregravid weight at enrollment. Women were asked to report the number of sex partners they had and the number of times per week they had vaginal intercourse in the 3 mo and 12 mo before the pregnancy.
Statistical analysis.
We used a Pearson chi-squared test to compare distributions of maternal characteristics by BV and vitamin D status and also vaginal flora status by vitamin D status. We compared log-transformed 25(OH)D by vaginal flora status using a Student's t test. To assess the independent association between maternal vitamin D status and BV, we used multivariable Poisson regression to estimate prevalence ratios (PR) using the “log” link (21). PR was selected instead of an odds ratio, because this was a cross-sectional analysis and BV was a common outcome (22).
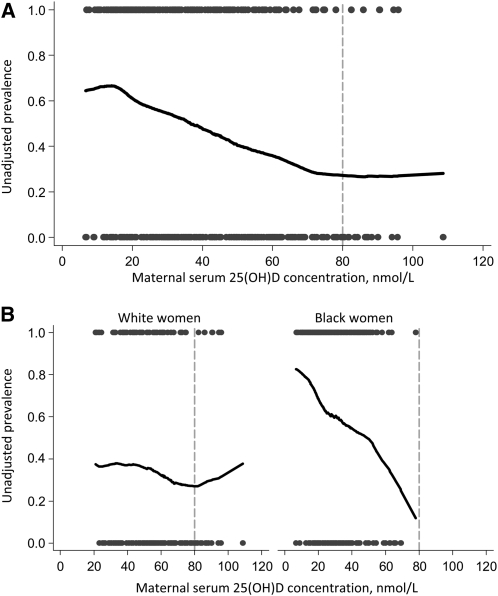
To assess the dose-response relation between maternal vitamin D status and the prevalence of BV, we inspected nonparametric curves produced using Lowess, locally-weighted regression (Fig. 1A). We then used published methods (23) to determine the most appropriate specification of 25(OH)D in our model. There was a negative, linear relation between 25(OH)D and the log-odds of BV up to a serum 25(OH)D concentration of 80 nmol/L, after which the log-odds appeared to plateau. Thus, 25(OH)D was specified in the model as a linear spline with a knot at 80 nmol/L. In the race-stratified models, a continuous, linear variable had the best fit (Fig. 1B). Theory-based causal diagrams (24) were used to determine which covariates should be considered potential confounders in the multivariable models. Potential confounders were maternal race/ethnicity, presence of sexually transmitted diseases (STD), age, parity, education, employment status, family income, prepregnancy BMI, marital status, smoking status, race of the father of the pregnancy, season, enrollment gestational age, number of sexual partners, and frequency of vaginal intercourse. Our goal was to fit parsimonious regression models and remove confounders if their inclusion did not satisfy our a priori change-in-estimate criterion (>10% change in the PR). Race was the only confounder in models of BV. All models presented here are adjusted for the presence of STD out of convention.
FIGURE 1 .
Lowess (locally-weighted regression) curves depicting the unadjusted association between maternal 25(OH)D concentration (nmol/L) at <16 wk and the prevalence of BV in the total population (A) and by race (B). The definition of vitamin D sufficiency is typically 25(OH)D >80 nmol/L (17,18), which is denoted with a dotted gray line. Data are truncated to the 5th–95th percentiles due to erratic behavior in the tails. The scatterplots illustrate the number of samples with BV (at 1.0 on the y-axis) and with normal vaginal flora (at 0.0 on the y-axis).
Values in the text are geometric means (95% CI). A P-value of 0.05 was considered significant. All analyses were conducted using Stata Software (StataCorp).
Results
Most women were 20–29 y old, multiparous, high-school educated, unmarried, unemployed, overweight or obese, and had a yearly household income <$10,000 (Table 1). Women most commonly reported having had 1 sexual partner and vaginal intercourse 1 or 2 times/wk.
TABLE 1.
