Summary
This review provides alternatives to two well established theories
regarding membrane energization by H+ V-ATPases. Firstly, we offer
an alternative to the notion that the H+ V-ATPase establishes a
protonmotive force (pmf) across the membrane into which it is inserted. The
term pmf, which was introduced by Peter Mitchell in 1961 in his chemiosmotic
hypothesis for the synthesis of ATP by H+ F-ATP synthases, has two
parts, the electrical potential difference across the phosphorylating
membrane, Δψ, and the pH difference between the bulk solutions on
either side of the membrane, ΔpH. The ΔpH term implies three
phases – a bulk fluid phase on the H+ input side, the
membrane phase and a bulk fluid phase on the H+ output side. The
Mitchell theory was applied to H+ V-ATPases largely by analogy with
H+ F-ATP synthases operating in reverse as H+ F-ATPases.
We suggest an alternative, voltage coupling model. Our model for V-ATPases is
based on Douglas B. Kell's 1979 `electrodic view' of ATP synthases in which
two phases are added to the Mitchell model – an unstirred layer on the
input side and another one on the output side of the membrane. In addition, we
replace the notion that H+ V-ATPases normally acidify the output
bulk solution with the hypothesis, which we introduced in 1992, that the
primary action of a H+ V-ATPase is to charge the membrane
capacitance and impose a Δψ across the membrane; the translocated
hydrogen ions (H+s) are retained at the outer fluid–membrane
interface by electrostatic attraction to the anions that were left behind. All
subsequent events, including establishing pH differences in the outside bulk
solution, are secondary. Using the surface of an electrode as a model, Kell's
`electrodic view' has five phases – the outer bulk fluid phase, an outer
fluid–membrane interface, the membrane phase, an inner
fluid–membrane interface and the inner bulk fluid phase. Light flash,
H+ releasing and binding experiments and other evidence provide
convincing support for Kell's electrodic view yet Mitchell's chemiosmotic
theory is the one that is accepted by most bioenergetics experts today. First
we discuss the interaction between H+ V-ATPase and the
K+/2H+ antiporter that forms the caterpillar
K+ pump, and use the Kell electrodic view to explain how the
H+s at the outer fluid–membrane interface can drive two
H+ from lumen to cell and one K+ from cell to lumen via
the antiporter even though the pH in the bulk fluid of the lumen is highly
alkaline. Exchange of outer bulk fluid K+ (or Na+) with
outer interface H+ in conjunction with (K+ or
Na+)/2H+ antiport, transforms the hydrogen ion
electrochemical potential difference,
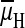 , to a K+
electrochemical potential difference,
, to a K+
electrochemical potential difference,
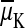 or a Na+
electrochemical potential difference,
or a Na+
electrochemical potential difference,
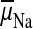 . The
. The
 or
or
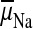 drives K+- or
Na+-coupled nutrient amino acid transporters (NATs), such as KAAT1
(K+ amino acid transporter 1), which moves Na+ and an
amino acid into the cell with no H+s involved. Examples in which
the voltage coupling model is used to interpret ion and amino acid transport
in caterpillar and larval mosquito midgut are discussed.
drives K+- or
Na+-coupled nutrient amino acid transporters (NATs), such as KAAT1
(K+ amino acid transporter 1), which moves Na+ and an
amino acid into the cell with no H+s involved. Examples in which
the voltage coupling model is used to interpret ion and amino acid transport
in caterpillar and larval mosquito midgut are discussed.
Keywords: electrogenic, electrophoretic, protonmotive force, electrochemical potential
“The obscure we see eventually, the completely apparent takes longer.”
Peter Mitchell, Nobel Lecture, 1978
Central role of the electrical potential difference as a membrane energizer in prokaryotes
Membrane potentials and pH differences in ATP synthesis and cation exchange
Peter Mitchell introduced the hypothesis that the proton electrochemical potential difference (pmf) established by the electron transport system provides the energy for ATP synthesis by the F1Fo ATP synthase in mitochondria, chloroplasts and bacteria (Mitchell, 1961). The pmf has two parts, the electrical potential difference, Δψ, across the membrane and the pH difference between the bulk solutions. The pH difference can be expressed in volts as RT/zF ln cH o/cH in, where cH o and cH in refer to the hydrogen ion concentrations in the bulk solutions outside and inside the coupling membrane, respectively. Mitchell referred to this type of coupling as `chemiosmotic' coupling. After several years of controversy, Mitchell's ATP synthesis by chemiosmotic coupling was accepted by the scientific community and became regarded as an established theory for which Mitchell was awarded the Nobel Prize in Chemistry in 1978. However, there have always been lingering doubts regarding the pH in the bulk fluid outside the coupling membranes, especially as applied to bacterial plasma membranes. R. J. P. Williams pointed out that the volume of the bulk solution outside a bacterial cell could be as large as the Pacific Ocean and that the H+ concentration there could not be increased by expulsion of H+ from bacteria (Williams, 1962). Williams, who was skeptical about the biologist's `membrane concept', argued that the hydrogen ions from the electron transport system remain within the outer regions of the ATP synthesizing entities (Williams, 1978). Harold (Harold, 1986) reviewed the entire topic of localized protons outside the ATP synthesizing membranes and observed that `in recent years, a growing number of investigators have proposed that protons translocated during respiration may be guided to the synthase without equilibrating with protons in the bulk phase' and notes that `there is much interest in localized protons or protonic microcircuits'. Kell reviewed the early literature and documented the advantages of his `electrodic view' over Mitchell's chemiosmotic theory (Kell, 1979).
The source of protons in the environment of alkalophilic bacteria is especially difficult to reconcile with the chemiosmotic theory because ATP synthesis is clearly driven by the proton electrochemical potential difference but the H+ concentration can be as low as 10–11 mol l–1 in the bulk fluid phase (e.g. Krulwich and Guffanti, 1989). However, the pmf drives H+ back into the cells and expels Na+ that leaks in from the caustic environment. A similar problem occurs in the case of midgut alkalinization in caterpillars and larval mosquitoes where an H+ V-ATPase uses energy from ATP hydrolysis to drive H+ from the cells towards the lumen even though the lumen H+ concentration can be less than 10–11 mol l–1 (Dow, 1984; Boudko et al., 2001).
During Na+ expulsion by alkalophilic bacteria, the hydrogen ion
electrochemical potential difference
(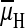 ) that is generated by the primary
electron transport system drives secondary cation exchangers such as the
Na+/2H+ antiporter, NhaA
(Krulwich et al., 1998;
Padan et al., 2005). NhaA has
been cloned, characterized, crystallized and its reaction mechanism determined
(Hunte et al., 2005;
Padan et al., 2005;
Padan et al., 2009). In the
case of caterpillar K+ secretion the
) that is generated by the primary
electron transport system drives secondary cation exchangers such as the
Na+/2H+ antiporter, NhaA
(Krulwich et al., 1998;
Padan et al., 2005). NhaA has
been cloned, characterized, crystallized and its reaction mechanism determined
(Hunte et al., 2005;
Padan et al., 2005;
Padan et al., 2009). In the
case of caterpillar K+ secretion the
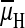 is generated by a primary
H+ V-ATPase (Wieczorek et al.,
1989), which drives a secondary K+/2H+
antiporter (Wieczorek et al.,
1991). The H+ V-ATPase is well established and widely
reviewed (e.g. Beyenbach and Wieczorek,
2006; Nelson and Harvey,
1999) and the K+/2H+ antiporter has been
well established biochemically (Azuma et
al., 1995; Grinstein and
Wieczorek, 1994; Wieczorek et
al., 1991) but only recently have attempts to clone the
K+/2H+ antiporter been fruitful as discussed below.
Membrane energization by the H+ V-ATPase is simpler to analyze than
that by the electron transport system because the source of H+s for
plasma membrane H+ V-ATPases is simply the cell cytoplasm, whereas
the source of H+s for the ATP synthase is a complex set of linked
redox reactions within inner mitochondrial, thylakoid or bacterial plasma
membranes.
is generated by a primary
H+ V-ATPase (Wieczorek et al.,
1989), which drives a secondary K+/2H+
antiporter (Wieczorek et al.,
1991). The H+ V-ATPase is well established and widely
reviewed (e.g. Beyenbach and Wieczorek,
2006; Nelson and Harvey,
1999) and the K+/2H+ antiporter has been
well established biochemically (Azuma et
al., 1995; Grinstein and
Wieczorek, 1994; Wieczorek et
al., 1991) but only recently have attempts to clone the
K+/2H+ antiporter been fruitful as discussed below.
Membrane energization by the H+ V-ATPase is simpler to analyze than
that by the electron transport system because the source of H+s for
plasma membrane H+ V-ATPases is simply the cell cytoplasm, whereas
the source of H+s for the ATP synthase is a complex set of linked
redox reactions within inner mitochondrial, thylakoid or bacterial plasma
membranes.
