Abstract
Integrins are involved in the binding and internalization of both enveloped and nonenveloped viruses. By using 3 distinct cell systems—CHO cells lacking expression of α5β1-integrin, HeLa cells treated with siRNA to α5-integrin, and mouse β1-integrin knockout fibroblasts, we show that α5β1-integrin is required for efficient infection by pseudovirions bearing the ebolavirus glycoprotein (GP). These integrins are necessary for viral entry but not for binding or internalization. Given the need for endosomal cathepsins B and L (CatB and CatL) to prime GPs for fusion, we investigated the status of CatB and CatL in integrin-positive and integrin-negative cell lines. α5β1-Integrin-deficient cells lacked the double-chain (DC) forms of CatB and CatL, and this correlated with decreased CatL activity in integrin-negative CHO cells. These data indicate that α5β1-integrin-negative cells may be refractory to infection by GP pseudovirions because they lack the necessary priming machinery (the double-chain forms of CatB and CatL). In support of this model, we show that GP pseudovirions that have been preprimed in vitro to generate the 19-kDa form of GP overcome the requirement for α5β1-integrin for infection. These results provide further support for the requirement for endosomal cathepsins for ebolavirus infection, identify the DC forms of these cathepsins as previously unrecognized factors that contribute to cell tropism of this virus, and reveal a previously undescribed role for integrins during viral entry as regulators of endosomal cathepsins, which are required to prime the entry proteins of ebolavirus and other pathogenic viruses.
Keywords: Ebola, filovirus, virus entry, proteolytic priming
Integrins are used by a variety of enveloped and nonenveloped viruses and bacteria to establish infection in host cells. Integrins are heterodimers composed of an α- and a β-subunit. They play an important role in a number of cellular functions, including cell adhesion, migration, proliferation, differentiation, and apoptosis. Integrins are attractive targets for pathogens because they are expressed on a wide variety of different cell types, where they initiate a cascade of signaling events that can facilitate endocytosis and intracellular trafficking of the pathogen (1, 2).
Integrins often serve as primary receptors, allowing viruses to bind to and infect host cells. This is the case with several picornaviruses and hantaviruses (2). For certain adenoviruses and reoviruses, the virus binds to a separate primary cell surface receptor, but interaction with an integrin coreceptor is necessary for internalization. Several herpesviruses have also been shown to bind to host cells via association with other receptors, but require interactions with integrins to initiate cell signaling cascades that promote virus endocytosis and trafficking. Finally, in addition to using integrins for virus internalization, rotavirus encodes an enterotoxin that binds to integrins on intestinal epithelial cells and induces a diarrheal response in mice (3).
β1-Integrins have been proposed to facilitate entry of the highly virulent filovirus, ebolavirus (EboV). Kawaoka and colleagues (4) found that β1-integrin expression was down-regulated in cells transfected with the EboV glycoprotein (GP). They hypothesized that GP interacts with β1-integrins, leading to its down-regulation, as is the case for the HIV glycoprotein gp120 and its receptor CD4. In support of this hypothesis, they found that treatment of target cells with Abs to β1-integrin or with soluble α5β1-integrin complexes reduced GP-pseudotyped virus infection ≈50%. Several other cell surface proteins have also been shown to influence GP-mediated entry, including C-type lectins and members of the Tyro3 family (5). So far no single cell surface protein has been found to be both necessary and sufficient for EboV entry. Thus, it is possible that viral entry involves a combination of receptor molecules and could vary by cell type.
Here, we confirmed a role for α5β1-integrin in EboV infection. Surprisingly, we found that rather than being needed for virus binding or internalization, α5β1-integrin is required for steps leading to fusion. We further determined that this requirement is at the level of endosomal cathepsins, which have been previously implicated in EboV entry (6, 7).
Results
Expression of α5β1-Integrin Enhances EboV GP-Mediated Infection.
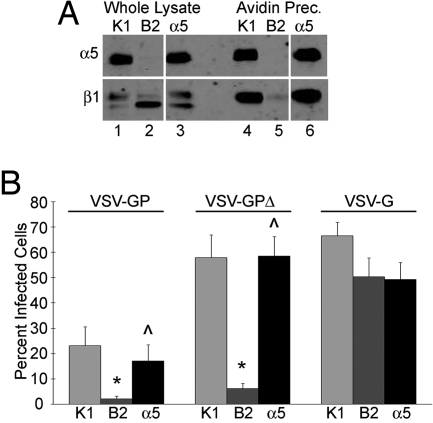
To examine the role of α5β1-integrin in EboV infection, we took advantage of a series of CHO cells that differ in their α5β1-integrin expression. CHO K1 cells express endogenous hamster α5β1-integrin. CHO B2 cells are a clone of CHO K1 cells that was selected for very low cell surface expression of α5β1. These cells still contain the β1-integrin chain and increased amounts of a pre-β1-integrin moiety, but lack detectable levels of α5-integrin and therefore do not express α5β1-integrin on their surface (8). CHO B2-α5 cells are a clone of CHO B2 cells engineered to stably express human α5-integrin, which can complex with hamster β1-integrin to promote surface expression of α5β1 (9). We confirmed expression of α5- and β1-integrins in these cells by surface biotinylating the cells and immunoblotting either the whole cell lysate or avidin-precipitated lysate for α5- and β1-integrins. As expected, only the CHO K1 and CHO B2-α5 cells express α5-integrin in whole cell lysates and on the surface (Fig. 1A, lanes 1, 3, 4, and 6). All 3 cell lines are positive for β1-integrin in the whole cell lysates (Fig. 1A, lanes 1–3), but the CHO B2 cells have greatly reduced β1-integrin expression on their surface (Fig. 1A, lane 5).
