Abstract
Background
The systemic response to injury is characterized by massive release of norepinephrine (NE) into the circulation as a result of global sympathetic activation. Multiple authors have demonstrated NE-mediated alterations in migration of circulating neutrophils to wounds. We hypothesized that NE further alters wound neutrophil phagocytic function through adrenergic signaling pathways.
Materials and Methods
A standard subcutaneous sponge wound model was employed. Murine wound neutrophils were harvested at 24 and 120 hours after injury and treated with physiologic (10−9M) and pharmacologic (10−6M) doses of norepinephrine. Phagocytosis of green fluorescent protein-labeled E. coli was assayed by flow cytometry. The signaling pathways mediating NE modulation of phagocytosis by wound neutrophils were defined by pharmacologic manipulation of alpha- and beta-adrenorecptors (ARs) and protein kinase A (PKA).
Results
Pharmacologic-dose NE, but not-physiologic-dose NE, suppressed the phagocytic efficiency of 120-hour wound neutrophils. This alteration in phagocytic efficiency appears to be mediated through alpha- and beta-ARs and downstream PKA. Phagocytosis by 24-hour wound neutrophils was not impacted by NE treatment.
Conclusions
The present study is the first to demonstrate NE-mediated alterations in the process of phagocytosis by wound neutrophils. We conclude that NE plays a temporally- and dose-defined immunomodulatory role in cutaneous wound healing through alterations in phagocytosis by wound neutrophils, and may represent a target for therapeutic manipulation of the innate immune response.
Keywords: Catecholamine, Norepinephrine, Neutrophil, Phagocytosis, Innate Immunity, Wound Healing
INTRODUCTION
Neutrophils are highly motile phagocytic cells that constitute the first line of defense of the innate immune system and are the predominant cellular component of the early inflammatory phase of wound healing(1). Following injury, they are recruited to the wound site from the circulation and function to protect the host against infection by phagocytosis of invading microorganisms, release of proteinases and generation of oxygen metabolites(2). Neutrophils are critical to the prevention of infection in wounds, playing a central role in host defense through phagocytosis and killing of pathogens at the site of injury(3).
While the human immune system is traditionally regarded as autonomous, the concept of nervous system modulation of immune function, or neuroimmunomodulation, dates back over one hundred years(4). Following wounding, there is destruction of noradrenergic nerve terminals innervating the injured tissue and release of norepinephrine into the peripheral circulation(5, 6). Further altering the post-injury catecholamine milieu in critically-ill patients is the routine usage of exogenous norepinephrine as a vasopressor agent for hemodynamic support in the intensive care unit. While there is considerable inter-patient variability in the degree of response to norepinephrine infusion because of disparate age, renal function, and hepatic function, it is clear that norepinephrine administration further increases circulating catecholamine levels, often to a degree that far exceeds physiologic levels(7). A growing body of literature indicates that norepinephrine and other adrenergic agonists can modulate many aspects of the immune response (initiative, proliferative and effector phases), altering production of and cellular responses to cytokines, lymphocyte proliferation, antibody secretion, and inflammatory gene expression(8–12). Additionally, the presence of α- and β-adrenoreceptors on neutrophils is well documented(13, 14). Previous studies have demonstrated that neutrophil adhesion to endothelial cells, requisite for localization to sites of injury, is inhibited by β-adrenergic stimulation(15, 16). Furthermore, adrenergic stimulation appears to inhibit neutrophil chemotaxis towards complement and lipopolysaccharide(17–19).
Once activated by tissue damage or infection, wound neutrophils that fail to encounter a pathogen to phagocytose will release their stored proteases into the extracellular space(20). This unrestrained activation results in the detrimental aspects of neutrophil infiltration, namely tissue liquefaction and pus production(2). Previous studies of peripheral blood neutrophils demonstrate alterations in phagocytosis(21–23) and oxidative burst activity(24) in response to elevated circulating catecholamines. However, there is increasing evidence in the literature that wound neutrophils are phenotypically different from their circulating counterparts(25, 26) and changes in the phenotype of wound neutrophils in response to catecholamines are poorly characterized.
The present study examines the role of norepinephrine in modulation of wound neutrophil phagocytic function. We demonstrate that pharmacologic doses of norepinephrine suppress the phagocytic efficiency of wound neutrophils through classical adrenergic receptor pathways. Additionally, this modulation of wound neutrophil function occurs at a time that correlates with clinical descriptions of the appearance of wound infections.
METHODS
Isolation of wound neutrophils
All animal procedures were reviewed and approved by the Loyola University Institutional Animal Use and Care Committee. To obtain wound neutrophils for in vitro analysis, a standard subcutaneous sponge model was employed(27). Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg). The dorsal side of the animals was shaved and scrubbed with betadine. A 2 cm longitudinal skin incision was made through the dermis and panniculus carnosus in a paraspinal location and two polyvinyl alcohol (PVA) sponges (20 x 5 x 2 mm, Rippey, El Dorado Hill, CA) were placed in dorsal subcutaneous pockets. The skin edges were approximated and closed with surgical clips. At 24 and 120 hours post-injury, the animals were sacrificed and the sponges retrieved and placed into 1 mL of Dulbecco’s PBS (D-PBS, Gibco BRL, Grand Island, NY) in a 17 x 100 mm polypropylene tube. Sponges were manually compressed to free inflammatory cells from the sponge matrix. The barrel of a 3 mL syringe was inserted into the top of each tube and the sponges placed into the syringe barrel. The syringe and tube were centrifuged at 200x g for 1–2 minutes to remove any residual liquid and cells from the sponges. Cells were counted using a hemocytometer and adjusted to a concentration of 1 x 106 cells/mL in Roswell Park Memorial Institute (RPMI)-1640 (Gibco BRL) media supplemented with 10% fetal bovine serum and placed in Teflon-coated tissue culture vials. To maximally simulate the wound environment and minimize post-harvest phenotypic alterations in cells, no further separation was undertaken and wound neutrophils were kept in suspension with other wound inflammatory cells (24h: Gr-1+/F4/80− Neutrophils 45±7.5%, 120h: Gr-1+/F4/80− Neutrophils 6.9±0.7%).