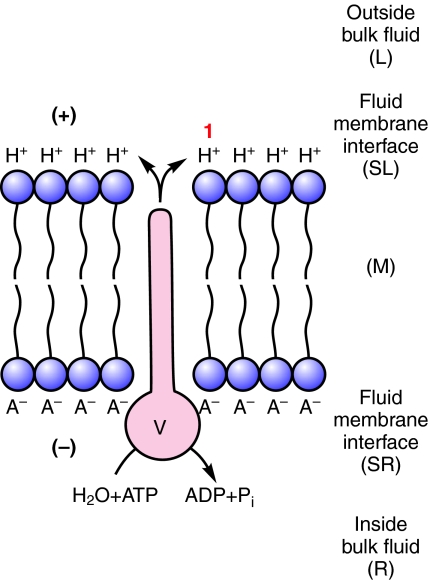
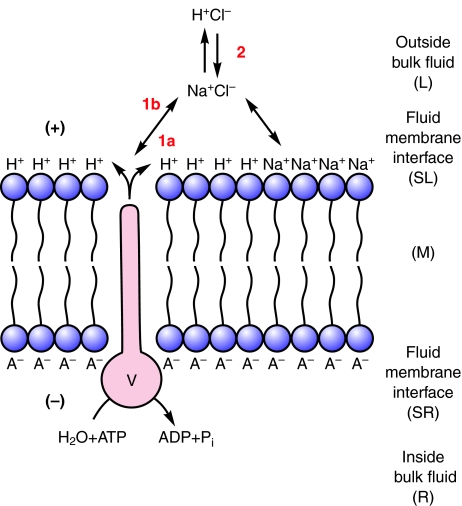
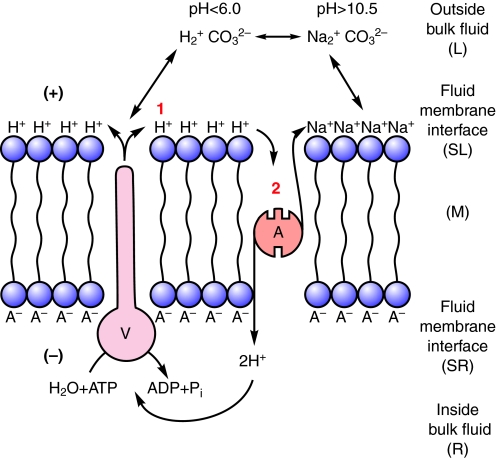
Let us consider membrane energization by an H+ V-ATPase more closely, assuming that H+ is the only ion translocated by the V-ATPase and that electroneutrality is preserved in the bulk solutions. A H+ V-ATPase, by itself in an ideal lipid bilayer that is impermeable to all charged solutes and with identical bulk solutions on either side would display but one activity upon addition of ATP: a H+ current would flow across the bilayer, charge the membrane capacitance and stop; the charge separation between the H+ on the output side and its former gegenion, A–, on the input side would appear as a membrane potential difference, Δψ, with size limited by the phosphorylation potential of ATP, ADP and inorganic phosphate (Pi) (Mandel et al., 1975) on the input side and the stoichiometric number of the H+ transported per ATP hydrolyzed (Fig. 1). Secondarily, the H+ would exchange with whatever cation is present in the outside bulk fluid. If the outside bulk fluid is simply H2O then exchange of H+ between the fluid–membrane interface and bulk fluid would not change the pH; if the outside fluid were NaCl then exchange of Na+ with H+ at the interface would acidify the fluid (Fig. 2). If Cl– were to follow the H+ then the output side would be more strongly acidified. If H+ from the bulk solution were exchanged for K+ or Na+ the output side would be alkalinized (Fig. 3). These deductions were made explicitly regarding H+ V-ATPases (Harvey, 1992) and had been implied much earlier in publications on prokaryotic ATP synthases and Na+/H+ antiporters which were summarized in a seminal paper by Kell (Kell, 1979). Kell's so-called `lectrodic view' (Figs 1, 2, 3, 4) is widely cited by bioenergeticists but largely ignored by epithelial transport physiologists.
Fig. 1.
H+ V-ATPase generates membrane potential. A H+ V-ATPase (V) is inserted into an ideal lipid bilayer (M) of a membrane; upon hydrolysis of ATP in the inside bulk fluid (R), H+ is translocated across the bilayer (M) to the fluid membrane interface (SL) and is separated from its gegenion, A–, which remains at the inner fluid membrane interface (SR). H+ is held at the fluid membrane interface (SL) by electrostatic attraction to its gegenion. A membrane potential is generated with the outside positive (+) to the inside (–).
Fig. 2.
H+ is replaced at the fluid membrane interface by Na+. If the outside bulk solution contains, say, NaCl at a concentration of, say, 10 mmol l–1 and the H+ concentration is, say, 10–4 mmol l–1 (pH 7) there would be 100,000 Na+s for every H+ in the outside bulk fluid, so H+ at the fluid membrane interface would move into the outside bulk phase, being replaced at the interface by Na+ and the outside bulk fluid would become acidic to the extent limited by the capacitance of the membrane.
Fig. 3.
Vm drives a K+ or
Na+/2H+ antiporter. The membrane potential
(Vm) established by the H+ V-ATPase drives two
H+ into the cell and one Na+ out to the fluid membrane
interface via a K+ or Na+/2H+
antiporter (A). The 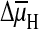 at the
interface is replaced by
at the
interface is replaced by 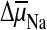 .
The voltage is changed but little and a steady state is established in which
H+ can recycle and Na+ can move out of the cells and
alkalinize the lumen as long as there is a K+ or Na+
salt and ATP in the inside bulk fluid. The pH of the outside bulk fluid
changes from <6.0 to >10.5 as H2CO3 is converted
to K2CO3 or Na2CO3.
.
The voltage is changed but little and a steady state is established in which
H+ can recycle and Na+ can move out of the cells and
alkalinize the lumen as long as there is a K+ or Na+
salt and ATP in the inside bulk fluid. The pH of the outside bulk fluid
changes from <6.0 to >10.5 as H2CO3 is converted
to K2CO3 or Na2CO3.
Fig. 4.
Vm also drives Na+ coupled amino acid symport. Vm drives Na+ that is stoichiometrically linked to an amino acid into the cell via a nutrient amino acid transporter (NAT, N). Although the membrane voltage is little changed, Na+ can recycle and amino acids can move into the cell as long as there is a sodium salt and an amino acid with affinity for the NAT in the outside bulk fluid. Although the energy for the symport process is ATP hydrolysis by the H+ V-ATPase there is no H+ involved in the symport per se, which is driven by the Na+ electrochemical potential difference
High H+ concentrations outside proton translocating membranes
If the 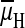 drives ATP synthases in
phosphorylating membranes, Na+/2H+ antiporters in
alkalophilic bacterial plasma membranes and K+/2H+
antiporters in caterpillar apical plasma membranes (the latter two from a
compartment with H+ concentration <10–11 mol
l–1) where does the high [H+]o come
from? The three problems would have a common solution if Kell's `electrodic
view' were applied – thus, Kell's cH L would refer
to the [H+] in the bulk fluid phase outside the membrane but there
is a higher cH SL at the bulk fluid–membrane
interface (Fig. 1). (Of course,
the H+s that make up the `higher cH SL' are the same
ones that make up the Δψ, which is another objection to the pmf
concept). Direct evidence for a separate outer fluid–membrane interface
phase and an outer bulk fluid phase is provided by Cherepanov, Mulkidjanian,
Junge and associates (Cherepanov et al.,
2003; Cherepanov et al.,
2004) (for a review, see
Mulkidjanian et al., 2005).
They used light flashes to activate enzymes that capture or eject hydrogen
ions either in the outer bulk fluid or at the surface of
H+-energized plasma membranes. The light activation technique
showed that H+s reach the membrane-bound enzymes in microseconds
whereas they reach enzymes in the bulk fluid only after milliseconds; thus
H+ appears along the membrane outer-face 1000 times faster than it
appears in the bulk fluid (Mulkidjanian et
al., 2005). These experimental results imply that Δψ and
ΔcH across the coupling membranes are more important than
ΔpH in the inside and outside bulk solutions as the driving forces for
H+ entry coupled to Na+ exit from cells via a
Na+/nH+ antiporter. The importance of
Δψ is consistent with evidence that the much studied Escherichia
coli Na+/2H+ antiporter, EcNhaA, is electrophoretic
(Taglicht et al., 1993). As
noted above, the source of H+ and its gegenions for eukaryotic
H+ V-ATPases is more obvious than that for the electron transport
system and the remainder of this paper will focus on the identification,
isolation, and characterization of the insect primary H+ V-ATPase
and two classes of secondary electrophoretic transporters, (Na+ or
K+)/nH+ antiporters (NHAs) and Na+-
or K+-coupled nutrient amino acid transporters (NATs) that are
driven primarily by the voltage generated by H+ V-ATPases.
drives ATP synthases in
phosphorylating membranes, Na+/2H+ antiporters in
alkalophilic bacterial plasma membranes and K+/2H+
antiporters in caterpillar apical plasma membranes (the latter two from a
compartment with H+ concentration <10–11 mol
l–1) where does the high [H+]o come
from? The three problems would have a common solution if Kell's `electrodic
view' were applied – thus, Kell's cH L would refer
to the [H+] in the bulk fluid phase outside the membrane but there
is a higher cH SL at the bulk fluid–membrane
interface (Fig. 1). (Of course,
the H+s that make up the `higher cH SL' are the same
ones that make up the Δψ, which is another objection to the pmf
concept). Direct evidence for a separate outer fluid–membrane interface
phase and an outer bulk fluid phase is provided by Cherepanov, Mulkidjanian,
Junge and associates (Cherepanov et al.,
2003; Cherepanov et al.,
2004) (for a review, see
Mulkidjanian et al., 2005).
