Summary
In the epididymis, spermatozoa acquire their ability to become motile and to fertilize an egg. A luminal acidic pH and a low bicarbonate concentration help keep spermatozoa in a quiescent state during their maturation and storage in this organ. Net proton secretion is crucial to maintain the acidity of the luminal fluid in the epididymis. A sub-population of epithelial cells, the clear cells, express high levels of the proton-pumping V-ATPase in their apical membrane and are important contributors to luminal acidification. This review describes selected aspects of V-ATPase regulation in clear cells. The assembly of a particular set of V-ATPase subunit isoforms governs the targeting of the pump to the apical plasma membrane. Regulation of V-ATPase-dependent proton secretion occurs via recycling mechanisms. The bicarbonate-activated adenylyl cyclase is involved in the non-hormonal regulation of V-ATPase recycling, following activation of bicarbonate secretion by principal cells. The V-ATPase is also regulated in a paracrine manner by luminal angiotensin II by activation of the angiotensin II type 2 receptor (AGTR2), which is located in basal cells. Basal cells have the remarkable property of extending long and slender cytoplasmic projections that cross the tight junction barrier to monitor the luminal environment. Clear cells are activated by a nitric oxide signal that originates from basal cells. Thus, a complex interplay between the different cell types present in the epithelium leads to activation of the luminal acidifying capacity of the epididymis, a process that is crucial for sperm maturation and storage.
Keywords: H+-ATPase, pseudostratified epithelia, basal cells, clear cells
Introduction
The establishment of male fertility is a complex process that requires concerted interactions between different tissues of the male reproductive tract and accessory glands, and between the different cell types that compose these organs. These include the production of a large number of spermatozoa by the testis, followed by several maturation steps that occur along the male excurrent duct. Morphologically and functionally distinct tissues are present in the male reproductive tract and include the testis, efferent ducts, epididymis and vas deferens. Spermatozoa produced by the testis are immature, and they cannot find and fertilize an egg. They acquire their motility and fertilizing capacity during their passage through the lumen of the epididymis, which is composed of one single convoluted tubule (Hinton and Palladino, 1995; Jones and Murdoch, 1996; Orgebin-Crist, 2003; Robaire and Viger, 1995; Yeung et al., 1993). Epithelial cells lining the epididymal duct play a vital role in establishing the optimal environment for the maturation and storage of spermatozoa (Da Silva et al., 2007b; Hinton and Palladino, 1995; Pastor-Soler et al., 2005; Robaire and Viger, 1995; Wong et al., 2002). The luminal fluid in which spermatozoa reside undergoes significant modifications as it moves from the proximal to the distal regions of the epididymis. For example, the establishment of a low pH and a low bicarbonate concentration in the epididymal lumen (Levine and Kelly, 1978; Levine and Marsh, 1971) is crucial for the maintenance of spermatozoa in a quiescent state during their maturation and storage (Acott and Carr, 1984; Carr et al., 1985). By preventing the activation of the calcium channel, CatSper1 (cation channel, sperm-associated 1), which is located in the sperm membrane and is involved in sperm capacitation, acidic pH contributes to the maintenance of sperm in a dormant state (Kirichok et al., 2006). Capacitation of sperm occurs after mixing with the prostatic and seminal vesicle fluids and is triggered by an influx of bicarbonate, which is abundant in these fluids, followed by activation of a bicarbonate-sensitive adenylyl cyclase (sAC) in sperm (Chen et al., 2000; Sinclair et al., 2000). The subsequent elevation of cAMP induces the phosphorylation of several proteins by protein kinase A, and downregulation of the epithelial sodium channel (EnaC) (Demarco et al., 2003; Hernandez-Gonzalez et al., 2006; Visconti et al., 1999) leading to capacitation.
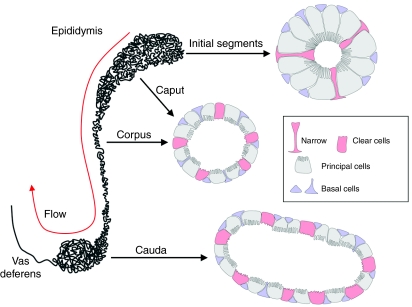
The epididymal epithelium is composed of four distinct cell types: principal, narrow, clear and basal cells. Principal and basal cells are present along the entire length of the epididymal tubule. Narrow cells are relatively low in number and are located exclusively in the initial segments. Clear cells are present in the caput, corpus and cauda epididymidis, as well as in the proximal vas deferens, and are absent from the initial segments (Fig. 1). Different sets of transporters, expressed in specific cell types in different segments of the epididymal tubule, participate in the progressive decrease in bicarbonate concentration and pH that occurs as the fluid flows through the lumen of the epididymis (reviewed by Da Silva et al., 2007b; Pastor-Soler et al., 2005). Significant bicarbonate reabsorption occurs in the initial segments and caput of the epididymis (Levine and Kelly, 1978; Levine and Marsh, 1971) via the sodium–hydrogen exchangers NHE2 and NHE3 (Bagnis et al., 2001; Cheng Chew et al., 2000) located in the apical membrane of principal cells, and the basolateral anion exchanger AE2 (Jensen et al., 1999b) and sodium–bicarbonate co-transporter NBC-e1 (also known as SLC4A4) (Jensen et al., 1999a). Clear cells, which express high levels of the vacuolar proton pumping ATPase, V-ATPase, are involved in luminal acidification in the distal epididymis (Breton et al., 1996; Brown et al., 1992; Herak-Kramberger et al., 2001; Pietrement et al., 2006). This review will focus on selected aspects of the regulation of V-ATPase-dependent proton secretion by these cells.
Fig. 1.
Schematic view of the epididymis. The epithelium lining the epididymis is composed of several cell types, including narrow, clear, principal and basal cells. Narrow and clear cells express high levels of the V-ATPase in their apical membrane and are important contributors to luminal acidification, especially in the distal region (cauda). Basal cells have the previously unrecognized property of sending narrow body projections that can contact the luminal side of the epithelium. Very few basal cells reaching the lumen were detected in the proximal regions including the initial segment and caput, but their number increased progressively in the corpus, to reach a maximum in the cauda.
Clear cells express the V-ATPase in their apical membrane
V-ATPase is a ubiquitous protein that acidifies intracellular organelles and it is also enriched in the plasma membrane of some specialized proton transporting cells. These include renal intercalated cells, osteoclasts, interdental cells of the inner ear, epithelial cells of the olfactory mucosa, and epididymal narrow and clear cells (Beyenbach and Wieczorek, 2006; Breton et al., 1996; Brown et al., 1992; Forgac, 2007; Paunescu et al., 2008; Pietrement et al., 2006; Stankovic et al., 1997; Sun-Wada et al., 2004; Wagner et al., 2004). The V-ATPase is composed of several subunits, which are assembled into two distinct V0 and V1 domains. The structure of this complex enzyme is described in detail in other reviews elsewhere in this issue (Saroussi and Nelson, 2009; Wieczorek et al., 2009). In mammals, the V0 domain contains five transmembrane subunits (a, d, e, c and c″) and the V1 domain contains eight cytosolic subunits (A to H) (see also Beyenbach and Wieczorek, 2006; Forgac, 2007; Wagner et al., 2004). Three copies of subunit A alternate with three copies of subunit B and form a large complex that is responsible for ATP binding and hydrolysis. This hydrolysis drives the rotation of a central rotor formed by subunits D, F and d with subsequent rotations of the c-c″ ring with respect to the static and larger subunit a. Two peripheral stalks formed by two sets of subunits G and E ensure the stability of subunit a together with the A3-B3 complex. Proton translocation occurs between the rotating c-c″ ring and the static subunit a.
