Abstract
HIV-1 infection induces a progressive disruption of the B cell compartment impairing long-term immune responses to routine immunizations. Depletion of specific memory B cell pools occurs during the 1st stages of the infection and cannot be reestablished by antiretroviral treatment. We reasoned that an early control of viral replication through treatment could preserve the normal development of the memory B cell compartment and responses to routine childhood vaccines. Accordingly, we evaluated the effects of different highly-active antiretroviral therapy (HAART) schedules in 70 HIV-1 vertically-infected pediatric subjects by B cell phenotypic analyses, antigen-specific B cell enzyme-linked immunosorbent spot (ELISpot) and ELISA for common vaccination and HIV-1 antigens. Initiation of HAART within the 1st year of life permits the normal development and maintenance of the memory B cell compartment. On the contrary, memory B cells from patients treated later in time are remarkably reduced and their function is compromised regardless of viral control. A cause for concern is that both late-treated HIV-1 controllers and noncontrollers loose protective antibody titers against common vaccination antigens. Timing of HAART initiation is the major factor predicting the longevity of B cell responses in vaccinated HIV-1-infected children.
Keywords: B cells memory, HAART, HIV-infected children, vaccination schedule
During HIV-1 infection, immunological functions are gradually lost with the depletion of CD4+ T cells (1). In parallel, several abnormalities occur in the B cell compartment including a progressive decline in total CD27+ memory B cells, hypergammaglobulinemia, impaired reactivity to immunization and loss of specific antibodies gained during the normal vaccination schedule (2–4). Highly-active antiretroviral therapy (HAART) reduces hypergammaglobulinemia (5) and increases the absolute B cell count (4, 6). A critical question is whether the introduction of HAART recovers and/or maintains the B cell compartment fully functional. Several authors suggested that phenotypic and functional alterations in the B cell compartment persist in HIV-1-infected patients despite HAART treatment (3, 4, 7, 8). Indeed, impaired humoral responses to vaccinations have been reported in HIV-1-infected children despite successful HAART (9, 10). However, no significantly different immunization schedules have been provided for these patients so far (11, 12). Responses to immunizations are complex, requiring the integration of several components of the immune system, including B- and T-lymphocytes, which are under development during the 1st year of life (13–15). HIV-1 may quantitatively and qualitatively affect these cells in a time-dependent manner. In previous articles, we and others showed that HAART initiated within the 1st year of life permits the normal development of an intact T cell repertoire (16–21). Even for the B cell compartment the detrimental elimination of antigen-specific pools of memory B cells occurs during the 1st stages of HIV-1 infection (22); thus, timing of treatment initiation may result in a different grade of disturbances of the memory B cell compartment. Here, we evaluated the effects of different HAART schedules on B cell responses in a cohort of 70 HIV-1 vertically-infected pediatric subjects. We reasoned that an early control of viral replication through treatment could preserve the normal development of the memory B cell compartment and responses to routine childhood vaccines. Our results show that HAART administered within the 1st year of life preserves the memory B cell compartment and its ability to mount specific secondary immune responses after antigenic challenge. On the contrary, patients treated later in time loose this ability suggesting that vaccine schedules might need to be revised in this latter group.
Results
Early Initiation of Highly-Active Antiretroviral Therapy Preserves Memory B cells in Vertically HIV-1 Infected Children.
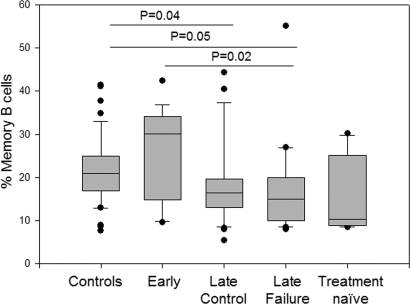
Memory B cells (CD19+CD27+) in the different HIV-1-infected groups and healthy controls were analyzed by flow cytometry. Early-treated patients maintained high percentages of memory B cells comparable to those observed in healthy controls whereas patients who started HAART later in life showed lower percentages (Fig. 1). A significant difference was found between the control group and both the late control and the late failure group (P = 0.04 and P = 0.05 respectively) and between the early and late failure group (P = 0.02) (Fig. 1). Memory B cell percentages did not show any correlation to age, Centers for Disease Control and Prevention (CDC) stage, CD4+ T cell/total B cell count and HIV-1 viral load. The same analysis was performed for IgM memory B cells (CD19+, CD27+, IgM+, IgD+) (23) and for switched memory B cells (CD19+, CD27+, IgM−, IgD−). However, despite a similar trend to the data on the total memory B cells was observed, results did not reach statistical significance.
