Abstract
Type I IFN-induced expression of dsRNA-activated protein kinase (PKR) during viral infection is a well-established antiviral mechanism. However, little is known about the expression of PKR in the context of p53 and about PKR involvement in p53-mediated tumor suppression. Here, we report that PKR is a p53 target gene and plays an important role in the tumor-suppressor function of p53. Activation of p53 by genotoxic stress induces a significant level of PKR expression by acting on the newly identified cis-acting element (ISRE), which is separated from the IFN-stimulated responsive element on the PKR promoter, resulting in translational inhibition and cell apoptosis. The genotoxin-mediated inhibition of translation is associated with the p53/PKR/elF2a (eukaryotic initiation factor-2α) pathway. To some extent, p53 activation induced by DNA damage facilitates cell apoptosis by activating PKR. PKR-knockdown human colon cancer cells grew rapidly in nude mice and proved resistant to anti-cancer drugs. These data indicate that p53-mediated tumor suppression can be attributed at least in part to the biological functions of PKR induced by p53 in genotoxic conditions.
Keywords: p53 tumor suppression, p53RE, translation inhibition and apoptosis
The p53 tumor suppressor plays a pivotal role in cellular homeostasis via the modulation of cell-cycle arrest, DNA repair, senescence, and apoptosis upon exposure to genotoxic stresses (1–7). Stress-mediated p53 activation results in the expression of a sizeable number of p53 target genes, including p21waf1/cip1 (8) for G0/G1 arrest, p21waf1/cip1 and 14–3-3σ for G2/M arrest (9), Bax, Noxa, Puma, Killer/DR5, Fas/Apo1, and others for p53-mediated apoptosis (10), a p53-inducible gene referred to as “TIGAR” for the control of glycolysis and cell survival (2), DRAM, a lysosomal protein that induces macroautophagy (11), and POMC/MSH keratinocyte-secreting hormone required for UV-induced pigmentation (12).
On the other hand, type I interferons (IFN-α/β), essential cytokines for antiviral activity, sometimes are referred to as “negative growth factors” (13) and manifest anti-oncogenic activities (14). In fact, type I IFNs have been used in the treatment of certain human cancers (15). A recent report demonstrated that the anti-oncogenic activities of IFNs are, in part, accompanied by an increase in the expression of p53 (16). However, the major proportion of the anti-oncogenic activity of type I IFN is most likely accompanied by the type I IFN-inducible dsRNA-activated protein kinase (PKR). IFN-α/β induces the expression of PKR through the activation of the highly conserved 13-bp sequence of the IFN-stimulated responsive element (ISRE) on the PKR promoter (17). PKR is one of the best-characterized protein kinases and is involved both in IFN-mediated antiviral activity (18) and in IFN-mediated anti-oncogenic activity (14, 16). Recently, it also was found that PKR or viral dsRNA down-regulates p53 (19, 20). Although the biological functions of PKR have been evaluated, it remains unclear whether the induction of PKR expression is exclusively dependent on type I IFN. If so, we would be unable to account for PKR-associated growth regulation or apoptosis in cells that lack type I IFN.
We found that PKR expression was markedly enhanced by genotoxin treatment in p53+/+ cells but not in p53−/− cells, regardless of viral infection or IFN treatment. Based on these observations, we investigated whether genotoxin-mediated PKR expression is associated with p53. Here we report that p53 induces PKR expression by acting on the newly identified cis-acting element (p53RE) on the PKR promoter. p53 activation caused by DNA-damaging stress results in a significant increase in the expression of PKR, followed by the induction of PKR-associated biological functions such as inhibition of translation and cell apoptosis, which have been studied in association with the tumor-suppressor functions of p53.
Results
p53 Induces a Significant Level of PKR Expression in an IFN-Independent Manner.
PKR, as well as other p53 target genes, was up-regulated in HCT116 (p53+/+) human colon cancer cells relative to isogenic HCT116 (p53−/−) cells (Fig. 1A). The transient expression of p53 induced PKR expression at both transcriptional (Fig. 1B) and translational levels in a dose-dependent manner (Fig. 1C); the quantity of PKR induced by p53 was equivalent to that induced by IFN-α and was equivalent to other p53 target genes (Fig. 1 A-C) such as p21waf1/cip1, Puma, or Bax (4, 7). In the immunocytochemistry and confocal studies, the p53-transfected HCT116 p53−/− cells were stained clearly by anti-PKR antibody (green), and the level of staining was quite similar to that of cells treated with IFN-α. The cells transfected with empty vector were barely stained (Fig. 1D). Even under physiological conditions, PKR was significantly induced by genotoxic stresses in p53wt cells but not in p53−/− (i.e., p53null) cells or in p53mutant cells (Fig. 1E; Fig. S1A), suggesting that endogenous p53 would be sufficient to activate PKR expression. Similar results were seen in p53+/+ and p53−/− mouse embryo fibroblast (MEF) cells at mRNA (Left) and protein (Right) levels (Fig. S1B). In addition, PKR expression induced by doxorubicin treatment was diminished markedly by PFTα, a p53-specific inhibitor (Fig. 1F) (21). These results indicate that p53 probably is involved in the enhancement of PKR expression. On the other hand, Takaoka et al. (16) found that IFN-induced p53 is crucial for host antiviral defense during vesicular stomatitis virus (VSV) infection. However, the underlying mechanism downstream of p53 has not been demonstrated. In the present study, as reported previously (16), type 1 IFN induced the expression of p53 in a dose-dependent manner (Fig. 1G). Also, VSV infection induced the expression of type I IFN regardless of p53 but induced PKR expression more efficiently in p53+/+ cells than in p53−/− cells (Fig. S2). The antiviral activity of p53 was abrogated by PKR knockdown (Fig. 1H). These results indicate that the antiviral function of IFN-induced p53 is associated with the expression of PKR downstream of p53.
