Abstract
Small molecule metabolites play important roles in Caenorhabditis elegans biology, but effective approaches for identifying their chemical structures are lacking. Recent studies revealed that a family of glycosides, the ascarosides, differentially regulate C. elegans development and behavior. Low concentrations of ascarosides attract males and thus appear to be part of the C. elegans sex pheromone, whereas higher concentrations induce developmental arrest at the dauer stage, an alternative, nonaging larval stage. The ascarosides act synergistically, which presented challenges for their identification via traditional activity-guided fractionation. As a result the chemical characterization of the dauer and male attracting pheromones remained incomplete. Here, we describe the identification of several additional pheromone components by using a recently developed NMR-spectroscopic approach, differential analysis by 2D NMR spectroscopy (DANS), which simplifies linking small molecule metabolites with their biological function. DANS-based comparison of wild-type C. elegans and a signaling-deficient mutant, daf-22, enabled identification of 3 known and 4 previously undescribed ascarosides, including a compound that features a p-aminobenzoic acid subunit. Biological testing of synthetic samples of these compounds revealed additional evidence for synergy and provided insights into structure–activity relationships. Using a combination of the three most active ascarosides allowed full reconstitution of the male-attracting activity of wild-type pheromone extract. Our results highlight the efficacy of DANS as a method for identifying small-molecule metabolites and placing them within a specific genetic context. This study further supports the hypothesis that ascarosides represent a structurally diverse set of nematode signaling molecules regulating major life history traits.
Keywords: dauer formation, differential analysis, metabolomics, NMR spectroscopy, sex pheromone
The importance of small-molecule signaling in the biology of Caenorhabditis elegans has started to emerge (1–3). Two families of endogenous small molecules have recently been shown to serve crucial functions in C. elegans endocrine and exocrine signaling: the dafachronic acids, bile-acid-like steroids that act as ligands of the nuclear hormone receptor DAF-12 (4), and the ascarosides, glycosides of the dideoxysugar ascarylose that regulate development and behavior in C. elegans (Fig. 1) (5–8).
Fig. 1.
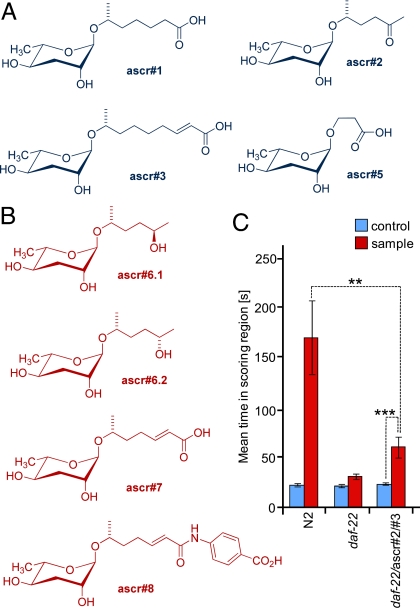
Structures and male-attracting activity of ascarosides in C. elegans. (A) Previously identified ascarosides via activity-guided fractionation (blue). (B) Additional ascarosides identified in this study via DANS (red). (C) Wild-type (N2) metabolite extract has strong male-attracting activity, whereas daf-22 mutant metabolite extract is inactive. A mixture of previously identified ascr#2 and ascr#3 in amounts corresponding to those present in the wild-type metabolite extract (20 fmol each) added to the inactive daf-22 metabolite extract resulted in significant male attraction, but was much less attractive than wild-type metabolite extract containing similar amounts of ascr#2 and ascr#3.
The ascarosides were initially discovered as major components of the dauer pheromone, a small molecule signal that induces developmental arrest of C. elegans larvae at the highly persistent, “non-aging” dauer stage (5–7). Dauer larvae can persist for many months under harsh environmental conditions before resuming development into adults with a normal lifespan of ≈15–20 days (9). As a result, the C. elegans dauer stage has been of great interest for the study of pathways regulating aging and development in eukaryotes. Genetic screens for mutants that either cannot attain the dauer stage or form dauer larvae constitutively have identified approximately 3 dozen DAF (DAuer Formation) genes (10). Subsequent studies revealed a significant role for genes of the dauer pathway in regulation of C. elegans adult lifespan, and orthologs of dauer genes in higher organisms appear to be involved in aging as well (11–13).
In addition to regulating developmental timing, the ascarosides ascr#2, ascr#3, and ascr#4 serve as a mating signal in C. elegans (Fig. 1) (8). A mixture of these 3 ascarosides acts as a potent male attractant at very low concentrations, whereas, at the much higher concentrations required to induce dauer formation, these compounds no longer attract males and instead deter hermaphrodites. Based on these results, male attraction and dauer formation in C. elegans appear as alternative responses to a common set of signaling molecules that thus connect reproductive and developmental pathways (8).