Fig. 1.
Early initiation of highly-active antiretroviral therapy preserves memory B cells in vertically HIV-1-infected children. Box plot analyses on the memory B cell percentages in controls and patients with different antiretroviral schedule.
Patients Treated Early Have the Capacity to Generate and Preserve Antigen-Specific Memory B Cells.
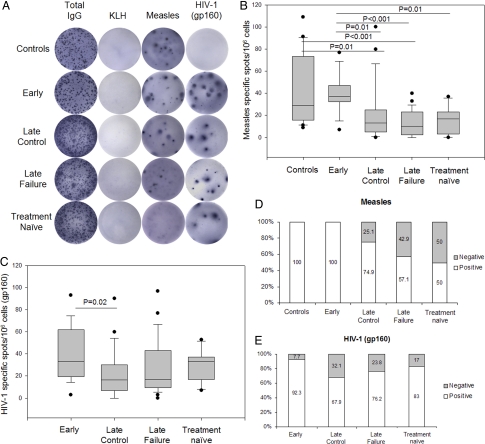
The presence of measles- and gp160-specific memory B cell pools in the different HIV-1-infected groups and healthy controls was analyzed by B cell enzyme-linked immunosorbent spot (ELISpot). A significant difference in the amount of measles-specific spots was found between early and late-treated patients regardless of viral control (P = 0.01 and P < 0.001 for the late control and failure group respectively) and between early and treatment naïve patients (P = 0.01) (Fig. 2 A and B). A progressive increase in the percentage of nonresponders to measles B cell ELISpot was observed among children starting HAART over the 1st year of life (25.1% for the late control group, 42.9% for the late failure group and 50% for the treatment naïve group) (Fig. 2D). No difference was found between early-treated patients and healthy controls (Fig. 2 B and D). The HIV-1 gp160-specific spot formation showed the same trend observed for the measles antigen although significance was only found between the early-treated and the late control groups (P = 0.02) (Fig. 2C). Accordingly, B cells from almost all early-treated patients (92.3%) showed the ability to produce gp160-specific spots compared with late-treated (67.9% for the late control group and 76.2% for the late failure group) and treatment naïve patients (83%) (Fig. 2E).
Fig. 2.
Early-treated patients generate and preserve antigen-specific memory B cells. (A) Representative ELISpot analyses. (B and C) Box plot analyses on the measles-specific and gp160-specific spot production in controls and patients with different antiretroviral schedule. (D and E) Percentages of patients responding to measles and HIV-1 gp160 ELISpot among groups. White bars, positive response; gray bars, negative response.
Early Initiation of Highly-Active Antiretroviral Therapy Leads to the Maintenance of Antibody Titers Above Protective Threshold in HIV-1 Infected Children.