Fig. 1.
p53 induces PKR expression. (A) Expression of endogenous PKR and other p53 target genes in HCT116 (p53+/+ or p53−/−) cells was assessed by Western blot analysis. (B) mRNA levels of PKR and other p53 target genes in the p53-transfected (3 μg pcDNA3-p53) or IFNα-treated (1,000U/ml for 24 h) HCT116 p53−/− cells and in untreated cells (UT) were examined by real-time quantitative RT-PCR. Results are expressed as means ± SEM (n = 3). (C) Western blot analysis of PKR and other p53 target genes in p53-transfected (1 or 3 μg of pcDNA3-p53) or IFNα-treated HCT116 p53−/− cells. (D) Immunocytochemistry of p53-transfected (1 μg pcDNA3-p53) or IFNα-treated (1,000U/ml for 24 h) HCT116 p53−/− cells after staining with anti-PKR antibody and anti-rabbit IgG-FITC (green) and anti-p53 antibody (DO-1) and anti-mouse IgG-Rhodamine (red), together with each isotype control IgG, as described in Materials and Methods. (E) PKR expression in p53 wild-type, mutant, and null cells under conditions of DNA damage (0.5 μM doxorubicin [Dox], 5 μM etoposide [Ets], and 1 mM hydroxyurea [HU]) for 12 h or 6 h after 20 J/m2 UV) and in untreated cells (UT). (F) PKR expression in RKO cells treated with IFN-α or doxorubicin (Dox) in the presence or absence of 20 μM pifithrin alpha (PFTα), a p53-specific inhibitor. (G) Western blot analysis of PKR and p53 in HCT116 p53+/+ and p53−/− cells treated with IFNα for 12 h. (H) HCT116 cells (p53+/+, p53−/−, p53+/+/sh-con, or sh-PKR) infected for 1 h with VSV (multiplicity of infection = 1) were cultured in presence or absence of doxorubicin (Dox, 1 μM). After 12 h, VSV in the culture supernatant was titrated by the 50% tissue culture infectious dose (TCID50) method. PKR-knockdown and p53-knockout was evaluated (Right). *, P < 0.01 and **, P < 0.02, as compared with indicated control, respectively.
p53 Acts Directly on the PKR Promoter.
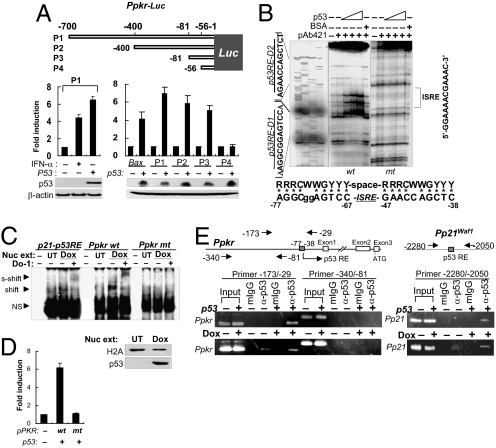
We then attempted to determine whether p53 acts directly on the PKR promoter. In the luciferase reporter assay, using PKR promoter (Ppkr)-conjugated luciferase (Ppkr-luc), transient expression of p53 induced strong luciferase activity in the Ppkrfull-luc–transfected cells (Fig. 2A, Lower left), suggesting that p53 may act on the PKR promoter. A series of promoter deletion assays showed that the putative p53-responsive region is located around -81/-1 of the 5′-franking region of the PKR promoter (Fig. 2A, Lower right). Further promoter deletion assays narrowed this region to -81/-38 (Fig. S3).
Fig. 2.
p53 acts directly on the PKR promoter. (A) Promoter deletion assay using luciferase reporters (Ppkr-Luc). Luciferase activities were assessed in p53-transfected HCT116 (p53−/−) cells. Results are reported as mean ± SEM (n = 3). (B) DNase I footprinting with 32P-labeled wild-type (wt) and mutant (mt, sequence shown in Fig. S4) PKR promoter fragments (-160/-29) and p53 protein (BaculoV-p53). Consensus p53RE sequence is aligned with the identified Ppkr-p53RE sequence (Lower). (C) EMSA using nuclear extracts (Nuc ext) obtained from doxorubicin-treated HCT116 p53+/+ cells (Dox), 32P-labeled PKR81/-29/p21 promoter fragments, and α-p53 antibody (DO-1). Nuclear extracts were assessed with anti-histone 2A (H2A) antibody and DO-1 (Lower). UT, untreated; NS, nonspecific. (D) Luciferase reporter assay with wild-type (wt) and mutant (mt) PKR promoters (Ppkr) used in EMSA in panel C and in Fig. S4B. Luciferase assay was performed 48 h after transfection of HCT116 p53+/+ cells with wild-type or mutant PKR promoter (Ppkr-81/-29)-attached luciferase reporters together with p53-expressing plasmid (1 μg pCDNA-p53). Data shown are from 3 independent experiments and are expressed as means ± SEM. (E) ChIP assay with chromatins obtained from p53-transfected HCT116 (p53−/−) cells or from doxorubicin-treated (0.5 μM for 12 h) p53+/+ cells (Dox), and DO-1 (α-p53) or control mouse IgG (mIgG).