Several subunits of the V-ATPase are encoded by more than one gene (reviewed by Beyenbach and Wieczorek, 2006; Forgac, 2007; Wagner et al., 2004). In mammals, the a subunit has four isoforms, subunits B, H and d have two isoforms, and subunits C and G have three isoforms. In addition, one E isoform, originally designated as ATP6E1, is expressed exclusively in the testis whereas its homolog, originally designated as ATP6E2, is expressed ubiquitously (Imai-Senga et al., 2002; Sun-Wada et al., 2002). For simplicity, the ubiquitously expressed E isoform will be referred to as subunit E throughout this review. Differential expression of a particular set of isoforms in different cell types controls the sub-cellular localization of the V-ATPase holo-enzyme (Hurtado-Lorenzo et al., 2006; Kawasaki-Nishi et al., 2001a; Kawasaki-Nishi et al., 2001b; Pietrement et al., 2006; Sun-Wada et al., 2003; Sun-Wada et al., 2004; Toyomura et al., 2003). In the epididymis, subunits A, B1, B2, C1, C2, G1, G3, E, a1, a4, d1 and d2 are all enriched in the apical domain of narrow and clear cells (Da Silva et al., 2007a; Paunescu et al., 2004; Pietrement et al., 2006). In addition, subunits A and a2 were detected in intracellular structures closely associated with the trans-Golgi network of all epithelial cells (Pietrement et al., 2006). Surprisingly, subunit d1 was observed in the apical membrane of principal cells in the apparent absence of other V-ATPase subunits, indicating a potential role for this subunit that might be distinct from its V-ATPase-related function (Pietrement et al., 2006).
V-ATPase isoform compensatory function
Different sub-cellular localization patterns for the a and B isoforms were observed in the apical domain of clear cells. A close co-localization of a4 and B1 with subunit E was detected in sub-apical vesicles and apical microvilli in contrast to a1 and B2, which were detected in sub-apical vesicles only and not in microvilli (Figs 2 and 3) (Paunescu et al., 2004; Pietrement et al., 2006). These results indicate that subunits a4 and B1 are the predominant isoforms responsible for proton secretion across the apical membrane of clear cells, whereas a1 and B2 might serve as back-up isoforms in cases of deficient or absent a4 and B1. In humans harboring mutations in ATP6V1B1 and ATP6V0A4, the genes that encode the B1 and a4 subunits, respectively, defective proton secretion by intercalated cells results in the development of systemic acidosis, a disease that is known as distal renal tubular acidosis (dRTA) (Karet et al., 1999; Stover et al., 2002). By contrast, although humans with B1 mutations develop deafness, most of those with a4 mutations have intact hearing, despite the fact that both B1 and a4 are expressed in the inner ear (Stover et al., 2002). Similarly, although a4 is the predominant isoform in the apical membrane of renal proximal tubule cells (Hurtado-Lorenzo et al., 2006), humans harboring a4 mutations do not develop proximal tubular acidosis. These cases further indicate the possibility that a given subunit isoform might constitute a backup for its counterpart. For example, can another a isoform replace the mutated a4 in the proximal tubule and inner ear? Such isoform replacement occurs in the absence of B1 in B1-knockout (KO) mice, where the compensatory insertion of B2 with the plasma membrane-bound V-ATPase allows V-ATPase-dependent proton transport to occur across the membrane of epididymal clear cells and renal intercalated cells (Da Silva et al., 2007a; Paunescu et al., 2007). Accordingly, male mice lacking the B1 subunit do not develop dRTA and are not infertile (Da Silva et al., 2007a). By contrast, in humans harboring B1 mutations, B2 replacement does not appear to take place, which results in the development of dRTA. Whether or not B2 is able to assemble into the holoenzyme in the presence of deficient B1, or whether the mutated B1 subunit by itself impairs V-ATPase trafficking, as was shown in cell cultures (Yang et al., 2006), are questions that will require further investigation. Future follow-up studies will also be necessary to determine whether or not human males with B1 and/or a4 mutations will develop infertility.
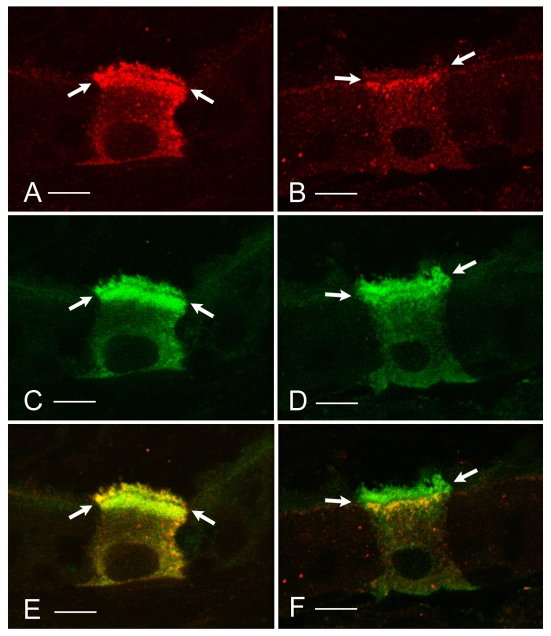
Fig. 2.
Immunolocalization of the a1 and a4 subunits of the V-ATPase, and comparison with the E subunit, a marker of all V-ATPase holoenzymes in clear cells. 5 μm sections of rat cauda epididymidis were stained for a1 (A; green) or a4 (B; green). The sections were double-labeled for E (C,D; red). a1 is located in sub-apical vesicles, where it colocalizes with E (yellow staining in the merged image shown in E), but it is absent from microvilli that are only labeled for the E subunit (red staining in E). a4 colocalizes with E in both sub-apical vesicles and apical microvilli (yellow-orange staining in the merged image shown in F). Scale bars, 5 μm. Reproduced from Pietrement et al. (Pietrement et al., 2006) with permission from Biology of Reproduction.
Fig. 3.
Immunolocalization of the B1 and B2 subunits of the V-ATPase, and comparison with the E subunit, a marker of all V-ATPase holoenzymes in clear cells. 5 μm sections of mouse cauda epididymidis were stained for B1 (A; red) or B2 (B; red). The sections were double-labeled for E (C,D; green). B1 colocalizes with E in both sub-apical vesicles and apical microvilli (yellow staining in the merged image in E). B2 is located in sub-apical vesicles, where it partially colocalizes with E (orange staining in the merged image in F), but it is absent from microvilli that are only labeled for the E subunit (green staining in F). Scale bars, 5 μm. Reproduced from Paunescu et al. (Paunescu et al., 2004) with permission from American Journal of Physiology – Cell Physiology.