Maternal characteristics in the total population and by BV and vitamin D status1
| Total population | BV | 25(OH)D <37.5 nmol/L | |
|---|---|---|---|
| Maternal race | n (%) | % | |
| Non-Hispanic white | 209 (44.6) | 27.3† | 25.8† |
| Non-Hispanic black | 260 (55.4) | 52.3 | 73.5 |
| Maternal age | |||
| <20 y | 58 (12.4) | 46.6 | 43.1 |
| 20–29 y | 332 (70.9) | 41.9 | 55.1 |
| ≥30 y | 78 (16.7) | 34.6 | 47.4 |
| Parity | |||
| 0 | 80 (17.1) | 30.0† | 33.8† |
| 1 | 195 (41.8) | 39.5 | 48.2 |
| ≥2 | 192 (41.1) | 47.9 | 63.5 |
| Maternal education | |||
| Less than high school | 116 (24.8) | 50.0 | 57.8 |
| High school or equivalent | 302 (64.7) | 41.1 | 52.0 |
| More than high school | 49 (10.5) | 26.5 | 42.9 |
| Marital status | |||
| Unmarried | 397 (84.7) | 44.8† | 55.7† |
| Married | 72 (15.4) | 20.8 | 33.3 |
| Employment status | |||
| Unemployed | 245 (52.2) | 43.7 | 52.2 |
| Employed part-time | 102 (21.8) | 40.2 | 49.0 |
| Employed full-time | 122 (26.0) | 36.9 | 54.9 |
| Family's yearly income | |||
| <$10,000 | 199 (43.1) | 46.2 | 57.8 |
| $10,000 to <$25,000 | 159 (34.4) | 41.5 | 52.8 |
| $25,000 to <$50,000 | 83 (18.0) | 32.5 | 44.6 |
| ≥$50,000 | 21 (4.5) | 28.6 | 33.3 |
| Prepregnancy BMI | |||
| <18.5 kg/m2 | 7 (1.5) | 0* | 42.9† |
| 18.5–24.9 kg/m2 | 167 (35.8) | 39.5 | 41.9 |
| 25.0–29.9 kg/m2 | 130 (27.8) | 41.5 | 46.2 |
| ≥30.0 kg/m2 | 163 (34.9) | 44.2 | 68.2 |
| Smoking status since becoming pregnant | |||
| Nonsmoker | 231 (50.3) | 35.1* | 57.1* |
| Smoker | 228 (49.7) | 47.8 | 47.8 |
| Sexual partners in the past 3 mo | |||
| 1 | 406 (92.1) | 40.4 | 53.2 |
| ≥2 | 35 (7.9) | 54.3 | 48.6 |
| Sexual partners in the past 12 mo | |||
| 1 | 287 (65.1) | 38.0 | 54.0 |
| 2 | 89 (20.2) | 46.1 | 50.6 |
| ≥3 | 65 (14.7) | 50.8 | 50.8 |
| Frequency of vaginal intercourse in the past 3 mo | |||
| 1/wk | 91 (20.7) | 35.2 | 52.8 |
| 2/wk | 102 (23.2) | 38.2 | 49.0 |
| 3/wk | 72 (16.4) | 48.6 | 66.7 |
| 4/wk | 64 (14.6) | 42.2 | 45.3 |
| 5/wk | 31 (7.1) | 41.9 | 64.5 |
| ≥6/wk | 80 (18.2) | 45.0 | 47.5 |
| Season at enrollment | |||
| Winter | 130 (27.7) | 34.6 | 69.2† |
| Spring | 87 (18.6) | 51.7 | 67.8 |
| Summer | 108 (23.0) | 35.2 | 38.9 |
| Autumn | 144 (30.4) | 45.1 | 37.5 |
Values are percent unless otherwise noted. Symbols indicate difference in the distribution: *P < 0.05, **P < 0.01, †P < 0.001.
Approximately 41% of all women had BV, 15% had intermediate flora, and 44% had normal flora. More than one-half of the sample had a serum 25(OH)D concentration <37.5 nmol/L (52%) and 41% had concentrations of 37.5 to ≤80 nmol/L. Of the 30 women (6.4%) with serum 25(OH)D concentrations >80 nmol/L, only 1 was black. BV and vitamin D deficiency were associated with black race, multiparity, being unmarried, obesity, and smoking.
