Abstract
Background: Selenium, a potential cancer prevention agent currently being tested against prostate cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT), plays an integral role in thyroid metabolism. The effects of long-term selenium supplementation on thyroid hormone concentrations are unknown.
Objective: The objective was to investigate the effects of long-term selenium supplementation on thyroid hormone concentrations.
Design: Twenty-eight healthy adults took 200 μg selenomethionine/d for 28 mo. The thyroid hormones triiodothyronine (T3), thyroxine (T4), and thyrotropin (TSH) were measured in plasma for 4 mo before supplementation and quarterly during supplementation. The assay methods were changed midstudy; the results of the 2 methods were not comparable. Therefore, one analysis was conducted based on the results of the first method, and a second analysis was based on all of the data, adjusted for the change. Serial data collection permitted a test for trends rather than simply a difference between initial and final values.
Results: By 9 mo, mean (±SEM) plasma selenium concentrations had increased from 1.78 ± 0.07 μmol/L at baseline to 2.85 ± 0.11 μmol/L for men and from 1.64 ± 0.04 to 3.32 ± 0.1.2 μmol/L for women. T3 concentrations in men increased 5% per year (P = 0.01). T4 and TSH concentrations were unchanged.
Conclusions: Selenium supplementation produced no clinically significant changes in thyroid hormone concentrations. A small but statistically significant increase in T3 concentrations was noted in men, with no corresponding decreases in TSH. A subset of SELECT subjects might be monitored periodically for changes during long-term selenium supplementation.
INTRODUCTION
Selenium is essential to the metabolism of thyroid hormones. The thyroid is rich in selenium and has several selenoproteins (1–3): 2 isoforms of iodothyronine 5′-deiodinase (5′DI-I and 5′DI-II), which catalyze the conversion of thyroxine (T4) to triiodothyronine (T3); 3 isoforms of glutathione peroxidase, 2 of which (GPX1 and GPX4) protect thyrocytes from hydrogen peroxide produced in the iodination of thyroglobulin; GPX3, which is secreted into the lumen, where it appears to regulate hydrogen peroxide concentrations (4); thioredoxin reductase type 1; and selenoprotein P (5). In addition, the extrathyroidal conversion of T4 to T3 depends on selenium-containing iodothyronine 5′-deiodinase isoforms 5′DI-II and 5′DI-III (6–9).
Studies in animal models have shown serum concentrations of T3 and selenium to be positively correlated (10) and selenium deprivation to increase circulating concentrations of T4 and/or decrease those of T3 (2, 5–16). The 5′DIs may be more sensitive than other selenoproteins to restricted selenium supply, because humans with an inherited defect in a selenocysteine insertion sequence binding protein (SBP2) involved in selenoprotein biosynthesis have markedly impaired thyroid hormone metabolism but GPX1 and selenoprotein P are less affected (17).
Inverse relations have been observed between plasma concentrations of selenium and thyroid volume, thyroid echostructure, and serum concentrations of T3 (19), T4 (20–24) and thyrotropin (TSH) (24). Low plasma selenium concentrations have been noted in patients with iodine-deficiency goiter (24). Selenium supplementation has yielded mixed results on thyroid hormone–related endpoints: no effects in healthy subjects (22, 25, 26) or patients with autoimmune thyroiditis (27–30), increased serum T4 concentrations in children of moderate selenium and iodine status (31, 32), increases in serum T3 and TSH concentrations and/or decreases in T4 in healthy subjects (33) and patients with autoimmune thyroid disease (27, 34) or phenylketonuria (35), and increases in both T3 and T4 with decreased TSH in patients with Graves disease (36).
Two selenium-supplementation trials have been performed in healthy, selenium-adequate males. Hawkes and Keim (33) fed 11 men diets providing 14 or 297 μg Se/d. By 100 d, the high-selenium group showed a small decrease in serum T3 and a small increase in TSH; small decreases in T4 concentrations were observed in both groups. Hawkes et al (37) followed up with a randomized, placebo-controlled study with 24 healthy men supplemented with 0 or 300 μg Se/d for 48 wk and found no significant effect on any of the thyroid hormones.
The work reported here was ancillary to a stable-isotope study to characterize the effects of long-term (2 y) selenium supplementation on the kinetics of selenium metabolism. In view of the reports of Rayman et al (26) and Hawkes and Keim (33), we conducted this work to exploit the advantages of our long-term supplementation trial to test the hypothesis that changes in selenium status affect circulating concentrations of thyroid hormones.