Regulation of V-ATPase-dependent proton secretion via recycling mechanisms
Clear cells significantly increase in number from the proximal to the distal regions of the epididymis, and they are most numerous in the cauda epididymidis (Fig. 4). Their contribution to luminal acidification is, therefore, higher in the distal epididymis than in the proximal epididymis, where bicarbonate reabsorption by principal cells occurs. As mentioned above, proton secretion by clear cells is achieved by apical V-ATPase, which works in conjunction with basolateral bicarbonate transporters (Breton et al., 1998), and cytosolic carbonic anhydrase II (Breton et al., 1996; Breton et al., 1999; Da Silva et al., 2007b). The V-ATPase inhibitors bafilomycin and concanamycin A abolish net proton secretion, as measured with an extracellular proton-selective electrode in cut-open vas deferens, a segment that also contains V-ATPase-rich clear cells, indicating the contribution of V-ATPase to luminal acidification (Breton et al., 1998; Breton et al., 2000a; Breton et al., 1996; Shum et al., 2008). Although clear cells express the basolateral transporters, NBCe-1 and AE2, functional analysis showed that proton secretion in these cells is independent of Cl–, but is SITS sensitive, indicating the potential participation of NBCe-1 and not AE2 in this process (Breton et al., 1998).
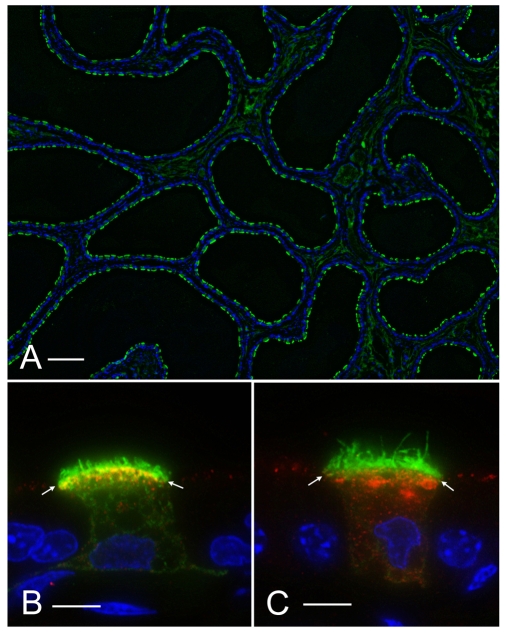
Fig. 4.
Relative numbers of clear cells in the rat caput (A) versus cauda (B) epididymidis. Rat epididymis was stained for the V-ATPase B1 subunit (green) to label clear cells, and NHERF1 (red), using antibodies that we have previously characterized (Pietrement et al., 2008). Nuclei and spermatozoa were stained with DAPI (blue). NHERF1 is located in the apical membrane of both principal cells and clear cells. B1-positive clear cells are much more numerous in the cauda (B) than in the caput (A) epididymidis. Scale bars, 50 μm.
Our laboratory has shown that the accumulation of V-ATPase in the apical membrane of clear cells is regulated by V-ATPase recycling between sub-apical vesicles and the apical plasma membrane, a process that is accompanied by extensive elongation of microvilli and increased net proton secretion (Beaulieu et al., 2005; Breton et al., 2000a; Pastor-Soler et al., 2003; Pastor-Soler et al., 2008; Shum et al., 2008). Cleavage of the SNARE protein, cellubrevin, inhibits V-ATPase-dependent proton secretion in isolated vas deferens, as well as in renal intercalated cells (Breton et al., 2000a; Rothenberger et al., 2007). The actin cytoskeleton also plays a key role in the regulation of V-ATPase recycling in clear cells (Beaulieu et al., 2005). Subunits B1, B2 and C of the V-ATPase interact directly with actin (Chen et al., 2004; Holliday et al., 2000; Vitavska et al., 2003). In addition, B1 can interact indirectly with the actin cytoskeleton via its association with NHERF1 (Fig. 4), a PDZ protein that contains a merlin-ezrin-radixin-moesin (MERM) actin-binding domain (Breton et al., 2000b). We have shown that clear cells express very high levels of the actin-capping and -severing protein, gelsolin (Beaulieu et al., 2005). Inhibition of actin polymerization using a permeant peptide that prevents uncapping of gelsolin from the barbed end of actin filaments, induced a marked accumulation of the V-ATPase in clear cell microvilli. These results indicate that gelsolin-dependent actin depolymerization in clear cells favors either the inhibition of V-ATPase endocytosis or stimulation of exocytosis, leading to the accumulation of V-ATPase in the plasma membrane (Beaulieu et al., 2005).
Non-hormonal regulation of V-ATPase recycling (crosstalk between principal cells and clear cells)
Principal cells of the cauda epididymidis and vas deferens secrete bicarbonate following basolateral adrenergic and hormonal stimulation (Carlin et al., 2003; Hagedorn et al., 2007; Leung and Wong, 1992; Pierucci-Alves and Schultz, 2008; Sedlacek et al., 2001; Wong, 1988). This process depends on the presence of CFTR (Wong, 1998), which is located in the apical membrane of principal cells (Pietrement et al., 2008) (Fig. 5). Acute bicarbonate secretion upon stimulation of the epididymal epithelium was proposed to help prime spermatozoa prior to ejaculation (Carlin et al., 2003). However, a sustained increase in luminal pH and bicarbonate concentration following stimulation of bicarbonate secretion might be detrimental to epididymal sperm survival. We proposed that clear cells are responsible for the re-establishment of the luminal resting acidic pH and low bicarbonate concentration that sperm require to remain inactive during their storage period (reviewed by Da Silva et al., 2007b; Pastor-Soler et al., 2005). To test this hypothesis, we developed an in vivo rat epididymis luminal microperfusion procedure for the study of luminal factors in the regulation of the epididymal epithelium (Fig. 6A). We showed that clear cells respond to an increase in luminal pH from the resting value of pH 6.6 to the alkaline pH of 7.8 by accumulating V-ATPase in their apical microvilli (Beaulieu et al., 2005; Pastor-Soler et al., 2003). A similar response was elicited when clear cells were luminally perfused with a bicarbonate-containing solution, compared with a phosphate-containing perfusate at constant pH (Pastor-Soler et al., 2003). We identified the bicarbonate-activated adenylyl cyclase, sAC, as the sensor responsible for the response of rat epididymal clear cells to variations in bicarbonate concentration (Pastor-Soler et al., 2003). V-ATPase apical membrane accumulation is induced by cAMP (Fig. 6B,C) and is dependent on the activity of protein kinase A (Pastor-Soler et al., 2008). Clear cells are, therefore, in a position to re-establish luminal low bicarbonate and pH conditions following an increase in bicarbonate secretion by principal cells. These results indicate a concerted interaction between principal cells, whose role would be to temporally prime spermatozoa during sexual arousal prior to ejaculation, and clear cells, which would then contribute to the re-establishment of the acidic conditions essential for keeping sperm in a quiescent state during their storage period in the epididymis.
Fig. 5.