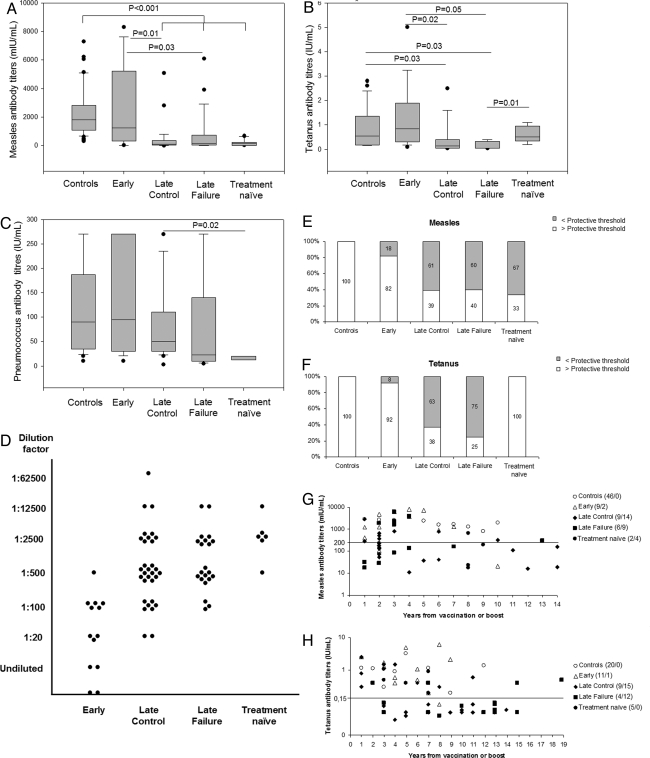
The levels of plasma antibodies against routine vaccination antigens including measles, tetanus toxoid and pneumococcus were measured in vertically HIV-1-infected patients compared with healthy controls (Fig. 3 A–C). Antibody titers against measles were lower in patients starting HAART over 1 year of age compared with healthy controls (P < 0.001) and early-treated patients (P = 0.01 for the late control and P = 0.03 for the late failure group) (Fig. 3A). Antitetanus antibodies were also higher in the early-treated compared with the late-treated patients (P = 0.02 for the late control group and P = 0.05 for the late failure group respectively) (Fig. 3B). Interestingly, a significant difference was also found between the late failure and the treatment naïve group (P = 0.01). A similar trend was observed for the levels of pneumoccoccus-specific antibodies although only the results between the late control group and the treatment naïve patients reached statistical significance (P = 0.02) (Fig. 3C). Moreover, antibody titers against HIV-1 were measured (Fig. 3D) and the late-treated groups and the treatment naïve group showed higher concentrations of anti-gp41 antibodies compared with early-treated patients (Fig. 3D). Among this latter group, 2 children who started treatment within the 1st year of life resulted persistently seronegative with this standard HIV-1 gp41 diagnostic ELISA technique (Fig. 3D). Finally, we evaluated the percentages of patients carrying antibody titers above the protective threshold of 0.2 units/mL for measles (24) and 0.15 units/mL for tetanus (25). All healthy controls and the majority of early-treated patients maintained antibody titers against measles and tetanus above protective threshold overtime (82% and 92% respectively). Conversely, more than half of the patients starting treatment after 1 year from birth presented with antibodies below protective threshold (Fig. 3 E and F). In the treatment naïve group, the majority of the patients had specific antibody titers below protective threshold for measles (67%) but not for tetanus (Fig. 3 E and F). The time after vaccination or boost for measles and tetanus antigens had a minimal influence on antibody titers in healthy controls and early-treated patients who maintained high antibody titers up to 10 and 7 years. Conversely, patients treated later than 1 year of life presented with antibody levels below protective threshold already 1 year after vaccination or booster dose (Fig. 3 G and H).
Fig. 3.
Early initiation of treatment permits the maintenance of anti measles and tetanus antibody titers above protective threshold in HIV-1 vertically-infected children. (A–C) Box plot analyses on the measles-, tetanus-, and pneumococcus-specific antibody titers. (D) Gp41-specific antibody detection. (E and F) Percentages of patients with measles- and tetanus-specific antibody titers above protective threshold. White bars, positive response; gray bars, negative response. (G and H) Scatter plot analyses on the measles- and tetanus-specific antibody titers in relation to years from vaccination or boost; the line indicates protective threshold. Shown are data from single time point sampling.
Discussion
In the present study, we identified timing of HAART initiation as the major factor predicting the longevity of memory B cell responses and immune protection in vaccinated HIV-1-infected children.