SUBJECTS AND METHODS
Subjects
Thirty-four subjects (17 men and 17 women) were recruited from the Cornell University community, Ithaca, NY. Interested individuals aged 20–60 y were screened on the basis of medical history, clinical laboratory tests, and health consciousness. Volunteers were excluded if they were pregnant or planning to become pregnant during the experimental period, taking or having a history of taking supplements containing >25 μg Se/d or 100 IU vitamin E/d in the past year or having a history of taking them in the past year, current or former smokers, being in a rigorous exercise/weight-reduction program, and ranking below 50% on a Health Consciousness Scale (38). All subjects gave informed consent. Each was given a membership in the Cornell Wellness Program or comparable community-based personal health/fitness program. In addition, each was paid for completing each phase of the study and received data on their blood work. Baseline characteristics of the 28 study participants (13 men and 15 women), who completed the study and are included in this report, are shown in Table 1. Early in the study, one male subject was diagnosed with Hashimoto disease—an autoimmune disorder frequently resulting in hypothyroidism; he was excluded from these analyses.
TABLE 1.
Characteristics of the subjects at baseline1
| Characteristic | Men (n = 13) | Women (n = 15) |
| Age (y) | 41.1 ± 3.8 | 41.0 ± 2.9 |
| Weight (kg) | 81.6 ± 4.3 | 66.1 ± 2.3 |
| Height (m) | 1.8 ± 0.02 | 1.6 ± 0.01 |
| BMI (kg/m2) | 26.1 ± 1.3 | 24.8 ± 0.9 |
| Plasma selenium (μmol/L) | 17.6 ± 0.7 | 16.3 ± 0.4 |
| T3 (nmol/L) | 1.5 ± 0.03 | 1.5 ± 0.04 |
| T4 (nmol/L) | 98.5 ± 3 | 110.6 ± 4 |
| TSH (μIU/mL) | 1.77 ± 0.25 | 1.82 ± 0.18 |
All values are means ± SEMs. SI units: plasma selenium, 1 μmol = 7.896 μg/mL; triiodothyronine (T3), 1 nmol = 0.651 ng/mL; thyroxine (T4), 1 nmol = 0.078 μg/dL. TSH, thyrotropin.
The scientific and subject safety aspects of the project were reviewed and approved by the Cornell University Human Subjects Committee, the National Cancer Institute Special Studies Review Board, the US Department of Agriculture Human Studies Oversight Review Board, and the University of North Dakota Human Subjects Committee (for the Grand Forks Human Nutrition Research Center).
Experimental design
This work was conducted as an ancillary study nested within a larger study to determine the effects of long-term selenium supplementation (28 mo) on the kinetics of selenium metabolism using multiple stable isotopes of that element. After a 4-mo baseline period, subjects took 200 μg Se/d as selenomethionine (SeMet) for 24 mo during which time selenium and thyroid hormones were measured periodically in plasma and serum, respectively.
Subjects were enrolled in the study in groups of 1–4 periodically from January through October 2000. Sample size was based on power considerations for the larger study; post hoc calculations of actual statistical power for the present study showed that the capability to detect 5% differences were as follows: for T3, 88% power for both men and women; for T4, 70% and 60% power for men and women, respectively; and for TSH, 11% and 20% power for men and women, respectively.
Sample collection and preparation
Plasma selenium (total), T3, T4, and TSH were measured in 5 baseline blood samples and then quarterly for 28 mo. Data are presented graphically as a function of the time of selenium supplementation, beginning with the presupplementation sampling (−4 mo). Thus, time points refer to duration of supplementation and not calendar time.
All samples were collected before 1000 h from fasting subjects. This avoided the known circadian pattern of TSH secretion (with 50% differences between peak values at the onset of sleep and nadirs during the afternoon) (39). Serum was separated within an hour and was stored (4°C) for up to 5 d before analyses of thyroid hormones. Plasma was prepared from whole blood by low-speed centrifugation and was held at −75°C for the analyses of total selenium.
