Abstract
Background: Genetically engineered “Golden Rice” contains up to 35 μg β-carotene per gram of rice. It is important to determine the vitamin A equivalency of Golden Rice β-carotene to project the potential effect of this biofortified grain in rice-consuming populations that commonly exhibit low vitamin A status.
Objective: The objective was to determine the vitamin A value of intrinsically labeled dietary Golden Rice in humans.
Design: Golden Rice plants were grown hydroponically with heavy water (deuterium oxide) to generate deuterium-labeled [2H]β-carotene in the rice grains. Golden Rice servings of 65–98 g (130–200 g cooked rice) containing 0.99–1.53 mg β-carotene were fed to 5 healthy adult volunteers (3 women and 2 men) with 10 g butter. A reference dose of [13C10]retinyl acetate (0.4–1.0 mg) in oil was given to each volunteer 1 wk before ingestion of the Golden Rice dose. Blood samples were collected over 36 d.
Results: Our results showed that the mean (±SD) area under the curve for the total serum response to [2H]retinol was 39.9 ± 20.7 μg·d after the Golden Rice dose. Compared with that of the [13C10]retinyl acetate reference dose (84.7 ± 34.6 μg·d), Golden Rice β-carotene provided 0.24–0.94 mg retinol. Thus, the conversion factor of Golden Rice β-carotene to retinol is 3.8 ± 1.7 to 1 with a range of 1.9–6.4 to 1 by weight, or 2.0 ± 0.9 to 1 with a range of 1.0–3.4 to 1 by moles.
Conclusion: β-Carotene derived from Golden Rice is effectively converted to vitamin A in humans. This trial was registered at clinicaltrials.gov as NCT00680355.
INTRODUCTION
The intake of vitamin A provides humans with an important nutrient for vision, growth, reproduction, cellular differentiation and proliferation, and integrity of the immune system. Vitamin A deficiency can result in visual or ocular malfunctions such as night blindness and xerophthalmia (1) and can reduce immune responsiveness (2), which can result in an increased incidence or severity of respiratory infections, gastrointestinal infections (3), and measles (4). Vitamin A can be obtained from food, either as preformed vitamin A in animal products (eg, eggs and dairy products) or as provitamin A carotenoids, mainly β-carotene in plant products (eg, dark-green leafy vegetables and fruit).
Clinical and subclinical vitamin A deficiency is still a problem, affecting 250 million schoolchildren worldwide (5, 6). To prevent clinical vitamin A deficiency in developing countries, chemically synthesized vitamin A supplements have been distributed periodically to deficient populations (7–9). This has been shown to be an efficient and generally safe strategy. However, supplementation programs with a periodic mass distribution have been difficult to sustain because of high distribution costs. Recently, food-based interventions to increase the availability of provitamin A–rich foods and their consumption have been suggested as a realistic and sustainable alternative to overcome vitamin A deficiency globally (10). However, the efficacy of carotenoid-rich foods in the prevention of vitamin A deficiency has been questioned in several recent studies, which reported little or no nutritional benefit of vitamin A from the increased consumption of dark-green or yellow vegetables (11, 12). Recently, studies have shown that the equivalency of vegetable provitamin A carotenoids to vitamin A is in the range of 10–27 μg all-trans β-carotene to 1 μg retinol activity (13–16). These studies showed that food matrices greatly affect the bioavailability of vitamin A and carotenoids.
In recent years, scientists have introduced the biosynthetic pathway for provitamin A carotenoids into staple foods, including genetically engineered Golden Rice, which contains 1.6–35 μg β-carotene per gram of dry rice. Golden Rice–1, which was transformed with a construct containing a phytoene synthase gene from daffodil, contains 1.6 μg carotenoids (0.8 μg β-carotene) per gram of dry rice (17). Golden Rice–2 was transformed with a construct containing a phytoene synthase gene from maize and contains up to 35 μg β-carotene per gram of dry rice (18). Because the vitamin A equivalency of various foods and supplements varies from 2 μg β-carotene to 1 μg retinol (when provided as a β-carotene supplement in oil) to 27 μg β-carotene to 1 μg retinol (when provided as vegetable β-carotene) (11, 13), and this equivalency is matrix dependent, it is important to determine the vitamin A equivalency of β-carotene from Golden Rice. This information is critical for the purpose of designing informed, food-based nutritional strategies for rice-eating regions throughout the world where vitamin A deficiency is common. Because vitamin A is homeostatically regulated in the circulation of healthy subjects and it is impossible to distinguish the newly formed vitamin A from endogenous vitamin A (19), we chose intrinsic labeling of the provitamin A carotene as the optimal approach to determine its vitamin A equivalence. We produced intrinsically labeled Golden Rice, fed the rice to healthy volunteers, and used an isotope reference method to determine the conversion factor of Golden Rice β-carotene to vitamin A.