Rat cauda epididymidis double-stained for CFTR (green) and the V-ATPase E subunit (red). Intense CFTR labeling is detected in the apical membrane of principal cells. Clear cells, identified by their positive labeling for the V-ATPase E subunit, do not express CFTR. The rabbit anti-CFTR antibody used here was purchased from Alomone Laboratory (Cat. no. ACL-006) and has been previously characterized in our laboratory (Pietrement et al., 2008). Sperm and nuclei were labeled with DAPI (blue). Scale bars, 15 μm. Lu, lumen.
Fig. 6.
Rat cauda epididymidis perfused in vivo and stained for the V-ATPase B1 subunit (green). Nuclei were stained with DAPI (blue). (A) Numerous B1-positive clear cells were detected. Luminal spermatozoa are absent from these perfused tubules. (B) Higher magnification of a clear cell perfused with a control phosphate-buffered solution adjusted to pH 6.6 and containing the endocytic marker, HRP. Double-labeling for HRP (red) and the V-ATPase B1 subunit (green) was performed. The V-ATPase is distributed between sub-apical vesicles and short microvilli. The yellow staining indicates partial colocalization of the V-ATPase with HRP in endosomes. (C) Clear cell perfused with an `activation' buffer containing bicarbonate and cpt-cAMP. The V-ATPase is mainly located in longer microvilli (green) and no colocalization with HRP-labeled endosomes is detected (red). The staining was performed as previously characterized (Shum et al., 2008). Scale bars, 150 μm (A), 5 μm (B,C).
Hormonal regulation of V-ATPase recycling (crosstalk between basal cells and clear cells)
The previous section illustrates how clear cells can respond, in a hormone-independent manner, to variations in their extracellular environment by the participation of bicarbonate-sensitive sAC. The following section describes the paracrine regulation of clear cells by the hormone angiotensin II (ANGII).
All components of the renin-angiotensin system (RAS) are present in the lumen of the epididymis, and play a key role in male fertility (Esther et al., 1996; Hagaman et al., 1998; Krege et al., 1995; Leung and Sernia, 2003; Ramaraj et al., 1998; Saez et al., 2004; Speth et al., 1999; Wong and Uchendu, 1990). High concentrations of angiotensin I (ANGI) and ANGII have been detected in the lumen of the epididymis (Wong and Uchendu, 1990). Principal cells produce ANGI, which is then secreted into the lumen (Wong and Uchendu, 1990). ANGI is converted to ANGII by the angiotensin I converting enzyme (ACE). Importantly, ACE KO male mice are infertile (Esther et al., 1996; Krege et al., 1995). ACE exists in two forms, the testicular form of ACE (tACE) also known as germinal ACE, which is expressed exclusively in spermatozoa (Langford et al., 1993; Sibony et al., 1994), and the somatic form of ACE (sACE) (Corvol et al., 1995). The reduction of male fertility in ACE KO mice is due to the absence of tACE and not sACE, as their fertility is restored after re-insertion of the tACE gene (Hagaman et al., 1998). ACE KO males are infertile because of the poor quality of their spermatozoa, which are normal in number but are unable to move up the female reproductive tract and fertilize an egg (Esther et al., 1996; Hagaman et al., 1998; Krege et al., 1995). Thus, a defect in sperm function rather than production is the leading cause of infertility in these mice. tACE is attached to the membrane of immature spermatozoa and it is released into the luminal fluid as sperm transit through the proximal regions of the epididymis (Gatti et al., 1999; Metayer et al., 2002; Thimon et al., 2005). It was postulated that luminal tACE might play a role in the regulation of the epididymal epithelium (Thimon et al., 2005). Absence of tACE might, therefore, impair the function of the epididymis and ultimately the maturation of spermatozoa as they transit through this organ. To test this hypothesis, we examined the role of ANGII, the product of ACE, on the acidification capacity of the epididymis.
We showed that in vivo luminal perfusion of rat epididymis with ANGII elicited a marked accumulation of V-ATPase in clear cell microvilli (Shum et al., 2008). This effect was accompanied by a significant increase in V-ATPase-dependent proton secretion in the cut-open vas deferens. These results were consistent with the activation of V-ATPase by ANGII that had been previously reported in renal intercalated cells (Pech et al., 2008; Rothenberger et al., 2007). In addition, we showed that the nitric oxide-cGMP pathway was responsible for V-ATPase activation in clear cells through the participation of the ANGII type II receptor (AGTR2) (Shum et al., 2008). However, RT-PCR analysis of clear cells isolated by fluorescence activated cell sorting (FACS) from transgenic mice that express EGFP in clear cells exclusively (Fig. 7A) (Miller et al., 2005) did not detect AGTR2 mRNA in these cells (Fig. 7B). In addition, AGTR2 protein was absent from rat epididymal clear cells, as demonstrated by immunofluorescence (Fig. 8A′,A″, arrowheads) (Shum et al., 2008). By contrast, a strong labeling for AGTR2 was detected in basal cells, which were found unexpectedly to extend narrow body projections that reach up toward the luminal border of the epithelium (Fig. 8A′,A″, arrows). Three-dimensional confocal microscopy confirmed that basal cells express AGTR2 (Fig. 8B, arrows) and that they produce a slender body extension that infiltrates between other epithelial cells towards the lumen. Interestingly, double labeling for claudin-1, a basal cell marker (Gregory et al., 2001) and ZO1, a tight junction (TJ) marker, showed that basal cells preferentially reach and sometimes cross the TJs at the tripartite junction between other epithelial cells (Fig. 9, arrows). In addition, while some basal cells did not interact with TJs (Fig. 10A), others showed various degrees of interactions from partial (Fig. 10B,C) to complete (Fig. 10D,E) with formation of a new TJ between themselves and adjacent epithelial cells. Similar patterns of interaction between basal cells and TJs were also seen in other tissues including the rat trachea and coagulating gland (Shum et al., 2008).
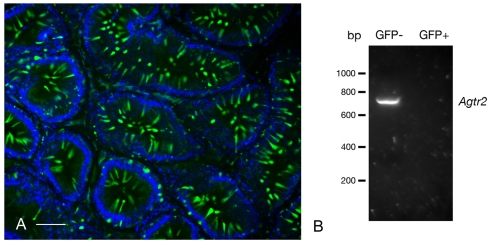
Fig. 7.
(A) Mouse caput epididymidis from a B1-EGFP transgenic mouse. Numerous EGFP-positive (green) clear cells are detected (see also Miller et al., 2005). Nuclei were stained with DAPI (blue). (B) RT-PCR detection of AGTR2 in clear cells isolated by FACS from B1-EGFP mouse epididymidis (GFP+) and in all other epididymal cell types (GFP–). AGTR2 was detected in the GFP-negative cell population, but not in the GFP-positive clear cells.
Fig. 8.
Expression of AGTR2 in basal cells. (A′,A″) Two examples of AGTR2 (green) and V-ATPase (red) labeling in rat cauda epididymidis. Arrows indicate AGTR2-positive basal cells, which send body projections towards the lumen. Arrowheads indicate nearby V-ATPase-positive clear cells. Nuclei were stained with DAPI (blue). (B) Three-dimensional (3D) reconstruction showing AGTR2-positive basal cells (green; arrows). One basal cell sends a projection between principal cells. Two clear cells, stained apically for the V-ATPase (red), are visible (arrowheads). The 3D mosaic was assembled from a stack of 0.1 μm interval optical Z sections obtained by laser scanning confocal microscopy. Lu, lumen. Scale bars, 5 μm. Reproduced from Shum et al. (Shum et al., 2008) with permission from Cell.