Cell treatment protocols
To determine if norepinephrine modulates phagocytosis by wound neutrophils, cells were initially divided into three groups (n=6 animals per group) and received no treatment, physiologic norepinephrine (10−9 M, Sigma, St. Louis, MO), or pharmacologic norepinephrine (10−6 M, Sigma). Cells were then maintained in culture overnight (18 hours) prior to assaying phagocytosis. To determine if norepinephrine-mediated changes in phagocytosis were adrenoreceptor dependent, cells were divided into three groups (n=5–6 animals per group) and received non-selective α-adrenergic blockade (phentolamine, 10−6 M, Sigma), non-selective β-adrenergic blockade (propranolol, 10−6 M, Sigma), or combined α- and β-adrenergic blockade (phentolamine and propranolol). Cells were pre-treated with adrenergic blockade for two hours prior to norepinephrine treatment as above (total of nine treatment groups). To determine if norepinephrine-mediated changes in phagocytosis involve the protein kinase A (PKA) signaling pathway, cells were incubated with H-89 (10−5 M, Sigma), a specific inhibitor of PKA, for two hours prior to norepinephrine treatment as above. The dosages and treatment durations employed were chosen to allow ease of comparison with previous studies(22, 23, 28).
E.coli Phagocytosis Assay
E.coli K-12 bacteria constitutively expressing green fluorescent protein (GFP-E.coli, a kind gift from ConjuGon, Inc., Madison, WI) were grown in LB broth containing chloramphenicol (20 μg/mL) in a shaking incubator at 37°C overnight. The following morning, bacteria were pelleted (1850x g for 15 minutes) and washed in 1X PBS (30 mL) three times. The bacteria were then re-suspended and diluted in 1X PBS to a final O.D. of 1.3–1.5 at λ=665 nm (~108 bacteria/mL). The bacteria were kept protected from light at room temperature until used to assay phagocytosis.
On the day prior to assay, inflammatory cells were isolated from retrieved subcutaneous sponges, as described above. Cells were incubated overnight at 37°C in Teflon-coated tissue culture vials. On the day of the assay, cells were washed twice with D-PBS and re-suspended in 1 mL of D-PBS with 0.1% glucose. An aliquot (100 μL) of the bacterial suspension was added to the cells, the cell-bacteria mixture pelleted (200x g x 5 min) to obtain maximal contact, and allowed to incubate at 37°C for 1 hour. From the time of addition of bacteria, all steps were carried out protected from light. The phagocytic process was arrested by the addition of 1 mL ice-cold PBS. The cells were washed twice with ice-cold PBS, resuspended in lysozyme (1 mg/mL in PBS, Amresco, Solon, OH) and incubated at 4°C for 20 minutes on an orbital shaker to remove adherent extracellular bacteria. Cells were then washed twice with ice-cold PBS.
For quantitative analysis, cells were stained with rat anti-mouse F4/80-APC (1:50 dilution, eBioscience, San Diego, CA) and rat anti-mouse Gr-1-PE (1:50 dilution, eBioscience) and maintained on ice until analyzed by flow cytometry (FACSCalibur or FACSCanto, Becton Dickinson, San Jose, CA). Neutrophils were identified by flow cytometry as Gr-1+F4/80− cells. Flow cytometry data analysis (FlowJo v6.4.2, TreeStar, Ashland, OR) included comparison of the percentage of cells that engaged in phagocytosis as well as comparison of the mean fluorescence intensity (MFI) of the GFP channel in the phagocytosis-positive cells (Phagocytic Index; proportional to number of bacteria ingested per cell; normalized to 100% in the control group to allow comparisons of data from multiple experiments).
For qualitative analysis, the post-phagocytosis cell suspension was centrifuged in a cytocentrifuge (Shandon Cytospin 2; Shandon, Oakland, CA), mounted with an anti-fade mounting medium (Vectashield, Vector Laboratories, Burlingame, CA) and maintained at 4°C until imaged by confocal microscopy (Zeiss LSM 510 microscope, Carl Zeiss, Thornwood, NJ).
Statistical Analysis
The mean and standard error of mean were calculated for each experimental group. Statistical analysis was performed using GraphPad Prism (Version 4.0, GraphPad Software, San Diego, CA). Data that were described over time were analyzed by analysis of variance (ANOVA) followed by Bonferonni post-comparison testing. Data described at single time points were analyzed by unpaired Student’s t test. Results with a p value less than 0.05 were considered statistically significant.