Assessment of selenium and thyroid hormone status
Selenium was measured by automated electrothermal atomic absorption spectrophotometry with a reduced (with hydroxylamine and ascorbic acid) palladium matrix modifier and an instrument (Varian Spectra 600; Varian Instruments, Walnut Creek, CA) equipped with L'Vov platforms and automated Zeeman-effect background correction. Body mass index (BMI; in kg/m2) was calculated from self-reported subject body weight and height. Thyroid hormones, T3, T4, and TSH were measured at the Cayuga Medical Center (Ithaca, NY) by radioimmunoassay with the use of commercial kits. The Abbott AxSYM (Abbott Diagnostics, Abbott Park, IL) kit lists analytic sensitivities as 0.15 ng/mL for T3, 1.0 μg/dL for T4, and 0.02 μIU/mL for TSH. The Beckman Access (Beckman Coulter Inc, Fullerton, CA) kit lists analytic sensitivities of 0.1 ng/mL for T3, 0.50 μg/dL for T4, and 0.01 μIU/mL for TSH. During the course of the study in (August 2002), Cayuga Medical Center changed thyroid hormone assays from the Abbott AxSYM to Beckman Access. Measurements of the same samples by both methods showed virtually complete agreement for T4 and TSH, but major differences for T3; the Beckman Access method yielded higher values. However, the sample size of the comparison study was insufficient to yield a calibration equation sufficiently accurate to be useful.
Data analysis
Our objective was to estimate the magnitude and direction of changes, if any, in the concentrations of T3, T4, and TSH associated with selenium supplementation. We used mixed models to allow repeated measurements from the same subject to be correlated. Because TSH is not normally distributed, it is typically shown in log-normal units, and the geometric, rather than the arithmetic, mean is given. We followed this practice.
In our models, the dependent variable was hormone concentration, and the main exposure was length of supplementation (S). For each hormone, we fit 3 regression models, all of which included the following covariates: age at baseline (age), BMI, and seasonal effect (season). Seasonal effect was defined as follows: season 1, winter (December to February); season 2, spring (March to May); season 3, summer (June to August); and season 4, fall (September to November). The first model included only the above covariates. The second model assumed a linear effect of selenium supplementation over time by including S as a covariate. The third model assumed a quadratic effect by including S and S2 as covariates. We used likelihood ratio tests to determine whether there was a linear and/or a quadratic effect on hormone concentrations and to test the significance of the other covariates.
As noted above, the effect of supplementation is confounded in our data by what we call an “assay effect,” that is, the effect of changing the assay method from Abbott AxSYM to Beckman Access on about 10 August 2002. We used 2 approaches in our modeling to take this confounding into account:
1) Censored approach: We excluded samples drawn after 10 August 2002 and used only those observations obtained with the Abbott AxSYM method. The advantage of this approach was that it eliminated changes due to the switch in test kits. The disadvantage was that it excluded some data: at 12 mo the data set included 15 women and 13 men; this number dropped to 11 women and 6 men at 18 mo (Table 2); consequently, this approach had less power to detect statistically significant changes.
2) Assay-modeled approach: With this approach, we used all the data and modeled the effect of changing the assay method by adding a covariate to the models that equaled 0 if the measurement was made before 10 August 2002, and 1 if the measurement was made afterward. The advantage of this approach was that it used data from the entire study and thus had more power to detect statistically significant changes. The disadvantage was that it relied on a model that may not adequately describe the complexities of the relation between the 2 assay methods.
TABLE 2.
Numbers of subjects used in the analyses by the censored and assay-modeled approaches1
| Women |
Men |
|||
| Month | Censored approach | Assay-modeled approach | Censored approach | Assay-modeled approach |
| −4 | 15 | 15 | 13 | 13 |
| −3 | 9 | 9 | 10 | 10 |
| −2 | 14 | 14 | 11 | 11 |
| −1 | 15 | 15 | 12 | 12 |
| 0 | 15 | 15 | 12 | 12 |
| 3 | 14 | 14 | 13 | 13 |
| 6 | 15 | 15 | 13 | 13 |
| 9 | 15 | 15 | 13 | 13 |
| 12 | 15 | 15 | 13 | 13 |
| 15 | 14 | 15 | 9 | 13 |
| 18 | 11 | 15 | 6 | 13 |
| 21 | 6 | 15 | 2 | 11 |
| 24 | 4 | 14 | 2 | 12 |
| 28 | 0 | 15 | 0 | 12 |
With the censored approach, only subjects whose assays were completed before October 2002 were included in the model. With the assay-modeled approach, all subjects for whom assays were completed during the entire study period were included in the model.
To determine whether the treatment effect, S, was statistically significant at any point during the supplementation period, we compared the likelihood ratio statistics with and without S for each study duration time point.