Fig. 1.
Expression of α5β1-integrin enhances EboV GP-mediated infection of CHO cells. (A) Total lysates and surface proteins of CHO K1 (K1), CHO B2 (B2), and CHO B2-α5 (α5) cells were immunoblotted for α5 (Upper) and β1 (Lower) integrin. Panels shown are from the same blot and same exposure; gaps indicate where lanes were removed. (B) The 3 CHO cell lines were infected with VSV-GP, VSV-GPΔ, or VSV-G, and the percentage of infected cells expressing GFP was measured by flow cytometry. Data shown are the averages from 10 experiments. Error bars indicate SEM. *, P ≤ 0.02 relative to CHO K1 cells. ⋀, P ≤ 0.05 relative to CHO B2 cells.
Using VSV pseudotypes encoding GFP that express full-length or mucin domain-deleted EboV GP or VSV G (VSV-GP, VSV-GPΔ, and VSV-G, respectively), we infected this panel of CHO cells and determined the percentage of infected cells by flow cytometry. Although we observed consistently higher infection with VSV-GPΔ virus as compared with VSV-GP, in both cases there was an ≈90% decrease in infection of the CHO B2 cells compared with CHO K1 cells (Fig. 1B). Reexpression of surface α5β1-integrin rescued infection by both VSV-GP and VSV-GPΔ. Infection by VSV-G was not significantly different across the 3 cell lines. These results support a previous report that indicated an important role for α5β1-integrin in EboV GP-mediated infection (4).
To further confirm that α5β1 expression is important for EboV GP-mediated infection, we used siRNA duplexes to knock down expression of α5- and β1-integrin in HeLa cells. Expression of α5-integrin was reduced by 80% in whole cell lysates and 50% on the cell surface in α5 siRNA-treated cells (Fig. 2A Upper, lanes 2 and 5). This correlated well with a 50% decrease in infection by VSV-GPΔ (Fig. 2B). β1-Integrin expression was not reduced, and in fact was increased, on the surface of the α5 siRNA-treated cells (Fig. 2A Middle, lane 5), suggesting that β1 is still present on the cell surface in complex with other α subunits. The residual infection in the α5 siRNA-treated HeLa cells could be due to these other αxβ1 complexes, and/or to the remaining 50% of α5β1 still present on the surface of these cells.
Fig. 2.
Knockdown of α5-integrin in HeLa cells reduces EboV GP-mediated infection. HeLa cells transfected with a nontargeting control siRNA oligonucleotide (Con) or siRNA oligonucleotides targeting α5- or β1-integrin were biotinylated and lysed for avidin precipitation and immunoblotting (A) or infected with VSV-GPΔ or VSV-G (B). (A) Representative immunoblots, presented as in Fig. 1, with densitometry values presented below showing the amount of protein, normalized for GAPDH loading, relative to the control siRNA-treated cells. (B) Data shown are the averages from 5 experiments. Error bars indicate SEM. *, P ≤ 0.0008 relative to control siRNA-treated cells.
In β1-targeted siRNA-treated cells, expression of β1-integrin was reduced by 80% in whole cell lysates and by 60% on the cell surface (Fig. 2A Middle, lanes 3 and 6); however, expression of α5-integrin was reduced by only 10% on the cell surface (Fig. 2A Top, lane 6). Because α5-integrin is not known to form a complex with any other subunit besides β1, this result suggests that significant levels (≈90%) of α5β1-integrin are still present on the surface of β1 siRNA-treated cells, likely explaining why VSV-GP infection of the β1 siRNA-treated cells was not reduced (Fig. 2B Left, black bar). Infection by VSV-G was not significantly inhibited by either siRNA treatment.
Integrin Expression Is Not Required for EboV GP-Mediated Binding or Internalization.