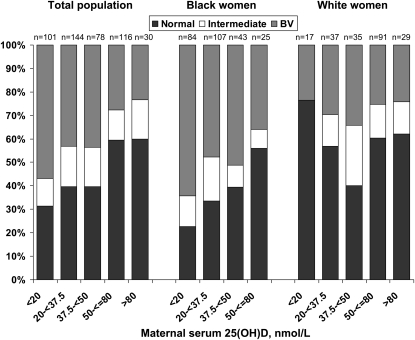
Women with BV had lower unadjusted mean 25(OH)D concentrations compared with women with normal vaginal flora {geometric mean [95% CI: 29.5 (27.1, 32.0) vs. 40.1 (37.0, 43.5) nmol/L]; P < 0.01}. Women with intermediate flora did not differ [34.9 (30.3, 40.1) nmol/L]. These associations remained after adjusting for race (data not shown). The prevalence of BV decreased as vitamin D status improved (Fig. 2; P < 0.001). Approximately 57% of the women with a serum 25(OH)D concentration <20 nmol/L had BV compared with 23% of women with a serum 25(OH)D concentration >80 nmol/L.
FIGURE 2 .
Distribution of BV, intermediate vaginal flora, and normal vaginal flora by maternal vitamin D status category in the cohort and by race. There was 1 black woman with 25(OH)D >80 nmol/L who was excluded from this graph.
There was a dose-response relation between 25(OH)D and prevalence of BV. The prevalence declined as 25(OH)D increased to ∼80 nmol/L and then reached a plateau (Fig. 1A). After adjustment for race and presence of STD, 25(OH)D concentrations of 20 and 50 nmol/L were associated with ∼65% and 26% increases in the likelihood of BV compared with a serum 25(OH)D concentration of 75 nmol/L (Table 2). Beyond 80 nmol/L, PR were not significant and the sample size was substantially reduced.
TABLE 2.
Association between maternal serum 25(OH)D at <16 wk and BV in the total sample of pregnant women and stratified by race
| Adjusted PR (95% CI)1 | |
|---|---|
| Total population2 | |
| Linear spline with knot at 80 nmol/L | |
| 20 nmol/L | 1.65 (1.01, 2.69) |
| 50 nmol/L | 1.26 (1.01, 1.57) |
| 75 nmol/L | Referent |
| 90 nmol/L | 1.32 (0.84, 2.09) |
| Black women | |
| Linear, continuous | |
| 15 nmol/L increase in 25(OH)D3 | 0.82 (0.68, 0.99) |
| 30 nmol/L increase in 25(OH)D4 | 0.68 (0.47, 0.98) |
| White women | |
| Linear, continuous | |
| 25 nmol/L increase in 25(OH)D3 | 0.96 (0.72, 1.27) |
| 50 nmol/L increase in 25(OH)D4 | 0.92 (0.52, 1.61) |
Adjusted for the presence of other sexually transmitted diseases.
Values were selected to approximate the 25th, 75th, 90th, and 97th percentiles of the 25(OH)D distribution.
1 SD increase in 25(OH)D.
2 SD increases in 25(OH)D.
In subgroup analyses, BV was more common as vitamin D status worsened among black women (P < 0.05) but not among whites (Fig. 2). Moreover, there was a linear, monotonic dose-response relation between 25(OH)D and the prevalence of BV among black women before (Fig. 1B) and after confounder adjustment (Table 2). In contrast, 25(OH)D and BV were not associated among whites (Fig. 1B; Table 2). Nevertheless, the relatively small sample of white women with severe vitamin D deficiency [n = 17 with 25(OH)D <20 nmol/L) may have limited the inferences that could be drawn from this group.