RESULTS
Selenium status
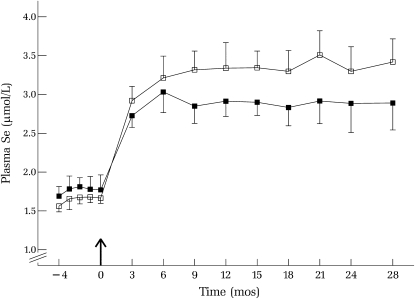
Selenium supplementation increased plasma selenium from a mean (±SEM) of 1.78 ± 0.07 μmol/L for men and 1.64 ± 0.04 μmol/L for women at baseline to 2.85 ± 0.11 μmol/L for men and 3.32 ± 0.12 μmol/L for women by month 9, after which plasma selenium concentrations were stable (Figure 1). Plasma selenium concentrations increased more in women than in men, which may be due in part to their lower body weights (Table 1). Because men and women received the same selenium dose (200 μg Se/d), the effective selenium dose (per unit body weight) was greater in women than in men.
FIGURE 1.
Mean (±SEM) plasma selenium concentrations during baseline (−4 to 0 mo) and over 28 mo of selenium supplementation (200 μg/d) in men (▪) and women (□); n varied over time (see Table 2). The arrow indicates the start of selenium intervention.
Thyroid hormone concentrations
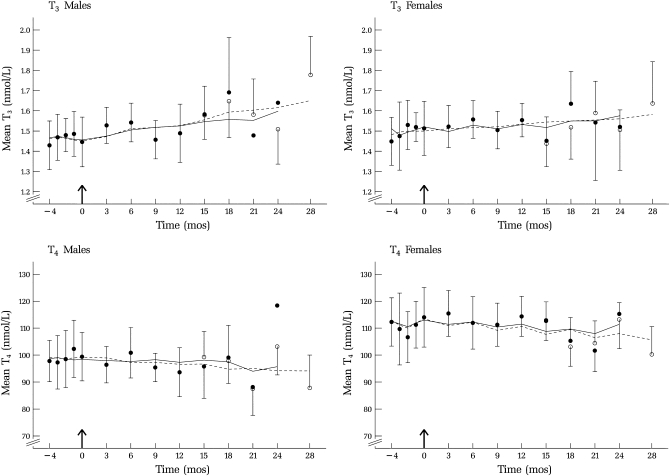
Plots of mean thyroid hormone concentrations by sex and month of selenium supplementation are shown in Figures 2 and 3. Because different assay methods were used to analyze the data during these 2 time periods, the data represent both the effect of supplementation and an “assay effect” (the effect of changing assays). Also indicated in those figures are projected values (open circles) for the case in which all samples had been analyzed by the same method.
FIGURE 2.
Mean (±SEM) triiodothyronine (T3) and thyroxine (T4) concentrations during baseline (−4 to 0 mo) and over 28 mo of selenium supplementation (200 μg/d); n varied over time (see Table 2). Arithmetic means are shown with 95% CIs (vertical bars) for points with >2 subjects. One-sided 95% CIs are shown as estimated by the censored (solid line) and the assay-modeled (dashed line) approaches adjusted for age, BMI, and season. Data collected before October 2002 (•) and estimates of data collected after 12 mo (○) are shown as indicated in the text. T3 concentrations increased significantly (5% per year; P < 0.001) in men, becoming significant after 18 mo of selenium supplementation.
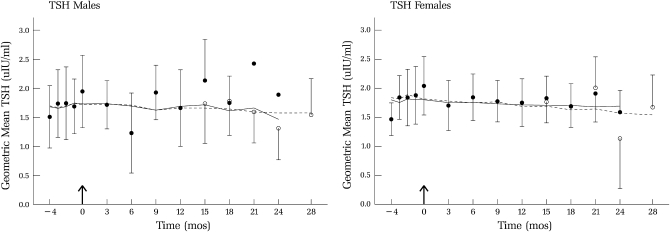
FIGURE 3.
Geometric mean (±SEM) thyrotropin (TSH) concentrations during baseline (−4 to 0 mo) and over 28 mo of selenium supplementation (200 μg/d) in men and women; n varied over time (see Table 2). Data collected before October 2002 (•) and estimates of data collected after month 12 (○) are shown as indicated in the text.