RESULTS
Norepinephrine suppresses wound neutrophil phagocytic efficiency in a temporally defined fashion
To determine if exogenous norepinephrine is able to suppress neutrophil phagocytosis, inflammatory cells were isolated from subcutaneous sponges 24 and 120 hours post-wounding and treated with physiologic norepinephrine, pharmacologic norepinephrine or with no treatment. After 18 hours of pre-treatment, phagocytosis of GFP-E.coli was assayed by flow cytometry. Norepinephrine treatment did not affect the viability of neutrophils in vitro (data not shown). Norepinephrine treatment did not modulate the percentage of neutrophils that undertook phagocytosis at either time point (24 hour: No Treatment 55 ± 3.2, Physiologic NE 59 ± 2.0, Pharmacologic 55 ± 4.3, p=NS, Figure 1A; 120 hour: No Treatment 29 ± 2.1, Physiologic NE 28 ± 5.1, Pharmacologic 19 ± 4.0, p=NS, Figure 2A). However, as a group, a smaller percentage of 120-hour neutrophils undertook phagocytosis as compared to those from 24-hour wounds (p<0.0001). Examination of phagocytic index, a measure of the number of bacteria ingested per cell, demonstrated that norepinephrine treatment did not alter the phagocytic efficiency of neutrophils from 24-hour wounds (No Treatment 100 ± 3.3, Physiologic NE 101 ± 4.6, Pharmacologic NE 102 ± 4.7; p=NS, Figure 1B). In contrast, pharmacologic NE, but not physiologic NE, decreased the phagocytic efficiency of 120-hour wound neutrophils (No Treatment 100 ± 4.1, Physiologic NE 92 ± 10, Pharmacologic NE 73 ± 7.1, p<0.05; Figure 2B and C). Due to this modulation, the intracellular mechanism mediating the suppression of phagocytic efficiency of 120-hour wound neutrophils by pharmacologic NE was investigated.
Figure 1. Norepinephrine does not modulate the phagocytic function of neutrophils from 24 hour wounds.
Animals (n=6 per group) underwent cutaneous incisional wounding with implantation of subcutaneous PVA sponges. Wound neutrophils were isolated from sponges harvested at 24 hours post-injury, placed in culture for 18 hours with media alone (Control), physiologic NE (Phys NE, 10−9 M) or pharmacologic NE (Pharm NE, 10−6 M), and phagocytosis of GFP-E.coli was assessed by flow cytometry. (A) Norepinephrine-treatment did not alter the percentage of neutrophils that engaged in phagocytosis (p=NS). (B) Representative phagocytosis histograms for control, physiologic NE-treated and pharmacologic NE-treated wound neutrophils. The height of each curve is proportional to the number of neutrophils ingesting GFP-E.coli of a given mean fluorescence intensity (MFI), where MFI increases in proportion to the number of bacteria. A shift in this curve to the right indicates more bacteria ingested per phagocyte and a shift to the left indicates fewer bacteria ingested per phagocyte. The geometric mean of each curve is calculated to provide the phagocytic index (C) and is analyzed by two-way ANOVA (p=NS).
Figure 2. Norepinephrine suppresses the phagocytic efficiency of neutrophils from 120-hour wounds.
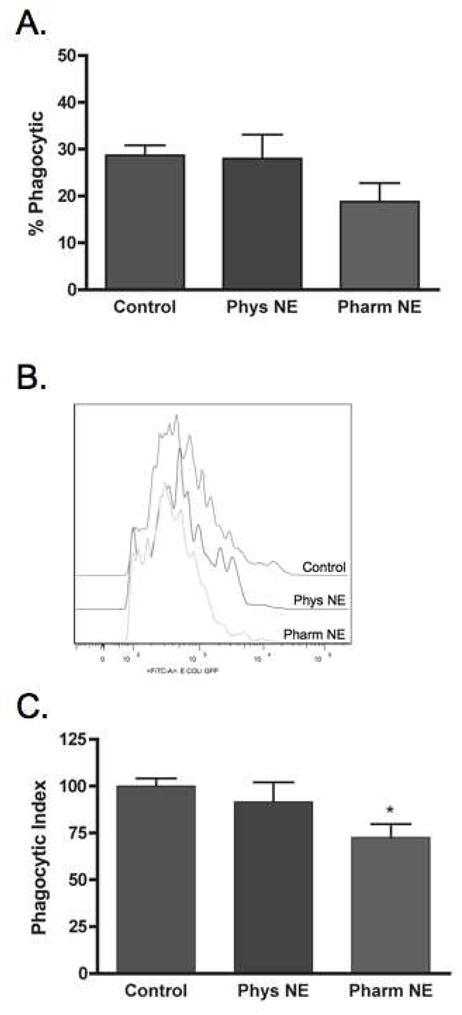
Animals (n=6 per group) underwent cutaneous incisional wounding with implantation of subcutaneous PVA sponges. Wound neutrophils were isolated from sponges harvested at 120 hours post-injury, placed in culture for 18 hours with media alone (Control), physiologic NE (Phys NE, 10−9 M), or pharmacologic NE (Pharm NE, 10−6 M), and phagocytosis of GFP-E.coli was assessed by flow cytometry. (A) Norepinephrine-treatment did not alter the percentage of neutrophils that engaged in phagocytosis (p=NS). (B) Representative phagocytosis histograms for control, physiologic NE-treated and pharmacologic NE-treated wound neutrophils. Pharmacologic-dose norepinephrine treatment resulted in a shift of the phagocytosis curve to the left, indicating fewer bacteria ingested per cell. The geometric mean of each curve was calculated to provide the phagocytic index (C) and was analyzed by two-way ANOVA followed by Bonferonni post-comparison testing. Pharmacologic-dose norepinephrine suppressed the phagocytic efficiency of 120-hour wound neutrophils (*p<0.05).