During the natural course of HIV-1 infection, B cells undergo phenotypic alterations and loss of functionality, including depletion of memory B cell pools, which are responsible for the maintenance of serologic memory (3, 26, 27). Antiretroviral therapy permits only partial restoration of the memory B cell compartment (4). In adults, most beneficial effects are obtained when therapy is applied during primary HIV-1 infection (PHI) (22). Perinatal HIV-1 infection is acquired in the milieu of a developing immune system and consequently, in the absence of antiretroviral treatment, PHI in children results in higher levels of HIV-1 viremia compared with adults (28). The decline in plasma virus levels requires considerably longer time during childhood, thus resulting in higher systemic viral exposure during the first 2 years of life (21). The maturation process of the immune system in presence of active HIV-1 replication remains poorly studied. Timing of viral suppression through HAART might be crucial in defining the stage of immune dysfunction (27).
We found that initiation of HAART within the 1st year of life in HIV-1 vertically-infected children is able to preserve the normal percentages of memory B cells. To assess whether this preserved memory B cell compartment is fully functional and able to differentiate into specific antibody secreting cells upon polyclonal stimulation in vitro, we performed B cell ELISpot assays against measles, an antigen directly correlated to the memory B cell percentage (29) and against HIV-1 gp160. Specimens from all of the early-treated patients were able to produce spots against measles and HIV-1. Taken together these results suggest that in early-treated patients the memory B cell compartment is fully functional for a representative common vaccination antigen as measles and for an antigen present since birth as the HIV-1 gp160. Moreover, we found that early-treated patients presented low or undetectable anti-HIV-1 antibody levels in the standard laboratory test against the HIV-1 gp41. This is a common phenomenon among HIV-1-infected infants receiving HAART early in life (21, 30). The apparent paradox of HIV-1-infected individuals remaining seronegative with standard serologic diagnostic procedures is a peculiarity of HIV-1 infection when HAART is started during PHI (31, 32). Therefore, the HIV-1-specific B cell ELISpot, may represent an additional important diagnostic tool for evaluating the presence and quality of HIV-1-specific antibodies and memory B cells in this population. Indeed, in this study, specimens obtained from early-treated patients led to high levels of HIV-1-specific spot forming cells despite low or undetectable titers of anti-HIV-1 antibodies.
To assess whether an early application of HAART also allows the immune system to respond and to maintain protective antibody titers upon common routine childhood vaccinations, we analyzed humoral responses to measles, tetanus and pneumococci. Noteworthy, the early-treated group developed and maintained antibody levels above protective threshold after measles and tetanus vaccination whereas the late-treated patients were below threshold despite successful HAART. These data are not due to the natural antibody decay because patients in the different groups have similar median years from vaccination or boost. This suggests that the maintenance of specific antibody titers observed in this study is related to a better maturation and preservation of the memory B cell compartment as a direct consequence of an early application of HAART. Interestingly, patients naïve to treatment maintained high levels of antitetanus antibodies. This is in accordance with a previous report showing that a preserved response to in vitro stimulation to tetanus toxoid is associated with long-term non progressive status in HIV-1 vertically-infected children (33).
However, the waning of immunity observed in the late-treated patients may be also related to HIV-1-associated B cell exhaustion. This phenomenon has already been indicated for a population of tissue-like memory B cells in peripheral blood, lacking the expression of CD27 and presenting low levels of surface CD21 (34). In this population, the chemokine and inhibitory receptor profiles are similar to those described on the antigen-specific T cells as a result of their exhaustion (34). Furthermore, these B cells presented low Ig diversity compared with memory B cells, indicating that they may be part of a dysfunctional memory B cell compartment (34). In this respect, we have recently shown that during chronic HIV-1 infection, both CD27+ and CD27- B cells present alterations in the expression of the receptor-ligand pair CXCR5/CXCL13 and have altered migration patterns probably as a result of B cell hyperactivation and exhaustion (35). This may lead to defective B/T cell contacts during germinal center reactions thus impairing the maintenance of specific antibodies during secondary immune responses (35).