Likelihood ratio tests indicated no statistically significant quadratic effects of selenium supplementation for any of the thyroid hormones; results presented are based on the second model (linear effect). The estimated effect of 1 mo of selenium supplementation on hormone concentrations for both the censored and assay-modeled analytic approaches, denoted as S, is shown in Table 3 for the 3 thyroid hormones.
TABLE 3.
Estimates of the monthly effect (S) of selenium supplementation on plasma thyroid hormone concentrations1
| Thyroid hormone concentration |
||||
| Censored approach |
Assay-modeled approach |
|||
| Hormone and sex | S | P | S | P |
| T3 (nmol/L) | ||||
| Women | 0.001 ± 0.002 | 0.69 | 0.003 ± 0.003 | 0.3 |
| Men | 0.006 ± 0.002 | 0.02 | 0.007 ± 0.002 | 0.01 |
| T4 (nmol/L) | ||||
| Women | −0.12 ± 0.20 | 0.54 | −0.23 ± 0.18 | 0.21 |
| Men | −0.05 ± 0.17 | 0.76 | −0.20 ± 0.16 | 0.2 |
| TSH (μIU/mL) | ||||
| Women | −0.004 ± 0.004 | 0.28 | −0.006 ± 0.005 | 0.21 |
| Men | −0.003 ± 0.006 | 0.66 | −0.003 ± 0.005 | 0.54 |
All values are means ± SEMs. Covariates included S, age, BMI, and season. T3, triiodothyronine; T4, thyroxine; TSH, thyrotropin.
Effect of supplementation
For T3, estimates of the supplementation effect, S, were significant for men with both the censored approach (0.006 ± 0.002 nmol · L−1 · mo−1; P = 0.02) and the assay-modeled approach (0.007 ± 0.002 nmol · L−1 · mo−1; P = 0.01). The latter corresponds to an average increase of ≈5% per year. (Figure 2). The supplementation effect became statistically significant at 15 mo with both the censored and the assay-modeled approach. For women, estimated increases were also positive but smaller, 0.001 ± 0.002 and 0.003 ± 0.003 nmol · L−1 · mo−1 for the 2 approaches, respectively, and were not statistically significant.
There was no effect of selenium supplementation on T4 for either men or women, but concentrations were higher for women than for men (Figure 2); this difference persisted throughout supplementation. There were no statistically significant effects of supplementation on T4 concentrations for either men or women, despite the slight decline in values (Figure 2). Similarly, concentrations of TSH (Figure 3) were not affected by selenium supplementation for either sex. For both T4 and TSH, for both sexes, S was slightly negative but not significantly different from zero.
Neither age at baseline nor BMI was statistically significant predictors of thyroid hormone concentrations. There were significant seasonal effects only among women. For T3, concentrations were higher in the fall than in the spring with the assay-modeled approach. For T4, concentrations were higher in the fall than in the winter or summer with both analytic approaches; they were higher in the spring than in the winter with the assay-modeled approach. In contrast, no seasonal effects were detected in TSH concentrations for either sex. Despite the fact that seasonal effects were sometimes statistically significant, adding seasonal effects to the models had a negligible effect on the estimated effects of selenium supplementation.
DISCUSSION
To date, all of the long-term studies of the effects of selenium supplementation on thyroid hormone metabolism have been in subjects with a low or deficient selenium status. The present study differs from previous studies in as much as the subjects were adequate in selenium by the standard of having plasma selenium concentrations >80 ng/mL, which Hill et al (40) calculated to be associated with maximal expression of plasma selenoproteins. Subjects in the present study had presupplementation plasma selenium concentrations of ≈130 ng/mL; this concentration corresponds to the 50th percentile for healthy Americans (41) and was very similar to the baseline concentration of subjects in studies by Hawkes and Keim (33). Furthermore, these concentrations are consistent with full expression of the iodothyronine deiodinases.
Our study used a before-after design to compare thyroid hormone concentrations before and after selenium supplementation. This design is more powerful than a comparison between 2 separate groups because it eliminates between-group variability by comparing each subject to him or herself. By reducing variance in this way, smaller differences, such as the increase in T3 in men, are detectable.
This study was the first to track the change in plasma selenium in response to long-term selenium supplementation over a relatively short time (3 mo). The increase in plasma selenium in response to selenium supplementation indicated that the selenium supplement was bioavailable. That the magnitude of that response (≈90 ng/mL) was greater than that observed in the Nutritional Prevention of Cancer with Selenium Trial (≈76 ng/mL) may reflect differences in the selenium supplements used: L-selenomethionine (present study) compared with high-selenium brewers' yeast (42). Nevertheless, both studies showed that a supplementation period of ≥6 mo is required to bring healthy Americans to new plateaus in plasma selenium—a longer period than used by Hawkes and Keim (33), but not by Rayman et al (26) (6 mo) or Hawkes et al (37) (11 mo).