MATERIALS AND METHODS
Production of intrinsically labeled Golden Rice
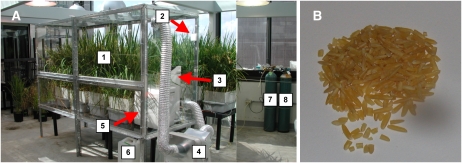
Rice seeds were imbibed and germinated on cheesecloth suspended over distilled water. After 4 d, seedlings were planted in trays suspended over 20-L tubs of nutrient solution containing the following macronutrients: KNO3, 1 mmol/L; KH2PO4, 1 mmol/L; Ca(NO3)2, 1 mmol/L; MgSO4, 1 mmol/L; K2SiO4, 0.1 mmol/L; CaCl2, 25μmol/L; H3BO3, 25 μmol/L; MnSO4, 2 μmol/L; ZnSO4, 2 μmol/L; CuSO4, 0.5 μmol/L; H2MoO4, 0.5 μmol/L; and NiSO4, 0.1 μmol/L. No deuterium oxide was added. Iron was added in chelated form as Fe(III)HEDTA (N-hydroxyethyl-ethylenediamine-triacetic acid) at 20 μmol/L. MES buffer (adjusted with potassium hydroxide) was added at 2 mmol/L to maintain the nutrient solution pH between 5.4 and 5.8. Plants were maintained on this solution until flowering (≈3.5 mo after planting) in a greenhouse in Houston, TX; the nutrient solution was topped off, as needed, with a refill solution containing the same nutrients as above, but without additional MES buffer or K2SiO4. A complete change-out of the solution (using starting solution) was performed at 6-wk intervals. Approximately 7 d after flowering, the plants were transferred to a new nutrient solution containing 23 atom% 2H2O, 20 μmol/L Fe(III)HEDTA, 2 mmol/L MES buffer, and the following macronutrients: KNO3, 5 mmol/L; KH2PO4, 2 mmol/L; Ca(NO3)2, 2 mmol/L; MgSO4, 1 mmol/L; K2SiO4, 0.1 mmol/L; CaCl2, 25 μmol/L; H3BO3, 25 μmol/L; MnSO4, 2 μmol/L; ZnSO4, 2 μmol/L; CuSO4, 0.5 μmol/L; H2MoO4, 0.5 μmol/L; and NiSO4, 0.1 μmol/L. The 23 atom% 2H2O allowed us to achieve a target peak enrichment of M + 9 [original mass of β-carotene (M) plus 9 atoms of 2H] for the Golden Rice β-carotene (determined empirically in pilot studies). At this time, plants were also placed in a clear plastic-walled labeling system (Figure 1), which maintained an elevated 2H2O concentration in the gas atmosphere surrounding the plants and panicles. Plants were maintained on this media, with the solution topped off as needed (using the same 2H2O solution) until the panicles had matured (≈3 wk later). During this labeling period, the temperature within the labeling system was maintained between 26 and 31°C, and the relative humidity was maintained between 45% and 55%. At maturity, whole panicles were collected and stored at −20°C until further processing. For processing, seeds were de-hulled with an Impeller Husker (model FC2K; Yamamoto Co, Ltd, Yamagata-ken, Japan); seeds were subsequently polished in small batches for 30 s with an electric grain polisher (model “Pearlest;” Kett Electric Laboratory, Tokyo, Japan). Polished seeds were stored at −80°C until shipped to Boston, where they were cooked and analyzed before the clinical studies.
FIGURE 1.
Photograph of the labeling chamber (A) and system components used to produce polished Golden Rice-2 (B). Components of the labeling system: 1, Golden Rice-2 in deuterated nutrient solution at seed fill stage; 2, carbon dioxide sensor; 3, tower fan for air mixing; 4, air conditioning unit; 5, dehumidifier; 6, collection tub for transpired heavy water from plants (collected from dehumidifier); 7, carbon dioxide supply; 8, water-free air.
Rice preparation
The rice was cooked by using a rice cooker (SR-G18FG; Panasonic, Chachoengsao, Thailand) by adding water in a quantity of 150% weight of the rice. The rice was cooked for 30 min. Our analysis showed that the total amount of Golden Rice β-carotene in the dose was the same before and after it was cooked (0.99 or 1.53 mg β-carotene in a dose). The cooked rice was divided into portions (130 g cooked rice containing 0.99 mg β-carotene or 200 g cooked rice containing 1.53 mg β-carotene) and kept at −15°C until served within 1–3 mo. On the day of feeding, the rice was brought to room temperature and then heated with a microwave oven [60 s by using a Panasonic NN-Sensor 953 (Genius) 1350-W microwave oven, 2.2 cubic feet capacity].
Volunteers and study design
The study protocol was approved by the Tufts Medical Center Institutional Review Board. Persons who had not taken vitamin A or β-carotene supplements within the past month and who were not disqualified based on several exclusion criteria were eligible to become study volunteers. Potential subjects were accepted into the study if they had none of the following conditions: severe or symptomatic cardiac disease or hypertension; history of bleeding disorders; chronic history of gastric, intestinal, liver, pancreatic, or renal disease; any portion of the stomach or the intestine removed (other than an appendectomy); history of intestinal obstruction, malabsorption, or use of antacid drugs; cancer (active or use of medications for a history of cancer treatment within the past 5 y); history of chronic alcoholism; a convulsive disorder; or abnormal results in screening blood or urine samples. Five volunteers (2 men and 3 women) from the Boston area were admitted to participate in the study after they were interviewed and signed the Informed Consent Form for the study.