To verify the location of p53RE on the PKR promoter, we conducted a DNase I footprinting assay. Two protected regions and their sequences were identified on positions -77/-67(p53RE-D1) and -47/-38 (p53RE-D2) of the PKR promoter, whereas these footprints were not detected by mutation in the region (Fig. 2B and Fig. S4A). As shown at the bottom of Fig. 2B, the putative pkr-p53RE sequence located on both sides of ISRE has some mutations at each site and an unusually long spacer (19 bp instead of less than 13 bp) between the 2 half-sites, as compared with the well-established p53RE consensus sequence (22).
In an EMSA using baculovirus-expressed p53 protein (BaculoV-p53) and Ppkr oligonucleotide (Ppkr-81/-29) encompassing the putative p53-responsve region (pkr-p53RE), the Ppkr oligonucleotide was shifted by p53 binding and also was supershifted by anti-p53 Ab (DO-1), whereas the mutant oligonucleotide containing pkr-p53RE was not shifted or supershifted (Fig. S4 A and B). In the cross-competitive EMSA, p53 binding to 32P-labeled Ppkr-81/-29 was not blocked completely by the unlabeled p21-p53RE competitor until a 500-fold molar excess was used, whereas the p53/32P-labeled p21-p53RE binding was blocked completely at only a 25-fold molar excess of the unlabeled Ppkr-81/-29 competitor (Fig. S4B). In the EMSA with nuclear extracts and Ppkr-81/-29 oligonucleotides, p53-mediated band shift and anti-p53 antibody-mediated supershift also were observed in doxorubicin-treated HCT116 p53+/+ cells (Fig. 2C). In the luciferase reporter assay, the Ppkr-81/-29 promoter was sufficiently active to induce luciferase activity, but the promoter encompassing mutant pkr-p53RE was not active (Fig. 2D). These combined data indicate that p53 activates the PKR promoter via direct, specific, and high-affinity binding to the p53RE.
To ascertain whether p53 binds to the pkr-p53RE under physiological conditions, we conducted a ChIP assay. As shown in Fig. 2E, Ppkr-specific ChIP bands were detected clearly only when p53-expressing samples (p53-transfected p53−/− cells or genotoxin-treated p53+/+ cells) were precipitated by anti-p53 antibody. The ChIP band was notably thicker in the samples obtained from doxorubicin-treated p53+/+cells than in those from untreated control (Fig. 2E, Bottom). These data suggest that p53 binds directly to the pkr-p53RE and activates the PKR promoter, resulting in the induction of PKR expression in response to DNA damage.
p53 Activates the PKR Promoter Independently of ISRE.
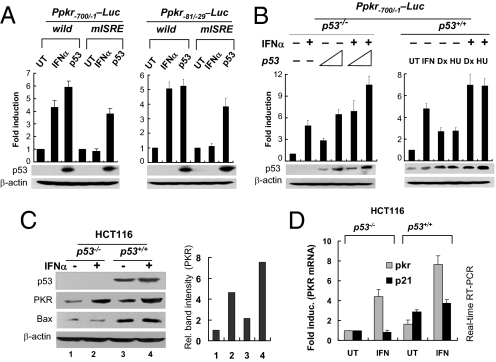
To determine whether p53-mediated PKR expression is dependent on the ISRE, we conducted a reporter assay with PKR promoter harboring a mutant ISRE (mISRE) (Fig. S4A). Transient expression of p53 induced substantial amounts of luciferase activity in the reporter assay with the mISRE-harboring PKR promoter (Ppkr-Luc), whereas IFNα treatment showed no luciferase activity (Fig. 3A). These results indicate that Ppkr-p53RE is not associated with ISRE. However, p53, expressed by transfection of pCDNA-p53 (Fig. 3B, Left) or physiologically induced by genotoxin-treatment (Fig. 3B, Right), and IFN-α showed mutual additive effects on Ppkr-mediated reporter expression levels. In accordance with the results from the reporter assay, endogenous PKR expression itself also was additively enhanced by both p53 and IFN-α, rather determined by protein levels (Fig. 3C and Fig. S5) and mRNA levels (Fig. 3D) of each alone. These data indicate that PKR can be induced not only by IFN but also by p53 noncompetitively.
Fig. 3.
p53 activates PKR promoter independently of ISRE. (A) Activity of the PKR promoters harboring mutant ISRE (mISRE) was assessed by luciferase assays in untreated (UT), p53-transfected (3 μg pcDNA3-p53) or IFNα-treated (1,000U/ml for 24 h) HCT116 p53−/− cells. Results are expressed as means ± SEM (n = 3). (B) Additive effects of p53 and IFN-α on the activity of the PKR promoter, accessed in p53-transfected HCT116 (p53−/−) cells (Left), or in genotoxin-treated (0.5 μM doxorubicin [Dx], 1 mM hydroxyurea [HU] for 12 h) HCT116 (p53+/+) cells in the presence (+) or absence (−) of IFN-α (Right). HU, hydroxyurea; UT, untreated Results are reported as means ± SEM (n = 3). (C, D) Additive effects of p53 and type 1 IFN on the expression of PKR at protein (C) and mRNA (D) levels in HCT116 (p53+/+ or p53−/−) cells were assessed by Western blotting and real-time RT-PCR, respectively. Results are reported as means ± SEM (n = 3). UT, untreated.