Fig. 9.
Basal cells reach the tight-junctions at the intersection between three epithelial cells. (A′,A″,A′″) Three different rotations of a three-dimensional reconstruction of an epididymis section stained for claudin-1 (red), a marker of basal cells, and the tight-junction protein ZO1 (green). Arrows indicate the tri-cellular corners where basal cells reach the tight-junctions. Scale bars, 10 μm. Reproduced from Shum et al. (Shum et al., 2008) with permission from Cell.
Fig. 10.
Basal cells cross the tight-junctions to reach the lumen (Lu). (A–D) Three-dimensional reconstructions of the apical region of basal cells from epididymis sections double stained for claudin-1 (red; a marker for basal cells) and ZO1 (green; a marker for tight junctions) showing different patterns of interaction. (A) No colocalization between claudin-1 and ZO1 (arrow); (B) partial colocalization of claudin-1 with ZO1 (yellow staining; arrows); (C) basal cell that penetrates the tight-junction (arrow); (D) basal cell forming a ZO1-stained tight junction (green) with adjacent cells (arrows). (E) Conventional microscopy image of the basal cell shown in D (arrow). A clear cell expressing apical V-ATPase (blue) is seen (arrowhead). The nuclei are also stained blue with DAPI. Scale bars, 5 μm. Reproduced from Shum et al. (Shum et al., 2008) with permission from Cell.
Altogether our results provide evidence that basal cells can actually reach the luminal side of an epithelium (Shum et al., 2008). This previously unrecognized property of basal cells now places them in a central position to survey the lumen of the epididymis, a property that might be present in other biological systems, including the upper respiratory tract. In the epididymis, one function of basal cells would be to scan the lumen for the presence of ANGII. Activation of AGTR2 in basal cells by luminal ANGII results in the increase of V-ATPase-dependent proton secretion in adjacent clear cells by production of nitric oxide in basal cells, which diffuses out and activates soluble guanylate cyclase in clear cells (Shum et al., 2008). The subsequent production of cGMP leads to the apical accumulation of V-ATPase in a manner similar to the effect elicited by cAMP. Our proposed model of basal–clear cell crosstalk is illustrated in Fig. 11. According to this model, luminal sampling of ANGII by basal cells followed by activation of proton secretion in clear cells would ensure that the luminal fluid is maintained at the acidic physiological pH range that is crucial for sperm maturation and storage in the epididymis. A similar crosstalk mechanism has also been proposed between basal cells and principal cells, via activation of basal cells by basolateral lysylbradykinin followed by activation of anion secretion in principal cells (Cheung et al., 2005; Leung et al., 2004).
Fig. 11.
Schematic representation of cell–cell crosstalk in the epididymal epithelium. Basal cells extend a slender body projection toward the lumen, and form a new tight junction with adjacent epithelial cells. Luminal ANGII triggers the production of nitric oxide (NO) by activation of AGTR2 in basal cells. The NO then diffuses out of basal cells and acts locally on clear cells to produce cGMP by activation of the soluble guanylate cyclase (sGC), which is enriched in these cells. cGMP induces the accumulation of V-ATPase in microvilli, which results in the increase of proton secretion. Modified from Shum et al. (Shum et al., 2008) and reproduced with permission from Cell.
In the epididymal lumen, a significant amount of ANGII might originate from the enzymatic activity of tACE, which would act on secreted ANGI. Thus, shedding of tACE from the sperm membrane during their transit through the epididymis might increase the availability of ANGII near the apical surface of the epithelium and provide a means by which spermatozoa modulate surrounding epithelial cells. Consequently, decreased levels of ANGII in the epididymal lumen of ACE KO male mice might impair the acidifying capacity of the epididymis with detrimental consequences on sperm quality. The importance of luminal acidification in the establishment of male fertility was recently illustrated by the fact that FOXI1 KO male mice, which have abnormally elevated epididymal luminal pH, are infertile as a result of the inability of their sperm to fertilize an egg (Blomqvist et al., 2006). Because angiotensinogen KO male mice are fertile (Hagaman et al., 1998), further studies will be required to determine whether the concentration of ANGII is reduced in the epididymal lumen of ACE KO mice, and whether these mice have impaired luminal acidification.
Conclusions
The epididymis is the main site for post-testicular sperm maturation and storage and is, therefore, a major player in the establishment of male fertility. The generation of an acidic luminal environment in the epididymis is essential for keeping sperm in a dormant, immotile state during their transit in this organ. A growing body of evidence indicates that epithelial cells lining the epididymal tubule have developed an elaborate network of cell-cell and cell-sperm `crosstalk' to regulate transepithelial transport. A sub-population of epithelial cells, the clear cells, express high levels of V-ATPase in their apical membrane and are important contributors to luminal acidification. Targeting of the V-ATPase to the plasma membrane depends upon the assembly of a particular set of V-ATPase subunit isoforms. In addition, V-ATPase-dependent proton secretion in clear cells is regulated by recycling of the V-ATPase to and from the apical plasma membrane. The luminal environment modulates this process, and proton secretion increases following a rise in luminal pH or luminal bicarbonate concentration, through the activation of sAC, which is enriched in clear cells. Proton secretion in clear cells is also modulated in a paracrine manner via ANGII, which is locally produced in the luminal fluid. This triggers a complex communication network between basal cells, which have the previously unrecognized ability to send narrow body projections across the tight-junction barrier to reach the lumen, and clear cells. Activation of AGTR2 by luminal ANGII induces the production of nitric oxide in basal cells, which then diffuses out to trigger the production of cGMP in clear cells, followed by apical membrane V-ATPase accumulation and subsequent increase in proton secretion. Thus, concerted interactions between different cell types take place in the epididymis for the fine control of an optimum acidic luminal environment that is critical for male fertility.
We would like to thank Eric Hill for his excellent technical assistance. This work was supported by National Institutes of Health Grants HD40793, DK38452 and HD045821. The work performed in the Microscopy Core Facility of the Massachusetts General Hospital Program in Membrane Biology was supported by Center for the Study of Inflammatory Bowel Disease Grant DK43351 and Boston Area Diabetes and Endocrinology Research Center Award DK57521. Deposited in PMC for release after 12 months.