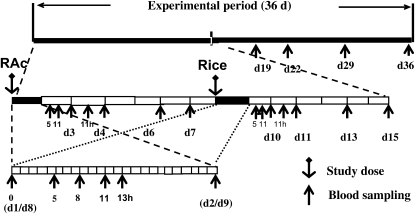
The full study for each volunteer lasted 36 d to draw several blood samples and to study blood response curves. On day 1 of the study, the volunteers consumed [13C10]retinyl acetate (Mretinol + 10) in an oil capsule as a reference dose. We first tested the use of 0.43 mg [13C10]retinyl acetate as a reference dose in one of the subjects. Subsequently, we used 0.99 mg [13C10]retinyl acetate as the reference dose for the other 4 volunteers to ensure successful detection of labeled retinol in each volunteer, even those with a higher body mass. The [13C10]retinyl retinyl acetate in an oil capsule was given together with 200 g cooked white rice, 10 g butter, 50 g peeled cucumbers, 0.2 g salt, 5 g vinegar, and a 500-mL bottle of water at breakfast (time 0). The total calorie content of the meal was ≈450 kcal (23% from fat). A second standardized meal (lunch) was eaten by all volunteers 4 h after the breakfast meal; this second meal contained 60 g turkey meat, 50 g white bread, 20 g roasted cashew, and 100 g cucumber (peeled) salad with 15 g corn oil and 5 g vinegar (total energy: 600 kcal, 40% from fat). On day 8 of the study, the volunteers consumed the same breakfast meal as on day 1, but 200 g white rice was replaced with labeled Golden Rice (either 130 g cooked Golden Rice together with 70 g cooked white rice containing 0.99 mg β-carotene or 200 g cooked Golden Rice containing 1.53 mg β-carotene). Also on day 8, the standardized lunch (as above) was eaten by all of the volunteers 4 h after the breakfast meal. The amount of Golden Rice in the breakfast meal varied because we were trying to study as many subjects as possible with a limited amount of intrinsically labeled rice. Our results showed that our method can effectively assess the vitamin A equivalency of β-carotene doses as low as 1 mg in rice.
A total of 30 serum samples (10 mL each) were obtained from each subject at the following time points: day 1 at 0 (just before the breakfast dose), 5, 8, 11, and 13 h (after the dose); day 2 at 0 (24 h after the dose taken before the day 2 breakfast), 5, and 11 h (after the day 2 breakfast); day 3 at 0 (48 h after the dose taken and before the day 3 breakfast) and 11 h (after the day 3 breakfast); days 4, 6, and 7 at 0 h (before each day's breakfast); day 8 at 0 (before the Golden Rice breakfast dose), 5, 8, 11, and 13 h (after the Golden Rice dose taken); day 9 at 0 (24 h after the dose taken and before the day 9 breakfast), 5, and 11 h (after the breakfast); day 10 at 0 (48 h after the dose taken and before the day 10 breakfast) and 11 h (after the day's breakfast); days 11, 13, 15, 19, 22, 29, and 36 at 0 h (before each day's breakfast) (Figure 2). Fasting serum samples were collected at the 0-h time points. The serum samples were kept at −70°C until analyzed. The retinol (Mretinol + 10) derived from the [13C10]retinyl acetate dose, the retinol (Mretinol + 5) formed from the labeled Golden Rice β-carotene, and the intact Golden Rice β-carotene (M + 9) were followed in all samples up until the end of the study.
FIGURE 2.
Experimental design for the Golden Rice human study. Three sets of dashed lines are used to amplify the overall time period to progressively larger scales. The first order (upper scale) includes the entire 36-d study and sampling period. The second order (middle scale) represents days 1–15 of more frequent blood sampling. Each cell on the horizontal time axis represents a 24-h period, with the division line representing the fasting blood collection time (0 h). The cells in black represent days 1 and 8, the days on which the tracers were ingested. The third order (lower scale) is common for these 2 dosing and high-multiple blood-sampling days (days 1 and 8), with each cell on the time axis representing 1 h. The 0 time represents the first blood sampling of the day, and all other numbers represent the times of subsequent sampling (in h) relative to the time of first sampling. The anchor symbols represent the oral dosing of the tracer. The arrows indicate the times at which blood samples were collected. d, day in the study; h, hour after study dose or after fasting blood sample, RAc, retinyl acetate.
[13C10 ]Vitamin A as an isotope reference
To quantify the amount of vitamin A formed from the Golden Rice β-carotene, a known amount of vitamin A that is differently labeled can be used as a reference dose. We used 1 mg [13C10]vitamin A [in the present study: Mretinol = retinol – H2O = m/z (mass/charge, a unit for mass spectrometry) 268, M [13C10]retinol = m/z 268 + 10 = m/z 278] in an oil capsule as a reference dose given 1 wk before the Golden Rice meal. Our initial test showed that our method can trace the vitamin A body response after ingestion of 0.43 mg [13C10]vitamin A—a physiologic dose.