Discussion
In this cohort of low-income pregnant women, we found a dose-response association between 25(OH)D and prevalence of BV. The likelihood of BV decreased as vitamin D status improved to levels indicating vitamin D sufficiency [25(OH)D = 80 nmol/L], beyond which the prevalence reached a plateau. Nonetheless, our small number of women with optimal vitamin D status limited our ability to study the BV risk curve beyond 80 nmol/L with good precision.
We know of no other studies that have investigated the relation between serum 25(OH)D and BV. In a recent report of 1520 nonpregnant women, past-year dietary intakes of vitamin D, as ascertained by FFQ, were not associated with “prevalent” BV or BV that was persistent across 2 study visits (25). Nevertheless, dietary vitamin D is a poor measure of overall vitamin D status, because 90% of 25(OH)D is obtained through sunlight exposure (26). Also, the nutrient database used to obtain vitamin D intake estimates is incomplete (27). Our exposure assessment using serum 25(OH)D avoids these and other limitations inherent in dietary assessment and captures recent (past 3 wk) vitamin D exposure.
The disruption of normal vaginal flora that occurs with BV is accompanied by important changes in the innate immunity of the vagina. Neutrophils are critical elements for lower genital tract immunity and neutrophil degranulation products, such as defensins, have been implicated in the pathogenesis of BV-associated adverse pregnancy outcome (28–30). Vitamin D may influence how the host recognizes pathogens and how the immune system responds to prevent and control microbial invasion (31). 1,25-Dihydroxyvitamin D, the hormonally active form of vitamin D, is important in regulating the production and function of innate antimicrobial defense molecules, such as cathelicidin, which is a neutrophil degranulation product that protects against invasive bacterial infection (32,33). Its relation to defensin production and other aspects of neutrophil function also may be relevant (13,34).
Cytokine production may be another factor linking vitamin D to BV and lower genital tract immunity. Among pregnant women with BV, cervical concentrations of proinflammatory cytokines are all greater than those among women without BV (35). Vitamin D has been linked to cytokine expression in a number of disease states and in vitro model systems, with the preponderance of evidence suggesting that it is immunomodulatory and antiinflammatory while still promoting the killing of pathogens (15).
We found that vitamin D deficiency was strongly associated with BV in black women but not in white women. This race difference was likely due to black women being overrepresented in the lower 25(OH)D range, where we saw a strong log-linear association between vitamin D status and BV risk, and white women being overrepresented in the upper range of the 25(OH)D distribution, where the risk may be leveling off. Larger studies of black and white women with a broad range of vitamin D status will be needed in the future to determine whether race modifies this effect.
The cross-sectional nature of this analysis limited our ability to determine the temporal relation between vitamin D and BV. Although we attempted to control for the many covariates related to vitamin D and BV, our effect estimates may have been further biased by other unmeasured or unknown confounders and/or measurement error in the covariate data we collected. We also lacked data on parathyroid hormone concentrations or other functional indicators of vitamin D status.
Our study had many notable strengths. We used the Nugent score from a Gram-stained vaginal smear (19), which is the microbiologic gold-standard for detection of BV. Furthermore, our identification of BV was in the first trimester, which may be the time of greatest clinical importance for subsequent adverse birth outcomes. Unlike many studies of risk factors for BV, which ascertained symptomatic patients only, we screened all pregnant women in the cohort, which increases the generalizability of our findings to low-income pregnant women seeking care in obstetric clinics. An additional strength is our measurement of serum 25(OH)D using a DiaSorin RIA kit, which detects 100% of 25(OH)-ergocalciferol and 25(OH)-cholecalciferol.
Our findings suggest that vitamin D deficiency is associated with BV at <16 wk of pregnancy. A better understanding of the vitamin D-BV relation will be ascertained with prospective studies of “incident” BV infections, persistent infections, and infections that spontaneously resolve. It is also of considerable importance to explore the effect of maternal vitamin D on particular organisms or flora patterns other than BV that are linked to adverse outcomes (36,37). If our results are replicated in other studies, vitamin D deficiency may contribute to the racial disparity in the prevalence of BV and other adverse outcomes of pregnancy.
Acknowledgments
We thank Tanya Bobo for her assistance.