They used light flashes to activate enzymes that capture or eject hydrogen
ions either in the outer bulk fluid or at the surface of
H+-energized plasma membranes. The light activation technique
showed that H+s reach the membrane-bound enzymes in microseconds
whereas they reach enzymes in the bulk fluid only after milliseconds; thus
H+ appears along the membrane outer-face 1000 times faster than it
appears in the bulk fluid (Mulkidjanian et
al., 2005). These experimental results imply that Δψ and
ΔcH across the coupling membranes are more important than
ΔpH in the inside and outside bulk solutions as the driving forces for
H+ entry coupled to Na+ exit from cells via a
Na+/nH+ antiporter. The importance of
Δψ is consistent with evidence that the much studied Escherichia
coli Na+/2H+ antiporter, EcNhaA, is electrophoretic
(Taglicht et al., 1993). As
noted above, the source of H+ and its gegenions for eukaryotic
H+ V-ATPases is more obvious than that for the electron transport
system and the remainder of this paper will focus on the identification,
isolation, and characterization of the insect primary H+ V-ATPase
and two classes of secondary electrophoretic transporters, (Na+ or
K+)/nH+ antiporters (NHAs) and Na+-
or K+-coupled nutrient amino acid transporters (NATs) that are
driven primarily by the voltage generated by H+ V-ATPases.
H+ V-ATPases as membrane energizers in eukaryotes
Initially, vacuolar-type ATPases were thought to energize vacuolar membranes and only a few specialized plasma membranes (Cidon and Nelson, 1982; Nelson, 1987); now they are recognized to be widely distributed plasma membrane energizers (Beyenbach and Wieczorek, 2006; Nelson and Harvey, 1999; Wieczorek et al., 1999) especially in freshwater organisms and insects.
Role of the Δψ component in H+ V-ATPase-generated electrochemical forces
As discussed above a H+ V-ATPase residing by itself in an
ideally impermeable lipid bilayer would first generate a membrane potential
difference, Δψ, across the ATPase-containing membrane
(Harvey, 1992). The
translocated H+ would transiently be held at the
membrane–bulk solution interface by the electrostatic attraction of the
anion from which it was separated during H+ translocation
(Fig. 1). If the H+
concentration in the external solution were, say, 10–7 mol
l–1 (pH 7) and the Na+ concentration were
10–2 mol l–1, there would be 100,000
Na+s for every H+ bombarding the external membrane; so
H+s sequestered at the membrane–bulk solution interface would
be exchanged for Na+ from the bulk solution and the H+
electrochemical potential difference,
 , would be replaced by a
Na+ electrochemical potential difference,
, would be replaced by a
Na+ electrochemical potential difference,
 (see
Fig. 2). This exchange would
take time and contribute to the delayed appearance of H+ in the
bulk fluid outside H+-ejecting sources. A similar argument applies
for any other ionic species in the bulk solutions. Thus, the motive force for
any ionic species, k, is given by Gibbs's electrochemical potential, in which
(see
Fig. 2). This exchange would
take time and contribute to the delayed appearance of H+ in the
bulk fluid outside H+-ejecting sources. A similar argument applies
for any other ionic species in the bulk solutions. Thus, the motive force for
any ionic species, k, is given by Gibbs's electrochemical potential, in which
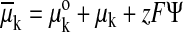 ,
where: μko is the standard chemical potential of k,
μk is the chemical potential of k which is given by RT
ln ck, where ck is the concentration of the
ion, z is the valency, F is Faraday's number and ψ is
the electrical potential on each side of a membrane. The more convenient ion
`concentration' rather than `activity' can be used because the activity
coefficient can be regarded as the same on both sides of the membrane and the
ratios of activities and concentrations in the equations to follow are
identical. The driving force for any ionic species is the difference in
electrochemical potential across the membrane. To calculate it, assume that
μko is the same on both sides of the membrane and
cancels out; by convention the reference potential is outside so:
Δψi–ψo. As noted above, Mitchell
called the driving force for hydrogen ions the protonmotive force (pmf) but we
will use the more explicit term `electrochemical potential difference',
Δμk (in volts), for any other ionic species, as
follows.
,
where: μko is the standard chemical potential of k,
μk is the chemical potential of k which is given by RT
ln ck, where ck is the concentration of the
ion, z is the valency, F is Faraday's number and ψ is
the electrical potential on each side of a membrane. The more convenient ion
`concentration' rather than `activity' can be used because the activity
coefficient can be regarded as the same on both sides of the membrane and the
ratios of activities and concentrations in the equations to follow are
identical. The driving force for any ionic species is the difference in
electrochemical potential across the membrane. To calculate it, assume that
μko is the same on both sides of the membrane and
cancels out; by convention the reference potential is outside so:
Δψi–ψo. As noted above, Mitchell
called the driving force for hydrogen ions the protonmotive force (pmf) but we
will use the more explicit term `electrochemical potential difference',
Δμk (in volts), for any other ionic species, as
follows.
For hydrogen ions the electrochemical potential difference is
 ln
(cH o/cH i) (when
ΔμH is given as pH the `ln' must be replaced by `log') at
30°C, RT/zF ln10≈60 mV so the expression becomes:
ln
(cH o/cH i) (when
ΔμH is given as pH the `ln' must be replaced by `log') at
30°C, RT/zF ln10≈60 mV so the expression becomes:
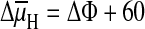 mV log
(cH o/cH i).
mV log
(cH o/cH i).
For sodium ions the difference in electrochemical potential is
 mV log
(cNa o/cNa i).
mV log
(cNa o/cNa i).
For potassium ions it is
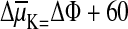 mV log
(cK o/cK i).
mV log
(cK o/cK i).
For chloride ions it is
 mV log
(cCl o/cCl i).
mV log
(cCl o/cCl i).
For any ion, k, the difference in electrochemical potential is,
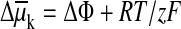 ln
(ck o/ck i).
ln
(ck o/ck i).
Clearly the electrical potential term, Δψ, applies equally to all ionic species. However, the chemical potential term, RT/zF ln (ck o/ck i), would depend upon the ionic species made available by pumps, transporters, channels or other conductances in the membrane. As discussed above, a Cl– channel would allow Cl– to accompany H+ into the output bulk solution and acidify it, as in lysosomes and other intracellular vacuoles as well as in the lumen of renal tubules and many other organs. As we will see below, a K+/2H+ antiporter would allow the voltage to drive two H+s back into the cells across the membrane in exchange for one K+ and alkalinize rather than acidify the side toward which the V-ATPase is translocating H+ (Fig. 3). Moreover, a Na+-coupled nutrient amino acid transporter (NAT) would allow the voltage to drive Na+ along with an amino acid into the cell with no involvement of H+ (Fig. 4). The bottom line is that the H+ V-ATPase is a powerful and versatile voltage generator not simply a pH gradient generator.
The importance of plasma membrane H+ V-ATPases
The H+ V-ATPase was first isolated and characterized from intracellular vacuoles; hence the name vacuolar-type H+-translocating ATPase (Cidon and Nelson, 1986; Uchida et al., 1985). Its role in vesicle acidification was established early so the notion that H+ V-ATPases acidify the side to which the H+s are translocated was emphasized rather than its role in generating Δψ. Soon after, the role of H+ V-ATPase in energizing animal cell plasma membranes such as osteoclasts, kidney tubules, ocular ciliary epithelium, fish gills, frog skin and more became apparent (Nelson and Harvey, 1999; Wieczorek et al., 1999 and references therein). Among the clearest examples of H+ V-ATPase-voltage-driven secondary transport are the K+ pumps of insect epithelia, especially those in Malpighian tubules, salivary glands, sensory sensilla and midgut (Beyenbach and Wieczorek, 2006; Harvey and Wieczorek, 1997; Wieczorek et al., 2009) and we will examine one of them in depth.
K+ pumps in insect ion-transporting epithelia
The first hint of what was later to be recognized as a V-ATPase was uncovered in 1953 by Arthur Ramsay who showed that K+ is more concentrated in urine than in the hemolymph of the blood-sucking insect, Rhodnius prolixus, as well as in six other insect species, and proposed that K+ is `actively transported' from the hemolymph into the tubule lumen (Ramsay, 1953a). Simon Maddrell, John Wood, Michael O'Donnell and colleagues studied this process for many years and developed the concept of a `common ion pump' (Maddrell and O'Donnell, 1992; Maddrell, 1981). Simultaneously, efforts to isolate and characterize the K+ pump were underway. Brij Gupta and Michael Berridge had located the active K+ transport mechanism in the apical plasma membrane of blowfly salivary glands (Gupta et al., 1978) where they also identified transport particles similar to those that they had first discovered in rectal papillae (Gupta and Berridge, 1966). Electrical studies by Küppers and Thurm located the key voltage step on the apical membrane of cells in the Dipteran sensory sensilla, which also contained similar particles (Küppers and Thurm, 1979). The K+ pump had been shown earlier to be independent of Na+ by tracer flux measurements on the isolated and short-circuited caterpillar midgut (Harvey and Nedergaard, 1964). A hint that it was localized in the goblet cell apical membrane (GCAM) was the presence of particles that Anderson and Harvey (Anderson and Harvey, 1966) recognized to be similar to the `elementary particles' on mitochondrial inner membranes and to the particles discovered by Gupta and Berridge (Gupta and Berridge, 1966). Electrical studies showed that the K+ pump is electrogenic and localized on the apical plasma membrane of the midgut epithelial cells (e.g. Dow, 1992; Moffett and Koch, 1988a; Moffett and Koch, 1988b; Wood et al., 1969). Intensive studies during the 1970s were guided by the hypothesis that the postulated K+-transport particles and their thermodynamic relationships are similar to those of the elementary particles on mitochondrial inner membranes, which led to the suggestion that they both be called portasomes (reviewed by Harvey, 1980; Harvey et al., 1981). Cioffi and Harvey (Cioffi and Harvey, 1981) showed that the portasome-containing goblet cell apical membranes in posterior midgut do not enclose mitochondria (which would contaminate prospective isolates); nevertheless, posterior midgut transports K+. Based on this information the K+-pump-containing goblet cell apical membrane (GCAM) was isolated by a novel assay based on ultrastructural features, mainly portasomes (Cioffi and Wolfersberger, 1983; Harvey et al., 1983). Dow et al. (Dow et al., 1984) confirmed that the K+-pump is on the GCAM by X-ray microanalysis (see Fig. 5).