Blood sample analysis
An HPLC instrument equipped with a C18 column was used to separate the serum retinol (20). The fractions containing retinol in the HPLC eluent were collected individually and derivatized for gas chromatography/electron capture negative chemical ionization–mass spectrometry (GC/ECNCI-MS) (21) to measure retinol enrichment from the reference vitamin A dose (Mretinol + 10 = m/z 278) or Golden Rice β-carotene (Mretinol + 5 = m/z 273) dose. The total enrichment of labeled retinol was determined by the evaluation of negative ions at Mretinol [m/z 268–270 (13C0 − 13C2)], Mretinol + 5 [m/z 273–277 (2H5 – 2H9)], and Mretinol + 10 [m/z 278–280 (13C10 – 13C12)]. The whole enrichment of the retinol from the Golden Rice β-carotene was calculated as 2 times the sum of the enrichment of Mretinol + 5, Mretinol + 6, Mretinol + 7, Mretinol + 8, and Mretinol + 9 based on the assumption of the symmetric distribution of the labeled Golden Rice β-carotene.
Concentrations of serum carotenoids and retinoids were determined using HPLC equipped with a C30 column (22). For enrichment of intact β-carotene, liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry (23) was used to determine the absorption of intact β-carotene after consumption of the cooked Golden Rice.
Areas under the curve of labeled retinol or β-carotene in the serum
Total serum responses (nmol) to the [2H]β-carotene dose and the [13C10]retinyl acetate dose were determined by multiplying the total serum volume (0.0435 L/kg body wt) by the concentration of [2H]β-carotene and [2H]retinol and [13C10]retinol in the circulation (nmol/L, determined for each time point of serum sampling by adding all of the enrichment masses). Areas under the curve (AUCs) for serum labeled retinol or β-carotene responses (in nmol·d) after the [2H]β-carotene dose and the [13C10]retinyl acetate dose were calculated by using the curves of total serum responses (in nmol; y axis) compared with time (in d; x axis) via Integral-Curve of Kaleidagraph (Synergy Software, Reading, PA). The conversion of the AUC unit from nmol·d to μg·d was done by using Mretinol = 291 for [2H5]retinol and M = 296 for [2H10]retinol. Because of the 7-d delay in the administration of the Golden Rice dose, the AUCs were calculated for 21 d after each labeled tracer.
Retinol equivalence calculations
The AUC of serum [2H]retinol response (from the labeled Golden Rice) was compared with the AUC of the vitamin A reference dose (0.4–1.0 mg [13C10]retinyl acetate; molecular mass = 336). The amount of 2H retinol was calculated as follows:
 |
Conversion factor calculations
The amount of a given oral dose of Golden Rice β-carotene (0.99–1.53 mg) compared with the amount of vitamin A derived from the β-carotene dose was defined as the β-carotene to vitamin A conversion factor. Thus, the conversion factor of Golden Rice β-carotene (calculated β-carotene from all-trans β-carotene plus one-half of all other provitamin A carotenoids) to vitamin A was determined as follows:
 |
where 536 is the molecular mass of β-carotene, and 286 is the molecular mass of retinol.
Statistical analyses
Statistical analyses was performed to assess the significance of differences between vitamin A conversion factors by sex and to determine correlations between conversion factors and the BMI of each subject. Systat version 10.2 (Systat Software Inc) was used for data analysis.
RESULTS
Confirmation of intrinsically labeled Golden Rice with enriched β-carotene
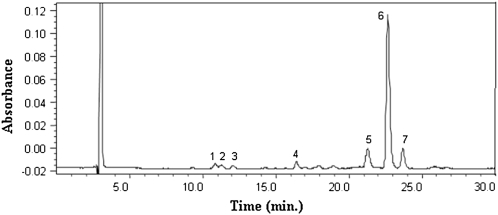
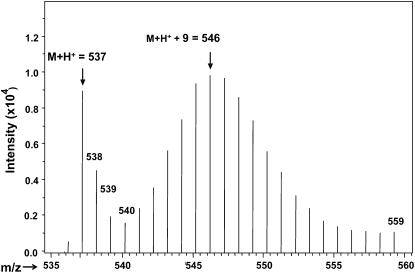
We grew Golden Rice hydroponically with mineral nutrients and introduced 23 atom% heavy water (2H2O) to the hydroponic medium after flowering (Figure 1) to intrinsically label the Golden Rice β-carotene with deuterium (2H). Our HPLC analysis showed that, in the grains, all-trans β-carotene was the dominant carotenoid (≈20 μg β-carotene in a gram of dry rice) together with small amounts of lutein, anhydrolutein, zeaxanthin, cryptoxanthin, 13-cis β-carotene, and 9-cis β-carotene (Figure 3). With this labeling method, the intrinsically labeled Golden Rice β-carotene showed a protonated molecule of m/z of Mβc + H+ = 536 + 1 (representing unlabeled β-carotene) and a range of isotopomers with the most abundant showing an enrichment of 9 deuterium at Menrich-βc = Mβc + H+ + 9 mass units (Figure 4) as analyzed by using the liquid chromatography/mass spectrometry with a positive atmospheric pressure chemical ionization interface (the total m/z was Mβc + 9 + H+ = 536 + 9 + 1 = m/z 546) (23). This labeled Golden Rice β-carotene produces retinol with a most abundant peak mass at the ionized retinol plus 5 mass units derived from deuterium minus water (the result of ionization in the mass ionization chamber).
FIGURE 3.