Norepinephrine-mediated suppression of wound neutrophil phagocytosis is adrenoreceptor dependent
Inflammatory cells were isolated from subcutaneous sponges 120 hours post-wounding and treated with α- or β-adrenergic blockade for two hours prior to treatment with pharmacologic norepinephrine. After 18 hours of pre-treatment, phagocytosis of GFP-E.coli was assayed by flow cytometry. Neither α-adrenergic blockade nor β-adrenergic blockade had any effect on wound neutrophil phagocytosis (data not shown). Pre-treatment with α-adrenergic blockade partially blocked the suppression of wound neutrophil phagocytosis by pharmacologic NE (Untreated Control 100 ± 3.0, Pharmacologic NE 75 ± 5.6, α-Blockade/Pharmacologic NE 81 ± 8.5, p=NS vs. Untreated control, Figure 3). Pre-treatment with β-adrenergic blockade similarly resulted in partial reversal of norepinephrine-mediated suppression of wound neutrophil phagocytic efficiency (β-Blockade/Pharmacologic NE 82 ± 7.0, p=NS vs. Untreated Control, Figure 3). Combined α- and β-adrenergic blockade pre-treatment had an additive effect and resulted in near-total blockade of norepinephrine-mediated suppression (α+β-Blockade/Pharmacologic NE 92 ± 3.8, p=NS vs. Untreated Control, Figure 3).
Figure 3. Norepinephrine-mediated suppression of wound neutrophil phagocytosis is adrenoreceptor dependent.

Animals (n=6 per group) underwent cutaneous incisional wounding with implantation of subcutaneous PVA sponges. Wound neutrophils were isolated from sponges harvested at 120 hours post-injury and received pre-treatment with alpha-adrenergic blockade, beta-adrenergic blockade, or combined alpha- and beta-adrenergic blockade. Following pre-treatment for two hours, cells were placed in culture for 18 hours with pharmacologic NE (10−6 M), and phagocytosis of GFP-E.coli was assessed by flow cytometry and compared to cells that were incubated in media alone, or with pharmacologic NE alone. (A) Representative phagocytosis histograms for control, pharmacologic NE only-treated, alpha-blockade & pharmacologic NE-treated, beta-blockade & pharmacologic NE-treated, and combined alpha/beta-adrenergic blockade & pharmacologic NE-treated wound neutrophils. Pharmacologic-dose norepinephrine treatment resulted in a shift of the phagocytosis curve to the left, indicating fewer bacteria ingested per cell. Neither alpha- nor beta-adrenergic blockade alone altered wound neutrophil phagocytosis (p=NS, data not shown). Alpha-adrenergic blockade alone and beta-adrenergic blockade partially prevented the suppression of wound neutrophil phagocytosis by pharmacologic norepinephrine (p=NS vs. Untreated Control). Combined alpha- and beta-adrenergic blockade resulted in near-total abrogation of norepinephrine-mediated suppression of wound neutrophil phagocytosis (p=NS vs. Untreated Control). (B) Quantitative representation of the results in (A), determined by calculating the geometric mean fluorescence intensity of GFP-E.coli and analyzed by two-way ANOVA followed by Bonferonni post-comparison testing (*p<0.05 vs. Untreated Control).
Norepinephrine-mediated suppression of wound neutrophil phagocytosis involves protein kinase A (PKA)
Catecholamine signaling via the α- and β-adrenoreceptors increases intracellular cAMP and the primary downstream target of cAMP is protein kinase A (PKA). To evaluate whether NE-mediated suppression of wound neutrophil function involves the cAMP/PKA pathway, inflammatory cells were isolated from subcutaneous sponges 120-hours post-wounding and treated with H-89, a potent inhibitor of protein kinase A (PKA), for two hours prior to treatment with pharmacologic norepinephrine. After 18 hours of pre-treatment, phagocytosis of GFP-E.coli was assayed by flow cytometry. PKA-inhibition alone, in the absence of norepinephrine, had no effect on wound neutrophil phagocytosis (data not shown). Pre-treatment with PKA-inhibitor blocked the suppression of wound neutrophil phagocytosis by pharmacologic NE (Untreated Control 100 ± 4.1 vs. H-89 + Pharmacologic NE 91 ± 2.9, p=NS vs. Untreated control, Figure 4).
Figure 4. Norepinephrine-mediated suppression of wound neutrophil phagocytosis involves protein kinase A (PKA).

Animals (n=6 per group) underwent cutaneous incisional wounding with implantation of subcutaneous PVA sponges. Wound neutrophils were isolated from sponges harvested at 120 hours post-injury and received pre-treatment with the PKA inhibitor H-89. Following pre-treatment for two hours, cells were placed in culture for 18 hours with pharmacologic NE (H-89+NE, 10−6 M), and phagocytosis of GFP-E.coli was assessed by flow cytometry and compared to cells that were incubated in media alone (Control), or with pharmacologic NE alone (Pharm NE). (A) Representative phagocytosis histograms for control, pharmacologic NE only-treated, and PKA-blockade & pharmacologic NE-treated wound neutrophils. Pharmacologic-dose norepinephrine treatment resulted in a shift of the phagocytosis curve to the left, indicating fewer bacteria ingested per cell. PKA-blockade alone had no effect on wound neutrophil phagocytosis (p=NS, data not shown). PKA inhibition prevented norepinephrine-mediated suppression of wound neutrophil phagocytosis (p=NS vs. Untreated Control). (B) Quantitative representation of the results in (A), determined by calculating the geometric mean fluorescence intensity of GFP-E.coli and analyzed by two-way ANOVA followed by Bonferonni post-comparison testing (*p<0.05 vs. Untreated Control).