PKR Plays an Important Role in the p53-Mediated Inhibition of Translation Under Conditions of DNA Damage.
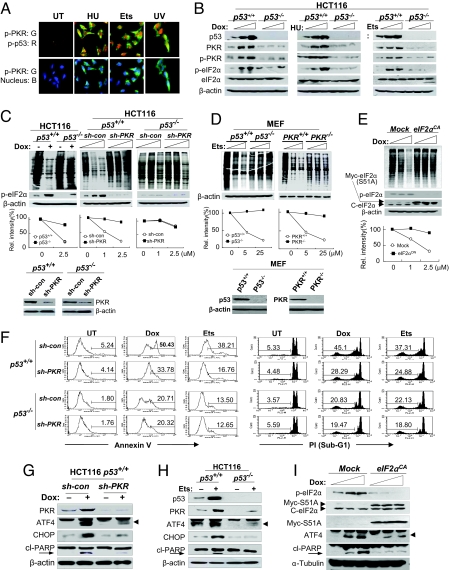
When the cells were treated with genotoxins, the PKR in the cytoplasm was largely phosphorylated in the immunohistochemistry (Fig. 4A). Genotoxin treatment enhanced PKR activation and eukaryotic initiation factor-2α (eIF2α) phosphorylation at Ser-51 in a dose-dependent manner in p53+/+ cells, whereas no enhancement was observed in isogenic p53−/− cells (Fig. 4B) or in p53KD (si-p53) cells (Fig. S6), suggesting that p53 also is involved in PKR activation and eIF2α phosphorylation in response to DNA damage stresses.
Fig. 4.
PKR plays a role in the p53-mediated inhibition of translation and apoptosis. (A) Genotoxin-treated (5 μM etoposide [Ets] and 1 mM hydroxyurea [HU] for 12 h) RKO cells were examined by immunocytochemistry with phospho-PKR and phospho-p53 antibodies and each isotype control (control data not shown). (B) Phospho-PKR and phospho-eIF2α were assessed in HCT116 (p53+/+ or p53−/−) cells following genotoxin-treatment (0–1 μM doxorubicin [Dox], 0–5 μM etoposide [Ets], and 0–2 mM hydroxyurea [HU] for 12 h). (C, D) A metabolic labeling assay performed with p53+/+, p53−/−, PKR+/+, and PKRKD isogenic HCT116 cells and p53+/+, p53−/−, PKR+/+, and PKR−/− MEF cells after genotoxin treatment with increasing concentrations for 12 h (Top). Relative band intensities are represented by histograms (Middle). PKR-knockdown, p53-knockout, and PKR knockout were confirmed (Bottom). Dox, doxorubicin; Ets, etoposide. (E) A metabolic labeling assay was performed with normal and eIF2α constitutively active cells (eIF2αCA by permanent expression of eIF2α/S51/A) after doxorubicin (Dox) treatment with increasing concentrations for 12 h. Expression of cellular (c-eIF2α) and mutant eIF2α (Myc-S51A) was confirmed. (F) Cell apoptosis was assessed by flow cytometry in untreated (UT) PKRKD (sh-PKR) HCT116 (p53+/+ and p53−/−) cells and after genotoxin treatment. Early-stage apoptosis was assessed by annexin V staining of genotoxin-treated (0.5 μM doxorubicin [Dox] and 5 μM etoposide [Ets] for 36 h) cells (Left). Later-stage apoptosis was assessed by measuring subG1 cells after propidium iodide (PI) staining of genotoxin-treated (0.5 μM doxorubicin [Dox] and 5 μM etoposide [Ets] for 48 h) cells, as described in Materials and Methods. (G, H) PKR-associated pro-apoptotic molecules and cleaved PARP were assessed in PKRKD (sh-PKR) isogenic HCT116 (p53+/+ and p53−/−) cells after genotoxin treatment (0.5 μM doxorubicin [Dox] and 5 μM etoposide [Ets]) for 12 h. (I) Phospho-eIF2α, ATF4, and cleaved PARP were determined in normal and eIF2αCA cells after genotoxin treatment with increasing concentrations for 12 h. Dox, doxorubicin.
Type I IFN-mediated inhibition of translation has been well defined with regard to PKR-mediated eIF2α phosphorylation (23). However, DNA damage/p53-mediated inhibition of translation has not yet been demonstrated clearly. When cells were treated with doxorubicin, inhibition of translation was observed clearly by the pulse–chase metabolic labeling assay in p53+/+ HCT116 cells, but only minor inhibitions were detected in p53−/− HCT116 cells (Fig. 4C, Left). On the other hand, genotoxin-mediated inhibition of translation in p53+/+ HCT116 cells was markedly obliterated by PKR-knockdown (sh-PKR) (Fig. 4C, Middle), as was observed in the isogenic p53−/− cells (Fig. 4C, Left) and other p53KD (si-p53) RKO cells (Fig. S7A). However, additional PKR-knockdown (sh-PKR) to the HCT116 p53−/− cells did not increase the resistance of p53−/− cells to the genotoxin-mediated inhibition of translation (Fig. 4C, Right). These results were supported further by the data from PKR-knockout (PKR−/−) MEF cells. Translation was markedly inhibited by etoposide in p53+/+ MEF cells, whereas no inhibition was observed in p53−/− MEF cells (Fig. 4D, Left). The difference between p53+/+ and p53−/− MEF cells in genotoxin-mediated inhibition of translation also was observed between PKR+/+ and PKR−/− MEF (p53+/+) cells (Fig. 4D, Right). Genotoxin-mediated inhibition of translation also was obliterated in eIF2α constitutively active mutant (eIF2αCA) (Fig. 4E). Taken together, our present findings suggest that inhibition of translation mediated by DNA damage is associated with a p53/PKR/eIF2α-phosphorylation pathway.