In both the dauer and mating assays, the ascarosides showed strong synergism: mixtures of ascarosides were potently active at concentrations at which individual components effected no response (6–8). Although biologically fascinating, the ascarosides' synergistic properties resulted in tremendous logistical challenges for their identification via activity-guided fractionation, as this required to combinatorially recombine chromatographic fractions to assess activity. Despite these efforts, biological testing of the ascarosides identified so far did not always reproduce activity of the original pheromone extracts, and it seemed likely that important components of both the dauer and mating pheromones remained to be identified (5–8).
The discovery of the ascarosides shows that a systematic characterization of structures and functions of small molecules in C. elegans and other model organisms will be critical for advancing our understanding of many biological processes (14). However, the armamentarium of traditional natural product chemistry appears ill-suited for this purpose, given the complexity of metabolomes and the scope of assigning functions to hundreds if not thousands of individual components, many of which represent previously undescribed chemical structures (15). We have developed an NMR-based approach that simplifies linking naturally occurring small molecules with their biological function and offers considerable advantages for the detection of synergism as well as for the characterization of chemically labile signaling molecules (16–18). Central to our method is the use of genetic information to detect individual compounds in the C. elegans metabolome that could be of interest within a specific biological context. Here, we demonstrate the use of this method, differential analysis by 2D NMR spectroscopy (DANS), for the identification of additional components of the mating and dauer pheromones of C. elegans. Ultimately, these investigations allowed full reconstitution of the male-attracting activity of wild-type pheromone extract to a daf-22 mutant.
Results
NMR-Spectroscopic Comparison of Wild Type with a Signaling-Deficient Mutant.
For the purpose of identifying missing components of the dauer and mating pheromones, the C. elegans mutant strain daf-22 offered a unique opportunity (19). daf-22-derived metabolite extracts had been shown to have little dauer-inducing activity and are not significantly active in the male attraction assay (Fig. 1C). Addition of physiologically relevant amounts of the pheromone components ascr#2 and ascr#3 to daf-22 metabolite extract does not fully reconstitute activity in the male attraction assay, indicating that additional pheromone components of perhaps yet undetermined structure are also not produced in daf-22 worms (Fig. 1C). Thus, it seemed likely that the biosynthesis of some or all of the mating and dauer pheromone components is abolished in daf-22 worms. Recent studies indicate that daf-22 encodes a homolog of human sterol carrier protein SCPx that participates in peroxisomal fatty acid β-oxidation of long-chained fatty-acid-like precursors of ascarosides (8, 20).
Therefore, a careful comparison of the daf-22 metabolome with that of wild-type worms should reveal the missing daf-22-dependent pheromone components among compounds present in wild-type worms but absent in daf-22. Previous experience with identification of novel secondary products in unfractionated microbial extracts suggested that DANS, a method based on overlaying 2D-NMR spectra, could be used to directly detect the daf-22-dependent compounds (17, 18). DANS, as shown previously, can provide detailed structural information even for minor components in complex metabolite extracts.
To obtain material for NMR-spectroscopic analysis, liquid cultures of wild-type (N2 Bristol) and daf-22(m130) worms were grown by using standard protocols and subsequently extracted as described previously (see Materials and Methods) (8). The resulting metabolite extracts were used to acquire a specific type of high-resolution 2D NMR spectrum, dqfCOSY. dqfCOSY spectra are particularly information rich and offer good dynamic range, which often permits characterization of even very minor components (16, 21). For differential analysis of the dqfCOSY spectra, the daf-22-derived spectrum was superimposed onto the wild-type spectrum, using a specific algorithm that suppressed signals present in both mutant and wild-type spectra. (Fig. 2A and Materials and Methods) (17). The algorithm chosen for this overlay allowed suppression of signals even in cases where compounds occurred at significantly different concentrations in the daf-22 and wild-type samples. As a result, only signals present in the wild-type spectrum but entirely absent from the daf-22 spectrum remained unaltered in the overlay (Fig. 2 A–C). The DANS algorithm can be fine-tuned to reveal less severe differences as well; however, for the current study we considered exclusively compounds consistently not expressed in daf-22 mutant worms.
Fig. 2.
DANS-based comparison of daf-22 and wild-type metabolomes. (A) Schematic representation of DANS. C. elegans wild-type metabolite extract has dauer-inducing and male-attracting activity, whereas daf-22 metabolite extract is inactive (8, 19). Comparison of wild-type and daf-22 metabolomes via DANS reveals candidate molecules for biological evaluation (red). (B) Aliphatic section of the dqfCOSY spectrum of C. elegans wild-type metabolite extract prior to differential analysis, revealing the complexity of this extract. (C) DANS overlay of the wild-type spectrum in Fig. 2B with the corresponding region of the daf-22 spectrum. Signals present in both wild-type and daf-22 spectra cancel or change color (blue), whereas signals representing compounds only present in wild type remain unaffected (brown), most prominently signals representing part of the side chains of ascr#2 and ascr#3.