Two sources of variation were avoided in this study. Diurnal changes, which have been reported for TSH concentrations (39), were avoided by drawing blood only in the morning after subjects had fasted. Because subjects entered the study throughout the year, quarterly blood collections were staggered throughout the year so that any changes in TSH concentrations due to season were prevented (43) and were not concentrated in any specific quarterly draw, but rather occurred in different quarters across the study population.
Because of the change in assay midway through the study, we used 2 approaches to analyze these data. The censored approach, using the first method alone, was the most conservative, requiring no assumptions about the change in assay method; however, it did not make use of all the data. The assay-modeled approach used all the data; however, it relied on the correctness of our model of the relation between the 2 assay methods. We note that the magnitude and direction of the model parameters were similar for the 2 approaches for all 3 thyroid hormones. Furthermore, similarities between the models were visually apparent in the plots for each hormone. Together, these data support the validity of the analysis.
Our study differed in several respects from the studies of Rayman et al (26) and Hawkes et al (37). We used SeMet as the intervention agent, whereas they used selenium-enriched yeast. We supplemented for 28 mo, whereas those studies supplemented for 6 and 12 mo, respectively. We used 5 baseline samples; each of the other studies used only a single baseline sample.
Neither of the previous intervention studies found any evidence that selenium supplementation affected thyroid hormone concentrations in subjects of baseline selenium status that was either low (37) or comparable with that of most Americans (26). A comparison between the present results and those of the other studies shows overall agreement in that none of these studies found differences related to selenium supplementation in any of the thyroid hormone concentrations in either sex at either 6 or 12 mo. The present study, which was longer than either of the other studies, detected a statistically significant increase in T3 concentration over 28 mo; however, that this effect was not accompanied by a change in TSH concentration indicates that it was not clinically significant.
These results indicate that, in healthy subjects of adequate selenium status, a period of ≥6 mo is required to reach a new steady state plasma selenium concentration when supplemented with 200 μg Se/d as SeMet. This finding is consistent with the results of the Nutritional Prevention of Cancer Trial (42). These results also show that such supplementation does not produce clinically significant changes in thyroid hormone concentrations. This was as expected, in as much as the baseline selenium status appears to have been sufficient for the maximal expression of selenoenzymes, including the selenium-dependent iodothyronine 5′-deiodinases. However, men responded to selenium supplementation with small but statistically significant increases in circulating T3 concentrations with no corresponding decreases in TSH. Considering the fairly long period of the selenium-supplementation period (28 mo), these findings cannot be considered clinically significant.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Marie Brindak (Cornell University), who participated in the subject recruitment and management and in the sample collection, processing, and distribution. We are grateful for the lifelong contributions of our colleague AD Hill, who died recently.
The authors' responsibilities were as follows—GFC, BBHP, PRT, and OAL: conceived and planned this study; GFC: responsible for recruiting and managing the human subjects and for coordinating the collection, processing, and analysis of the samples; WKC: responsible for subject health surveillance and performed some blood collections; KYP and ADH: performed the plasma selenium analyses; JEM: managed the data and assisted in the statistical analyses; BHP and DNM: conducted the statistical analysis; and GFC and BHP: prepared the article. None of the authors had any conflicts of interests.