References
- Acott, T. S. and Carr, D. W. (1984). Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol. Reprod. 30, 926-935. [DOI] [PubMed] [Google Scholar]
- Bagnis, C., Marsolais, M., Biemesderfer, D., Laprade, R. and Breton, S. (2001). Na(+)/H(+)-exchange activity and immunolocalization of NHE3 in rat epididymis. Am. J. Physiol. Renal Physiol. 280, F426-F436. [DOI] [PubMed] [Google Scholar]
- Beaulieu, V., Da Silva, N., Pastor-Soler, N., Brown, C. R., Smith, P. J., Brown, D. and Breton, S. (2005). Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+ATPase (V-ATPase) recycling. J. Biol. Chem. 280, 8452-8463. [DOI] [PubMed] [Google Scholar]
- Beyenbach, K. W. and Wieczorek, H. (2006). The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209, 577-589. [DOI] [PubMed] [Google Scholar]
- Blomqvist, S. R., Vidarsson, H., Soder, O. and Enerback, S. (2006). Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J. 25, 4131-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton, S., Smith, P. J. S., Lui, B. and Brown, D. (1996). Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat. Med. 2, 470-472. [DOI] [PubMed] [Google Scholar]
- Breton, S., Hammer, K., Smith, P. J. S. and Brown, D. (1998). Proton secretion in the male reproductive tract: involvement of chloride-independent bicarbonate transport. Am. J. Physiol. Cell Physiol. 275, C1134-C1142. [DOI] [PubMed] [Google Scholar]
- Breton, S., Tyszkowski, R., Sabolic, I. and Brown, D. (1999). Postnatal development of H+ ATPase (proton-pump)-rich cells in rat epididymis. Histochem. Cell Biol. 111, 97-105. [DOI] [PubMed] [Google Scholar]
- Breton, S., Nsumu, N. N., Galli, T., Sabolic, I., Smith, P. J. and Brown, D. (2000a). Tetanus toxin-mediated cleavage of cellubrevin inhibits proton secretion in the male reproductive tract. Am. J. Physiol. Renal Physiol. 278, F717-F725. [DOI] [PubMed] [Google Scholar]
- Breton, S., Wiederhold, T., Marshansky, V., Nsumu, N. N., Ramesh, V. and Brown, D. (2000b). The B1 subunit of the H+ATPase is a PDZ domain-binding protein. Colocalization with NHE-RF in renal B-intercalated cells. J. Biol. Chem. 275, 18219-18224. [DOI] [PubMed] [Google Scholar]
- Brown, D., Lui, B., Gluck, S. and Sabolic, I. (1992). A plasma membrane proton ATPase in specialized cells of rat epididymis. Am. J. Physiol. 263, C913-C916. [DOI] [PubMed] [Google Scholar]
- Carlin, R. W., Lee, J. H., Marcus, D. C. and Schultz, B. D. (2003). Adenosine stimulates anion secretion across cultured and native adult human vas deferens epithelia. Biol. Reprod. 68, 1027-1034. [DOI] [PubMed] [Google Scholar]
- Carr, D. W., Usselman, M. C. and Acott, T. S. (1985). Effects of pH, lactate, and viscoelastic drag on sperm motility: a species comparison. Biol. Reprod. 33, 588-595. [DOI] [PubMed] [Google Scholar]
- Chen, S. H., Bubb, M. R., Yarmola, E. G., Zuo, J., Jiang, J., Lee, B. S., Lu, M., Gluck, S. L., Hurst, I. R. and Holliday, L. S. (2004). Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J. Biol. Chem. 279, 7988-7998. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Cann, M. J., Litvin, T. N., Iourgenko, V., Sinclair, M. L., Levin, L. R. and Buck, J. (2000). Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625-628. [DOI] [PubMed] [Google Scholar]
- Cheng Chew, S. B., Leung, G. P. H., Leung, P. Y., Tse, C. M. and Wong, P. Y. D. (2000). Polarized distribution of NHE1 and NHE2 in the rat epididymis. Biol. Reprod. 62, 755-758. [DOI] [PubMed] [Google Scholar]
- Cheung, K. H., Leung, G. P., Leung, M. C., Shum, W. W., Zhou, W. L. and Wong, P. Y. (2005). Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J. Gen. Physiol. 125, 443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol, P., Williams, T. A. and Soubrier, F. (1995). Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 248, 283-305. [DOI] [PubMed] [Google Scholar]
- Da Silva, N., Shum, W. W., El-Annan, J., Paunescu, T. G., McKee, M., Smith, P. J., Brown, D. and Breton, S. (2007a). Relocalization of the V-ATPase B2 subunit to the apical membrane of epididymal clear cells of mice deficient in the B1 subunit. Am. J. Physiol. Cell Physiol. 293, C199-C210. [DOI] [PubMed] [Google Scholar]
- Da Silva, N., Shum, W. W. C. and Breton, S. (2007b). Regulation of V-ATPase-dependent luminal acidification in the epididymis. Asian J. Androl. 9, 476-482. [DOI] [PubMed] [Google Scholar]
- Demarco, I. A., Espinosa, F., Edwards, J., Sosnik, J., De La Vega-Beltran, J. L., Hockensmith, J. W., Kopf, G. S., Darszon, A. and Visconti, P. E. (2003). Involvement of a Na+/HCO3– cotransporter in mouse sperm capacitation. J. Biol. Chem. 278, 7001-7009. [DOI] [PubMed] [Google Scholar]
- Esther, C. R., Jr, Howard, T. E., Marino, E. M., Goddard, J. M., Capecchi, M. R. and Bernstein, K. E. (1996). Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab. Invest. 74, 953-965. [PubMed] [Google Scholar]
- Forgac, M. (2007). Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917-929. [DOI] [PubMed] [Google Scholar]
- Gatti, J. L., Druart, X., Guerin, Y., Dacheux, F. and Dacheux, J. L. (1999). A 105- to 94-kilodalton protein in the epididymal fluids of domestic mammals is angiotensin I-converting enzyme (ACE): evidence that sperm are the source of this ACE. Biol. Reprod. 60, 937-945. [DOI] [PubMed] [Google Scholar]
- Gregory, M., Dufresne, J., Hermo, L. and Cyr, D. (2001). Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology 142, 854-863. [DOI] [PubMed] [Google Scholar]
- Hagaman, J. R., Moyer, J. S., Bachman, E. S., Sibony, M., Magyar, P. L., Welch, J. E., Smithies, O., Krege, J. H. and O'Brien, D. A. (1998). Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. USA 95, 2552-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn, T. M., Carlin, R. W. and Schultz, B. D. (2007). Oxytocin and vasopressin stimulate anion secretion by human and porcine vas deferens epithelia. Biol. Reprod. 77, 416-424. [DOI] [PubMed] [Google Scholar]
- Herak-Kramberger, C. M., Breton, S., Brown, D., Kraus, O. and Sabolic, I. (2001). Distribution of the vacuolar H+ ATPase along the rat and human male reproductive tract. Biol. Reprod. 64, 1699-1707. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez, E. O., Sosnik, J., Edwards, J., Acevedo, J. J., Mendoza-Lujambio, I., Lopez-Gonzalez, I., Demarco, I., Wertheimer, E., Darszon, A. and Visconti, P. E. (2006). Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J. Biol. Chem. 281, 5623-5633. [DOI] [PubMed] [Google Scholar]