DANS Revealed Known Pheromone Components and New Metabolites.
The NMR-spectroscopic analyses immediately revealed that signals diagnostic for the side chains of ascr#2, ascr#3, and ascr#5 were present in wild-type but absent in daf-22-derived spectra, indicating that daf-22 worms do not produce these compounds (Fig. 2 B and C; also see supporting information (SI) Appendix, Figs. S1–S4) (6). The absence of NMR-spectroscopic signals representing ascr#2, ascr#3, and ascr#5 from daf-22 spectra was among the strongest differences detected via DANS, which directly corresponds to the earlier finding that these compounds constitute major components of the dauer and mating pheromones. In fact, additional 2D NMR spectra (HSQC and HMBC) of the wild-type metabolite extract permitted full assignment of the structures of ascr#2 and ascr#5, suggesting that these compounds could have been identified solely via DANS, completely avoiding activity-guided fractionation (see SI Appendix, Figs. S5–S8). ascr#3 could likely have been identified in this manner as well, but, because of its structurally more complicated side chain, would have required additional TOCSY spectra.
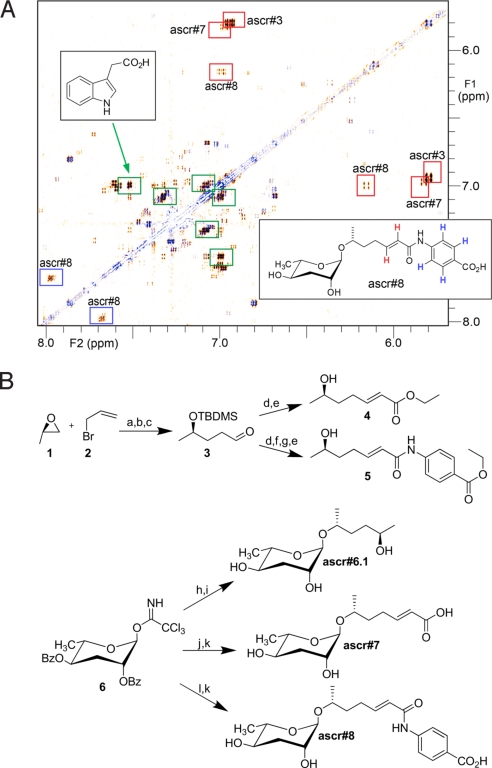
In addition to signals representing these known compounds, DANS reproducibly revealed several additional structural fragments that appeared to be daf-22 dependent (Fig. 3A). By using additional HSQC and HMBC spectra, the most abundant of these fragments were shown to represent 3-substituted indole derivatives, most prominently indole-3-acetic acid. Two other structural fragments closely resembled the side chain of ascr#3, featuring a trans-double bond likely conjugated to a carbonyl and adjacent to at least 1 CH2 group. The daf-22 dependence of these structural fragments as well as their similarities to the side chain of ascr#3 suggested that these two fragments belong to ascarosides that could represent additional components of the mating or dauer pheromones. Another daf-22-dependent fragment appeared to represent an aromatic subunit, perhaps a 1,4-disubstituted benzene.
Fig. 3.
Identification of daf-22-dependent metabolites ascr#6.1, ascr#7, and ascr#8. (A) DANS overlay of daf-22 and wild-type spectra, aromatic region, showing signals representing carbonyl-conjugated double bonds of ascr#3, ascr#7, and ascr#8 (red), the p-substituted aromatic ring in ascr#8 (blue), and several indole derivatives, of which the major component is indole-3-acetic acid (green). Signals corresponding to the methyl esters of ascr#3 and ascr#8, artifacts that arose from storage in methanol, have been removed for clarity. (B) Synthesis of ascr#6.1, ascr#7, and ascr#8. a, Mg/THF, 82%; b, TBDMSCl/DMF, 71%; c, O3/(CH3)2S, 54%; d, (CH3O)2POCH2CO2CH3/LiCl/DIEA/CH3CN, 90%; e, 40% HF/H2O, 88%; f, LiOH/dioxane 60 °C, 93%; g, (I) Oxalylchloride/DCM/DMF (II) p-aminobenzoic acid/DIEA/DCM, 31%; h, TMSOTF/hexanediol/DCM, 72%; i, KOH, 93%; j, TMSOTF and 4 in DCM– 36%; k, LiOH/THF, 100%; l: TMSOTF and 5 in DCM – 89%.