REFERENCES
- 1.Dickson RC, Tomlinson RH. Selenium in blood and human tissues. Clin Chim Acta 1967;16:311–21 [DOI] [PubMed] [Google Scholar]
- 2.Glattre E, Mracova A, Lener J, Vobecky M, Egertova E, Mysliveckova M. Study of distribution and interaction of arsenic and selenium in rat thyroid. Biol Trace Elem Res 1995;49:177–85 [DOI] [PubMed] [Google Scholar]
- 3.Murillo M, Carrion N, Quintana M, et al. Determination of selenium and iodine in human thyroids. J Trace Elem Med Biol 2005;19:23–7 [DOI] [PubMed] [Google Scholar]
- 4.Howie AF, Walker SW, Akesson B, Arthur JR, Beckett GJ. Thyroidal extracellular glutathione peroxidase: a potential regulator of thyroid hormone synthesis. Biochem J 1995;308:713–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman MB, Köhrle J. The impact of iron and selenium deficiency on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid 2002;12:867–78 [DOI] [PubMed] [Google Scholar]
- 6.Beckett GJ, Hutchinson AR, Nicol F, Arthur JR. A role for selenium in the hepatic 5”-deiodination of thyroxine. J Endocrinol 1988;117:287–94 [Google Scholar]
- 7.Davey JC, Becker KB, Schneider MJ, St Germain DL, Galton VA. Cloning of a cDNA for the type II iodothyronine deiodinase. J Biol Chem 1995;270:26786–9 [DOI] [PubMed] [Google Scholar]
- 8.Dhingra S, Singh U, Bansal MP. Effect of selenium depletion and supplementation on the kinetics of type I 5′-iodothyronine deiodinase and T3/T4 in rats. Biol Trace Elem Res 2004;97:95–104 [DOI] [PubMed] [Google Scholar]
- 9.Ramauge M, Pallud S, Esfandiari A, et al. Evidence that the type III iodothyronine deiodinase in rat astrocyte is a selenoprotein. Endocrinology 1996;137:3021–5 [DOI] [PubMed] [Google Scholar]
- 10.Arthur JR, Beckett GJ. Selenium deficiency and thyroid hormone metabolism. Selenium in biology and medicine. New York, NY: Springer-Verlag, 1988:90–5 [Google Scholar]
- 11.Bates JM, Spate VL, Morris JS, St Germain DL, Galton VA. Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during development. Endocrinology 2000;141:2490–500 [DOI] [PubMed] [Google Scholar]
- 12.Chang WP, Combs GF, Jr, Scanes CG, Marsh JA. The effects of dietary vitamin E and selenium deficiencies on plasma thyroid hormone and thymic hormone concentrations in the chicken. Dev Comp Immunol 2005;29:265–73 [DOI] [PubMed] [Google Scholar]
- 13.Hotz CS, Fitzpatrick DW, Trick KD, L'Abbe MR. Dietary iodine and selenium interact to affect thyroid hormone metabolism in rats. J Nutr 1997;127:1214–8 [DOI] [PubMed] [Google Scholar]
- 14.Jianhua H, Ohtsuka A, Hayashi K. Selenium influences growth via thyroid hormone status in broiler chickens. Br J Nutr 2000;84:727–32 [PubMed] [Google Scholar]
- 15.Voudouri AE, Chadio SE, Menegatos JG, Zervas GP, Nicol F, Arthur JR. Selenoenzyme activities in selenium- and iodine-deficient sheep. Biol Trace Elem Res 2003;94:213–24 [DOI] [PubMed] [Google Scholar]
- 16.Wu HY, Xia YM, Ha PC, Chen XS. Changes in myocardial thyroid hormone metabolism and alpha-glycerophosphate dehydrogenase activity in rats deficient in iodine and selenium. Br J Nutr 1997;78:671–6 [DOI] [PubMed] [Google Scholar]
- 17.Dumitrescu AM, Laio XH, Abdullah MSY, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 2005;37:1247–52 [DOI] [PubMed] [Google Scholar]
- 18.Derumeaux H, Valeix P, Castetbon K, et al. Association of selenium with thyroid volume and echostructure in 35- to 6-year-old French adults. Eur J Endocrinol 2003;148:309–15 [DOI] [PubMed] [Google Scholar]
- 19.Strain JJ, Bokje E, van't Veer P, et al. Thyroid hormones and selenium status in breast cancer. Nutr Cancer 1997;27:48–52 [DOI] [PubMed] [Google Scholar]
- 20.Ravaglia G, Forti P, Maioli F, et al. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab 2000;85:2260–5 [DOI] [PubMed] [Google Scholar]
- 21.Olivieri O, Girelli D, Azzini M, et al. Low selenium status in the elderly influences thyroid hormones. Clin Sci 1995;89:637–42 [DOI] [PubMed] [Google Scholar]
- 22.Thomson CD. Selenium and iodine interactions with thyroid status. Asia Pac J Clin Nutr 2003;12(suppl):S1415023604 [Google Scholar]