DISCUSSION
Neutrophils are the first inflammatory cells to arrive to the site of injury where their primary role is phagocytosis of pathogens and debris(29). While their absolute necessity for the healing of sterile surgical wounds is debated(30, 31), there is clear evidence that neutrophil dysfunction leads to increased infectious complications(32). Previous studies have demonstrated phenotypic alterations of circulating neutrophils in response to catecholamine stimulation(17, 21–24, 33–38), however the effect of these stimuli on recruited wound neutrophils is unknown(39). The present study is the first to demonstrate norepinephrine-mediated alterations in the process of phagocytosis by wound neutrophils.
We have demonstrated that exogenous pharmacologic-dose norepinephrine decreased the phagocytic efficiency of neutrophils isolated from wounds 120 hours post-injury. Our results also indicate that norepinephrine-mediated suppression of wound neutrophil phagocytosis involves both the α- and β-adrenergic receptors and intracellular signaling via protein kinase A, the primary target of cAMP (Figure 5). Previous reports of catecholamine modulation of neutrophil phagocytosis have focused on exercise as a trigger of the stress response(40–42). Studies of peripheral blood neutrophils isolated from human subjects before and after exercise, as well as examination of exogenous catecholamine treatment on these cells, demonstrate that norepinephrine stimulates phagocytosis, and that this stimulation involves both α- and β-adrenoreceptor activation(22, 23). Indeed, there is a large body of literature demonstrating catecholamine-mediated stimulation of adrenoreceptors, elevation of intracellular cAMP and signaling via its associated protein kinases in neutrophils(13, 15, 17, 24, 33, 37, 38, 43, 44).
Figure 5. Putative model of norepinephrine-mediated alterations in 120-hour wound neutrophil phagocytosis.
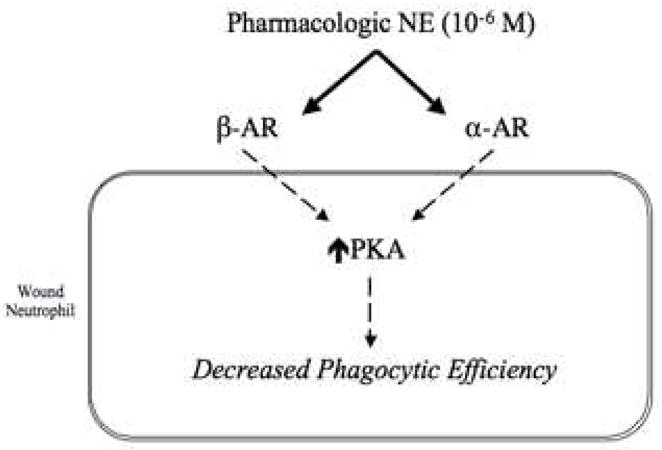
Based on the results of experiments including stimulation of neutrophils with norepinephrine and pharmacologic blockade of both α- and β-adrenergic subtypes, as well as the PKA signaling cascade, a putative model of norepinephrine-mediated modulation of wound neutrophil phagocytic efficiency is proposed. At a pharmacologic dose of NE, both adrenergic receptor subtypes appear to be involved in the reduction of wound neutrophil phagocytic efficiency, and the intracellular effects of NE treatment appear to be mediated via PKA. Future experiments to determine the mechanistic changes downstream of PKA that lead to alterations in phagocytic efficiency are warranted.
We observed that neutrophils isolated from 24-hour wounds, unlike those from 120-hour wounds, did not demonstrate norepinephrine-responsiveness of their phagocytic processes. This is in contrast to circulating 24-hour and 120-hour neutrophils from restraint-stressed animals, which display quantitatively similar alterations in phagocytosis at both time points(45). A number of possibilities for this temporal difference in the wound neutrophil response to norepinephrine exist. When comparing wounds versus time, a smaller percentage of cells from 120-hour wounds undertook phagocytosis than those at 24-hours. It is possible that this group of cells represents a subset of total wound neutrophils that exhibit norepinephrine-responsiveness(46), but does not comprise a large enough percentage of the 24-hour wound population for this difference to be detected. However, a previous, selective examination of neutrophil subsets from whole blood failed to demonstrate differences in catecholamine response(28). Alternatively, there is evidence that the pattern of gene expression of wound neutrophils differs from that of circulating cells, and these changes may evolve over time in the wound(26). Additionally, a temporal difference in catecholamine response may reflect a change in the neutrophils’ function in the wound over time. There is evidence that macrophage-produced tumor necrosis factor primes neutrophils for enhanced responsiveness to IL-8 through negative modulation of cAMP(47). In wound neutrophils from later time points, the altered catecholamine-responsiveness of phagocytosis may reflect these changes in cAMP signaling. The molecular basis for this difference between circulating and tissue neutrophils is unclear, but may be related to inflammatory priming(48). Circulating neutrophils demonstrate increased surface expression of L-selectin, a marker of cellular maturity, in response to catecholamines. However, there is shedding of this marker upon infiltration to the wound and during ongoing inflammation(49).