The two suspected ascarosides were present at ≈10–20% of the concentrations of ascr#3, based on comparing relative intensities of the dqfCOSY signals representing the double-bond protons in these structures. This suggested that the compounds of interest were present in quantities of <30 μg/50 mg of extract, the typical amount used for acquisition of the NMR spectra (6). Because of their lower concentrations, full NMR-spectroscopic characterization of these two daf-22-dependent compounds was not possible at this stage and required enrichment via chromatography. In contrast to traditional methodology for the isolation of bioactive natural products, this chromatographic fractionation did not have to rely on bioassays, because the NMR-spectroscopic signals identified via DANS could be used to track the daf-22-dependent compounds.
In two chromatographic steps, an enriched metabolite sample was obtained, whose dqfCOSY-NMR spectra contained two of the remaining daf-22-dependent signals detected in the original metabolite sample. This sample was extensively characterized by using additional 2D NMR spectroscopy and mass spectrometry, indicating the presence of 3 ascarosides (SI Appendix, Figs. S9–S11). Of these 3 components, 1 molecule included two of the daf-22-dependent fragments detected via DANS, the 1,4-disubstituted aromatic unit and a carbonyl-conjugated trans-double bond. Detailed spectroscopic analysis showed this compound to consist of a p-aminobenzoate (PABA) subunit attached to an unsaturated 7-carbon side chain; we named this compound ascr#8. ascr#8 copurified with two other novel ascarosides, which were identified as diastereomeric side-chain hydroxylated ascarosides, ascr#6.1 and ascr#6.2. Following identification of ascr#8, it became apparent that the one remaining unassigned daf-22-dependent fragment detected via DANS could perhaps represent an ascaroside with an unsaturated 7-carbon side chain lacking the PABA subunit, corresponding to a bis-norhomolog of ascr#3. This compound, a fourth previously unchararacterized ascaroside, was named ascr#7.
Syntheses of New Ascarosides.
To confirm these structural assignments, to determine the relative configuration of the oxygenated carbons in the side chains of these compounds, and to procure material to investigate biological activity, samples of ascr#6.1, ascr#7, and ascr#8 were synthesized as shown in Fig. 3B. The side chains of ascr#7 and ascr#8 were derived via Grignard reaction using allyl bromide and (R)-propylene oxide. The (R)-enantiomer was chosen for this reaction, because analysis of NOESY spectra suggested that the side-chain configurations in ascr#7 and ascr#8 would correspond to those of ascr#2 and ascr#3, both of which had been determined previously to be (R) (6). After protection and ozonolysis, the resulting aldehyde 3 was subjected to a Horner–Emmons reaction. Subsequent desilylation furnished the building block 4 needed for the synthesis of ascr#7, whereas careful alkaline hydrolysis followed by treatment with oxalyl chloride, coupling with p-aminobenzoic acid ethyl ester and subsequent desilylation produced the side chain 5 of ascr#8. Finally, dibenzoyl-protected ascarylose, prepared as described in ref. 6, was coupled with 4, 5, or (2R,5R)-hexanediol, and the resulting 3 products were deprotected to furnish enantiomerically pure samples of ascr#6.1, ascr#7, and ascr#8. Spectroscopic data of these synthetic samples were identical to those obtained for the daf-22-dependent metabolites and thus confirmed our spectroscopic assignments. The assignment of (2S)-configuration to ascr#6.2, which has not been synthesized, follows indirectly from the identification of its diastereomer, ascr#6.1, as (2R).
Mass Spectrometric Validation of DANS Results.
To validate the results obtained via DANS and to further confirm the structure of ascr#7, we analyzed the wild-type and daf-22 metabolite extracts via HPLC-electrospray-MS. We found that HPLC-MS was far less informative than NMR spectroscopy for comparison of these crude metabolite extracts, because amino acids, peptides, and various other compounds strongly dominated the UV and MS total ion current (TIC) chromatograms. Against this highly variable background, the presence or absence of metabolites of unknown molecular mass was difficult to determine, and peaks corresponding to ascr#6.1, ascr#7, and ascr#8 could only be identified by using the corresponding synthetic samples for reference (SI Appendix, Figs. S12–S16). In this manner, we confirmed the presence of ascr#6.1, ascr#7, and ascr#8 in wild-type metabolite extracts, in addition to the known ascr#1–3 and ascr#5. Subsequent analyses of daf-22 extracts produced no evidence for the presence of any of the ascarosides ascr#1 through ascr#8 (SI Appendix, Figs. S12–S16). Quantitative HPLC-MS analyses showed that wild-type liquid cultures contained variable concentrations of ascarosides, generally in the range of 10–100 nM for ascr#1, 100–200 nM for ascr#2, 100–200 nM for ascr#3, 10–20 nM for ascr#6.1 and ascr#6.2, 10–40 nM for ascr#7, and 10–70 nM for ascr#8. The particularly high variability in the measured concentrations of ascr#8 appeared to be a result of its susceptibility to hydrolysis. Metabolite samples that had been handled repeatedly at room temperature showed decreasing quantities of ascr#8 by NMR spectroscopy, and a methanolic solution of synthetic ascr#8 showed as much as 5% decomposition into the corresponding methyl ester and free p-aminobenzoic acid after 24 h at room temperature. Therefore, ascr#8 was likely more abundant in the wild-type liquid cultures than our HPLC-MS analysis suggested.