Integrins have been shown to be involved in infection by a wide range of viruses, most often at the stages of virus binding or internalization. The CHO cell lines described above were used to determine if α5β1-integrin is involved in EboV GP-mediated binding or internalization using a biochemical assay (see Methods). As shown in Fig. 3A, neither virus binding (lanes 1–3) nor internalization (lanes 7–9) was dependent on α5β1-integrin expression. To confirm these results, binding and internalization of VSV-GPΔ in CHO B2 and CHO B2-α5 cells was visualized by immunofluorescence. In agreement with the above results, we found that both cell lines bound and internalized equivalent amounts of virus (Fig. 3B and Fig. S1 A and B). As a further test, we compared binding of increasing amounts of a recombinant EboV receptor binding region (RBR) to CHO B2 and CHO B2-α5 cells. As shown previously (10, 11), this recombinant RBR protein binds specifically to cells that are susceptible to EboV GP-mediated infection and not to cells that are refractory to EboV infection (Fig. S1C). Consistent with the above data, there was no significant difference in RBR binding between the CHO B2 and CHO B2-α5 cells (Fig. 3C and Fig. S1C).
Fig. 3.
α5β1-Integrin regulates EboV GP-mediated entry after binding and after internalization. (A) VSV-GPΔ was bound to the panel of CHO cells at 4 °C, and the cells were either lysed directly (binding), treated with proteinase K to strip off bound virus and then lysed (no warm-up), or incubated at 37 °C for 2 h and then treated with proteinase K before lysing (internalization). Lysates were immunoblotted for the matrix protein of VSV (M). A representative blot from 8 separate experiments, presented as in Fig. 1, is shown, with densitometry values representing the amount of M protein, normalized for actin loading, relative to the parental CHO K1 cells. (B) VSV-GPΔ was bound to cells and internalized as described in Methods. Cells were permeabilized and stained with anti-EboV GP antibody (green) and phalloidin (red). Arrows indicate pseudovirions. See Fig. S1 A and B for larger version and quantitation. (C) Increasing concentrations of recombinant EboV RBR or control rabbit Fc (Con) were bound to cells, stained with AlexaFluor 488-conjugated Protein A, and analyzed by flow cytometry for the percentage of cells bound (Left) and for the mean fluorescence intensity (MFI) of cells (Right). (D) HIV-GPΔ and HIV-G were added to the panel of CHO cells, and fusion was measured as the percentage of blue cells. Results shown are the averages of normalized data from 8 experiments. The average percent infection in the CHO K1 cells was 26% for HIV-GPΔ and 64% for HIV-G. Error bars indicate SEM. *, P ≤ 6 × 10−10 relative to CHO K1 cells. ⋀, P ≤ 0.02 relative to CHO B2 cells.
α5β1-Integrin Expression Is Required at or Before EboV GP-Mediated Fusion.
After binding and internalization, the virus must undergo fusion with a cellular membrane. To determine if surface expression of α5β1-integrin is required for events leading up to EboV GP-mediated fusion, the 3 CHO cell lines were infected with HIV pseudotyped viruses containing β-lactamase (BlaM) and bearing EboV GPΔ (HIV-GPΔ). Upon fusion, the BlaM is released into the cytoplasm where it can cleave a fluorogenic substrate, resulting in a shift in fluorescence that can be measured by flow cytometry (12). This differs from the VSV pseudotype system used above in that neither transcription of viral mRNA nor translation of viral proteins is required to generate a positive signal. Our data with the HIV BlaM-containing pseudotypes closely mirrored what was found with the VSV GFP-encoding pseudotypes; EboV GP-mediated fusion was greatly reduced in the CHO B2 cells and was rescued by expression of α5β1-integrin (Fig. 3D). There were no significant differences in fusion across the 3 cell lines when HIV BlaM pseudotypes bearing VSV G (HIV-G) were used. Taken together, these data indicate that α5β1-integrin is not required for virus binding or internalization, but is required at or before virus fusion.
Expression of α5β1-Integrin Correlates with CatL Activity and the Presence of Double Chain (DC) Forms of CatB and CatL.
The endosomal cysteine proteases CatB and CatL are required for efficient EboV GP-mediated infection (6, 7). We previously showed that CatB and CatL cleave GP into a 19-kDa primed form, which is then triggered for fusion by an as yet unidentified mechanism. To examine if the block in viral fusion in the α5β1-integrin-negative CHO cells was at the stage of GP cleavage by cathepsins, we first looked at CatB and CatL activity in these cells. Using small fluorogenic peptide substrates for CatB and CatL, enzyme activity was measured in whole cell lysates of the 3 CHO cell lines. Surprisingly, CatL activity was reduced by >90% in the CHO B2 cells as compared with the parental CHO K1 cells (Fig. 4A). This activity was significantly rescued by stable transfection of α5 integrin. In contrast to CatL activity, CatB activity against a small peptide substrate was not significantly different across the panel of CHO cells (Fig. 4B).
Fig. 4.