If maintenance of specific antibody titers was only due to specific memory B cell formation, and to the ability of those memory B cells to differentiate into plasma cells upon reinfection, the relation between circulating antibody titers and specific memory B cells should be very stringent. However, the strength of this correlation varies considerably in several different studies (29, 36–38). This might be partially explained by a different sensitivity between B cell ELISpot and ELISA. Nevertheless, data in this study showed a clear trend toward a positive correlation between the specific antibody titers to measles and the number of measles-specific spots (R = 0.25, P = 0.04). Interestingly, the maintenance of antimeasles and antitetanus antibody titers above protective threshold occurs in the early-treated patients whereas this is not measurable for pneumococcal vaccination because its immunogenicity does not necessarily translate into long-term clinical protection (39). Antibody quantification by ELISA may not equate with antibody efficacy in vivo; protection may be related not only to antibody concentration but also to antibody avidity and function (39). Furthermore, to evaluate positive responses to pneumococcal polysaccharide vaccine (PPV) the use of arbitrary levels is less accurate than comparing fold increase from baseline (39) and the presence of opsonizing antibodies should be considered. Finally, post vaccination pneumococcal IgG levels seem to be reduced in treatment naïve and HAART-treated HIV-1-infected patients (40, 41). The mechanisms underlying this condition are poorly known. In this context, of particular interest is the observation that transitional B cells stimulated by CpG terminally differentiate into plasma cells producing natural antibodies with innate pneumoccoccal specificity (42). It has been recently shown that TLR9 responsiveness to CpG stimulation is diminished in HIV-1 disease (43). Despite these limitations, a positive but not significant trend of higher levels of pneumococcal antibody titers was observed in the early-treated group.
To obtain an optimal response upon vaccination, a preserved humoral and cellular response is required (12). In HIV-1 infection, this could be obtained through the application of an early antiretroviral therapy as we demonstrated in this study. Noteworthy, to reduce the morbidity and mortality caused by preventable infectious diseases, our data prompt clinicians to periodically check specific antibody levels, down to 1 year from vaccination or last boost, in HIV-1 vertically-infected children initiating HAART later than the 1st year of life. Additional booster vaccinations must be taken into account in this population.
Methods
Study Design, Subjects, and Vaccinations.
This study is a cross-sectional analysis of 70 HIV-1 vertically-infected pediatric subjects enrolled at the Children Hospital Bambino Gesù, Rome, Italy. Children who started HAART within the 1st year of life were defined as “Early”-treated patients whereas “Late”-treated patients include children treated later in life. This latter group was subsequently subdivided into “Late Control” and “Late Failure” with respect to viral control. Patients with no therapeutic history were defined as “Treatment naïve.” All children were treated according to pediatric HIV-1 guidelines (44). National routine vaccination protocol (www.ministerodellasalute.it) was applied for measles (Priorix, GlaxoSmithKline), tetanus (Hexavac, Sanofi Pasteur or Infanrix-hexa, GlaxoSmithKline for the first 3 doses then Boosterix, GlaxoSmithKline, for boosting) and Pneumococcus (Pneumo 23, Sanofi Pasteur). The clinical parameters of the patients enclosed in the study are reported in Table 1. Fifty age-matched healthy controls were also included in the study. Ethical permissions and research approval at the Ospedale pediatrico Bambino Gesù, Rome, Italy, and written informed consent from parents or legal guardians was obtained.
Table 1.