Interestingly, DANS indicated that certain other ascaroside derivatives are present in both wild-type and daf-22 metabolite extracts. In fact, daf-22 spectra showed evidence for at least two different types of ascarylose derivatives, whereas wild-type spectra revealed strong signals representing four or more different types of ascarylose-containing compounds (SI Appendix, Fig. S4). Chromatographic isolation of the ascarosides present in both daf-22 and wild-type extracts revealed highly lipophilic compounds featuring side chains of 29 and 31 carbon atoms, which are similar to lipids previously identified from egg shells of Ascaris sp. (see SI Appendix) (22–24). Thus, daf-22 metabolite extracts are distinguished from wild type by their lack of ascarosides with short side chains.
ascr#4, a previously identified component of the male attracting pheromone (8), was not detected in either the HPLC-MS or NMR-spectroscopic analyses. It is possible that ascr#4 does not get produced in significant quantities under our liquid culture conditions. Alternatively, it could be that in liquid culture ascr#4 is quickly hydrolyzed, resulting in formation of ascr#2. Because aqueous and methanolic solutions of ascr#4 are stable for extended periods of time at room temperature, deglucosylation of ascr#4 in liquid culture would likely occur under enzymatic control. We confirmed that our wild-type worms are capable of producing ascr#4 by incubating worms in M9 media as described in ref. 8. The resulting extracts contained ascr#4 as the most abundant ascaroside, in agreement with previously published results (8).
New Ascarosides Modulate Development and Behavior.
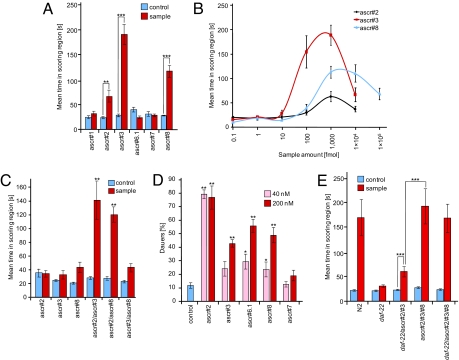
Behavioral experiments with synthetic samples of the new ascarosides, ascr#6.1, ascr#7, and ascr#8, indicate that ascr#8 is a strong male-specific attractant, whereas ascr#5, ascr#6.1, and ascr#7 have little or no activity in this assay (Fig. 4A and SI Appendix, Fig. S17). As shown in Fig. 4A, ascr#8 attracts C. elegans males more strongly than ascr#2 and slightly less than ascr#3. Dose–response curves indicate that ascr#8's activity maximum occurs over a broader concentration range, compared with ascr#3 (Fig. 4B). In previous experiments, we had shown that ascr#2 and ascr#3 strongly synergize in the mating assay (8). In analogous experiments with ascr#8, we found synergy for the combination of ascr#2 and ascr#8, whereas the combination of ascr#3 and ascr#8 did not result in increased activity (Fig. 4C).
Fig. 4.
Biological evaluation of daf-22-dependent ascarosides identified via DANS. (A) Differential activity of the identified ascarosides in the male attraction assay. ascr#3 showed maximal activity followed by ascr#8 and ascr#2. All other ascarosides did not exhibit significant activity. All compounds were assayed in amounts of 1 pmol. (B) Concentration dependence of male attraction for ascr#2, ascr#3, and ascr#8. ascr#8 shows a broader range of activity than ascr#2 and ascr#3. Data for ascr#2 and ascr#3 have been published (8). (C) Synergistic interactions between the three most active ascarosides. Combinations of ascr#2 and ascr#3 as well as ascr#2 and ascr#8 displayed strong synergy, whereas ascr#3 and ascr#8 did not. ascr#2 and ascr#8 were tested at 100 fmol and ascr#3 at 10 fmol (8). (D) Dauer induction of the different ascarosides. All compounds were assayed at concentrations of 40 nM and 200 nM, and each compound was tested on at least 5 different days. ascr#2 was the most potent dauer inducer and showed significant dauer induction at both concentrations tested. ascr#3, ascr#6.1, and ascr#8 show significant dauer induction only at 200 nM, whereas ascr#7 does not show significant dauer formation at both of the concentrations tested. (E) Reconstitution of male attraction activity of wild-type metabolite extract by combining synthetic ascarosides. A mixture of 20 fmol of ascr#2 and 20 fmol of ascr#3 resulted in significant male attraction, but was less attractive than wild-type metabolite extract containing similar amounts of ascr#2 and ascr#3. However, a ternary mixture of 20 fmol of each ascr#2, ascr#3, and ascr#8 was as active as wild-type metabolite extract. Adding daf-22 metabolite extract does not further increase activity of this ternary mixture. For each data point in Fig. 4 A–C and E, n ≥ 30 animals were used. Error bars, SEM; *, P < 0.01; **, P < 0.001; ***, P < 0.0001, unpaired test with Welch's correction (A and E) and one-factor ANOVA with Tukey–Kramer post test (C).