It was reported that, upon activation, mammalian target of rapamycin (mTOR) C1 increases the phosphorylation levels of p70S6 kinase and eIF4E-binding protein 1 (4EBP1), leading to an enhancement of translation (24), whereas, p53 inhibits the activity of mTOR by activating the AMP-activated protein kinase (AMPK) and tuberous sclerosis (TSC)1/TSC2 pathway (25). We examined whether genotoxin-induced inhibition of translation is associated with the p53-mediated mTORC1 inhibitory pathway. As shown in Fig. S7B, PKR-knockdown of p53+/+ cells abrogated the genotoxin-induced inhibition of translation (from 23% to 92%), as shown in p53−/− cells (96%), whereas treatment of p53+/+ cells with AMPK inhibitor to block the p53-mTORC1 inhibitory pathway attenuated genotoxin-induced inhibition of translation only weakly (from 23% to 33%). These data suggest that the p53/AMPK/mTORC1 inhibitory pathway is a minor route compared with the p53-PKR pathway in genotoxin-induced inhibition of translation.
PKR Plays an Important Role in the p53-Mediated Cell Apoptosis Under Conditions of DNA Damage.
On the other hand, it has been well documented that p53 plays a crucial role in genotoxin/UV-mediated apoptosis and that the p53-knockout/knockdown (p53KO/KD) cells are resistant to apoptosis mediated by DNA damage (26). The underlying mechanism remains uncertain, however. In the present study, the resistance of p53KO/KD cells to apoptosis mediated by DNA damage also was observed in the PKRKD(sh-PKR) HCT116 p53+/+ cells in the analyses of early and later-stage apoptosis, by examining annexin V-expressing cells and subG1 populations, respectively (Figs. 4F and S8A). However, additional PKR-knockdown in HCT116 p53−/− cells did not show further resistance to the apoptosis mediated by stress resulting from DNA damage (Fig. 4F). These data suggest that PKR plays an important role in genotoxin-induced p53-mediated cell apoptosis.
Although genotoxin/p53-mediated apoptosis has been well established with regard to anti-cancer activity, it remains unclear whether genotoxin-mediated apoptosis is functionally associated with the inhibition of translation. In the kinetic studies, genotoxin-mediated inhibition of translation was observed in the relatively early stage, and cell apoptosis occurred in the later stage in response to doxorubicin in PKR-competent (sh-con) cells, but inhibition of translation was delayed significantly, in parallel with cell apoptosis, under the same genotoxic condition in PKRKD(sh-PKR) cells (Fig. S8 B and C). These data suggest that even though PKR plays a role in genotoxin-mediated inhibition of translation and apoptosis, the genotoxin-mediated apoptosis is, to some extent, functionally associated with but temporally dissociated from PKR-mediated inhibition of translation.
The phosphorylation of eIF2α at Ser-51 leads to a significant reduction in protein synthesis, concomitant with induced expression of the basic leucine zipper (bZIP) regulator, activating transcription factor 4 (ATF4), and its target gene CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), resulting in caspase activation and cell apoptosis (27). In accordance with our recent report (28), enhancements of ATF4/CHOP, followed by caspase-mediated cleavage of poly(ADP-ribose) polymerase (PARP) (29) were detected readily in p53+/+PKR+/+ cells but were barely detectable in the p53+/+PKRKD (sh-PKR) cells under conditions of DNA damage (Fig. 4G). These phenomena also were observed in p53+/+ and p53−/− cells (Fig. 4H). ATF4 enhancement was not detected in eIF2αCA cells even after treatment with genotoxins (Fig. 4I), suggesting that the induction of ATF4 in response to genotoxins is dependent on the phosphorylation of eIF2α. ATF4-knockdown or PKR-knockdown (by si-RNA) HCT116 p53+/+ cells were found to be resistant to genotoxin-induced apoptosis, as compared with the control (si-con) p53+/+ cells, whereas additional knockdown of ATF4 or PKR in p53−/− cells did not increase the resistance of HCT116 p53−/− cells to genotoxin-induced apoptosis (Fig. S8D). These results strongly indicate that PKR has an important role in p53-mediated inhibition of translation and apoptosis under conditions of DNA damage.
PKR Contributes to the Tumor-Suppressor Function of p53.