Chromatogram of Golden Rice carotenoids. Each labeled chromatographic peak represents an identified carotenoid compound: 1, lutein; 2, anhydrolutein; 3, zeaxanthin; 4, cryptoxanthin; 5, 13-cis β-carotene; 6, all-trans β-carotene; 7, 9-cis β-carotene.
FIGURE 4.
Deuterium enrichment profile of Golden Rice β-carotene analyzed by liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry (positive ion mode). Hydroponic labeling does not produce uniform enrichment, but rather a range of isotopomers. The vertical axis represents the signal intensity. The horizontal axis represents the mass (M + H+ = mass plus hydrogen atom with positive charge) of the isotopomers. Unlabeled β-carotene is shown with a mass of 537. The most abundant isotopomer of labeled β-carotene (with 9 deuterium atoms) is represented by an m/z of 546. The enrichment of Golden Rice β-carotene is 86%.
Characteristics of volunteers
Five healthy nonsmokers (2 men and 3 women; age: 41–70 y; BMI: 22–29) were recruited as study volunteers. The characteristics of these subjects are presented in Table 1. Concentrations of carotenoids and retinoids in serum samples collected at baseline and analyzed by using HPLC (22) are presented in Table 2. These data showed that the vitamin A and carotenoid concentrations of the volunteers were in the normal range.
TABLE 1.
Characteristics of the 5 study subjects
| Subject no. | Sex | Age | Weight | BMI |
| y | kg | kg/m2 | ||
| 1 | F | 62 | 63.3 | 22.2 |
| 2 | F | 60 | 63.6 | 24.9 |
| 3 | F | 60 | 70.4 | 26.6 |
| 4 | M | 70 | 75.4 | 27.7 |
| 5 | M | 41 | 95.3 | 28.5 |
| Mean ± SD | 59 ± 11 | 73.6 ± 13.2 | 26.0 ± 2.5 |
TABLE 2.
Carotenoid and retinol concentrations in the serum of each subject at the beginning of the study1
| Subject no. | Lutein | Zeax | Crypt | 13-c-β-C | α-C | t-β-C | 9c-β-C | 15-c-lyco | 13-c-lyco | 9-c-lyco | t-lyco | 5,9-di-c-lyco | Retinol | RE |
| μmol/L | ||||||||||||||
| 1 | 0.19 | 0.05 | 0.08 | 0.04 | 0.15 | 0.46 | 0.01 | 0.06 | 0.09 | 0.08 | 0.12 | 0.15 | 2.06 | 0.04 |
| 2 | 0.22 | 0.07 | 0.13 | 0.01 | 0.14 | 0.27 | 0.01 | 0.04 | 0.07 | 0.06 | 0.09 | 0.11 | 1.65 | 0.03 |
| 3 | 1.18 | 0.05 | 0.46 | 0.01 | 0.16 | 0.97 | 0.01 | 0.07 | 0.28 | 0.13 | 0.32 | 0.26 | 2.85 | 0.04 |
| 4 | 0.12 | 0.05 | 0.04 | 0.03 | 0.09 | 0.23 | 0.01 | 0.04 | 0.06 | 0.05 | 0.08 | 0.11 | 2.74 | 0.02 |
| 5 | 0.16 | 0.08 | 0.12 | 0.01 | 0.13 | 0.31 | 0.04 | 0.07 | 0.13 | 0.09 | 0.22 | 0.21 | 1.92 | 0.04 |
| Mean | 0.38 | 0.06 | 0.16 | 0.02 | 0.13 | 0.45 | 0.01 | 0.06 | 0.13 | 0.08 | 0.17 | 0.17 | 2.24 | 0.03 |
| SD | 0.45 | 0.02 | 0.17 | 0.01 | 0.03 | 0.31 | 0.01 | 0.02 | 0.09 | 0.03 | 0.10 | 0.07 | 0.53 | 0.01 |
Zeax, zeaxanthin; Crypt, cryptoxanthin; β-C, β-carotene; α-C, α-carotene; lyco, lycopene; c, cis; t, trans; RE, retinyl esters.
Blood response to a Golden Rice meal
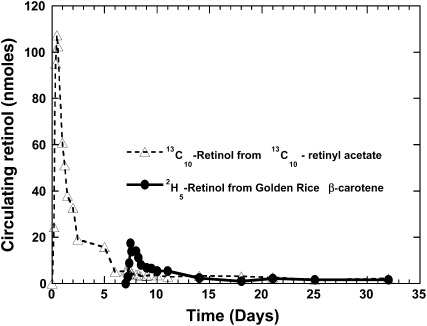
Retinols labeled at Mretinol + 5 or Mretinol + 10 (Mretinol = m/z 268, representing unlabeled endogenous retinol) were detected in serum extracts, as shown in the middle and bottom panels of Figure 5. The mass distribution of circulating retinol at baseline (day 1 at 0 h before the reference dose; top panel), the enrichment of Mretinol + 10 = m/z 278 from the reference dose [13C]retinyl acetate (day 1 at 13 h after the dose; middle panel), and the enrichment of Mretinol + 5 = m/z 273 and Mretinol + 10 = m/z 278 retinol (day 9 at 0 or 24 h after the Golden Rice meal; bottom panel) are shown in the figure. The kinetic responses of labeled Mretinol + 5 = m/z 273 and Mretinol + 10 = m/z 278 retinol up to 32 d from one subject are presented in Figure 6.