The model that we employed in order to investigate the catecholamine response of wound neutrophils is likely to influence the results that we have observed. In this system, wound inflammatory cells are isolated in aggregate, and no further attempts are made to separate individual cell types. As would be expected, neutrophils were the predominant cell type in the wounds at 24 hours (45±7.5%), with macrophages predominating at 120 hours (41±2%). While this is likely a better model of in vivo effects of catecholamines on these cell types, it does make comparison of our results to those of groups employing purified cell populations less straightforward. Injury and sepsis result in the production of marked amounts of both pro- and anti-inflammatory cytokines by neutrophils and macrophages and multiple studies have demonstrated alterations in the production of pro-inflammatory cytokines in response to catecholamines(50). While it is difficult to develop a consistent framework of catecholamine/cytokine/phagocytosis interactions because of the heterogeneity of cell types and doses used in various studies, recent work indicates that catecholamine effects on target cells are dependent upon the cellular level of cyclic AMP and alteration of gene transcription via cAMP-reponse element binding (CREB) protein(51–54).
A handful of studies have examined alterations in neutrophil phagocytosis following sympathectomy(55–57). While these studies differ in the technique of sympathectomy employed, in all cases the phagocytic function of peripheral blood neutrophils was assessed and compared between sympathectomized subjects and un-treated controls. Each group of investigators observed a decrease in phagocytosis by neutrophils in the sympathectomy group. These results further augment the observation that exogenous catecholamines enhance peripheral blood neutrophil phagocytic function(23). Additionally, these studies provide a nice counterpoint for our observation that, following sympathectomy, wound neutrophils demonstrated increased phagocytosis (unpublished observation), which is consistent with the present finding that exogenous norepinephrine suppresses wound neutrophil phagocytic function.
Following phagocytosis, neutrophils kill pathogens with antimicrobial proteins and production of reactive oxygen species such as superoxide, hydrogen peroxide and hypochlorous acid(29). Because these same fundamental biochemical processes that govern microbial killing by neutrophils are capable of damaging host cells and tissue(20), neutrophils are implicated as the mediators of tissue injury in a variety of inflammatory disorders(58). In addition to modulating the process of phagocytosis, catecholamines may represent a target for the manipulation of these potentially detrimental neutrophil functions(24), and further investigation of these processes is warranted.
Our results reinforce a role for norepinephrine in the modulation of the phagocytic processes of neutrophils. Importantly, our observations in concert with those of other groups, point towards important functional differences between circulating and wound neutrophils as well as temporal differences between early and late wound neutrophils. Examination of associated neutrophil functions, such as production of reactive oxygen species, as well as changes in the neutrophil transcriptome as a potential final common pathway for alterations in phagocytosis and catecholamine-responsiveness may yield targets for future work in the therapeutic manipulation of innate immunity to influence wound healing.
Acknowledgments
This work was supported by NIH T32-GM08750 (AG), R01-GM42577 (RLG), R01-GM55238 (LAD), and the Dr. Ralph and Marion C. Falk Medical Research Trust (AG, RLG, LAD).
Footnotes
Portions of this manuscript were presented at:
- Academic Surgical Congress – San Diego, California, February 2006
- American Academy of Pediatrics - Surgical Section, Atlanta, Georgia, October 2006
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ankush Gosain, Email: agosain@lumc.edu.
Richard L. Gamelli, Email: rgamell@lumc.edu.
Luisa A. DiPietro, Email: ldipiet@uic.edu.
References
- 1.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 4.Tonkoff W. Zur Kenntnis der Nerven der Lymphdrüsen. Anat Anz. 1899;16:456–459. [Google Scholar]
- 5.Woolf PD, McDonald JV, Feliciano DV, Kelly MM, Nichols D, Cox C. The catecholamine response to multisystem trauma. Arch Surg. 1992;127:899–903. doi: 10.1001/archsurg.1992.01420080033005. [DOI] [PubMed] [Google Scholar]
- 6.Zetterstrom BE, Palmerio C, Fine J. Protection Of Functional And Vascular Integrity Of The Spleen In Traumatic Shock By Denervation. Proc Soc Exp Biol Med. 1964;117:373–376. doi: 10.3181/00379727-117-29584. [DOI] [PubMed] [Google Scholar]
- 7.Beloeil H, Mazoit JX, Benhamou D, Duranteau J. Norepinephrine kinetics and dynamics in septic shock and trauma patients. Br J Anaesth. 2005;95:782–788. doi: 10.1093/bja/aei259. [DOI] [PubMed] [Google Scholar]