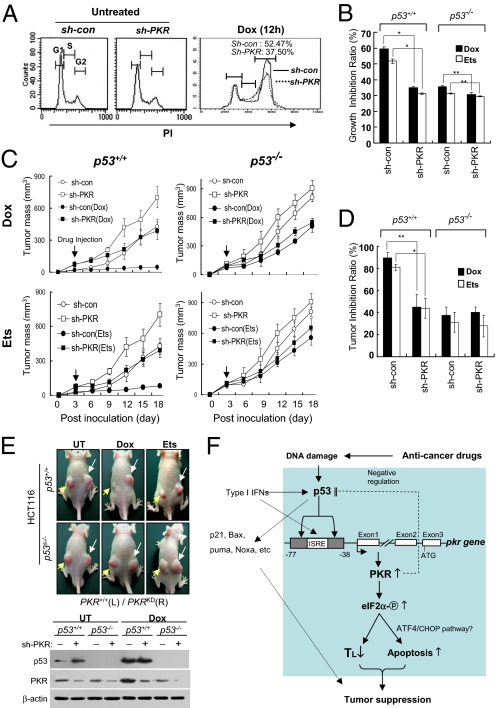
Finally, we attempted to determine whether PKR is involved in the tumor-suppressor function of p53. Once PKR was knocked down (sh-PKR), HCT116 p53+/+ cells became small and grew rapidly, and the growth rate of the p53+/+PKRKD cells was almost equivalent to that of p53−/− cells (Fig. S9 A and B). p53 has been reported to induce G2 arrest under genotoxic stresses (9), but the underlying mechanism remains unclear. In our present studies, doxorubicin-mediated G2 arrest was clearly attenuated by PKR-knockdown (by sh-PKR) in HCT116 p53+/+ cells (Fig. 5A), suggesting that PKR downstream of p53 plays an important role in G2 arrest under genotoxic conditions. The p53+/+PKRKD HCT116 cells (sh-PKR) were profoundly resistant to the growth-inhibitory effects of anti-cancer drugs such as doxorubicin and etoposide; their resistance was equivalent to that of p53−/− HCT116 cells under the same conditions (Fig. 5B and Fig. S9 C and D). These results suggest that PKR plays an important role in the p53-mediated inhibition of cell growth. We then evaluated the kinetics of tumor growth and the sensitivity of PKR-knockdown tumors to anti-cancer drugs in vivo. When inoculated into nude mice, the p53+/+PKRKD HCT116 cells were shown rapidly to establish solid tumors that grew faster than tumors established with control (sh-con) p53+/+ HCT116 cells. Furthermore, the tumors established with PKRKD cells were resistant to treatment with doxorubicin or etoposide, whereas tumors established with control (sh-con) cells were readily blocked by identical treatments (Fig. 5C, Left 2 panels). On the other hand, the growth rates and drug-resistance patterns of PKRKD tumors were similar to those of p53−/− tumors (Fig. 5C, Right 2 panels). In p53+/+ tumors, PKRKD (sh-PKR) tumors were more resistant to anti-cancer drugs than PKR-normal (sh-con) tumors, whereas the resistance of p53−/− tumors to anti-cancer drugs was not further affected by additional PKR knockdown (Fig. 5D). PKRKDp53+/+ tumors were larger and more resistant to genotoxin treatments than PKR+/+p53+/+ tumors (Fig. 5E, Top). However in p53−/− tumors, tumor growth and resistance to anti-cancer drugs were not further affected by additional PKR knockdown (Fig. 5E, Lower panels). These results suggest that PKR is involved in the p53-mediated tumor suppression downstream of p53.
Fig. 5.
PKR contributes to the tumor-suppressor function of p53. (A) Normal (sh-con) and PKRKD (sh-PKR) HCT116 cells were treated or not treated with 0.2 μM doxorubicin (Dox) for 12 h. Each sample was subjected to cell-cycle analysis by flow cytometry with CellQuest software (Adobe) after propidium iodide staining. (B) PKR-normal (sh-con) and PKRKD (sh-PKR) HCT116 (p53+/+ or p53−/−) cells were cultured in the presence or absence of 50 nM doxorubicin (Dox) or 1 μM etoposide (Ets), respectively, and the number of cells was recorded every day for 4 days (shown in Fig. S9C). Growth inhibition ratios [(1 − number of cells after drug treatment/number of cells without drug treatment) × 100] were calculated with the data obtained on day 4 in Fig. S9C. *, P < 0.01 and **, P < 0.05, as compared with the group of cells harboring control sh-RNA, respectively. Results are reported as means ± SEM (n = 5). (C) Nude mice were inoculated s.c. in the dorsal area (107 cells/injection, 4 mice/sample) with sh-con (left dorsal) and sh-PKR (right dorsal) HCT116 (p53+/+ or p53−/−) cells. Three days later, mice were treated i.p. once with doxorubicin (Dox) (2 mg kg−1) or etoposide (25 mg kg−1), and tumor growth was monitored for 18 days. Results are reported as means ± SEM (n = 4). (D) Inhibition ratios of tumor growth in tumor-bearing mice by doxorubicin (Dox) or etoposide (Ets) treatment were recalculated with the data on day 18 and are represented as means ± SEM (n = 4). *, P < 0.01 and **, P < 0.02, respectively, as compared with the mouse group bearing tumors expressing control sh-RNA. (E) Untreated (UT) and genotoxin-treated tumor-bearing mice were imaged on day 15 (Top). Yellow arrows and white arrows indicate the tumors established by inoculating sh-con (left dorsal) and sh-PKR (right dorsal) HCT116 (p53+/+ or p53−/−) cells, respectively. The expression of p53 and PKR was examined from each tumor (Bottom). (F) p53-induced PKR expression and associated tumor-suppression mechanisms are described together with other p53 target genes under conditions of DNA damage. Our findings described in this article are boxed.