Fig. 5.
Diagram of transverse section through the posterior midgut of fifth instar Manduca sexta larva showing two columnar cells enclosing a goblet cell [modified from Cioffi and Wolfersberger (Cioffi and Wolfersberger, 1983)]. pm, peritrophic membrane; CCAM, columnar cell apical membrane; LM, lateral membrane; GCAM, goblet cell apical membrane; BM, basal membrane; MV, microvilli; M, mitochondrion; SJ, septate junction; GC, goblet cavity; AMP, apical membrane projection; P, portasome (equivalent to V1 sector of H+ V-ATPase); BI, basal infolding; BL, basal lamina. The region in the small square is enlarged in C showing the CCAM with nutrient amino acid transporter (N) inserted into the membrane of a microvillus (equivalent to the BBM). The region in the large square is enlarged in B showing the GCAM with portasomes (V1 ATPase sectors) as round black dots with key thermodynamic parameters for the epithelium. Thermodynamic data for the electrical potential and chemical concentration differences (Dow and Peacock, 1989; Dow, 1992; Dow, 1984) were combined by Dow (Dow, 1992) into a revised view of pH and ion regulation in the caterpillar midgut that includes the H+ V-ATPase and K+/2H+ antiporter concept. Dow's model is combined with Cioffi's diagram of the ultrastructure of the anterior midgut epithelium (Cioffi and Wolfersberger, 1983) to describe the pathway by which K+ is translocated from the hemolymph to the goblet cell cytoplasm, then to the goblet cavity, and finally through the goblet valve to the lumen. The relevant point here is that the force which drives H+ from the goblet cavity back into the cell via the K+/2H+ antiporter is the 269 Δψ across the GCAM that was generated by the H+ V-ATPase. The antiport results in a [K+] of 190 mmol l–1 in the cavity compared with a [K+] of 130 mmol l–1 in the cell while the cavity pH is rendered slightly more alkaline than that of the cells (Chao et al., 1991). The sulfate groups projecting from the GCAM into the goblet cavity were deduced from X-ray microanalysis data (Dow et al., 1984). They provide strong anions so that the predominant ions in the cavity are 2K+ and SO42–. When K+ passes through the goblet valve into the lumen the predominant anion there is carbonate and the 2K+ CO32– accounts for the high lumen pH of 11. This route is difficult to envision in terms of Mitchell's protonmotive force, three-phase model but is predicted by the Kell and Harvey voltage coupled, five-phase model. Clearly it is the large membrane potential rather than the small pH difference (in the wrong direction) that is driving the K+/2H+ antiport across the GCAM.
H+ V-ATPase-K+/2H+ antiporter paradigm
The goblet cell apical membrane ATPase is a H+ V-ATPase
Although Cioffi, Wolfersberger and Harvey had isolated pure GCAM vesicles and knew that they contained the long sought K+ pump (Cioffi and Wolfersberger, 1983; Harvey et al., 1983), they were frustrated because two days work yielded sufficient enzyme for only two or three activity assays. The impasse was broken when Helmut Wieczorek appeared at their door; he had been trying to isolate the K+-ATPase from blowfly labella and had developed a micro assay that enabled one to do hundreds of assays on a tiny sample. Using Wieczorek's assay on the purified membranes the combined group determined that the ATPase activity was much higher in the isolated GCAM fraction than in the columnar cell apical membranes (a.k.a. microvilli, brush border membrane or BBM), lateral membranes or basal membranes (for locations see Fig. 5), moreover the GCAM ATPase was stimulated by K+ (Wieczorek et al., 1986). Abandoning sensory sensilla, Wieczorek and associates solubilized the caterpillar GCAM ATPase and made a paradigm-altering discovery – the GCAM ATPase is an H+ V-ATPase (Schweikl et al., 1989; Wieczorek et al., 1989). Starting with Gill and Ross (Gill and Ross, 1991) and continuing throughout the 1990s all of the caterpillar GCAM V-ATPase subunits have been cloned, localized and characterized (reviewed by Wieczorek et al., 2000) and attention has shifted to its structure (reviewed by Gruber et al., 2000), mechanism of action and regulation (reviewed by Beyenbach and Wieczorek, 2006; Wieczorek et al., 2009).
The insect K+-pump is an H+ V-ATPase-K+/2H+ antiporter hybrid
Had the focus on K+ rather than H+ led the field astray for two decades? The answer is no! K+ not H+ is the ion that is transported across the isolated midgut and accounts for all of the short-circuit current within experimental error (Cioffi and Harvey, 1981); moreover the output side is alkaline (pH 10–14) not acidic (Dow, 1984). Then, Wieczorek proposed the second paradigm-changing hypothesis – the H+ V-ATPase imposes a Δψ across the goblet cell apical membrane and the Δψ drives electrophoretic K+/nH+ antiport, explaining how K+, not H+, is transported (Wieczorek et al., 1991). Moffett and associates had pointed out earlier that the antiport must be electrophoretic (Chao et al., 1991) and Azuma et al. (Azuma et al., 1995) showed that, indeed, the antiport stoichiometry is one K+ for two H+.
The quest for the K+/2H+ antiporter
The quest for the insect K+ pump had taken nearly forty years, from the Ramsay `active K+ transport' concept in 1953 to the Wieczorek–Harvey `H+ V-ATPase-K+/2H+ antiporter' concept in 1991. Now a new quest began – to isolate the antiporter and determine its structure and properties. The new quest would be more difficult than the old one because, even though the antiporter is present in the same GCAM preparation that yielded the V-ATPase there is no equivalent of ATPase activity and portasomes to use as assay; antiporter activity must be measured in intact membrane vesicles (Wieczorek et al., 1991); moreover the turnover number of secondary transporters is an order of magnitude greater than that of primary pumps and their density is correspondingly lower. So membrane biochemistry was replaced by molecular biology. Wieczorek's brilliant group, especially Alexandra Lepier, and many other groups attempted for several years to clone the gene encoding the transporter. They were able to show that K+/2H+ antiport is insensitive to bafilomycin, a specific V-ATPase inhibitor, but is inhibited by amiloride or concanavalin A. Lepier et al. identified several glycosylated polypeptides in GCAM that are not subunits of the V-ATPase and thus would be candidates for the antiporter protein (Lepier et al., 1994). However, attempts to clone the gene encoding the antiporter by available techniques were increasingly frustrating and were largely abandoned (for a review see Grinstein and Wieczorek, 1994).
Genomes to the rescue
With the advent of the new millennium the Drosophila melanogaster genome was published (Adams et al., 2000) and a new strategy for cloning the antiporter emerged – the antiporter gene must be present in a genome and the trick is to find it. Two classes of membrane proteins, Na+/H+ exchangers (NHEs) and Na+/H+ antiporters (NHAs) were soon characterized. Metazoan NHEs use the inwardly directed Na+ gradient established by the Na+/K+ P-ATPase to drive Na+ into cells and expel metabolically produced H+ (Orlowski and Grinstein, 2004) whereas bacterial NHAs use the redox-generated voltage to drive H+ into cells and Na+ out, as discussed above. Nevertheless, nothing was known about genomic insect NHEs and NHAs so both types were candidates for the missing antiporter.
Within a year Giannakou and Dow (Giannakou and Dow, 2001) had identified three Na+/H+ exchanger (NHE) genes by cyber-screening, determined their positions relative to human and other genes in a phylogenetic tree, identified the genes in Southern blots, determined their primary sequences and amiloride binding regions of the encoded proteins, determined their transcription patterns by RT-PCR and unlatched the door to the antiporter's hiding place (Giannakou and Dow numbered the NHEs in order of their identification). Fluxes and fluid secretion in insect Malpighian tubules had been studied by electrophysiological methods (Beyenbach, 1995; Beyenbach et al., 2000) which served as a background for molecular cloning studies by Gill and associates that opened the door to the hiding place (reviewed by Pullikuth et al., 2003). Gill's group identified five genes and named them by their evolutionary relationships to characterized vertebrate counterparts. Later Brett et al. (Brett et al., 2005) placed the five exchangers in broad phylogenetic context and assigned new names; all three nomenclatures are listed in Table 1 for the reader's convenience.
Table 1.
Sodium-hydrogen exchanger nomenclature comparison
| Giannakou and Dow, 2001 | Pullikuth et al., 2003 | Brett et al., 2005 | CPA family |
|---|---|---|---|
| NHE2 | NHE8 | NHE1 | CPA1 |
| NHE1 | NHE3 | NHE2 | CPA1 |
| NHE3 | NHE6 | NHE3 | CPA1 |
| – | NHE10 | NHA1 | CPA2 |
| – | NHE9 | NHA2 | CPA2 |
AeNHE3 (Brett's NHE2) from Aedes aegypti was the first insect NHE to be cloned and its location identified in mosquitoes and characterized in yeast and fibroblasts (Pullikuth et al., 2006). The authors reported that AeNHE3 is present in basal membranes in almost all tissues of Ae. aegypti adults but they noted that splice variants might change the polarity of expression. They studied the relationship of AgNHE3 to V-ATPase and concluded that it is a basal, amiloride-insensitive mediator of transepithelial ion and fluid transport. Then Kang'ethe et al. (Kang'ethe et al., 2007) cloned and characterized AeNHE8 (Brett's NHE1) and reported that it mediates amiloride-sensitive exchange across Malpighian tubules. It is expressed in the apical membranes of Malpighian tubules, gastric caeca and rectum. They proposed that `AeNHE8 may be coupled to the inward H+ gradient across the Malpighian tubules and plays a role in the extrusion of excess sodium and potassium...'. However, Piermarini et al. were not able to confirm the apical localization in Malpighian tubules (Piermarini et al., 2009).