Clinical characteristics of the HIV-1 infected subjects
| Group | Early | Late Control | Late Failure | Treatment naïve | ||
|---|---|---|---|---|---|---|
| No. of Subjects | 13 | 30 | 21 | 6 | ||
| Male | 3 | 14 | 10 | 2 | ||
| Female | 10 | 16 | 11 | 4 | ||
| Age mean (±SD) | 6.8 (±3.2) | 13.7 (±4.1) | 15.8 (±4.1) | 11.8 (±4.3) | ||
| CDC status at diagnosis | N | 1 | 3 | 3 | 1 | |
| A | 3 | 8 | 1 | 1 | ||
| B | 1 | 11 | 8 | 4 | ||
| C | 8 | 8 | 9 | 0 | ||
| HIV-1 viral load mean copies/ml (±SD) | <50 (±0) | <05 (±0) | 7,519 (±15017) | 22,133 (±19473) | ||
| Months of HIV-1 viral load control Mean (±SD) | 53.9 (±26.7) | 48.3 (±24.3) | 0 (±0) | 0 (±0) | ||
| CD4+ counts median (range) | 817 (353–2,964) | 665 (225–1,208) | 399 (265–998) | 368 (298–906) | ||
| CD4+ %median (range) | 35 (25–46) | 33 (16–48) | 22 (10–41) | 25 (19–33) | ||
| CD19+ %median (range) | 16 (9–30) | 15 (10–29) | 15 (4–45) | 10 (7–16) | ||
| HAART initiation age mean (±SD) | 6.8 months (±3.0) | 7.4 years (±4.9) | 6.7 years (±3.9) | none | ||
| Years from vaccination or boost mean (±SD) | Measles | 4.2 (±2.7) | 4.7 (±4.5) | 3.7 (±3.0) | 6.1 (±3.2) | |
| Tetanus | 5.3 (±2.4) | 7.4 (±3.8) | 9.7 (±5.2) | 5.0 (±2.0) | ||
| Pneumococcus | 3.8 (±3.5) | 3.8 (±1.6) | 3.9 (±1.0) | 3.0 (±1.7) |
Cell Preparation and Flow Cytometry.
Peripheral blood mononuclear cells (PBMCs) and plasma were purified from Ficoll–Hypaque EDTA (EDTA)-treated blood (Amersham Pharmacia Biotech). PBMCs were stained with Fluorescein isothiocyanate (FITC)-, Phycoerythrin (PE)-, and Phycoerythrin-Cyanine 5 (PE-Cy5)-conjugated anti-human monoclonal antibodies binding to CD19, CD27, surface IgD and IgM (BD PharMingen), acquired using a 4-color FACScalibur instrument and analyzed by CellQuest Software (Becton Dickinson Immunocytometry Systems). A total of 25,000 live lymphocytes were gated and CD19+CD27+ memory B cell percentages were analyzed for all patients, and CD19+, CD27+, IgM+, IgD+ identified as IgM memory B cells (23) and CD19+, CD27+, IgM−, IgD− identified as switched memory B cells.
B Cell Enzyme-Linked Immunosorbent Spot.
Before loading PBMCs into precoated ELISpot plates, cells were polyclonally activated as described in ref. 3. ELISpot 96-well filtration plates (Millipore; MSIPS4510) were coated as described in ref. 3 and with 1 μg per well HIV-1 recombinant gp160 (MN strain) (Protein Sciences Corporation) subsequently loaded with 3 × 105 cells per well. Plates were then processed as described in ref. 3. Developed spots were counted with an AID Elispot reader using AID software version 3.2.3 (Cell Technology). For each antigen, a number of developed spots below or equal to the 25th percentile on the total raw data distribution was considered as a negative response (n = 3 for measles and n = 7 for HIV-1 gp160).
Quantification of Specific Antibodies in Plasma.
Plasma antibody titers against measles, tetanus, pneumococcus (Streptococcus pneumoniae) and gp41 were measured by ELISA reader (Labsystem Multiscan RC photometer) using: the Enzygnost antimeasles Virus/IgG ELISA kit (Dade Behring), VaccZymeTM antitetanus toxoid IgG, anti-PCP IgG kit (The Binding Site) and the Enzygnost HIV ½ Plus ELISA (Dade Behring) following manufacturer's instructions. The protective threshold was set at 0.2 units/mL for measles (24) and 0.15 units/mL for tetanus (25). For gp41-specific antibody detection, 6-fold serial dilutions of plasma were prepared (from undiluted to 1:62,500) and antibody levels for each sample were determined as the reciprocal of the highest serum dilution giving an OD at 450 nm above the cut-off value.
Statistical Analyses.