CatL activity levels and expression of the DC forms of CatB and CatL are reduced in the α5β1-negative CHO cells. CatL (A) and CatB (B) activity in cell lysates was measured against small fluorogenic peptide substrates. Results shown are the averages of 3 experiments. Error bars indicate SEM. *, P ≤ 0.001 relative to CHO K1 cells. ⋀, P ≤ 0.02 relative to CHO B2 cells. (C) Untransfected CHO cells and CHO cells transfected with a plasmid encoding human CatL were analyzed by immunoblotting for CatL. (D and E) Cell lysates and supernatants from equivalent numbers of cells transfected with plasmids encoding human CatB (D) or CatL (E) were concentrated and analyzed by immunoblotting for CatB or CatL as indicated. haDC, DC form of endogenous hamster CatL; huPro, huSC, and huDC, proform, SC form, and DC form, respectively, of overexpressed human CatB and CatL.
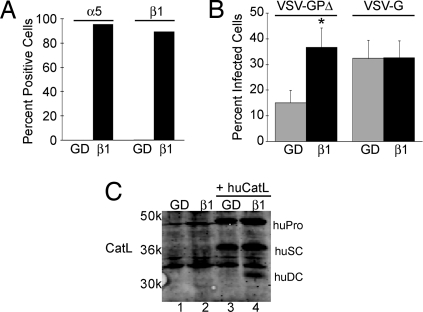
Next, we asked if the decrease in CatL activity seen in the CHO B2 cells correlated with a loss of CatL protein. We found that the CHO B2 cells were missing an ≈24-kDa protein that was detected by the CatL Ab (Fig. 4C, lane 2). The molecular mass of this missing band is consistent with it being the heavy chain of the mature DC form of hamster CatL (haDC), which is generated by sequential processing of the pro- and single chain (SC) forms. The presence or absence of this putative DC form of CatL mirrored the CatL activity data (Fig. 4A), and coincided with the pattern seen for EboV GP-mediated infection (Fig. 1B) and fusion (Fig. 3D) in these cells. To determine if overexpression of CatL could rescue infection and expression of the putative DC band in the CHO B2 cells, the CHO cell lines were transiently transfected with a plasmid encoding human CatL and either infected with VSV-GPΔ or lysed for immunoblotting. VSV-GPΔ infection was not rescued in the CHO B2 cells when CatL was ectopically expressed (Fig. S2). Strikingly, although these cells expressed high levels of the precursor (huPro) and SC (huSC) forms of CatL, they specifically lacked the presumptive DC form of the human enzyme (huDC), which runs at a slightly retarded electrophoretic mobility relative to the analogous hamster CatL species (Fig. 4C, lane 5).
We next examined CatB in the α5β1-integrin-negative cells. As was the case for CatL, the DC form of ectopically expressed CatB was also absent in the CHO B2 cells (Fig. 4D, lane 2), and ectopic expression of human CatB did not rescue infection (Fig. S2). However, in contrast to CatL, the absence of the DC form of CatB did not correlate with a statistically significant decrease in CatB activity in these cells, as measured with a small peptide substrate (Fig. 4B). This is in agreement with a study by Dermody and colleagues (13), who showed that CatB activity toward this peptide substrate correlated with expression of the SC, and not the DC, form of CatB.
To determine if the absence of the DC forms of these enzymes was due to increased secretion, supernatants from equivalent numbers of transfected cells were collected, concentrated, and analyzed by Western blot. The levels of secreted CatB and CatL were not significantly different between the CHO B2 cells and the α5β1-integrin-positive cells (Fig. 4 D and E, lanes 4–6). Moreover, the DC form of CatL was not trapped in high molecular mass or insoluble complexes after cell lysis, since DC CatL was not present when transfected cells were lysed in low pH or boiling sample buffer (Fig. S3 A and B). Similarly, pretreating the transfected cells for 24 h with the cysteine protease inhibitor E64d (10–200 μM) did not result in detection of DC CatL (Fig. S3C), ruling out the possibility that the DC form was being digested by a cysteine protease in the CHO B2 cells. The lack of the DC forms is also unlikely to be due to a defect in processing, because the pro- or SC forms of CatB and CatL did not significantly accumulate in the CHO B2 cells. Finally, bulk CatL protein is not grossly mislocalized in CHO B2 cells, because it colocalized predominantly with the late endosome and lysosome markers LBPA and LAMP1, as in the α5β1-positive cells (Figs. S4 and S5).
Expression of α5β1-Integrin Enhances Infection and Expression of the DC Form of CatL in GD25 Cells.