- 23.Chanoine JP. Selenium and thyroid function in infants, children and adolescents. Biofactors 2003;19:137–43 [DOI] [PubMed] [Google Scholar]
- 24.Tong YJ, Teng WP, Jin Y, et al. [An epidemiological study on the relationship between selenium and thyroid function in areas with different iodine intake.]. Zhonghua Yi Xue Za Zhi 2003;83:2036–9(in Chinese) [PubMed] [Google Scholar]
- 25.Zagrodzki P, Szmigiel H, Ratajczak R, et al. The role of selenium in iodine metabolism in children with goiter. Environ Health Perspect 2000;108:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayman MP, Thompson AJ, Bekaert B, et al. Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom. Am J Clin Nutr 2008;87:370–8 [DOI] [PubMed] [Google Scholar]
- 27.Gartner R, Gasnier BCH, Dietrich JW, et al. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibody concentrations. J Clin Endocrinol Metab 2002;87:1687–91 [DOI] [PubMed] [Google Scholar]
- 28.Duntas LH, Maqntzou E, Koutras DA. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur J Endocrinol 2003;148:389–93 [DOI] [PubMed] [Google Scholar]
- 29.Gartner R, Gasnier BCH. Selenium and treatment of autoimmune thyroiditis. Biofactors 2003;19:165–70 [DOI] [PubMed] [Google Scholar]
- 30.Angstwurm MWA, Schopohl J, Gaertner R. Selenium substitution has no direct effect on thyroid hormone metabolism in critically ill patients. Eur J Endocrinol 2004;151:47–54 [DOI] [PubMed] [Google Scholar]
- 31.Contempre B, Dual NL, Dumont JE, Nego B, Diplock AT, Vanderpas J. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin Endocrinol (Oxf) 1992;36:579–83 [DOI] [PubMed] [Google Scholar]
- 32.Thomson CD, McLachlan SK, Grant AM, Paterson E, Lillico AJ. The effect of selenium on thyroid status in a population with marginal selenium and iodine status. Br J Nutr 2005;94:962–8 [DOI] [PubMed] [Google Scholar]
- 33.Hawkes W C, Keim NL. Dietary selenium intake modulates thyroid hormone and energy metabolism in men. J Nutr 2003;133:3443–8 [DOI] [PubMed] [Google Scholar]
- 34.Moncayo R, Moncayo H. Nutritional treatment of incipient thyroid autoimmune disease: influence of selenium supplementation on thyroid function and morphology in children and young adults. Clin Nutr 2005;24:530–1 [DOI] [PubMed] [Google Scholar]
- 35.Calomme MR, Vanderpas JB, Françoi B, et al. Thyroid function parameters during a selenium repletion/depletion study of phenylketonuric subjects. Cell Mol Life Sci 1995;51:1208–15 [DOI] [PubMed] [Google Scholar]
- 36.Vrca VB, Skreb F, Cepelak I, Romic Z, Mayer L. Supplementation with antioxidants in the treatment of Graves' disease: the effect of glutathione peroxidase activity and concentration of selenium. Clin Chim Acta 2004;341:55–63 [DOI] [PubMed] [Google Scholar]
- 37.Hawkes CW, Keim NL, Richter D, et al. High-selenium yeast supplementation in free-living North American men: no effect on thyroid hormone metabolism or body composition. J Trace Elem Med Biol 2008;22:131–42 [DOI] [PubMed] [Google Scholar]
- 38.Gould SJ. Health consciousness and healthy behavior: the application of a new health consciousness scale. Am J Prev Med 1990;6:228–34 [PubMed] [Google Scholar]
- 39.Surks MI, Goswami G, Daniels GH. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab 2005;90:5489–96 [DOI] [PubMed] [Google Scholar]
- 40.Hill KE, Xia Y, Åkesson B, Boeglin ME, Burk RF. Selenoprotein P concentration in plasma as an index of selenium status in selenium-deficient and selenium supplemented Chinese subjects. J Nutr 1996;126:138–45 [DOI] [PubMed] [Google Scholar]
- 41.Niskar AS, Paschal DC, Kieszak SM, et al. Serum selenium levels in the US population: third National Health and Nutrition Examination Survey/1988-1994. Biol Trace Elem Res 2003;91:1–10 [DOI] [PubMed] [Google Scholar]
- 42.Clark LC, Combs G, Jr, Turnbull B, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996;276:1957–63 [PubMed] [Google Scholar]
- 43.Maes M, Mommen K, Hendrickx D, et al. Components of biological variation, including seasonality, in blood concentration of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin Endocrinol (Oxf) 1997;46:587–98 [DOI] [PubMed] [Google Scholar]