- Hinton, B. T. and Palladino, M. A. (1995). Epididymal epithelium: its contribution to the formation of a luminal fluid microenvironment. Microsc. Res. Tech. 30, 67-81. [DOI] [PubMed] [Google Scholar]
- Holliday, L. S., Lu, M., Lee, B. S., Nelson, R. D., Solivan, S., Zhang, L. and Gluck, S. L. (2000). The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J. Biol. Chem. 275, 32331-32337. [DOI] [PubMed] [Google Scholar]
- Hurtado-Lorenzo, A., Skinner, M., El Annan, J., Futai, M., Sun-Wada, G. H., Bourgoin, S., Casanova, J., Wildeman, A., Bechoua, S., Ausiello, D. A. et al. (2006). V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 8, 124-136. [DOI] [PubMed] [Google Scholar]
- Imai-Senga, Y., Sun-Wada, G. H., Wada, Y. and Futai, M. (2002). A human gene, ATP6E1, encoding a testis-specific isoform of H(+)-ATPase subunit E. Gene 289, 7-12. [DOI] [PubMed] [Google Scholar]
- Jensen, L. J., Schmitt, B. M., Berger, U. V., Nsumu, N. N., Boron, W. F., Hediger, M. A., Brown, D. and Breton, S. (1999a). Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol. Reprod. 60, 573-579. [DOI] [PubMed] [Google Scholar]
- Jensen, L. J., Stuart-Tilley, A. K., Peters, L. L., Lux, S. E., Alper, S. L. and Breton, S. (1999b). Immunolocalization of AE2 anion exchanger in rat and mouse epididymis. Biol. Reprod. 61, 973-980. [DOI] [PubMed] [Google Scholar]
- Jones, R. C. and Murdoch, R. N. (1996). Regulation of the motility and metabolism of spermatozoa for storage in the epididymis of eutheran and marsupial mammals. Reprod. Fertil. Dev. 8, 553-568. [DOI] [PubMed] [Google Scholar]
- Karet, F. E., Finberg, K. E., Nelson, R. D., Nayir, A., Mocan, H., Sanjad, S. A., Rodriguez-Soriano, J., Santos, F., Cremers, C. W., Di Pietro, A. et al. (1999). Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat. Genet. 21, 84-90. [DOI] [PubMed] [Google Scholar]
- Kawasaki-Nishi, S., Bowers, K., Nishi, T., Forgac, M. and Stevens, T. H. (2001a). The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J. Biol. Chem. 276, 47411-47420. [DOI] [PubMed] [Google Scholar]
- Kawasaki-Nishi, S., Nishi, T. and Forgac, M. (2001b). Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J. Biol. Chem. 276, 17941-17948. [DOI] [PubMed] [Google Scholar]
- Kirichok, Y., Navarro, B. and Clapham, D. E. (2006). Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737-740. [DOI] [PubMed] [Google Scholar]
- Krege, J. H., John, S. W., Langenbach, L. L., Hodgin, J. B., Hagaman, J. R., Bachman, E. S., Jennette, J. C., O'Brien, D. A. and Smithies, O. (1995). Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature 375, 146-148. [DOI] [PubMed] [Google Scholar]
- Langford, K. G., Zhou, Y., Russell, L. D., Wilcox, J. N. and Bernstein, K. E. (1993). Regulated expression of testis angiotensin-converting enzyme during spermatogenesis in mice. Biol. Reprod. 48, 1210-1218. [DOI] [PubMed] [Google Scholar]
- Leung, A. Y. and Wong, P. Y. (1992). Studies of transepithelial Cl- transport in cultured cauda epididymal cells of rats by the short-circuit current method. J. Physiol. 457, 391-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, G. P., Cheung, K. H., Leung, C. T., Tsang, M. W. and Wong, P. Y. (2004). Regulation of epididymal principal cell functions by basal cells: role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1). Mol. Cell Endocrinol. 216, 5-13. [DOI] [PubMed] [Google Scholar]
- Leung, P. S. and Sernia, C. (2003). The renin-angiotensin system and male reproduction: new functions for old hormones. J. Mol. Endocrinol. 30, 263-270. [DOI] [PubMed] [Google Scholar]
- Levine, N. and Kelly, H. (1978). Measurement of pH in the rat epididymis in vivo. J. Reprod. Fertil. 52, 333-335. [DOI] [PubMed] [Google Scholar]
- Levine, N. and Marsh, D. J. (1971). Micropuncture studies of the electrochemical aspects of fluid and electrolytes transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J. Physiol. (Lond.) 213, 557-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metayer, S., Dacheux, F., Dacheux, J. L. and Gatti, J. L. (2002). Germinal angiotensin I-converting enzyme is totally shed from the rodent sperm membrane during epididymal maturation. Biol. Reprod. 67, 1763-1767. [DOI] [PubMed] [Google Scholar]
- Miller, R. L., Zhang, P., Smith, M., Beaulieu, V., Paunescu, T. G., Brown, D., Breton, S. and Nelson, R. D. (2005). V-ATPase B1 subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am. J. Physiol. Cell Physiol. 288, C1134-C1144. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist, M. C. (2003). The epididymis in the post-genome era. In The Third International Conference on the Epididymis, vol. 3 (ed. B. T. Hinton and T. T. Turner), pp. 2-22. Charlottesville, VA: The Van Doren Company. [Google Scholar]
- Pastor-Soler, N., Beaulieu, V., Litvin, T. N., Da Silva, N., Chen, Y., Brown, D., Buck, J., Levin, L. R. and Breton, S. (2003). Bicarbonate regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 278, 49523-49529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler, N., Pietrement, C. and Breton, S. (2005). Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 20, 417-428. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler, N. M., Hallows, K. R., Smolak, C., Gong, F., Brown, D. and Breton, S. (2008). Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am. J. Physiol. Cell Physiol. 294, C488-C494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu, T., Da Silva, N., Marshansky, V., McKee, M., Breton, S. and Brown, D. (2004). Expression of the 56kDa B2 subunit isoform of the vacuolar H+ATPase in proton secreting cells of the kidney and epididymis. Am. J. Physiol. Cell Physiol. 287, C149-C162. [DOI] [PubMed] [Google Scholar]
- Paunescu, T., Russo, L., Da Silva, N., Kovacikova, J., Van Hoek, S., McKee, M., Wagner, C., Breton, S. and Brown, D. (2007). Compensatory membrane expression of the V-ATPase B2 subunit isoform in renal medullary intercalated cells of B1-deficient mice. Am. J. Physiol. Renal Physiol. 293, F1915-F1926. [DOI] [PubMed] [Google Scholar]
- Paunescu, T. G., Jones, A. C., Tyszkowski, R. and Brown, D. (2008). V-ATPase expression in the mouse olfactory epithelium. Am. J. Physiol. Cell Physiol. 295, C923-C930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech, V., Zheng, W., Pham, T. D., Verlander, J. W. and Wall, S. M. (2008). Angiotensin II activates H+-ATPase in type A intercalated cells. J. Am. Soc. Nephrol. 19, 84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Alves, F. and Schultz, B. D. (2008). Bradykinin-stimulated cyclooxygenase activity stimulates vas deferens epithelial anion secretion in vitro in swine and humans. Biol. Reprod. 79, 501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrement, C., Sun-Wada, G. H., Silva, N. D., McKee, M., Marshansky, V., Brown, D., Futai, M. and Breton, S. (2006). Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol. Reprod. 74, 185-194. [DOI] [PubMed] [Google Scholar]