As the correlation between α5β1-integrin expression, EboV GP-mediated fusion, and the presence of the DC form of CatL was highly unexpected, we used an additional cell line to confirm these findings. As shown in Fig. 5A, GD25 cells, which are fibroblastic cells derived from β1-deficient mouse embryonic stem cells, do not express α5- or β1-integrins on their surface. β1GD25 cells, which have been stably transfected with the mouse β1A subunit (14), express both α5- and β1-integrins on their surface. α5β1-Integrin expression again led to a significant increase in VSV-GPΔ, but not VSV-G, infection, in this cell system (Fig. 5B). Although we were unable to visualize endogenous CatL (Fig. 5C, lanes 1 and 2) or CatB in these cells, the DC form of ectopically expressed human CatL was only detectable in the cells that express α5β1 (Fig. 5C, lanes 3 and 4). This supports what was seen in the CHO cells; surface expression of α5β1-integrin correlates with the presence of the DC form of CatL and leads to an enhancement of EboV GP-mediated infection. The basal level of infection of GD25 cells by VSV-GPΔ that is independent of β1 expression may be due to other integrins, such as αVβ3 (not present in CHO B2 cells), that contribute to cathepsin regulation. Alternatively, GD25 cells may possess other proteases that can process GP at a low efficiency.
Fig. 5.
Expression of α5β1-integrin enhances EboV GP-mediated infection of GD25 cells. (A) GD25 (GD) and β1GD25 (β1) cells were stained with Abs specific for α5- and β1-integrins, and the percentage of positive cells was measured by flow cytometry. (B) GD25 and β1GD25 cells were infected with VSV-GPΔ or VSV-G, and the percentage of infected cells was analyzed by flow cytometry. Data shown are the averages from 13 experiments. Error bars indicate SEM. *, P ≤ 0.03 relative to GD25 cells. (C) GD25 and β1GD25 cells were either untransfected or transfected with a plasmid encoding human CatL, and lysates were analyzed by immunoblotting for CatL.
Prepriming of VSV-GP with Thermolysin Rescues Infection in the α5β1-Negative CHO Cells.
Previously, we found that in vitro treatment of VSV-GP with either a combination of CatB and CatL at low pH or thermolysin at neutral pH cleaves GP into a 20-kDa and subsequently a 19-kDa form. Virus bearing this cleaved form of GP (VSV-GP19k) has significantly overcome the block to infection imposed by siRNAs targeting CatB or CatB + CatL (6). To determine if the block in infection of the α5β1-negative CHO B2 cells was due to the reduction in CatL activity present in these cells, the panel of CHO cells was infected with VSV-GPΔ that had been pretreated with thermolysin to generate VSV-GP19k (Fig. 6A). While infection with mock-treated VSV-GPΔ was again significantly decreased in CHO B2 cells relative to CHO K1 and CHO B2-α5 cells (Fig. 6B Left), infection of CHO B2 cells by VSV-GP19k was significantly increased as compared with mock-treated virus and occurred at similar levels to those seen in each of the other cell lines (Fig. 6B Right). These results indicate that the primary block in infection in the α5β1-negative CHO B2 cells is cleavage of GP to its primed 19-kDa form.
Fig. 6.
Pretreatment of VSV-GPΔ to form VSV-GP19K rescues infection in the α5β1-integrin-negative CHO cells. VSV-GPΔ was either mock-treated (M; VSV-GPΔmock) or pretreated with thermolysin T to generate VSV-GP19k and analyzed by immunoblotting for GP1 (A) or used to infect the panel of CHO cells (B). Results shown in B are the averages from 9 experiments. Error bars indicate SEM. *, P ≤ 0.007 relative to CHO K1 cells. ⋀, P ≤ 0.01 relative to CHO B2 cells. **, P ≤ 0.04 relative to mock-treated VSV-GPΔ in the same cells.
Discussion
In this study, we show that expression of α5β1-integrin correlates with EboV GP-pseudotyped virus infection postbinding and internalization, but before fusion. Expression of α5β1-integrin also correlates with expression of the DC forms of CatB and CatL. Cleavage of VSV-GPΔ to VSV-GP19k rescues infection in α5β1-integrin-negative CHO B2 cells, indicating that the primary defect in infection of these cells is cathepsin processing of GP, although we have not formally ruled out an additional defect in virus trafficking. These studies provide further support for the requirement for active cathepsins for EboV fusion with host cells and identify the DC forms of CatB and CatL as previously unrecognized factors that contribute to cell tropism of this virus. These findings also identify a previously unrecognized role for integrins in virus entry: regulation of cathepsin expression and activity. This role for integrins in cathepsin regulation may not be restricted to α5β1-integrin, because a partial rescue in EboV GP-mediated infection and CatB and CatL DC expression was observed in CHO B2 cells engineered to express α4β1-integrin (Fig. S6).