ascr#8's dauer-inducing activity is similar to that of ascr#3, as shown in Fig. 4D. Interestingly, ascr#7, which differs from ascr#3 only in that it has a 2-carbon-shorter side chain, did not show any activity in either the dauer or male attraction assays at the concentrations tested (Fig. 4 A, and D). Meanwhile, ascr#8, the structurally most distinct ascaroside, closely mimics the activity profile of ascr#3 in both the mating and dauer assays.
Synthetic Ascaroside Blend Fully Reconstitutes Mating Activity.
Part of the motivation for the current investigations resulted from the finding that ascarosides that had been identified previously through activity-guided fractionation failed to fully reconstitute activity in the male attraction assay (8). As shown in Fig. 1C, mixtures of ascr#2 and ascr#3 are less active than a dilution of wild-type metabolite extract containing similar amounts of these two ascarosides. Because the third previously identified component of the male attracting pheromone, ascr#4 (8), was not present in significant quantities in the wild-type liquid culture extract, other components must account for its higher activity. To assess whether the suite of compounds identified via DANS would yield full activity, we prepared a near-physiological mixture of the three most abundant and individually most active ascarosides, ascr#2, ascr#3, and ascr#8. As shown in Fig. 4E, this mixture alone was as active in this male-attraction assay as wild-type metabolite extract at an equivalent dilution. Adding daf-22 metabolite extract did not further enhance activity, indicating that male-attracting activity of wild-type metabolite extract as measured in this assay originates exclusively from the presence of the daf-22-dependent compounds.
Discussion
The DANS-based comparison of C. elegans wild-type and daf-22 metabolite extracts led to the identification of four ascarosides, three of which were shown to contribute to regulating development and behavior. Their identification substantially increases our knowledge of small-molecule signaling in C. elegans and provides new opportunities to develop tools for investigating underlying pathways. Our results further support the earlier finding that different ascarosides serve different functions, and provide a fascinating glimpse at the complex structure–activity relationships of these endogenous signaling molecules. Given that the earlier characterized ascarosides show strong synergism (7, 8), a more detailed characterization of the roles that ascr#6 to ascr#8 play in C. elegans biology will require a much larger series of assays exploring various combinations and concentrations of all of the ascarosides identified so-far. Furthermore, it will be interesting to investigate whether ascaroside biosynthesis is regulated by environmental factors, as suggested by differences in the observed activity profiles of different ascarosides and variability of their concentrations in the metabolite extracts (7). In particular, the factors regulating the biosynthesis of ascr#4 will be of interest: large amounts of ascr#4 are consistently produced under some conditions (8), whereas this compound is consistently absent from liquid cultures.
DANS revealed that production of all short-chained ascarosides is strongly daf-22-dependent. None of the ascarosides ascr#1 to ascr#8 could be detected in the >10 separate daf-22 liquid cultures (see Materials and Methods) that were analyzed by dqfCOSY. The structural features of ascr#1 to ascr#8, specifically the occurrence of α-β-unsaturated carboxylic acids with 7 or 9 carbon atoms in conjunction with a 6-carbon 2-ketone, suggest that these compounds derive from β-oxidation of longer-chained precursors. In fact, it has recently been shown that DAF-22, a homolog of human sterol carrier protein SCPx, and DHS-28, a homolog of the human D-bifunctional protein, participate in the biosynthesis of ascarosides ascr#2, ascr#3, and ascr#5 via peroxisomal β-oxidation (20). However, the ultimate precursors from which DHS-28 and DAF-22 produce these shorter-chained ascarosides have not been determined. In our current study, we identified long-chained O-ascarylosyl-(2,ω-1)-alkanediols as the only ascarosides present in daf-22 media extracts. It is possible that these very lipophilic compounds constitute the substrates for the β-oxidation cascade producing ascarosides ascr#1 to ascr#8. Similar long-chained O-ascarylosyl-(2,ω-1)-alkanediols have been identified from several parasitic nematode species, where they occur in large quantities in the egg's internal lipid layer (22–24).