Before the final analysis, antibody data were censured by excluding non vaccinated subjects. Student t test or Mann–Whitney Rank Sum test were applied to analyze the CD27+ memory B cell percentages, the number of antigen-specific spots and the titers of plasma-specific antibodies with respect to treatment schedule. Linear regression analyses were performed to correlate age, CDC stage, CD4+ T cell/total B cell count and HIV-1 viral load to the above parameters.
Acknowledgments.
We thank Angela Aquilani and Veronica Santilli for their fundamental help and Professor Britta Wahren (Swedish Institute for Infectious Disease Control) for kindly providing the gp160 antigen. This work was supported by grants from the Swedish Medical Research Council; the Swedish International Development Agency; the Fp6 EuropeanUnion Europrise network of excellence; the Regione Autonoma della Sardegna, Cagliari, Italy; the Ministero della Salute, Rome, Italy; and the regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Hladik F, McElrath MJ. Setting the stage: Host invasion by HIV. Nat Rev. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagigi A, Nilsson A, De Milito A, Chiodi F. B-cell immunopathology during HIV-1 infection: Lessons to learn for HIV-1 vaccine design. Vaccine. 2008;26:3016–3025. doi: 10.1016/j.vaccine.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 3.Titanji K, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 4.Bekker V, et al. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: Loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118:e315–322. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. Combined antiretroviral therapy reduces hyperimmunoglobulinemia in HIV-1 infected children. AIDS. 2004;18:1423–1428. doi: 10.1097/01.aids.0000125985.94527.b2. [DOI] [PubMed] [Google Scholar]
- 6.Moir S, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 7.De Milito A. B lymphocyte dysfunctions in HIV infection. Curr HIV Res. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 8.D'Orsogna LJ, Krueger RG, McKinnon EJ, French MA. Circulating memory B cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS. 2007;21:1747–1752. doi: 10.1097/QAD.0b013e32828642c7. [DOI] [PubMed] [Google Scholar]
- 9.Abzug MJ, et al. Pertussis booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. Pediatrics. 2007;120:e1190–1202. doi: 10.1542/peds.2007-0729. [DOI] [PubMed] [Google Scholar]
- 10.Borkowsky W, et al. Cell-mediated and humoral immune responses in children infected with human immunodeficiency virus during the first four years of life. J Pediatr. 1992;120:371–375. doi: 10.1016/s0022-3476(05)80899-6. [DOI] [PubMed] [Google Scholar]
- 11.Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull WHO. 2003;81:61–70. [PMC free article] [PubMed] [Google Scholar]
- 12.Obaro SK, Pugatch D, Luzuriaga K. Immunogenicity and efficacy of childhood vaccines in HIV-1-infected children. Lancet Infect Dis. 2004;4:510–518. doi: 10.1016/S1473-3099(04)01106-5. [DOI] [PubMed] [Google Scholar]
- 13.Butler JE, et al. The piglet as a model for B cell and immune system development. Vet Immunol Immunopathol. 2008;15;128(1–3):147–170. doi: 10.1016/j.vetimm.2008.10.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaspan HB, Lawn SD, Safrit JT, Bekker LG. The maturing immune system: Implications for development and testing HIV-1 vaccines for children and adolescents. AIDS. 2006;20:483–494. doi: 10.1097/01.aids.0000210602.40267.60. [DOI] [PubMed] [Google Scholar]
- 15.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19(25–26):3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 16.Luzuriaga K, Sullivan JL. Prevention of mother-to-child transmission of HIV infection. Clin Infect Dis. 2005;40:466–467. doi: 10.1086/427294. [DOI] [PubMed] [Google Scholar]
- 17.Palma P, et al. Delayed early antiretroviral treatment is associated with an HIV-specific long-term cellular response in HIV-1 vertically infected infants. Vaccine. 2008;26:5196–5201. doi: 10.1016/j.vaccine.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Pantazis N, et al. The effect of antiretroviral treatment of different durations in primary HIV infection. AIDS. 2008;22:2441–2450. doi: 10.1097/QAD.0b013e328319ea4e. [DOI] [PubMed] [Google Scholar]
- 19.Romiti ML, et al. Kinetics of the T-cell receptor CD4 and CD8 V beta repertoire in HIV-1 vertically infected infants early treated with HAART. AIDS. 2001;15:2075–2084. doi: 10.1097/00002030-200111090-00002. [DOI] [PubMed] [Google Scholar]