- 8.Alaniz RC, Thomas SA, Perez-Melgosa M, Mueller K, Farr AG, Palmiter RD, Wilson CB. Dopamine beta-hydroxylase deficiency impairs cellular immunity. Proc Natl Acad Sci U S A. 1999;96:2274–2278. doi: 10.1073/pnas.96.5.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinstein DL, Heneka MT, Gavrilyuk V, Dello Russo C, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem Int. 2002;41:357–365. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 10.Frohman EM, Vayuvegula B, van den Noort S, Gupta S. Norepinephrine inhibits gamma-interferon-induced MHC class II (Ia) antigen expression on cultured brain astrocytes. J Neuroimmunol. 1988;17:89–101. doi: 10.1016/0165-5728(88)90017-3. [DOI] [PubMed] [Google Scholar]
- 11.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 12.Szelenyi J. Cytokines and the central nervous system. Brain Res Bull. 2001;54:329–338. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 13.Abraham E, Kaneko DJ, Shenkar R. Effects of endogenous and exogenous catecholamines on LPS-induced neutrophil trafficking and activation. Am J Physiol. 1999;276:L1–8. doi: 10.1152/ajplung.1999.276.1.L1. [DOI] [PubMed] [Google Scholar]
- 14.Carr DJ. Neuroendocrine peptide receptors on cells of the immune system. Chem Immunol. 1992;52:84–105. doi: 10.1159/000319386. [DOI] [PubMed] [Google Scholar]
- 15.Boxer LA, Allen JM, Baehner RL. Diminished polymorphonuclear leukocyte adherence. Function dependent on release of cyclic AMP by endothelial cells after stimulation of beta-receptors by epinephrine. J Clin Invest. 1980;66:268–274. doi: 10.1172/JCI109853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derian CK, Santulli RJ, Rao PE, Solomon HF, Barrett JA. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by cAMP modulators. J Immunol. 1995;154:308–317. [PubMed] [Google Scholar]
- 17.Silvestri M, Oddera S, Lantero S, Rossi GA. beta 2-agonist-induced inhibition of neutrophil chemotaxis is not associated with modification of LFA-1 and Mac-1 expression or with impairment of polymorphonuclear leukocyte antibacterial activity. Respir Med. 1999;93:416–423. doi: 10.1053/rmed.1999.0584. [DOI] [PubMed] [Google Scholar]
- 18.Deitch EA, Bridges RM. Stress hormones modulate neutrophil and lymphocyte activity in vitro. J Trauma. 1987;27:1146–1154. doi: 10.1097/00005373-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 19.McCorkle FM, Taylor RL, Jr, Denno KM, Jabe JM. Monoamines alter in vitro migration of chicken leukocytes. Dev Comp Immunol. 1990;14:85–93. doi: 10.1016/0145-305x(90)90010-c. [DOI] [PubMed] [Google Scholar]
- 20.Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega E. Neuroendocrine mediators in the modulation of phagocytosis by exercise: physiological implications. Exerc Immunol Rev. 2003;9:70–93. [PubMed] [Google Scholar]
- 22.Ortega E, Collazos ME, Maynar M, Barriga C, De la Fuente M. Stimulation of the phagocytic function of neutrophils in sedentary men after acute moderate exercise. Eur J Appl Physiol Occup Physiol. 1993;66:60–64. doi: 10.1007/BF00863401. [DOI] [PubMed] [Google Scholar]
- 23.Ortega E, Marchena JM, Garcia JJ, Barriga C, Rodriguez AB. Norepinephrine as mediator in the stimulation of phagocytosis induced by moderate exercise. Eur J Appl Physiol. 2005;93:714–718. doi: 10.1007/s00421-004-1245-8. [DOI] [PubMed] [Google Scholar]
- 24.Weiss M, Schneider EM, Tarnow J, Mettler S, Krone M, Teschemacher A, Lemoine H. Is inhibition of oxygen radical production of neutrophils by sympathomimetics mediated via beta-2 adrenoceptors? J Pharmacol Exp Ther. 1996;278:1105–1113. [PubMed] [Google Scholar]
- 25.Roy S, Khanna S, Yeh PE, Rink C, Malarkey WB, Kiecolt-Glaser J, Laskowski B, Glaser R, Sen CK. Wound site neutrophil transcriptome in response to psychological stress in young men. Gene Expr. 2005;12:273–287. doi: 10.3727/000000005783992025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theilgaard-Monch K, Knudsen S, Follin P, Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172:7684–7693. doi: 10.4049/jimmunol.172.12.7684. [DOI] [PubMed] [Google Scholar]
- 27.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 28.Wenisch C, Parschalk B, Weiss A, Zedwitz-Liebenstein K, Hahsler B, Wenisch H, Georgopoulos A, Graninger W. High-dose catecholamine treatment decreases polymorphonuclear leukocyte phagocytic capacity and reactive oxygen production. Clin Diagn Lab Immunol. 1996;3:423–428. doi: 10.1128/cdli.3.4.423-428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 30.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 31.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost. 2004;92:275–280. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 32.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns AM, Keogan M, Donaldson M, Brown DL, Park GR. Effects of inotropes on human leucocyte numbers, neutrophil function and lymphocyte subtypes. Br J Anaesth. 1997;78:530–535. doi: 10.1093/bja/78.5.530. [DOI] [PubMed] [Google Scholar]
- 34.Gosain A, Jones SB, Shankar R, Gamelli RL, DiPietro LA. Norepinephrine modulates the inflammatory and proliferative phases of wound healing. J Trauma. 2006;60:736–744. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 35.Moon BC, Girotti MJ, Dawson R, Wren SF. PMN superoxide radical production following a metabolic-endocrine simulation of trauma. Ann Surg. 1986;203:246–249. doi: 10.1097/00000658-198603000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qualliotine D, DeChatelet LR, McCall CE, Cooper MR. Stimulation of oxidative metabolism in polymorphonuclear leukocytes by catecholamines. J Reticuloendothel Soc. 1972;11:263–276. [PubMed] [Google Scholar]