ascr#8 differs from earlier identified ascarosides in that it incorporates an aromatic subunit derived from PABA, an essential vitamin for some microorganisms, and it is possible that the PABA moiety in ascr#8 is of bacterial origin. Interestingly, our analysis did not produce any evidence for the presence of a corresponding PABA derivative of ascr#3 (which would feature a 9-carbon side chain) even though ascr#3 is considerably more abundant than ascr#7. Nor did we find evidence for the PABA derivative of ascr#1, the saturated analog of ascr#7, even though ascr#1 is produced in larger amounts than ascr#7 (5). These findings indicate that ascaroside biosynthesis is tightly regulated and suggest that different compounds serve different functions in C. elegans biology. Our results from the mating and dauer assays support this hypothesis. Given the particularly high specificity of ascr#8 biosynthesis, it could be that this compound has important additional functions that remain to be determined. Furthermore, the biosynthesis of ascr#8 could depend on additional genes not required for ascr#1 to ascr#7.
In addition to the absence of ascarosides ascr#1 to ascr#8 in daf-22 worms, our analyses revealed stark differences in tryptophan metabolism. Several 3-substituted indole derivatives, including indole-3-acetic acid, that were produced in large amounts by wild-type C. elegans, could not be detected or occurred at much lower concentrations in daf-22 cultures. Whereas it is unlikely that biosynthesis of ascarosides and indole-3-acetic acid is directly linked via daf-22, it seems possible that the presence of ascarosides influences tryptophan metabolism.
Metabolism in C. elegans strongly depends on environmental conditions, and even small changes in temperature, nutrient conditions, or other factors can induce significant changes in relative concentrations of compounds (7). To minimize the impact of such variations, we specifically designed the algorithms used for DANS in this study to highlight only cases where a compound is completely absent (given the detection limit of the NMR-spectroscopic equipment) from the daf-22 spectra. Furthermore, our analyses occasionally revealed compounds whose presence or absence in wild-type or daf-22 metabolite extracts was not reproducible (for example, see SI Appendix, Fig. S2). Such compounds were excluded from the present analysis. Increase of NMR-spectroscopic sensitivity, or consideration of metabolites whose biosynthesis is less strongly daf-22-dependent, might reveal additional compounds relevant for phenotypic differences between wild-type and daf-22 worms.
It should be noted that, whereas ascr#2, ascr#3, ascr#5, ascr#7, and ascr#8 could easily be detected via DANS, ascr#1, ascr#6.1, and ascr#6.2 as minor components initially escaped detection, because their NMR-spectroscopic signals completely overlapped with signals of more abundant metabolites, including ascr#2 and ascr#3. Use of higher NMR-spectroscopic field strength for DANS could reduce, although not eliminate, the chances for such signal obstruction to occur. In cases where overlap is a concern, additional fractionation may be required to reduce sample complexity.
The current study shows that comparative NMR-spectroscopic methods such as DANS can be used to dissect changes in small-molecule production in response to genetic manipulation, and that this approach can complement or replace activity-guided fractionation for identifying biologically relevant small molecules. The primary benefit of DANS lies in the ability to quickly obtain structural information for metabolites that are good candidates for further evaluation in a specific biological context. It seems likely that application of DANS to metabolomes of other signaling-deficient C. elegans mutants will aid in the discovery of additional classes of endogenous compounds.
Materials and Methods
Analytical Instrumentation and Procedures.
NMR spectra were recorded on a Varian INOVA 600 NMR (600 MHz for 1H, 151 MHz for 13C). NMR spectra were processed using Varian VNMR and MestreLabs MestReC software packages. Additional processing of bitmaps derived from NMR spectra was performed by using Adobe Photoshop CS3 (17). HPLC-MS was performed using an Agilent 1100 Series HPLC system equipped with a diode array detector and connected to a Quattro II spectrometer (Micromass/Waters). Data acquisition and processing for the MS was controlled by the MassLynx software. Flash chromatography was performed by using an Teledyne ISCO CombiFlash system.
C. elegans Strains and General Culture Methods.
C. elegans variety Bristol, strain N2 (wild type), and mutant daf-22(m130) worms were grown at room temperature (22 °C) on NGM agar plates, which were made with Bacto agar (BD Biosciences) and seeded with OP50 bacteria grown overnight (25).
Preparation of Metabolite Extracts.
Metabolite extracts were prepared according to a previously described method that was modified as follows (8). Worms (N2 or daf-22) from 3 NGM plates were washed by using M9-medium into a 100-mL S-medium preculture where they were grown for 6 days at 22 °C on a rotary shaker. Subsequently, the culture was transferred into a 1-L Erlenmeyer flask containing 400 mL of S-medium for a combined volume of 500 mL of S-medium, which was then grown for an additional 14–16 days at 22 °C on a rotary shaker. Concentrated OP50 derived from 1 L of bacterial culture was added as food at days 1, 3, 5, and 7 after which they were fed every day. Subsequently, the cultures were centrifuged and the conditioned media and worm pellet were lyophilized separately. Residual solids were extracted with 95% ethanol (250 mL 3 times) at room temperature for 12 h. The resulting yellow suspension was filtered and the filtrate evaporated in vacuo at room temperature.