Discussion
Although many p53 target genes have been described, the precise mechanisms of p53-mediated tumor suppression remain uncertain. In the present study, we found that PKR is a p53 target gene, regardless of type I IFN or viral infection, and plays an important role in the tumor-suppressor function of p53 at least in part through inhibition of translation and induction of cell apoptosis. PKR was markedly induced by p53 without the aid of type I IFN (Fig. 1). Recently, more than 540 p53-binding loci and 98 p53 target genes were revealed in the human genome by a ChIP-and-PET (paired-end ditag) coupled screening strategy (30). However, PKR and also other p53 target genes, such as DRAM (11), TIGAR (31), POMC/MSH (12), human cell apoptosis susceptible protein (hCAS) (32), and others, were not listed in the report (30).
In accordance with the well-defined p53RE consensus sequence (22), we identified 2 p53RE domains (p53RE-D1 and p53RE-D2) near the ISRE region on the PKR promoter. The binding affinity of p53 to the p53RE on the PKR promoter seemed higher than that of p53 to p21-p53RE as determined by competitive EMSA and ChIP assays (Fig. 2 C and E and Fig. S4B). The higher affinity could be attributed to our use of a single 5′-p53RE of the p21 promoter (8) rather than both p21-p53REs, which are widely separated on the p21 promoter (33). p53-mediated activation of the PKR promoter remained largely unaffected by mutations on the ISRE (Fig. 3A), indicating that PKR can be induced by p53 independently of type 1 IFN. Thus PKR has a dual function, protecting cells both from DNA damage and from viral infection. Recently, Munoz-Fontela et al. demonstrated that IRF9 is a p53 direct target gene, thus causing p53-dependent up-regulation of ISRE-dependent genes (34). That finding would explain why p53-mediated PKR promoter activity was reduced slightly by ISRE mutation on the PKR promoter (Fig. 3A). Although genotoxin-induced/p53-mediated inhibition of translation has been reported as a tumor-suppressor function of p53 (35), the precise mechanism remains uncertain.
Our data demonstrate that DNA damage induces p53 expression, followed by the expression and activation of PKR, resulting in phosphor-eIF2α–mediated inhibition of translation and the induction of cell apoptosis (Fig. 4 and Figs. S6–S8). In other words, the genotoxin-mediated inhibition of translation is associated with a p53/PKR/eIF2α pathway. These results suggest that p53-induced PKR plays an important role in maintaining cell homeostasis by controlling the inefficient energy consumption of translation under conditions of DNA damage. In addition, well-addressed p53-mediated G2 arrest (9) was clearly attenuated by PKR knockdown in genotoxin-treated p53+/+ cells (Fig. 5A), and similar attenuation patterns were detected regularly in isogenic p53−/− cells in response to doxorubicin/etoposide (data not shown). These data suggest that PKR probably is involved in the inhibition of G2/M transitions downstream of p53. It has been demonstrated that the cells lacking p53 target genes, such as Puma or Noxa, are resistant to apoptosis induced by DNA damage (7). Interestingly, similar patterns of resistance to apoptosis induced by DNA damage were detected in PKRKD human cells (Fig. 4). However, the resistance of p53+/+PKRKD cells to apoptosis induced by DNA damage was not as strong as that of p53−/− cells (Fig. 4F), suggesting that, in addition to PKR, other pro-apoptotic p53 target genes may be involved in p53-mediated apoptosis in conditions of DNA damage. In supporting experiments, additional Puma-knockdown (by si-RNA) further attenuated the doxorubicin-mediated apoptosis of PKRKD (sh-PKR) HCT116 cells (Fig. S10).
Recently, the Koromilas group reported that PKR promotes the proteasomal degradation of p53 in association with glycogen synthase kinase-3 (GSK-3β) and Mdm-2, independently of translational control (19). Given this background information, we examined p53 levels in the presence or absence of PKR and found that p53 levels were slightly increased in p53+/+PKRKD HCT116 and RKO cells, as compared with p53+/+PKR+/+ cells, both under normal conditions and under genotoxic stress (Fig. S11A). The stability of p53 was slightly enhanced in PKRKD cells, as compared with PKR-competent cells (Fig. S11B). The treatment of cells with MG132, a proteasomal inhibitor, and Nutlin3, a Mdm2 inhibitor, abrogated the PKR-mediated down-regulation of p53, and the enhanced p53 augmented the expression of PKR and other p53 target genes only in p53wt cells (Figs. S11C and S12). These results indicate that p53-induced PKR conversely plays a role in the feedback down-regulation of p53. The PKR-mediated negative feedback of p53 seems to be a kind of homeostatic control of p53-mediated PKR enhancements. However, the PKR-mediated down-regulation of p53 may not be strong enough to obliterate the overexpression of p53 and down-stream tumor-suppressor functions under genotoxic conditions.
PKRKD cells grew faster than normal cells in vitro and in vivo. PKRKD human colon cancer cells and derived tumors proved to be resistant to anti-cancer drugs as shown in p53−/− cells and derived tumors (Fig. 5). In our recent tissue microarray analysis, we found that the PKR level was lower in many human p53-negtive (undetectable) tumor tissues than in normal tissues (data not shown). These results indicate that the tumor-suppressor functions of p53 are, to some degree, attributable to the functions of p53-induced PKR. Our findings suggest that PKR downstream of p53 may protect cells from tumorigenesis under conditions of DNA damage and facilitate p53+/+ tumors that are, in part, susceptible to anti-cancer drugs.
Based on our combined findings, we report that PKR, induced by p53 in response to the stress of DNA damage, plays an important role in the tumor-suppressor function of p53, at least in part through the activation of intracellular networks summarized in Fig. 5F.