- 37.Schopf RE, Lemmel EM. Control of the production of oxygen intermediates of human polymorphonuclear leukocytes and monocytes by beta-adrenergic receptors. J Immunopharmacol. 1983;5:203–216. doi: 10.3109/08923978309039106. [DOI] [PubMed] [Google Scholar]
- 38.Wahle M, Greulich T, Baerwald CG, Hantzschel H, Kaufmann A. Influence of catecholamines on cytokine production and expression of adhesion molecules of human neutrophils in vitro. Immunobiology. 2005;210:43–52. doi: 10.1016/j.imbio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Paty PB, Graeff RW, Waldman FM, Hunt TK, Mathes SJ. Biologic priming of neutrophils in subcutaneous wounds. Arch Surg. 1988;123:1509–1513. doi: 10.1001/archsurg.1988.01400360079013. [DOI] [PubMed] [Google Scholar]
- 40.Kjaer M. Epinephrine and some other hormonal responses to exercise in man: with special reference to physical training. Int J Sports Med. 1989;10:2–15. [PubMed] [Google Scholar]
- 41.Hoffman-Goetz L, Pedersen BK. Exercise and the immune system: a model of the stress response? Immunol Today. 1994;15:382–387. doi: 10.1016/0167-5699(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 42.Mazzeo RS, Rajkumar C, Jennings G, Esler M. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. J Appl Physiol. 1997;82:1869–1874. doi: 10.1152/jappl.1997.82.6.1869. [DOI] [PubMed] [Google Scholar]
- 43.Nielson CP. Beta-adrenergic modulation of the polymorphonuclear leukocyte respiratory burst is dependent upon the mechanism of cell activation. J Immunol. 1987;139:2392–2397. [PubMed] [Google Scholar]
- 44.Kopprasch S, Gatzweiler A, Graessler J, Schroder HE. Beta-adrenergic modulation of FMLP- and zymosan-induced intracellular and extracellular oxidant production by polymorphonuclear leukocytes. Mol Cell Biochem. 1997;168:133–139. doi: 10.1023/a:1006855020989. [DOI] [PubMed] [Google Scholar]
- 45.Shilov YI, Orlova EG. Adrenergic regulation of phagocytic activity of peripheral blood neutrophils, monocytes, and eosinophils in stressed rats. Bull Exp Biol Med. 2000;129:477–479. doi: 10.1007/BF02439808. [DOI] [PubMed] [Google Scholar]
- 46.Frey MJ, Mancini D, Fischberg D, Wilson JR, Molinoff PB. Effect of exercise duration on density and coupling of beta-adrenergic receptors on human mononuclear cells. J Appl Physiol. 1989;66:1494–1500. doi: 10.1152/jappl.1989.66.3.1494. [DOI] [PubMed] [Google Scholar]
- 47.Brandt E, Petersen F, Flad HD. Recombinant tumor necrosis factor-alpha potentiates neutrophil degranulation in response to host defense cytokines neutrophil-activating peptide 2 and IL-8 by modulating intracellular cyclic AMP levels. J Immunol. 1992;149:1356–1364. [PubMed] [Google Scholar]
- 48.Ahmed NA, Christou NV. Decreased neutrophil L-selectin expression in patients with systemic inflammatory response syndrome. Clin Invest Med. 1996;19:427–434. [PubMed] [Google Scholar]
- 49.McGill SN, Ahmed NA, Hu F, Michel RP, Christou NV. Shedding of L-selectin as a mechanism for reduced polymorphonuclear neutrophil exudation in patients with the systemic inflammatory response syndrome. Arch Surg. 1996;131:1141–1146. doi: 10.1001/archsurg.1996.01430230023005. discussion 1147. [DOI] [PubMed] [Google Scholar]
- 50.Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006;21:160–172. doi: 10.1177/0885066605284330. [DOI] [PubMed] [Google Scholar]
- 51.Dhingra VK, Uusaro A, Holmes CL, Walley KR. Attenuation of lung inflammation by adrenergic agonists in murine acute lung injury. Anesthesiology. 2001;95:947–953. doi: 10.1097/00000542-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 52.Haraguchi S, Good RA, Day NK. Immunosuppressive retroviral peptides: cAMP and cytokine patterns. Immunol Today. 1995;16:595–603. doi: 10.1016/0167-5699(95)80083-2. [DOI] [PubMed] [Google Scholar]
- 53.Misra UK, Pizzo SV. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J Biol Chem. 2005;280:38276–38289. doi: 10.1074/jbc.M507332200. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura A, Johns EJ, Imaizumi A, Abe T, Kohsaka T. Regulation of tumour necrosis factor and interleukin-6 gene transcription by beta2-adrenoceptor in the rat astrocytes. J Neuroimmunol. 1998;88:144–153. doi: 10.1016/s0165-5728(98)00109-x. [DOI] [PubMed] [Google Scholar]
- 55.Campagnolo DI, Bartlett JA, Keller SE, Sanchez W, Oza R. Impaired phagocytosis of Staphylococcus aureus in complete tetraplegics. Am J Phys Med Rehabil. 1997;76:276–280. doi: 10.1097/00002060-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Carter L, Ferrari JK, Davison JS, Befus D. Inhibition of neutrophil chemotaxis and activation following decentralization of the superior cervical ganglia. J Leukoc Biol. 1992;51:597–602. doi: 10.1002/jlb.51.6.597. [DOI] [PubMed] [Google Scholar]
- 57.Derevenco P, Marina C, Pavel T, Olteanu A, Junie M, Baciu I. Phagocytic response in rats following chemical sympathectomy with 6-hydroxydopamine. Rev Roum Physiol. 1992;29:57–62. [PubMed] [Google Scholar]
- 58.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]