Mosquito NHEs are not electrophoretic plasma membrane proteins
In a detailed study of an NHE that was cloned from Ae. aegypti adult Malpighian tubules, Piermarini, Beyenbach and associates were able to work around the pitfalls of an endogenous conductance that is activated by xenic cRNA and showed that AeNHE8 is an endosomal transporter (Piermarini et al., 2009). Using quantitative PCR (qPCR) and immunohistochemistry they showed that AeNHE8 is widely distributed in adult mosquito tissues and not especially prominent in Malpighian tubules. That it is not a plasma membrane protein was determined by western blots of Malpighian tubules and confirmed by labeling with an affinity-purified antibody that is specific to AeNHE8. The intracellular transporter was located in the principal cells in the distal, secretory region of Malpighian tubules. The prospect that AeNHE8 is contained in vesicles that fuse with the plasma membrane under conditions of diuresis was ruled out by feeding mosquitoes a blood meal and application of dibutyryl-cAMP to isolated tubules, both of which stimulate Na+ excretion but did not alter the localization of the transporter. Efforts to characterize the exchanger that was expressed heterologously in Xenopus laevis oocytes by two-electrode voltage clamp techniques were frustrated by the activation of well known Na+ conductances (Nessler et al., 2004; Reifarth et al., 1999; Tzounopoulos et al., 1995). However, Piermarini et al. were able to analyze the transporter by measuring changes in pHi with pH-selective electrodes. The Na+/H+ exchange was inhibited by ethyl isopropyl amiloride (EIPA). Na+ could be replaced partially by Li+ but only poorly by K+.
Piermarini et al. provided a comprehensive review of insect NHEs and concluded that none of the three NHEs in the Ae. aegypti genome was a reasonable candidate for the K+/2H+ antiporter. They noted that although NHAs have not been studied in Aedes the data from Dow's group on Drosophila (discussed below) show that both DmNHA1 and DmNHA2 are present on the brush border of principal cells and data from Anopheles gambiae obtained by Harvey's group show that AgNHA1 is present in Malpighian tubules (Okech et al., 2008). Piermarini et al. note that `NHAs are the best candidates for apical cation/H+ exchangers in Malpighian tubules of Aedes'. They conclude that the potential of AgNHA1 for mediating K+/2H+ or Na+/2H+ antiport (Rheault et al., 2007) makes NHAs even more attractive because they could use the high apical-membrane voltage that is established by the H+ V-ATPase (Day et al., 2008).
Drosophila NHAs are plasma membrane proteins
All three NHE genes are expressed in Drosophila melanogaster Malpighian tubules (Giannakou and Dow, 2001). However, none of them appear to be expressed near V-ATPases in plasma membranes, as revealed by a search of the FlyAtlas expression resource (Chintapalli et al., 2007). From their lack of success in identifying any of the NHEs at apical plasma membranes in Drosophila, Day et al. concluded the transporters most probably function in endosomes (Day et al., 2008). However, both of the two NHA genes are expressed in Drosophila (CG10806/Nha1) and (CG31052/Nha2) in the same CPA2 (NHA) family as bacterial electrophoretic antiporters (Brett et al., 2005). Using immunocytochemistry and over-expression of GFP-tagged NHA both DmNHA1 and DmNHA1 were found to often be present in the same membrane as V-ATPases (Day et al., 2008). These results prompted the authors to title their paper `Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa'. This pattern of association between NHAs and H+ V-ATPases is similar to that reported earlier for An gambiae (Okech et al., 2008; Rheault et al., 2007; Smith et al., 2008) and supports the notion that the voltage from electrogenic H+ V-ATPases drives cation exchange by electrophoretic NHA.
If NHEs are not located in plasma membranes then how are metabolic acids expelled from the cells. Since H+ V-ATPases are present and nutrient amino acid transporters (NATs) are very likely to be present in these cells, it has been proposed that in mosquito larval midgut V-ATPases transporting H+ outwardly across the same membrane in which Na+- or K+-coupled NATs are transporting Na+ inwardly, constitute NHEs; they have been called NHEVNATs (Harvey et al., 2009) and may be functional replacements for the missing NHEs.
Assuming that the H+ V-ATPase–K+/2H+ antiporter hypothesis is correct, the next question is – how does it work? We assume that the couple moves K+ into the goblet cell cavity where the H+ concentration is only 10–7.23 mol l–1 (Chao et al., 1991) but the K+ concentration is >10–1 mol l–1 (Dow et al., 1984) (Fig. 5). What is the source of the H+s that are driven from cavity to cell? The most obvious hypothesis is that the large >240 mV membrane potential (Dow and Peacock, 1989) across the GCAM is equivalent to a 10,000-fold concentration difference of a monovalent cation and can drive the electrophoretic antiport without regard to the concentrations of H+ and K+. But the membrane potential would also drive K+ toward the cells, placing the entire burden for H+ re-entry on the affinity of H+ for its binding site on the antiporter being much greater than the affinity of K+ for its site. An alternative hypothesis is that the GCAM is like ATP synthesizing membranes (Kell, 1979; Cherepanov et al., 2004; Mulkidjanian and Cherepanov, 2006) and the H+ concentration in the unstirred layer adjacent to the membrane lining the cavity is much higher than that in the bulk fluid.
ΔΨs drive K+- or Na+-amino acid symport without H+
As discussed above, ATP synthesis via the F1Fo ATPase in mitochondria, chloroplasts or bacteria as well as Na+/2H+ antiport by bacteria and (tentatively) insect plasma membranes are all driven by H+-coupled mechanisms. Thus, H+ is the ion that the electron transport system and the H+ V-ATPases charge the membranes with, and H+ is the ion that the synthases and antiporters translocate. But there are five well documented cases in which the voltage from a H+ V-ATPase drives K+- or Na+-coupled amino acid uptake and H+ is not involved in the secondary transport at all. Those cases are K+/amino acid symport by KAAT1 (K+ amino acid transporter 1) (Castagna et al., 1998) and CAATCH1 (cation amino acid transporter channel) (Feldman et al., 2000) from caterpillars and Na+/amino acid symport by AeAAT1i (Ae. aegypti amino acid transporter 1) (Boudko et al., 2005a), AgNAT6 (An. gambiae nutrient amino acid transporter 6) (Meleshkevitch et al., 2009) and AgNAT8 (Meleshkevitch et al., 2006) from mosquito larvae. Details have been reviewed recently (Harvey et al., 2009) and only a brief summary is presented here.
Caterpillars grow more than 1,000-fold in less than a month on a leafy diet that is rich in K+ and poor in Na+; they use amino acids both as substrates for protein synthesis and for energy. K+ rather than Na+ is the coupling cation but K+ gradients are insufficient to drive the symport (Dow et al., 1984) whereas large voltage gradients are present (Dow and Peacock, 1989) (Fig. 5). Amino acid uptake by isolated caterpillar midgut is K+ dependent and voltage driven (Nedergård, 1972). Electrophoretic K+-coupled amino acid transport across the apical plasma membrane of wild silkworm larval posterior midgut was demonstrated in brush border membrane vesicle studies by Giordana, Sacchi, Parenti and associates (Giordana et al., 1989; Hanozet et al., 1980). Much of their work was confirmed in studies on Manduca sexta by Wolfersberger, Harvey and associates (e.g. Hennigan et al., 1993a; Hennigan et al., 1993b). The uptake is clearly driven by the voltage generated by the H+ V-ATPase in adjacent goblet cells. The Italian and American groups, joined by Matthias Hediger, cloned KAAT1 (Castagna et al., 1998). Soon after CAATCH1, a second cation-coupled amino acid transporter was also cloned from caterpillar midgut (Feldman et al., 2000).
Mosquito larvae, unlike leaf-eating caterpillars, can live on highly varied diets found in habitats ranging from alkaline salt marshes to alkali-ion-dilute fresh water. The pH of their alimentary canal ranges from near neutrality in the foregut to >10.5 in anterior midgut and back to near neutrality in posterior midgut in the absence of diffusion barriers (Clements, 1992; Dadd, 1975; Ramsay, 1950). Fresh water mosquitoes take up Na+, use it for amino acid symport in the midgut and conserve it by reabsorption in the Malpighian tubules and hindgut (Clements, 1992; Ramsay, 1953b; Smith et al., 2008). A mosquito amino acid/Na+ symporter, AeAAT1i, that has high sequence identify with caterpillar KAAT1 and CAATCH1, was cloned from Ae. aegypti larvae (Boudko et al., 2005a). More recently AgNAT8 (Meleshkevitch et al., 2006) and AgNAT6 (Meleshkevitch et al., 2009) were cloned from An. gambiae larvae.