Materials and Methods
Animals, Cells, and Virus.
Information about animals, cells, and virus used for the present study is given in SI Materials and Methods.
Additional Materials and Methods.
DNA damage stresses and reagents, Western blot analysis, recombinant plasmids and mutagenesis, recombinant p53 protein, real-time quantitative RT-PCR analysis, immunocytochemistry, luciferase assays, EMSA, oligonucleotide probes for EMSA, DNase I footprinting, ChIP assay, construction of genetically modified cell lines, and related references are provided in SI Materials and Methods.
Statistical Analysis.
Statistical analysis was performed using Student's t test with GraphPad Instat Software. P < 0.05 was considered statistically significant.
Supporting data are available in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank H. W. Lee, J. C. Bell, and B. Vogelstein for providing p53−/− mouse MEF cells, PKR−/− MEF cells, and p53−/− HCT116 cells, respectively. We thank S. Y. Kim, Y. E. Choi, and J. E. Ha for their faithful support of this project. This work was supported by Specific Basement Grant R11–2002-098–01004–0 from the Korea Science and Engineering Foundation and by Bio New Drug Grants A060115 and A040010 from the Korean Ministry of Health and Welfare.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812148106/DCSupplemental.
References
- 1.Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102(1):55–66. doi: 10.1016/s0092-8674(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 2.Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ. Principles of tumor suppression. Cell. 2004;116(2):235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 6.el-Deiry WS. The role of p53 in chemosensitivity and radiosensitivity. Oncogene. 2003;22(47):7486–7495. doi: 10.1038/sj.onc.1206949. [DOI] [PubMed] [Google Scholar]
- 7.Villunger A, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302(5647):1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 8.el-Deiry WS, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 9.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20(15):1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 10.Sax JK, El-Deiry WS. p53 downstream targets and chemosensitivity. Cell Death Differ. 2003;10(4):413–417. doi: 10.1038/sj.cdd.4401227. [DOI] [PubMed] [Google Scholar]
- 11.Crighton D, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126(1):121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Cui R, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Biron CA. Interferons alpha and beta as immune regulators—a new look. Immunity. 2001;14(6):661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 14.Zamanian-Daryoush M, Der SD, Williams BR. Cell cycle regulation of the double stranded RNA activated protein kinase, PKR. Oncogene. 1999;18(2):315–326. doi: 10.1038/sj.onc.1202293. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill FR, Smyth JF. Interferons in cancer therapy: A reappraisal. Lancet. 1987;2(8554):317–319. doi: 10.1016/s0140-6736(87)90901-9. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka A, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424(6948):516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 17.Kuhen KL, Samuel CE. Isolation of the interferon-inducible RNA-dependent protein kinase Pkr promoter and identification of a novel DNA element within the 5′-flanking region of human and mouse Pkr genes. Virology. 1997;227(1):119–130. doi: 10.1006/viro.1996.8306. [DOI] [PubMed] [Google Scholar]
- 18.Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285(5424):107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 19.Baltzis D, et al. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem. 2007;282(43):31675–31687. doi: 10.1074/jbc.M704491200. [DOI] [PubMed] [Google Scholar]
- 20.Marques JT, et al. Down-regulation of p53 by double-stranded RNA modulates the antiviral response. J Virol. 2005;79(17):11105–11114. doi: 10.1128/JVI.79.17.11105-11114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komarov PG, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285(5434):1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 22.el-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, O'Hara EB, Trieselmann BA, Romano PR, Dever TE. The interferon-induced double-stranded RNA-activated protein kinase PKR will phosphorylate serine, threonine, or tyrosine at residue 51 in eukaryotic initiation factor 2alpha. J Biol Chem. 1999;274(45):32198–32203. doi: 10.1074/jbc.274.45.32198. [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Guan KL. Expanding mTOR signaling. Cell Research. 2007;17(8):666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 25.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102(23):8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhtar RS, et al. BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death. J Neurosci. 2006;26(27):7257–7264. doi: 10.1523/JNEUROSCI.0196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280(14):14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- 28.Lee E, Yoon C, Kim Y, Bae Y. The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis. FEBS Lett. 2007;581(22):4325–4332. doi: 10.1016/j.febslet.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006;27(10):2018–2027. doi: 10.1093/carcin/bgl043. [DOI] [PubMed] [Google Scholar]
- 30.Wei CL, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124(1):207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 31.Green DR, Chipuk JE. p53 and metabolism: Inside the TIGAR. Cell. 2006;126(1):30–32. doi: 10.1016/j.cell.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Ohkubo S, Tatsuno I, Prives C. hCAS/CSE1L associates with chromatin and regulates expression of select p53 target genes. Cell. 2007;130(4):638–650. doi: 10.1016/j.cell.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Resnick-Silverman L, St Clair S, Maurer M, Zhao K, Manfredi JJ. Identification of a novel class of genomic DNA-binding sites suggests a mechanism for selectivity in target gene activation by the tumor suppressor protein p53. Genes Dev. 1998;12(14):2102–2107. doi: 10.1101/gad.12.14.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz-Fontela C, et al. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med. 2008;205(8):1929–1938. doi: 10.1084/jem.20080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilleray V, Constantinou C, Clemens MJ. Regulation of protein synthesis by inducible wild-type p53 in human lung carcinoma cells. FEBS Lett. 2006;580(7):1766–1770. doi: 10.1016/j.febslet.2006.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.