FIGURE 5.
Enrichment mass spectrometric profile of serum retinol samples collected from one subject. Top panel: profile obtained before ingestion of the labeled dose; data are an average of 11.3–11.6 min on the gas chromatography–mass spectrometry (GC/MS) chromatogram for a sample collected on day 1, 0 h. Middle panel: profile obtained after ingestion of the reference dose; data are an average of 13.7–13.8 min on the GC/MS chromatogram for a sample collected on day 1, 13 h after the [13C10]retinyl acetate dose. Bottom panel: profile obtained after ingestion of the labeled Golden Rice dose; data are an average of 11.3–11.6 min on the GC/MS chromatogram for a sample collected on day 9, 24 h after the Golden Rice meal. Arrows indicate signal intensities that were greater than the abundance values on the y axis.
FIGURE 6.
Calculated labeled retinol species in the circulation of a representative volunteer, throughout the course of the study, after consumption of [13C10]retinyl acetate on day 1 and after a deuterium-labeled Golden Rice β-carotene dose on day 8.
The responses of the volunteers who consumed a reference dose on day 1 and a labeled Golden Rice dose (130 or 200 g cooked weight containing 0.99 or 1.53 mg labeled β-carotene, respectively) on day 8, together with 10 g butter, are presented in Table 3.
TABLE 3.
Subject responses to a reference dose of [13C10]retinyl acetate and a Golden Rice meal with [2H9]β-carotene1
| Conversion factor |
|||||||
| Subject no. | GR β-C | [13C10]RAc | AUC [2H5]retinol | AUC [13C10]retinol | Retinol equivalent | By weight | By mole |
| mg | mg | μg·d | μg·d | mg | |||
| 1 | 0.99 | 1.01 | 19.4 | 67.5 | 0.25 | 4.0 | 2.1 |
| 2 | 1.53 | 1.01 | 38.8 | 57.9 | 0.94 | 2.6 | 1.4 |
| 3 | 0.99 | 0.43 | 74.3 | 53.9 | 0.51 | 1.9 | 1.0 |
| 4 | 0.99 | 1.01 | 34.4 | 124.2 | 0.24 | 4.1 | 2.2 |
| 5 | 1.53 | 1.01 | 32.8 | 119.9 | 0.24 | 6.4 | 3.4 |
| Mean ± SD | 1.21 ± 0.30 | 0.89 ± 0.26 | 39.9 ± 20.5 | 84.7 ± 34.5 | 0.36 ± 0.17 | 3.8 ± 1.7 | 2.0 ± 0.9 |
GR β-C, Golden Rice β-carotene; RAc, retinyl acetate; AUC, area under the curve.
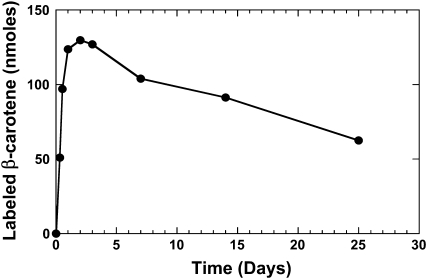
For Golden Rice β-carotene, analysis of serum samples by liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry (23) showed that the labeled β-carotene was absorbed intact after consumption of the cooked Golden Rice. Serum response kinetics of intact β-carotene are presented in Figure 7.
FIGURE 7.
Serial measurements of deuterium-labeled β-carotene in the circulation of a representative volunteer. Values are presented for the 25 d of monitoring after the oral dose of labeled Golden Rice.
Conversion factor of Golden Rice β-carotene to vitamin A
The AUC response of vitamin A (Mretinol + 5) formed from the dose of labeled Golden Rice β-carotene was compared with the AUC of the reference [13C10]vitamin A, up to 21 d (Table 3). The Golden Rice containing 0.99 mg β-carotene provided 0.24–0.51 mg retinol, and the Golden Rice containing 1.53 mg β-carotene provided 0.24–0.94 mg retinol. It should be noted that at these physiologic doses (0.99–1.53 mg β-carotene), it is unlikely that the differences in dose would influence the outcome of retinol equivalence per milligram of β-carotene. Acknowledging that we had a limited number of subjects (n = 5), statistical analysis showed that there was no difference in the calculated conversion factors between subjects taking 0.99 or 1.53 mg Golden Rice β-carotene. Altogether, our results show that the conversion factor of Golden Rice β-carotene to retinol is 3.8 ± 1.7 (mean ± SD) to 1 with a range of 1.9–6.4 to 1 by weight, or 2.0 ± 0.9 to 1 with a range of 1.0–3.4 to 1 by mol, as presented in Table 3. The conversion factors between men (n = 2) and women (n = 3) were not different. In addition, in these 5 subjects, there was no correlation between the conversion factors and BMIs.