A total of more than a dozen Na+-coupled amino acid transporters have been cloned by Gill's group (Umesh et al., 2003), Boudko and Harvey's group and others (reviewed by Boudko et al., 2005b). When expressed in Xenopus oocytes, the five NATs from mosquito larvae exhibited characteristic profiles for uptake of the 20 structural amino acids. Of most concern here, the application of amino acids induced large, amino-acid-specific, inward Na+ currents. Evidently the non-specific endogenous Na+ or K+ currents of oocytes were not an overwhelming problem because the amplitude of the amino acid-induced currents depended upon the specific amino acid, the pH of the bathing solution and the transmembrane voltage; thus, all of the cloned NATs appear to be electrophoretic transporters in which K+- or Na+-coupled amino acid uptake is driven by the voltage generated by H+ V-ATPases that are invariably present in the apical plasma membranes in mosquito posterior midgut cells.
H+ V-ATPase activity interpreted by Kell's electrodic model
The ΔpH in Mitchell's three-phase chemiosmotic theory (Mitchell, 1961; Mitchell, 1991) refers to the difference in H+ concentration between the outside bulk fluid (L) and the inside bulk fluid (R) (Kell, 1979). Primary ATP synthesis and secondary Na+/2H+ antiport in alkalophilic bacteria and K+ or Na+ antiport in larval insect midguts are hard to interpret by this three-phase model. By contrast, the ATP synthesis and cation exchange are easily interpreted by Kell's five-phase electrodic model (Kell, 1979; Kell, 1992). Similarly, V-ATPase energization of insect midguts is hard to interpret in terms of pH in the bulk fluid of cells and lumen but easy to interpret by the five-phase model (Figs 3 and 4). We postulate that the plasma membrane H+ V-ATPase translocates H+ from the cytoplasmic fluid (R) across the dielectric lipid bilayer (M) and charges the transmembrane capacitance (SL versus SR) creating a membrane potential, Δψ, with the outside of the cells positive to the inside. The H+ is held within the outside fluid membrane interface (Fig. 3, SL) by electrostatic attraction to the negative gegenion that is held within the inside interface phase (SR), but can exchange with any cation in the outside bulk fluid phase (L) and acidify it to an extent limited by the capacitance. The Δψ can drive an anion outwardly via a transporter or channel and drive H+ back inwardly via a transporter such as (tentatively) AgNHA1 (A in Figs 3 and 4) or channel in a steady-state flow while the charge separation is maintained. The Δψ can also drive a cation-coupled amino acid transport, e.g. via AgNAT8 (N in Fig. 4) into the cells. This coupling corresponds to Kell's `electrodic' coupling and might be called `voltage coupling'. Voltage coupling across the membrane's lipid bilayer appears to explain the H+ V-ATPase coupling process much more clearly than Mitchell's `protonmotive force' between two bulk phases.
Complexity of membrane energization and energy utilization
In these early days of the post-genomic era discrepancies are to be expected. Thus, the analyses of AeNHE8 by Gill's group and Beyenbach's group both used technically sound techniques but led to very different conclusions. Recall that Gill's group concluded that AeNHE8 (Brett's AeNHE1) is located in apical membranes of Malpighian tubules, gastric caeca and rectum whereas Beyenbach's group concluded that none of the three mosquito NHEs are located in plasma membranes but play roles in endosomes instead. But Gill's people believe that NHE8 is also localized in the apical membrane, in addition to being present in endosomes. This discrepancy may be due to detection of processed and unprocessed forms of NHE3. Similarly, Dow's group concluded that Drosophila H+ V-ATPase is located in apical membranes (Day et al., 2008), a conclusion supported by Tripathi and associates who provided direct electrophysiological evidence for V-ATPase-generated fluxes of H+ toward the basal membranes (Shanbhag and Tripathi, 2009). Again, new evidence from Onken et al. (Onken et al., 2009) shows that the apical region of the cytoplasm in epithelial cells of anterior midgut of mosquitoes has a pH as high as 8, which will lead to a re-evaluation of models of pH regulation in mosquito alimentary canal.
With thousands of genes in the insect genomes and discrepancies in reported results from identical mosquitoes it is increasingly clear that the analysis of Na+ and K+/H+ antiport (exchange) has just begun. Circuit diagrams for ion and pH regulation systems of epithelia will increasingly resemble those of modern color television sets whereas our current diagrams resemble those of crystal radio sets. In an initial attempt to deal with this complexity, explicit parameters in the 1992 version of the voltage coupling model (Harvey, 1992) were incorporated into circuit diagrams that enabled semi-quantitative computer simulations of ion and pH regulation as well as amino acid uptake in the caterpillar midgut (Martin and Harvey, 1994). Hopefully, the wealth of new experimental data along with the new insight provided by Kell's five-phase electrodic model will enable circuit diagrams to become ever more sophisticated and realistic.
This work was supported in part by research grants AI-52436 and AI-30464 from NIH and by funds from the Whitney Laboratory for Marine Bioscience, the Emerging Pathogens Institute and the Department of Epidemiology and Biostatistics, University of Florida. I thank my colleagues of the past half century who have contributed to the work reviewed here, especially Julian A. T. Dow for discussions on NHEs and NHAs in Drosophila. I thank the two JEB referees, Bernard A. Okech, Clifford L. Slayman, Francis G. Martin and Peter A. V. Anderson for many sharp criticisms and helpful discussions. Finally, I thank M. Lynn Milstead for much iteration of the illustrations. Deposited in PMC for release after 12 months.
References
- Adams, M. D., Celniker, S. E., Holt, R. A., Evans, C. A., Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P. W., Hoskins, R. A., Galle, R. F. et al. (2000). The genome sequence of Drosophila melanogaster. Science 287, 2185-2195. [DOI] [PubMed] [Google Scholar]
- Anderson, E. and Harvey, W. R. (1966). Active transport by the Cecropia midgut. II. Fine structure of the midgut epithelium. J. Cell Biol. 31, 107-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma, M., Harvey, W. R. and Wieczorek, H. (1995). Stoichiometry of K+/H+ antiport helps to explain extracellular pH 11 in a model epithelium. FEBS Lett. 361, 153-156. [DOI] [PubMed] [Google Scholar]
- Beyenbach, K. W. (1995). Mechanisms and regulation of electrolyte transport in Malpighian tubules. J. Insect Physiol. 41, 197-207. [Google Scholar]
- Beyenbach, K. W. and Wieczorek, H. (2006). The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209, 577-589. [DOI] [PubMed] [Google Scholar]
- Beyenbach, K. W., Pannabecker, T. L. and Nagel, W. (2000). Central role of the apical membrane H+-ATPase in electrogenesis and epithelial transport in Malpighian tubules. J. Exp. Biol. 203, 1459-1468. [DOI] [PubMed] [Google Scholar]
- Boudko, D. Y., Moroz, L. L., Linser, P. J., Trimarchi, J. R., Smith, P. J. S. and Harvey, W. R. (2001). In situ analysis of pH gradients in mosquito larvae using noninvasive, self-referencing, pH-sensitive microelectrodes. J. Exp. Biol. 204, 691-699. [DOI] [PubMed] [Google Scholar]
- Boudko, D. Y., Kohn, A. B., Meleshkevitch, E. A., Dasher, M. K., Seron, T. J., Stevens, B. R. and Harvey, W. R. (2005a). Ancestry and progeny of nutrient amino acid transporters. Proc. Natl. Acad. Sci. USA 102, 1360-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudko, D. Y., Stevens, B. R., Donly, B. C. and Harvey, W. R. (2005b). Nutrient amino acid and neurotransmitter transporters. In Comprehensive Molecular Insect Science, vol. 4 (ed. K. Latrou, L. Gilbert and S. Gill), pp. 255-309. Amsterdam: Elsevier. [Google Scholar]
- Brett, C. L., Donowitz, M. and Rao, R. (2005). Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. Cell Physiol. 288, C223-C239. [DOI] [PubMed] [Google Scholar]
- Castagna, M., Shayakul, C., Trotti, D., Sacchi, V. F., Harvey, W. R. and Hediger, M. A. (1998). Cloning and characterization of a potassium-coupled amino acid transporter. Proc. Natl. Acad. Sci. USA 95, 5395-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, A. C., Moffett, D. F. and Koch, A. (1991). Cytoplasmic pH and goblet cavity pH in the posterior midgut of the tobacco hornworm Manduca sexta. J. Exp. Biol. 155, 403-414. [Google Scholar]
- Cherepanov, D. A., Feniouk, B. A., Junge, W. and Mulkidjanian, A. Y. (2003). Low dielectric permittivity of water at the membrane interface: effect on the energy coupling mechanism in biological membranes. Biophys. J. 85, 1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov, D. A., Junge, W. and Mulkidjanian, A. Y. (2004). Proton transfer dynamics at the membrane/water interface: dependence on the fixed and mobile pH buffers, on the size and form of membrane particles, and on the interfacial potential barrier. Biophys. J. 86, 665-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli, V. R., Wang, J. and Dow, J. A. (2007). Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, 715-720. [DOI] [PubMed] [Google Scholar]
- Cidon, S. and Nelson, N. (1982). Properties of a novel ATPase enzyme in chromaffin granules. J. Bioenerg. Biomembr. 14, 499-512. [DOI] [PubMed] [Google Scholar]
- Cidon, S. and Nelson, N. (1986). Purification of N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J. Biol. Chem. 261, 9222-9227. [PubMed] [Google Scholar]
- Cioffi, M. and Harvey, W. R. (1981). Comparison of potassium transport in three structurally distinct regions of the insect midgut. J. Exp. Biol. 91, 103-116. [Google Scholar]
- Cioffi, M. and Wolfersberger, M. G. (1983). Isolation of separate apical, lateral and basal plasma membrane from cells of an insect epithelium: a procedure based on tissue organization and ultrastructure. Tissue Cell 15, 781-803. [DOI] [PubMed] [Google Scholar]
- Clements, A. N. (1992). The Biology of Mosquitoes. London: Chapman and Hall.