In addition to EboV, several other viruses have been shown to require endosomal cathepsin activity for cleavage of their fusion proteins. The F proteins of Hendra and Nipah viruses are cleaved by cathepsins before viral release, and this cleavage is required to prime the fusion protein (15). CatL cleavage of the spike proteins of MHV-2 and SARS after endocytosis has been shown to promote fusion (16, 17). CatB and CatL are also required for productive infection by nonenveloped mammalian reoviruses (18). For reoviruses, it has been proposed that CatL or CatB cleavage promotes disassembly of virions to infectious subvirion particles (ISVPs) within the endosome in cell culture and non-intestinal tissues, as is seen extracellularly with intestinal proteases (such as chymotrypsin) in natural enteric reovirus infections. Interestingly, reovirus also requires β1-integrins for efficient infection. Using the β1-integrin positive and negative GD25 cells described above, Dermody and colleagues (19) found that expression of β1-integrins significantly enhanced reovirus infection. Furthermore, they showed that pretreating reovirus with chymotrypsin to generate ISVPs relieved the need for β1-integrin, similar to what we have shown here for thermolysin-generated VSV-GP19k. In contrast to what we found for EboV pseudotypes, internalization of reovirus was also reduced in the β1 knockout cells. However, since the block in internalization of reovirus in GD25 cells was not complete and could not account for the full decrease in infection of these cells (19), it is possible that loss of expression of the DC form of CatL may have also contributed to the reduction in reovirus infection.
The NPXY motifs within the β1-integrin cytoplasmic tail have been implicated in the process of reovirus trafficking (20). GD25 cells stably expressing β1-integrins containing mutations in these motifs (β1GD25-NPXF) were able to internalize reovirus, but the virus was trafficked to organelles resembling secondary lysosomes and did not generate a productive infection. A similar phenomenon has recently been demonstrated for EboV entry in the presence of inhibitors of the PI3K-Akt signaling pathway (21). PI3K is activated in response to integrin ligation in a wide range of cellular processes, and during the entry of several viruses (2, 22). PI3K may also be involved in crosstalk between integrins and Tyro3 family members, which have been implicated in ebolavirus entry (5). Davey and colleagues showed that PI3K was activated during EboV entry and that, while EboV was still able to bind to cells and be internalized in the presence of PI3K inhibitors, the virus accumulated in cytosolic compartments and did not undergo fusion with the endosomal membrane. The observed defects in infection by reovirus in the NPXF-expressing cells and by EboV in PI3K inhibitor-treated cells may have been due to defects in expression of the DC form of CatL in addition to the proposed defects in trafficking. Under these circumstances, uncleaved virus particles may be unable to escape the endosome and therefore may accumulate further down the endosomal pathway than in normally functioning cells.
One of the surprising outcomes of this study is that the DC form of CatL appears to be regulated by α5β1-integrin and, at least in the CHO cells, the DC form of CatB is similarly regulated. The absence of the DC forms of these enzymes in the α5β1-integrin-negative cells does not appear to be due to a defect in processing, because there is no significant accumulation of the precursor forms of the cathepsins. The defect is also not due to a mutation in the CatB or CatL genes, because the DC forms of CatB and CatL are also missing when human cathepsins are ectopically expressed in these cells. Finally, secretion of these enzymes is not enhanced in the absence of α5β1-integrin. Instead, we suggest that surface expression of α5β1-integrin helps to stabilize the DC forms of CatB and CatL. For example, it is possible that the α5β1-integrin-negative cells have increased activity of an E64d-insensitive protease that degrades the DC forms of CatB and CatL. Alternatively, α5β1-integrin expression may be required for expression of a chaperone protein that can form a complex with the DC forms of the cathepsins and stabilize them. Finally, CatB and CatL may form a complex with α5β1-integrin itself, as has been shown for secreted cathepsin X (CatX) and αVβ3-integrin (23). While neither CatB nor CatL contains an RGD motif, as is found in CatX, it is possible that they can interact with α5β1-integrin through an RGD-independent mechanism, or via interactions with another molecule, and that this interaction either stabilizes or regulates trafficking of the DC form of the enzymes. Although the bulk of the CatL protein was found in late endosomes and lysosomes irrespective of α5β1-integrin surface expression, it is possible that the DC forms of the enzymes may be trafficked to a different location in the α5β1-integrin-negative cells. Such mislocalization could cause the enzymes to be rapidly degraded by bringing them into contact with a cellular protease that they do not normally encounter or by preventing them from interacting with cellular factors that stabilize them.
It has recently been shown that miRNA targeting of the non-receptor tyrosine kinase Abl also results in specific loss of the DC form of CatL (24). Because the activity and localization of Abl has been shown to be regulated by adhesion through α5 integrins (25), it is possible that Abl may contribute to the regulation of CatL by α5β1-integrins. Abl can also activate the PI3K-Akt pathway (26), which is required for EboV entry (21). It is therefore possible that Tyro3 family members, PI3K, Akt, and Abl are all involved in the link between α5β1-integrin and the DC forms of cathepsins.
Based on our data and those of Dermody and colleagues, it appears that at least 2 different viruses have evolved similar entry mechanisms in which they take advantage of widely expressed integrins on the cell surface to promote virus penetration within an endocytic organelle containing active cathepsins. While integrins are well known to regulate binding and signaling activities at the cell surface that lead to viral entry, to our knowledge this is the first clear evidence that integrin expression can also regulate virus entry at subsequent steps within intracellular organelles, in this case by controlling endosomal cathepsins. These results raise the possibility that integrin expression may influence other organelle functions that could be important for infection by a variety of viruses.