DISCUSSION
Golden Rice is a bioengineered crop with yellow-colored endosperm that contains β-carotene (provitamin A). To produce Golden Rice, 2 enzymes are introduced into the endosperm [phytoene synthase (psy) and phytoene desaturase (crtl)] via an endosperm-specific glutelin (Gtl) promoter (15), to establish a β-carotene biosynthetic pathway in the rice grains. This is the first study on the vitamin A value of Golden Rice in humans, and our analysis showed a very efficient bioconversion of β-carotene to vitamin A (Table 3). Using a conversion factor in which 3.8 μg Golden Rice β-carotene provides 1 μg retinol, along with the level of Golden Rice β-carotene being 20–30 μg/g uncooked rice, we project that 100 g uncooked rice provides 500–800 μg retinol. This represents 80–100% of the estimated average requirement (EAR) for men and women and 55–70% of the Recommended Dietary Allowance (RDA, derived from the EAR) for men and women, as set by the US National Academy of Science (24). In the United States, the EAR and RDA for vitamin A were set based on the amount needed to provide 4 mo of vitamin A storage in the body. For children, additional study of Golden Rice β-carotene conversion to retinol is needed. However, we speculate that 50 g uncooked Golden Rice, which is a reasonable serving size for children aged 4–8 y in rice-eating regions, who eat ≈130–200 g rice/d (25), would be able to provide >90% of vitamin A EAR (275 μg retinol/d) or >60% of the RDA (400 μg retinol/d) (24).
In this study, we used a combination of state-of-the-art approaches to determine the vitamin A equivalence of Golden Rice in humans. Plants were grown hydroponically in heavy water to intrinsically label Golden Rice β-carotene with deuterium. The subjects were fed both the labeled rice and a reference dose of differently labeled retinol. Sensitive mass spectrometry approaches were used to analyze both β-carotene and retinol enrichment in serum with stable isotope labeling, which allowed us to easily discern Golden Rice and reference dose molecules from preexisting, endogenous β-carotene and retinol. Our subsequent results, based on a small number of US volunteers, showed the effective conversion of Golden Rice β-carotene, even though all individuals were of normal vitamin A status. Whether this conversion efficiency is a good approximation for rice-eating populations with marginal-to-severe vitamin A deficiency, or perhaps is a conservative underestimate, has yet to be determined. To provide information for public health or public policy purposes, a larger long-term trial targeting individuals with marginal vitamin A status is needed. For instance, an isotope dilution approach could be used to evaluate changes in whole-body vitamin A stores (26) after an extended feeding period of Golden Rice with incorporation of the rice into daily diets.
The food matrix plays an important role in determining the bioavailability of vitamin A from provitamin A carotenoids in a particular food. Rice has a simple and easily digestible food matrix, which allows for a high bioavailability and bioconversion of β-carotene to vitamin A. Similarly, spirulina, with its simple food matrix, has also shown a highly efficient conversion factor for β-carotene to vitamin A of 4.5 to 1 by weight in humans (27). To combat vitamin A deficiency, consumption of locally available vegetables, fruit, and other plant foods, such as algae products, should be encouraged. Each of these plant foods can contribute to vitamin A nutrition, although the conversion of the provitamin A carotenoids within them may not be equivalent. Conversion factors for provitamin A carotenoids from various plants have been reported as 12 to 1 for fruit (14, 28), 13 to 1 for sweet potato (15), 15 to 1 for carrots (16), and 10 to 1 (15), 21 to 1 (16), 26 to 1 (14), 27 to 1 (13), and 28 to 1 (28) for green leafy vegetables. Thus, comparatively speaking, Golden Rice has a very favorable conversion ratio.
It should be noted that we closely monitored our subjects for any possible adverse effects after the consumption of Golden Rice and found no evidence of any problems, including allergic reactions or gastrointestinal disturbance. Although this attests to the probable safety of Golden Rice, we acknowledge that only a single serving was fed to each study subject. A much longer exposure with a larger cumulative consumption of Golden Rice would be needed to make definitive assertions regarding the inherent safety of this food for human use.
Staple foods should not only provide energy but also nutrients in a bioavailable form. Thus, Golden Rice may be a cost-effective staple food for combating vitamin A deficiency in rice-eating populations (29). Other provitamin A–containing staple foods, such as corn, cassava, sweet potato, and sorghum should be developed to serve other vitamin A–deficient populations with different food cultures.
Acknowledgments
We thank the Metabolic Research Unit of the Jean Mayer USDA Human Nutrition Research Center on Aging for recruiting our volunteers and for performing the human study procedures and David Dworak and Chee-Ming Li of the USDA/ARS Children's Nutrition Research Center for helping to label and produce the Golden Rice.
The authors' responsibilities were as follows—GT: designed the study, supervised the data collection, analyzed the data, and wrote the manuscript; JQ: collected and analyzed the samples; GGD: supervised the mass spectrometric analysis and revised the manuscript; RMR: supervised the human study as the study physician and revised the manuscript; and MAG: designed the production methods for the labeled Golden Rice, harvested the labeled Golden Rice for the study, and revised the manuscript. No financial benefit was obtained from this research study.