- Pietrement, C., Da Silva, N., Silberstein, C., James, M., Marsolais, M., Van Hoek, A., Brown, D., Pastor-Soler, N., Ameen, N., Laprade, R. et al. (2008). Role of NHERF1, cystic fibrosis transmembrane conductance regulator, and cAMP in the regulation of aquaporin 9. J. Biol. Chem. 283, 2986-2996. [DOI] [PubMed] [Google Scholar]
- Ramaraj, P., Kessler, S. P., Colmenares, C. and Sen, G. C. (1998). Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J. Clin. Invest. 102, 371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire, B. and Viger, R. S. (1995). Regulation of epididymal epithelial cell functions. Biol. Reprod. 52, 226-236. [DOI] [PubMed] [Google Scholar]
- Rothenberger, F., Velic, A., Stehberger, P. A., Kovacikova, J. and Wagner, C. A. (2007). Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J. Am. Soc. Nephrol. 18, 2085-2093. [DOI] [PubMed] [Google Scholar]
- Saez, F., Legare, C., Laflamme, J. and Sullivan, R. (2004). Vasectomy-dependent dysregulation of a local renin-angiotensin system in the epididymis of the cynomolgus monkey (Macaca fascicularis). J. Androl. 25, 784-796. [DOI] [PubMed] [Google Scholar]
- Saroussi, S. and Nelson, N. (2009). The little we know on the structure and machinery of V-ATPase. J. Exp. Biol. 212, 1604-1610. [DOI] [PubMed] [Google Scholar]
- Sedlacek, R. L., Carlin, R. W., Singh, A. K. and Schultz, B. D. (2001). Neurotransmitter-stimulated ion transport by cultured porcine vas deferens epithelium. Am. J. Physiol. Renal Physiol. 281, F557-F570. [DOI] [PubMed] [Google Scholar]
- Shum, W. W. C., Da Silva, N., McKee, M., Smith, P. J. S., Brown, D. and Breton, S. (2008). Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 135, 1108-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibony, M., Segretain, D. and Gasc, J. M. (1994). Angiotensin-converting enzyme in murine testis: step-specific expression of the germinal isoform during spermiogenesis. Biol. Reprod. 50, 1015-1026. [DOI] [PubMed] [Google Scholar]
- Sinclair, M. L., Wang, X. Y., Mattia, M., Conti, M., Buck, J., Wolgemuth, D. J. and Levin, L. R. (2000). Specific expression of soluble adenylyl cyclase in male germ cells. Mol. Reprod. Dev. 56, 6-11. [DOI] [PubMed] [Google Scholar]
- Speth, R. C., Daubert, D. L. and Grove, K. L. (1999). Angiotensin II: a reproductive hormone too? Regul. Pept. 79, 25-40. [DOI] [PubMed] [Google Scholar]
- Stankovic, K. M., Brown, D., Alper, S. L. and Adams, J. C. (1997). Localization of pH regulating proteins H+ATPase and Cl–/HCO3– exchanger in the guinea pig inner ear. Hear. Res. 114, 21-34. [DOI] [PubMed] [Google Scholar]
- Stover, E. H., Borthwick, K. J., Bavalia, C., Eady, N., Fritz, D. M., Rungroj, N., Giersch, A. B., Morton, C. C., Axon, P. R., Akil, I. et al. (2002). Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J. Med. Genet. 39, 796-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun-Wada, G. H., Imai-Senga, Y., Yamamoto, A., Murata, Y., Hirata, T., Wada, Y. and Futai, M. (2002). A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J. Biol. Chem. 277, 18098-18105. [DOI] [PubMed] [Google Scholar]
- Sun-Wada, G. H., Murata, Y., Namba, M., Yamamoto, A., Wada, Y. and Futai, M. (2003). Mouse proton pump ATPase C subunit isoforms (C2-a and C2-b) specifically expressed in kidney and lung. J. Biol. Chem. 278, 44843-44851. [DOI] [PubMed] [Google Scholar]
- Sun-Wada, G. H., Wada, Y. and Futai, M. (2004). Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim. Biophys. Acta 1658, 106-114. [DOI] [PubMed] [Google Scholar]
- Thimon, V., Metayer, S., Belghazi, M., Dacheux, F., Dacheux, J. L. and Gatti, J. L. (2005). Shedding of the germinal angiotensin I-converting enzyme (gACE) involves a serine protease and is activated by epididymal fluid. Biol. Reprod. 73, 881-890. [DOI] [PubMed] [Google Scholar]
- Toyomura, T., Murata, Y., Yamamoto, A., Oka, T., Sun-Wada, G. H., Wada, Y. and Futai, M. (2003). From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J. Biol. Chem. 278, 22023-22030. [DOI] [PubMed] [Google Scholar]
- Visconti, P. E., Stewart-Savage, J., Blasco, A., Battaglia, L., Miranda, P., Kopf, G. S. and Tezon, J. G. (1999). Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol. Reprod. 61, 76-84. [DOI] [PubMed] [Google Scholar]
- Vitavska, O., Wieczorek, H. and Merzendorfer, H. (2003). A novel role for subunit C in mediating binding of the H+-V-ATPase to the actin cytoskeleton. J. Biol. Chem. 278, 18499-18505. [DOI] [PubMed] [Google Scholar]
- Wagner, C. A., Finberg, K. E., Breton, S., Marshansky, V., Brown, D. and Geibel, J. P. (2004). Renal vacuolar H+-ATPase. Physiol. Rev. 84, 1263-1314. [DOI] [PubMed] [Google Scholar]
- Wieczorek, H., Beyenbach, K. W., Huss, M. and Vitavska, O. (2009). Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 212, 1611-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, P. Y. (1988). Mechanism of adrenergic stimulation of anion secretion in cultured rat epididymal epithelium. Am. J. Physiol. 254, F121-F133. [DOI] [PubMed] [Google Scholar]
- Wong, P. Y. (1998). CFTR gene and male fertility. Mol. Hum. Reprod. 4, 107-110. [DOI] [PubMed] [Google Scholar]
- Wong, P. Y. and Uchendu, C. N. (1990). The role of angiotensin-converting enzyme in the rat epididymis. J. Endocrinol. 125, 457-465. [DOI] [PubMed] [Google Scholar]
- Wong, P. Y. D., Gong, X. D., Leung, G. P. H. and Cheuk, B. L. Y. (2002). Formation of the epididymal fluid microenvironment. In The Epididymis: From Molecules to Clinical Practice (ed. B. Robaire and B. T. Hinton), pp. 119-130. New York: Kluwer Academic/Plenum Publishers.
- Yang, Q., Li, G., Singh, S. K., Alexander, E. A. and Schwartz, J. H. (2006). Vacuolar H+-ATPase B1 subunit mutations that cause inherited distal renal tubular acidosis affect proton pump assembly and trafficking in inner medullary collecting duct cells. J. Am. Soc. Nephrol. 17, 1858-1866. [DOI] [PubMed] [Google Scholar]
- Yeung, C. H., Cooper, T. G., Oberpenning, F., Schulze, H. and Nieschlag, E. (1993). Changes in movement characteristics of human spermatozoa along the length of the epididymis. Biol. Reprod. 49, 274-280. [DOI] [PubMed] [Google Scholar]