Materials and Methods
Cells.
CHO K1, CHO B2, and CHO B2-α5 cells (A. Rick Horwitz, University of Virginia, Charlottesville, VA) were maintained as described in ref. 27. HeLa cells were obtained from American Type Culture Collection and maintained in the recommended growth media. GD25 and β1GD25 cells (Deane Mosher, University of Wisconsin, Madison, WI) were maintained as described in ref. 14.
Measuring Integrin Expression.
In Fig. 1, cells were biotinylated, lysed, and avidin precipitated as described in ref. 27. Eluted proteins were analyzed under non-reducing conditions by immunoblotting with Abs specific for human α5 (AB1928; Chemicon) or β1 (Doug DeSimone, University of Virginia) integrins. In Fig. 5, cells were fixed and stained with Abs specific for mouse α5 (BD Biosciences) or β1 (19656; Santa Cruz Biotechnology) integrins.
Viruses and Infections.
VSV-GP, VSV-GPΔ, and VSV-G were made essentially as described in (6, 28) and infections were performed at approximate multiplicities of infection (m.o.i.) of 1–2 as described in ref. 6. HIV-GPΔ and HIV-G were produced and infections were performed essentially as described in ref. 29. The BlaM-Vpr and HIVΔEnv plasmids were provided by Christopher Broder (Uniformed Services University, Bethesda, MD). Fusion was measured as the shift in fluorescence from green to blue using a CyAn ADP LX 9 Color flow cytometer (DakoCytomation).
siRNA.
HeLa cells were transfected with control non-targeting siRNA oligonucleotides or SMARTpool oligonucleotides consisting of 4 different siRNA oligonucleotides targeting α5 or β1 integrin (Dharmacon) using oligofectamine (Invitrogen). Forty-eight hours posttransfection cells were infected with VSV-GPΔ or biotinylated and lysed for immunoblotting. Proteins were visualized using the Odyssey infrared imaging system and densitometry was performed using the Odyssey software (LiCor).
Binding and Internalization.
VSV-GPΔ was bound to cells at an approximate m.o.i. of 2 at 4 °C. After 2 h unbound virus was washed off and cells were either lysed to measure bound virus, or warmed to 37 °C for 2 h to allow internalization and then treated with 1 mg/mL proteinase K before lysing to remove uninternalized virus. Parallel wells were treated with proteinase K without the warm-up step to confirm that proteinase K effectively removed virus that was bound but not internalized. Lysates were analyzed by immunoblotting using an Ab specific for the matrix protein (M) of VSV (Michael Whitt, University of Tennessee, Memphis, TN). For immunofluorescence, virus was bound and internalized as above at an approximate m.o.i. of 15. Cells were fixed in 2% PFA, permeabilized with 0.05% Saponin, and stained with a monoclonal Ab to GP (Lisa Hensley, U.S. Army Medical Research Institute of Infectious Diseases, Frederick, MD) followed by anti-mouse AlexaFluor 488. Texas-Red conjugated phalloidin was used to visualize actin. Images were collected with a Nikon C1 laser scanning confocal unit attached to a Nikon Eclipse TE2000-E microscope. Binding of the recombinant EboV receptor binding region was performed as described in (10, 11).
Cathepsin Activity Assays and Immunoblotting.
Innozyme CatB and CatL activity assay kits (Calbiochem) were used to measure CatB and CatL enzyme activities against small fluorogenic substrate peptides. To examine protein levels of CatB and CatL, cells were either left untransfected or transfected with plasmids encoding human CatL or CatB (Origene) using FuGENE 6 (Roche). Forty-eight hours posttransfection the cells were lysed and equivalent amounts of protein, measured using the bicinchoninic acid assay (Pierce), were analyzed by immunoblotting with Abs specific for CatL or CatB (Athens Research and Technology). Alternatively, to visualize secreted CatB and CatL, transfection media was replaced with serum-free DMEM 24 h posttransfection. After an additional 24-h incubation, supernatants from equivalent cell numbers were collected and the cells were lysed for immunoblotting as above. Collected supernatants were centrifuged to remove cellular debris and proteins in the supernatant were precipitated using CHCl3/MeOH containing salmon sperm DNA before analyzing by immunoblotting.
In vitro proteolysis of GP. VSV-GP was mock-treated or treated with 0.5 mg/mL thermolysin (Sigma) for 30 min at 37 °C as described in ref. 6. The reactions were neutralized by the addition of 10 mM EDTA. Virus samples were analyzed by immunoblotting with a polyclonal GP1 Ab raised against sGP-Fc (Paul Bates, University of Pennsylvania, Philadelphia, PA) or used for infection.
Supplementary Material
Acknowledgments.