- Dadd, R. H. (1975). Alkalinity within the midgut of mosquito larvae with alkaline-active digestive enzymes. J. Insect Physiol. 21, 1847-1853. [DOI] [PubMed] [Google Scholar]
- Day, J. P., Wan, S., Allan, A. K., Kean, L., Davies, S. A., Gray, J. V. and Dow, J. A. (2008). Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J. Cell Sci. 121, 2612-2619. [DOI] [PubMed] [Google Scholar]
- Dow, J. A. T. (1984). Extremely high pH in biological systems: a model for carbonate transport. Am. J. Physiol. 246, R633-R636. [DOI] [PubMed] [Google Scholar]
- Dow, J. A. T. (1992). pH gradients in lepidopteran midgut. J. Exp. Biol. 172, 355-375. [DOI] [PubMed] [Google Scholar]
- Dow, J. A. T. and Peacock, J. M. (1989). Microelectrode evidence for the electrical isolation of goblet cell cavities in Manduca sexta middle midgut. J. Exp. Biol. 143, 101-114. [Google Scholar]
- Dow, J. A. T., Gupta, B. L., Hall, T. A. and Harvey, W. R. (1984). X-ray microanalysis of elements in frozen-hydrated sections of an electrogenic K+ transport system: the posterior midgut of tobacco hornworm (Manduca sexta) in vivo and in vitro. J. Membr. Biol. 77, 223-241. [DOI] [PubMed] [Google Scholar]
- Feldman, D. H., Harvey, W. R. and Stevens, B. R. (2000). A novel electrogenic amino acid transporter is activated by K+ or Na+, is alkaline pH-dependent, and is Cl–-Independent. J. Biol. Chem. 275, 24518-24526. [DOI] [PubMed] [Google Scholar]
- Giannakou, M. E. and Dow, J. A. (2001). Characterization of the Drosophila melanogaster alkali-metal/proton exchanger (NHE) gene family. J. Exp. Biol. 204, 3703-3716. [DOI] [PubMed] [Google Scholar]
- Gill, S. S. and Ross, L. S. (1991). Molecular cloning and characterization of the B-subunit of a vacuolar H+-ATPase from the midgut and Malpighian tubules of Helicoverpa-virescens. Arch. Biochem. Biophys. 291, 92-99. [DOI] [PubMed] [Google Scholar]
- Giordana, B., Sacchi, V. F., Parenti, P. and Hanozet, G. M. (1989). Amino acid transport systems in intestinal brush-border membranes from lepidopteran larvae. Am. J. Physiol. 257, R494-R500. [DOI] [PubMed] [Google Scholar]
- Grinstein, S. and Wieczorek, H. (1994). Cation antiports of animal plasma membranes. J. Exp. Biol. 196, 307-318. [DOI] [PubMed] [Google Scholar]
- Gruber, G., Radermacher, M., Ruiz, T., Godovac-Zimmermann, J., Canas, B., Kleine-Kohlbrecher, D., Huss, M., Harvey, W. R. and Wieczorek, H. (2000). Three-dimensional structure and subunit topology of the V(1) ATPase from Manduca sexta midgut. Biochemistry 39, 8609-8616. [DOI] [PubMed] [Google Scholar]
- Gupta, B. L. and Berridge, M. J. (1966). A coat of repeating subunits on the cytoplasmic surface of the plasma membrane in the rectal papillae of the blowfly, Calliphora erythrocephala (Meig.), studied in situ by electron microscopy. J. Cell Biol. 29, 376-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, B. L., Berridge, M. J., Hall, T. A. and Moreton, R. B. (1978). Electron microprobe and ion-selective microelectrode studies of fluid secretion in the salivary glands of Calliphora. J. Exp. Biol. 72, 261-284. [DOI] [PubMed] [Google Scholar]
- Hanozet, G. M., Giordana, B. and Sacchi, V. F. (1980). K+-dependent phenylalanine uptake in membrane vesicles isolated from the midgut of Philosamia cynthia larvae. Biochim. Biophys. Acta 596, 481-486. [DOI] [PubMed] [Google Scholar]
- Harold, F. (1986). The Vital Force: A Study of Bioenergetics. New York: Freeman.
- Harvey, W. R. (1980). Water and ions in the gut. In Insect Biology in the Future (ed. M. Locke and D. S. Smith). New York: Academic Press.
- Harvey, W. R. (1992). Physiology of V-ATPases. J. Exp. Biol. 172, 1-17. [PubMed] [Google Scholar]
- Harvey, W. R. and Nedergaard, S. (1964). Sodium-independent active transport of potassium in the isolated midgut of Cecropia silkworm. Proc. Natl. Acad. Sci. USA 51, 757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, W. R. and Wieczorek, H. (1997). Animal plasma membrane energization by chemiosmotic H+ V-ATPases. J. Exp. Biol. 200, 203-216. [DOI] [PubMed] [Google Scholar]
- Harvey, W. R., Cioffi, M. and Wolfersberger, M. G. (1981). Portasomes as coupling factors in active ion transport and oxidative phosphorylation. Am. Zool. 21, 775-791. [Google Scholar]
- Harvey, W. R., Cioffi, M., Dow, J. A. and Wolfersberger, M. G. (1983). Potassium ion transport ATPase in insect epithelia. J. Exp. Biol. 106, 91-117. [DOI] [PubMed] [Google Scholar]
- Harvey, W. R., Boudko, D. Y., Rheault, M. R. and Okech, B. A. (2009). NHEVNAT: an H+ V-ATPase electrically coupled to a Na+:nutrient amino acid transporter (NAT) forms an Na+/H+ exchanger (NHE). J. Exp. Biol. 212, 347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan, B. B., Wolfersberger, M. G. and Harvey, W. R. (1993a). Neutral amino acid symport in larval Manduca sexta midgut brush-border membrane vesicles deduced from cation-dependent uptake of leucine, alanine, and phenylalanine. Biochim. Biophys. Acta 1148, 216-222. [DOI] [PubMed] [Google Scholar]
- Hennigan, B. B., Wolfersberger, M. G., Parthasarathy, R. and Harvey, W. R. (1993b). Cation-dependent leucine, alanine, and phenylalanine uptake at pH 10 in brush-border membrane vesicles from larval Manduca sexta midgut. Biochim. Biophys. Acta 1148, 209-215. [DOI] [PubMed] [Google Scholar]
- Hunte, C., Screpanti, E., Venturi, M., Rimon, A., Padan, E. and Michel, H. (2005). Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435, 1197-1202. [DOI] [PubMed] [Google Scholar]
- Kang'ethe, W., Aimanova, K. G., Pullikuth, A. K. and Gill, S. S. (2007). NHE8 mediates amiloride-sensitive Na+/H+ exchange across mosquito Malpighian tubules and catalyzes Na+ and K+ transport in reconstituted proteoliposomes. Am. J. Physiol. Renal Physiol. 292, F1501-F1512. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. (1979). On the functional proton current pathway of electron transport phosphorylation: an electrodic view. Biochim. Biophys. Acta 549, 55-99. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. (1992). The protonmotive force as an intermediate in electron transport-linked phosphorylation: problems and prospects. Curr. Top. Cell. Regul. 33, 279-289. [DOI] [PubMed] [Google Scholar]
- Krulwich, T. A. and Guffanti, A. A. (1989). The Na+ cycle of extreme alkalophiles: a secondary Na+/H+ antiporter and Na+/solute symporters. J. Bioenerg. Biomembr. 21, 663-677. [DOI] [PubMed] [Google Scholar]
- Krulwich, T. A., Ito, M., Hicks, D. B., Gilmour, R. and Guffanti, A. A. (1998). pH homeostasis and ATP synthesis: studies of two processes that necessitate inward proton translocation in extremely alkaliphilic Bacillus species. Extremophiles 2, 217-222. [DOI] [PubMed] [Google Scholar]
- Küppers, J. and Thurm, U. (1979). Active ion transport by a sensory epithelium. I. Transepithelial short circuit current, potential difference, and their dependence on metabolism. J. Comp. Phyisol. 134, 131-136. [Google Scholar]
- Lepier, A., Azuma, M., Harvey, W. R. and Wieczorek, H. (1994). K+/H+ antiport in the tobacco hornworm midgut: the K(+)-transporting component of the K+ pump. J. Exp. Biol. 196, 361-373. [DOI] [PubMed] [Google Scholar]
- Maddrell, S. H. P. (1981). The functional design of the insect excretory system. J. Exp. Biol. 90, 1-15. [Google Scholar]
- Maddrell, S. H. and O'Donnell, M. J. (1992). Insect malpighian tubules: V-ATPase action in ion and fluid transport. J. Exp. Biol. 172, 417-429. [DOI] [PubMed] [Google Scholar]
- Mandel, L. J., Moffett, D. F. and Jöbsis, F. F. (1975). Redox state of respiratory chain enzymes and potassium transport in silkworm mid-gut. Biochim. Biophys. Acta 408, 123-134. [DOI] [PubMed] [Google Scholar]
- Martin, F. G. and Harvey, W. R. (1994). Ionic circuit analysis of K+/H+ antiport and amino acid/K+ symport energized by a proton-motive force in Manduca sexta larval midgut vesicles. J. Exp. Biol. 196, 77-92. [DOI] [PubMed] [Google Scholar]
- Meleshkevitch, E. A., Assis-Nascimento, P., Popova, L. B., Miller, M. M., Kohn, A. B., Phung, E. N., Mandal, A., Harvey, W. R. and Boudko, D. Y. (2006). Molecular characterization of the first aromatic nutrient transporter from the sodium neurotransmitter symporter family. J. Exp. Biol. 209, 3183-3198. [DOI] [PubMed] [Google Scholar]