REFERENCES
- 1.Sommer A. Nutritional blindness: xerophthalmia and keratomalacia. New York, NY: Oxford University Press, 1982 [Google Scholar]
- 2.Ross AC, Stephensen CB. Vitamin A and retinoids in antiviral responses. FASEB J 1996;10:979–85 [PubMed] [Google Scholar]
- 3.Usha N, Sankaranarayanan A, Walia B, Ganguly N. Assessment of preclinical vitamin A deficiency in children with persistent diarrhea. J Pediatr Gastroenterol Nutr 1991;13:168–75 [DOI] [PubMed] [Google Scholar]
- 4.Sommer A, West K., Jr Vitamin A deficiency. Health, survival, and vision. New York, NY: Oxford University Press, 1996 [Google Scholar]
- 5.Underwood BA, Arthur P. The contribution of vitamin A to public health. FASEB J 1996;10:1040–8 [PubMed] [Google Scholar]
- 6.World Health Organization Global prevalence of vitamin A deficiency. Geneva, Switzerland: WHO, 1995, (WHO/NUT/95.3.) [Google Scholar]
- 7.World Health Organization, Vitamin A supplements: a guide to their use in the treatment of vitamin A deficiency and xerophthalmia. Geneva, Switzerland: WHO, 1997 [Google Scholar]
- 8.West KP, Jr, Pokhrel RP, Katz J, et al. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 1991;338:67–71 [DOI] [PubMed] [Google Scholar]
- 9.Ribaya-Mercado JD, Solomons NW, Medrano Y, et al. Use of the deuterated-retinol-dilution technique to monitor vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. Am J Clin Nutr 2004;80:1291–8 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Vitamin and mineral requirements in human nutrition. 2nd ed Available from http://whqlibdoc.who.int/publications/2004/9241546123_chap2.pdf(cited 29 March 2009)
- 11.de Pee S, West C, Muhila K, Hautvast G. Lack of improvement in vitamin A status with increased consumption of dark green leafy vegetables. Lancet 1995;346:75–81 [DOI] [PubMed] [Google Scholar]
- 12.Bulux J, de Serrano JQ, Giuliano A, et al. Plasma response of children to short-term chronic β-carotene supplementation. Am J Clin Nutr 1994;59:1369–75 [DOI] [PubMed] [Google Scholar]
- 13.Tang G, Gu X, Xu Q, et al. Green and yellow vegetables can maintain vitamin A nutrition of Chinese children. Am J Clin Nutr 1999;70:1069–76 [DOI] [PubMed] [Google Scholar]
- 14.de Pee S, West CE, Permaesih D, Martuti S, Muhilal Y, Hautvast JG. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 1998;68:1058–67 [DOI] [PubMed] [Google Scholar]
- 15.Haskell MJ, Jamil KM, Hassan F, et al. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutr 2004;80:705–14 [DOI] [PubMed] [Google Scholar]
- 16.Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MG. Spinach or carrot can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterium-labeled vegetables. Am J Clin Nutr 2005;82:821–8 [DOI] [PubMed] [Google Scholar]
- 17.Ye X, Al-Babili S, Kloti A, et al. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000;287:303–5 [DOI] [PubMed] [Google Scholar]
- 18.Paine JA, Shipton CA, Chaggar S, et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol 2005;23:482–7 [DOI] [PubMed] [Google Scholar]
- 19.Grusak MA. Intrinsic stable isotope labeling of plants for nutritional investigations in humans. J Nutr Biochem 1997;8:164–71 [Google Scholar]
- 20.Tang G, Dolnikowski GG, Blanco MC, Fox JG, Russell RM. Serum carotenoids and retinoids in ferrets fed canthaxanthin. J Nutr Biochem 1993;4:58–63 [Google Scholar]
- 21.Tang G, Qin J, Dolnikowski G. Deuterium enrichment of retinol in humans determined by gas chromatography electron capture negative chemical ionization mass spectrometry. J Nutr Biochem 1998;9:408–14 [Google Scholar]
- 22.Yeum KJ, Booth SL, Sadowski JA, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr 1996;64:594–602 [DOI] [PubMed] [Google Scholar]
- 23.Tang G, Andrien BA, Jr, Dolnikowski G, Russell RM. Atmospheric pressure chemical ionization and electron capture negative chemical ionization mass spectrometry in studying beta-carotene conversion to retinol in humans. Methods Enzymol 1997;282:140–54 [DOI] [PubMed] [Google Scholar]
- 24.Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc Washington, DC: Food and Nutrition Board, Institute of Medicine, National Academy Press, 2001 [Google Scholar]
- 25.Keyou G, ed The dietary and nutritional status of Chinese population—children and adolescents (1992 National Nutrition Survey). Vol 2 Beijing, China: Institute of Nutrition and Food Hygiene, Chinese Academy of Preventive Medicine, People's Medical Publishing House, 1992 [Google Scholar]
- 26.Furr HC, Green MH, Haskell M, et al. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr 2005;8:596–607 [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Wang Y, Wang Z, et al. Vitamin A equivalence of spirulina β-carotene in Chinese adults as assessed by using a stable-isotope reference method. Am J Clin Nutr 2008;87:1730–7 [DOI] [PubMed] [Google Scholar]
- 28.Khan NC, West CE, de Pee S, et al. The contribution of plant foods to the vitamin A supply of lactating women in Vietnam: a randomized controlled trial. Am J Clin Nutr 2007;85:1112–20 [DOI] [PubMed] [Google Scholar]
- 29.Stein AJ, Sachdev HPS, Qaim M. Genetic engineering for the poor: Golden Rice and public health in India. World Dev 2008;36:144–58 [Google Scholar]