We thank our colleagues for generous gifts of reagents, as indicated in Materials and Methods, and Edward Park for excellent technical assistance. This work was supported by National Institutes of Health (NIH) Grants AI22470 and U54 AI7168 (to J.M.W.) and in part by NIH Grant AI050733 (to A.H.B.). K.L.S., C.J.S., and D.D. were supported in part by National Institutes of Health Training Grants (5T32 AI07046 and 5T32 AI055432) to the University of Virginia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807578106/DCSupplemental.
References
- 1.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Stewart PL, Nemerow GR. Cell integrins: Commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007;15:500–507. doi: 10.1016/j.tim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Seo N-S, et al. Integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci USA. 2008;105:8811–8818. doi: 10.1073/pnas.0803934105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takada A, et al. Downregulation of β1 integrins by Ebola virus glycoprotein: Implication for virus entry. Virology. 2000;278:20–26. doi: 10.1006/viro.2000.0601. [DOI] [PubMed] [Google Scholar]
- 5.Dolnik O, Kolesnikova L, Becker S. Filoviruses: Interactions with the host cell. Cell Mol Life Sci. 2008;65:756–776. doi: 10.1007/s00018-007-7406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schornberg K, et al. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J Virol. 2006;80:4174–4178. doi: 10.1128/JVI.80.8.4174-4178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiner C, et al. Isolation and characterization of Chinese hamster ovary cell variants deficient in the expression of fibronectin receptor. J Cell Biol. 1989;109:3157–3167. doi: 10.1083/jcb.109.6.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z, Huang K, Horwitz AF. Identification of a domain on the integrin α5 subunit implicated in cell spreading and signaling. J Biol Chem. 1998;273:31670–31679. doi: 10.1074/jbc.273.48.31670. [DOI] [PubMed] [Google Scholar]
- 10.Dube D, et al. Cell adhesion promotes ebola virus envelope glycoprotein-mediated binding and infection. J Virol. 2008;82:7238–7242. doi: 10.1128/JVI.00425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dube D, et al. The primed ebolavirus glycoprotein (19 kilodalton GP1,2): Sequence and residues critical for host cell binding. J Virol. 2009;83:2883–2891. doi: 10.1128/JVI.01956-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavrois M, de Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20:1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- 13.Ebert DH, Kopecky-Bromberg SA, Dermody TS. Cathepsin B is inhibited in mutant cells selected during persistent reovirus infection. J Biol Chem. 2004;279:3837–3851. doi: 10.1074/jbc.M310048200. [DOI] [PubMed] [Google Scholar]
- 14.Wennerberg K, et al. β1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diederich S, Thiel L, Maisner A. Role of endocytosis and cathepsin-mediated activation in Nipah virus entry. Virology. 2008;375:391–400. doi: 10.1016/j.virol.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Z, et al. Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry. J Virol. 2006;80:5768–5776. doi: 10.1128/JVI.00442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch BJ, Bartelink W, Rottier PJM. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol. 2008;82:8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiff LA, Nibert ML, Tyler KL. In: Fields Virology. Fields BN, Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1853–1915. [Google Scholar]
- 19.Maginnis MS, et al. β1 integrin mediates internalization of mammalian reovirus. J Virol. 2006;80:2760–2770. doi: 10.1128/JVI.80.6.2760-2770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maginnis MS, et al. NPXY motifs in the β1 iontegrin cytoplasmic tail are required for functional reovirus entry. J Virol. 2008;82:3181–3191. doi: 10.1128/JVI.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeed MF, Kolokoltsov AA, Freiberg AN, Holbrook MR, Davey RA. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 2008;4:e1000141. doi: 10.1371/journal.ppat.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 23.Lechner AM, et al. RGD-dependent binding of procathepsin X to integrin αvβ3 mediates cell-adhesive properties. J Biol Chem. 2006;281:39588–39597. doi: 10.1074/jbc.M513439200. [DOI] [PubMed] [Google Scholar]
- 24.Yogalingam G, Pendergast AM. ABL kinases regulate autophagy by promoting the trafficking and function of lysosomal components. J Biol Chem. 2008;283:35941–35953. doi: 10.1074/jbc.M804543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JY. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci USA. 1996;93:15174–15179. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharas MG, Fruman DA. ABL oncogenes and phosphoinositide 3-kinase: Mechanism of activation and downstream effectors. Cancer Res. 2005;65:2047–2053. doi: 10.1158/0008-5472.CAN-04-3888. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Bridges LC, White JM. Selective modulation of integrin-mediated cell migration by distinct ADAM family members. Mol Biol Cell. 2005;16:4982–4991. doi: 10.1091/mbc.E05-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takada A, et al. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci USA. 1997;94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonezawa A, Cavrois M, Greene WC. Studies of Ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: Involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J Virol. 2005;79:918–926. doi: 10.1128/JVI.79.2.918-926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.