Supported by the NIH grants K01 MH074092 and R01 HD056999 to L. M. Bodnar and R01 HD041663 and R01 HD052732 to H. N. Simhan.
Author disclosures: L. M. Bodnar, M. A. Krohn, and H. N. Simhan, no conflicts of interest.
Abbreviations used: BV, bacterial vaginosis; 25(OH)D, 25-hydroxyvitamin D; PR, prevalence ratio; STD, sexually transmitted disease.
References
- 1.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–20. [DOI] [PubMed] [Google Scholar]
- 2.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DB, Macones G. Bacterial vaginosis in pregnancy: current findings and future directions. Epidemiol Rev. 2002;24:102–8. [DOI] [PubMed] [Google Scholar]
- 4.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG II, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–42. [DOI] [PubMed] [Google Scholar]
- 5.Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams JD, Thom E, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1995;173:1231–5. [DOI] [PubMed] [Google Scholar]
- 6.Brocklehurst P, Hannah M, McDonald H. Interventions for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2000;CD000262. [DOI] [PubMed]
- 7.Vallor AC, Antonio MA, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis. 2001;184:1431–6. [DOI] [PubMed] [Google Scholar]
- 8.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–7. [DOI] [PubMed] [Google Scholar]
- 9.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–92. [DOI] [PubMed] [Google Scholar]
- 10.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. [DOI] [PubMed] [Google Scholar]
- 12.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478–85. [DOI] [PubMed] [Google Scholar]
- 13.Hewison M. Vitamin D and innate immunity. Curr Opin Investig Drugs. 2008;9:485–90. [PubMed] [Google Scholar]
- 14.Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol. 2005;233:115–24. [DOI] [PubMed] [Google Scholar]
- 15.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–4. [DOI] [PubMed] [Google Scholar]
- 16.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. [DOI] [PubMed] [Google Scholar]
- 19.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simhan HN, Bodnar LM, Krohn MA. Paternal race and bacterial vaginosis during the first trimester of pregnancy. Am J Obstet Gynecol. 2008;198:196 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 22.Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med. 1998;55:272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witte JS, Greenland S. A nested approach to evaluating dose-response and trend. Ann Epidemiol. 1997;7:188–93. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 25.Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, Klebanoff MA. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr. 2007;137:2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:S1678–88. [DOI] [PubMed] [Google Scholar]
- 27.Holden JM, Lemar LE. Assessing vitamin D contents in foods and supplements: challenges and needs. Am J Clin Nutr. 2008;88:S551–3. [DOI] [PubMed] [Google Scholar]
- 28.Simhan HN, Caritis SN, Krohn MA, Hillier SL. The vaginal inflammatory milieu and the risk of early premature preterm rupture of membranes. Am J Obstet Gynecol. 2005;192:213–8. [DOI] [PubMed] [Google Scholar]
- 29.Simhan HNMD, Caritis SNMD, Krohn MAP, Hillier SLP. Elevated vaginal pH and neutrophils are associated strongly with early spontaneous preterm birth. Am J Obstet Gynecol. 2003;189:1150–4. [DOI] [PubMed] [Google Scholar]
- 30.Balu RB, Savitz DA, Ananth CV, Hartmann KE, Miller WC, Thorp JM, Heine RP. Bacterial vaginosis, vaginal fluid neutrophil defensins, and preterm birth. Obstet Gynecol. 2003;101:862–8. [DOI] [PubMed] [Google Scholar]
- 31.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. [DOI] [PubMed] [Google Scholar]
- 33.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. [DOI] [PubMed] [Google Scholar]
- 34.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yudin MH, Landers DV, Meyn L, Hillier SL. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstet Gynecol. 2003;102:527–34. [DOI] [PubMed] [Google Scholar]
- 36.Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M, Yamada H, Sakuragi N, Minakami H. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol. 2006;44:51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards RK, Ferguson RJ, Reyes L, Brown M, Theriaque DW, Duff P. Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J Matern Fetal Neonatal Med. 2006;19:357–63. [DOI] [PubMed] [Google Scholar]