- 20.Violari A, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanchetta M, et al. Early therapy in HIV-1-infected children: Effect on HIV-1 dynamics and HIV-1-specific immune response. Antivir Ther. 2008;13:47–55. [PubMed] [Google Scholar]
- 22.Titanji K, et al. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS. 2005;19:1947–1955. doi: 10.1097/01.aids.0000191231.54170.89. [DOI] [PubMed] [Google Scholar]
- 23.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen RT, et al. Measles antibody: Reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 25.Dietz V, Galazka A, van Loon F, Cochi S. Factors affecting the immunogenicity and potency of tetanus toxoid: Implications for the elimination of neonatal and non-neonatal tetanus as public health problems. Bull WHO. 1997;75:81–93. [PMC free article] [PubMed] [Google Scholar]
- 26.De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS. 2001;15:957–964. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 27.Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122:12–19. doi: 10.1016/j.jaci.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prendergast A, Tudor-Williams G, Jeena P, Burchett S, Goulder P. International perspectives, progress, and future challenges of paediatric HIV infection. Lancet. 2007;370:68–80. doi: 10.1016/S0140-6736(07)61051-4. [DOI] [PubMed] [Google Scholar]
- 29.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 30.Luzuriaga K, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: Control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol. 2000;74:6984–6991. doi: 10.1128/jvi.74.15.6984-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassutto S, Johnston MN, Rosenberg ES. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin Infect Dis. 2005;40:868–873. doi: 10.1086/428127. [DOI] [PubMed] [Google Scholar]
- 32.Vigano A, et al. Failure to eradicate HIV despite fully successful HAART initiated in the first days of life. J Pediatr. 2006;148:389–391. doi: 10.1016/j.jpeds.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Resino S, Correa R, Bellon JM, Munoz-Fernandez MA. Preserved immune system in long-term asymptomatic vertically HIV-1 infected children. Clin Exp Immunol. 2003;132:105–112. doi: 10.1046/j.1365-2249.2003.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moir S, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cagigi A, et al. Altered expression of the receptor-ligand pair CXCR5/CXCL13 in B-cells during chronic HIV-1 infection. Blood. 2008;112:4401–4410. doi: 10.1182/blood-2008-02-140426. [DOI] [PubMed] [Google Scholar]
- 36.Leyendeckers H, et al. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol. 1999;29:1406–1417. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 37.Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20(3–4):498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 38.Mamani-Matsuda M, et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood. 2008;205:1331–1342. doi: 10.1182/blood-2007-11-123844. [DOI] [PubMed] [Google Scholar]
- 39.Kamchaisatian W, Wanwatsuntikul W, Sleasman JW, Tangsinmankong N. Validation of current joint American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology guidelines for antibody response to the 23-valent pneumococcal vaccine using a population of HIV-infected children. J Allergy Clin Immunol. 2006;118:1336–1341. doi: 10.1016/j.jaci.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Barradas MC, et al. Antibody to capsular polysaccharides of Streptococcus pneumoniae after vaccination of human immunodeficiency virus-infected subjects with 23-valent pneumococcal vaccine. J Infect Dis. 1992;165:553–556. doi: 10.1093/infdis/165.3.553. [DOI] [PubMed] [Google Scholar]
- 41.Luzuriaga K, et al. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350:2471–2480. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 42.Capolunghi F, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 43.Jiang W, et al. Impaired naive and memory B cell responsiveness to TLR9 stimulation in HIV-infection. J Virol. 2008;82:7837–7845. doi: 10.1128/JVI.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharland M, Blanche S, Castelli G, Ramos J, Gibb DM. PENTA guidelines for the use of antiretroviral therapy, 2004. HIV Med. 2004;5(Suppl 2):61–86. doi: 10.1111/j.1468-1293.2004.00227.x. [DOI] [PubMed] [Google Scholar]