For mating assays, metabolite extract derived from a 100-mL liquid culture was redissolved in 1 mL of methanol. 10-μL aliquots of this mixture were diluted into 10 mL of water of which 1-μL samples were assayed.
Statistical Analysis.
For Fig. 1C and 4 A and E we used unpaired t tests with Welch's correction. For Fig. 4C, we used one-factor ANOVA with Tukey–Kramer multiple comparison post test to compare holding times of the different ascarosides tested. For the dauer formation assay, one-factor ANOVA with Dunnett's post test was used, since all ascarosides were compared with a common control (***, P < 0.001; **, P < 0.01; *, P < 0.05).
Supplementary Material
Acknowledgments.
We thank Art Edison and Fatma Kaplan for helpful discussions, Olena Vatamaniuk for assistance with C. elegans liquid cultures, and Ivan Keresztes for assistance with NMR spectroscopy. This work was supported in part by National Institutes of Health Grant P41 GM079571 (to F.C.S.) and by the Howard Hughes Medical Institute (J.S., P.W.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7685.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811918106/DCSupplemental.
References
- 1.Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–399. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 2.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder FC. Small molecule signaling in Caenorhabditis elegans. ACS Chem Biol. 2006;1:198–200. doi: 10.1021/cb600173t. [DOI] [PubMed] [Google Scholar]
- 4.Motola DL, et al. Identification of Ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Jeong PY, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 6.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 7.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci USA. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan J, et al. A blend of small molecules regulates both mating and development in. Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden JW, Riddle DL. A Caenorhabditis elegans dauer-inducing pheromone and an antagonistic component of the food supply. J Chem Ecol. 1984;10:1265–1280. doi: 10.1007/BF00988553. [DOI] [PubMed] [Google Scholar]
- 10.Riddle DL, Albert PS. In: C. elegans II. Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 739–768. [Google Scholar]
- 11.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang RA. C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 12.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 13.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 14.Beckstead RB, Thummel CS. Indicted: Worms caught using steroids. Cell. 2006;124:1137–1140. doi: 10.1016/j.cell.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Rochfort S. Metabolomics reviewed: A New “omics” platform technology for systems biology and implications for natural products research. J Nat Prod. 2005;68:1813–1820. doi: 10.1021/np050255w. [DOI] [PubMed] [Google Scholar]
- 16.Taggi AE, Meinwald J, Schroeder FC. A new approach to natural products discovery exemplified by the identification of sulfated nucleosides in spider venom. J Am Chem Soc. 2004;126:10364–10369. doi: 10.1021/ja047416n. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder FC, et al. Differential analysis of 2D NMR spectra: New natural products from a pilot-scale fungal extract library. Angew Chem Int Ed. 2007;46:901–904. doi: 10.1002/anie.200603821. [DOI] [PubMed] [Google Scholar]
- 18.Butcher RA, et al. The identification of bacillaene, the product of the PksX megacomplex in. Bacillus subtilis. Proc Natl Acad Sci USA. 2007;104:1506–1509. doi: 10.1073/pnas.0610503104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden JW, Riddle DL. A gene affecting production of the Caenorhabditis elegans dauer-inducing pheromone. Mol Gen Genet. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- 20.Butcher RA, et al. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci USA. 2009;106:1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronquist M, Meinwald J, Eisner T, Schroeder FC. Exploring uncharted terrain in nature's structure space using capillary NMR spectroscopy: 13 steroids from 50 fireflies. J Am Chem Soc. 2005;127:10810–10811. doi: 10.1021/ja053617v. [DOI] [PubMed] [Google Scholar]
- 22.Fairbairn D. The biochemistry of Ascaris. Exp Parasitol. 1957;6:491–554. doi: 10.1016/0014-4894(57)90037-1. [DOI] [PubMed] [Google Scholar]
- 23.Jezyk PF, Fairbairn D. Ascarosides and ascaroside esters in Ascaris lumbricoides (Nematoda) Comp Biochem Physiol. 1967;23:691–705. doi: 10.1016/0010-406x(67)90334-9. [DOI] [PubMed] [Google Scholar]
- 24.Bartley JP, Bennett EA, Darben PA. Structure of the ascarosides from. Ascaris suum J Nat Prod. 1996;59:921–926. doi: 10.1021/np960236+. [DOI] [PubMed] [Google Scholar]
- 25.Stiernagle TC. In: C. elegans: A Practical Approach. Hope I, editor. Cambridge, UK: Oxford Univ Press; 1999. pp. 55–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.