- Meleshkevitch, E. A., Robinson, M., Popova, L. B., Miller, M. M., Harvey, W. R. and Boudko, D. Y. (2009). Cloning and functional expression of the first eukaryotic Na+-tryptophan symporter, AgNAT6. J. Exp. Biol. 212, 1559-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, P. (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144-148. [DOI] [PubMed] [Google Scholar]
- Mitchell, P. (1991). Foundations of vectorial metabolism and osmochemistry. Biosci. Rep. 11, 297-344; discussion 345-346. [DOI] [PubMed] [Google Scholar]
- Moffett, D. F. and Koch, A. R. (1988a). Electrophysiology of K+ transport by midgut epithelium of lepidopteran insect larvae. I. The transbasal electrochemical gradient. J. Exp. Biol. 135, 25-38. [Google Scholar]
- Moffett, D. F. and Koch, A. R. (1988b). Electrophysiology of K+ transport by midgut epithelium of lepidopteran insect larvae. II. The transapical electrochemical gradients. J. Exp. Biol. 135, 39-49. [Google Scholar]
- Mulkidjanian, A. Y. and Cherepanov, D. A. (2006). Probing biological interfaces by tracing proton passage across them. Photochem. Photobiol. Sci. 5, 577-587. [DOI] [PubMed] [Google Scholar]
- Mulkidjanian, A. Y., Cherepanov, D. A., Heberle, J. and Junge, W. (2005). Proton transfer dynamics at membrane/water interface and mechanism of biological energy conversion. Biochemistry (Mosc.) 70, 251-256. [DOI] [PubMed] [Google Scholar]
- Nedergaard, S. (1972). Active transport of a-aminoisobutyric acid by the isolated midgut of Hyalophora cecropia. J. Exp. Biol. 56, 167-172. [Google Scholar]
- Nelson, N. (1987). The vacuolar proton-ATPase of eukaryotic cells. BioEssays 7, 251-254. [DOI] [PubMed] [Google Scholar]
- Nelson, N. and Harvey, W. R. (1999). Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol. Rev. 79, 361-385. [DOI] [PubMed] [Google Scholar]
- Nessler, S., Friedrich, O., Bakouh, N., Fink, R. H., Sanchez, C. P., Planelles, G. and Lanzer, M. (2004). Evidence for activation of endogenous transporters in Xenopus laevis oocytes expressing the Plasmodium falciparum chloroquine resistance transporter, PfCRT. J. Biol. Chem. 279, 39438-39446. [DOI] [PubMed] [Google Scholar]
- Okech, B. A., Boudko, D. Y., Linser, P. J. and Harvey, W. R. (2008). Cationic pathway of pH regulation in larvae of Anopheles gambiae. J. Exp. Biol. 211, 957-968. [DOI] [PubMed] [Google Scholar]
- Onken, H. and Moffett, D. F. (2009). Revisiting the cellular mechanisms of strong luminal alkalinization in the anterior midgut of larval mosquitoes. J. Exp. Biol. 212, 373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski, J. and Grinstein, S. (2004). Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 447, 549-565. [DOI] [PubMed] [Google Scholar]
- Padan, E., Bibi, E., Ito, M. and Krulwich, T. A. (2005). Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717, 67-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan, E., Kozachkov, L., Herz, K. and Rimon, A. (2009). NhaA crystal structure: functional–structural insights. J. Exp. Biol. 212 1593-1603. [DOI] [PubMed] [Google Scholar]
- Piermarini, P. M., Weihrauch, D., Meyer, H., Huss, M. and Beyenbach, K. W. (2009). NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow-fever mosquito Aedes aegypti. Am. J. Physiol. Renal Physiol. 296, F730-F750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullikuth, A. K., Filippov, V. and Gill, S. S. (2003). Phylogeny and cloning of ion transporters in mosquitoes. J. Exp. Biol. 206, 3857-3868. [DOI] [PubMed] [Google Scholar]
- Pullikuth, A. K., Aimanova, K., Kang'ethe, W., Sanders, H. R. and Gill, S. S. (2006). Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J. Exp. Biol. 209, 3529-3544. [DOI] [PubMed] [Google Scholar]
- Ramsay, J. A. (1950). Osmotic regulation in mosquito larvae. J. Exp. Biol. 27, 145-157. [DOI] [PubMed] [Google Scholar]
- Ramsay, J. A. (1953a). Active transport of potassium by the malpighian tubules of insects. J. Exp. Biol. 30, 358-369. [Google Scholar]
- Ramsay, J. A. (1953b). Exchanges of sodium and potassium in mosquito larvae. J. Exp. Biol. 30, 79-89. [Google Scholar]
- Reifarth, F. W., Clauss, W. and Weber, W. M. (1999). Stretch-independent activation of the mechanosensitive cation channel in oocytes of Xenopus laevis. Biochim. Biophys. Acta 1417, 63-76. [DOI] [PubMed] [Google Scholar]
- Rheault, M. R., Okech, B. A., Keen, S. B., Miller, M. M., Meleshkevitch, E. A., Linser, P. J., Boudko, D. Y. and Harvey, W. R. (2007). Molecular cloning, phylogeny and localization of AgNHA1: the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J. Exp. Biol. 210, 3848-3861. [DOI] [PubMed] [Google Scholar]
- Schweikl, H., Klein, U., Schindlbeck, M. and Wieczorek, H. (1989). A vacuolar-type ATPase, partially purified from potassium transporting plasma membranes of tobacco hornworm midgut. J. Biol. Chem. 264, 11136-11142. [PubMed] [Google Scholar]
- Shanbhag, S. and Tripathi, S. (2009). Review. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosphilia midgut. J. Exp. Biol. 212, 1731-1744. [DOI] [PubMed] [Google Scholar]
- Smith, K. E., Vanekeris, L. A., Okech, B. A., Harvey, W. R. and Linser, P. J. (2008). Larval anopheline mosquito recta exhibit a dramatic change in localization patterns of ion transport proteins in response to shifting salinity: a comparison between anopheline and culicine larvae. J. Exp. Biol. 211, 3067-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglicht, D., Padan, E. and Schuldiner, S. (1993). Proton-sodium stoichiometry of NhaA, an electrogenic antiporter from Escherichia coli. J. Biol. Chem. 268, 5382-5387. [PubMed] [Google Scholar]
- Tzounopoulos, T., Maylie, J. and Adelman, J. P. (1995). Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys. J. 69, 904-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, E., Ohsumi, Y. and Anraku, Y. (1985). Purification and properties of H+-translocating Mg2+-adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J. Biol. Chem. 260, 1090-1095. [PubMed] [Google Scholar]
- Umesh, A., Cohen, B. N., Ross, L. S. and Gill, S. S. (2003). Functional characterization of a glutamate/aspartate transporter from the mosquito Aedes aegypti. J. Exp. Biol. 206, 2241-2255. [DOI] [PubMed] [Google Scholar]
- Wieczorek, H., Wolfersberger, M. G., Cioffi, M. and Harvey, W. R. (1986). Cation-stimulated ATPase activity in purified plasma membranes from tobacco hornworm midgut. Biochim. Biophys. Acta 857, 271-281. [DOI] [PubMed] [Google Scholar]
- Wieczorek, H., Weerth, S., Schindlbeck, M. and Klein, U. (1989). A vacuolar-type proton pump in a vesicle fraction enriched with potassium transporting plasma membranes from tobacco hornworm midgut. J. Biol. Chem. 264, 11143-11148. [PubMed] [Google Scholar]
- Wieczorek, H., Putzenlechner, M., Zeiske, W. and Klein, U. (1991). A vacuolar-type proton pump energizes K+/H+ antiport in an animal plasma membrane. J. Biol. Chem. 266, 15340-15347. [PubMed] [Google Scholar]
- Wieczorek, H., Brown, D., Grinstein, S., Ehrenfeld, J. and Harvey, W. R. (1999). Animal plasma membrane energization by proton-motive V-ATPases. BioEssays 21, 637-648. [DOI] [PubMed] [Google Scholar]
- Wieczorek, H., Grber, G., Harvey, W. R., Huss, M., Merzendorfer, H. and Zeiske, W. (2000). Structure and regulation of insect plasma membrane H(+)V-ATPase. J. Exp. Biol. 203, 127-135. [DOI] [PubMed] [Google Scholar]
- Wieczorek, H., Beyenbach, K. W., Huss, M. and Vitavska, O. (2009). Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 212, 1611-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, R. J. P. (1962). Possible functions of chains of catalysts. J. Theor. Biol. 3, 209-229. [DOI] [PubMed] [Google Scholar]
- Williams, R. J. (1978). The multifarious couplings of energy transduction. Biochim. Biophys. Acta 505, 1-44. [DOI] [PubMed] [Google Scholar]
- Wood, J. L., Farrand, P. S. and Harvey, W. R. (1969). Active transport of potassium by the cecropia midgut. VI. Microelectrode potential profile. J. Exp. Biol. 50, 169-178. [DOI] [PubMed] [Google Scholar]