Abstract
The ability to remember contexts associated with aversive and rewarding experiences provides a clear adaptive advantage to animals foraging in the wild. The present experiments investigated whether hormonal signals released during feeding might enhance memory of recently experienced contextual information. Oleoylethanolamide (OEA) is an endogenous lipid mediator that is released when dietary fat enters the small intestine. OEA mediates fat-induced satiety by engaging type-α peroxisome proliferator-activated receptors (PPAR-α) in the gut and recruiting local afferents of the vagus nerve. Here we show that post-training administration of OEA in rats improves retention in the inhibitory avoidance and Morris water maze tasks. These effects are blocked by infusions of lidocaine into the nucleus tractus solitarii (NTS) and by propranolol infused into the basolateral complex of the amygdala (BLA). These findings suggest that the memory-enhancing signal generated by OEA activates the brain via afferent autonomic fibers and stimulates noradrenergic transmission in the BLA. The actions of OEA are mimicked by PPAR-α agonists and abolished in mutant mice lacking PPAR-α. The results indicate that OEA, acting as a PPAR-α agonist, facilitates memory consolidation through noradrenergic activation of the BLA, a mechanism that is also critically involved in memory enhancement induced by emotional arousal.
Keywords: fatty acid ethanolamide, lipid, PPAR-α
Food ingestion stimulates mucosal cells in the vertebrate small intestine to produce the bioactive fatty-acid amide oleoylethanolamide (OEA) (1−3), a potent endogenous agonist of type-α peroxisome proliferator-activated receptors (PPAR-α) (4). Acting as a local messenger within the gut (5), newly formed OEA stimulates enterocytes to express PPAR-α–regulated genes involved in lipid absorption, such as fatty-acid transporters and binding proteins (4, 6), and engages afferent fibers of the vagus nerve to delay further eating (1, 7, 8). OEA production in enterocytes is selectively triggered by dietary fat, not protein or carbohydrate, and requires both the cellular internalization of food-derived oleic acid and the targeting of this fatty acid to the enzyme pathway responsible for OEA biosynthesis (8). Small-intestinal OEA signaling appears to serve, therefore, as a peripheral fat-sensing mechanism that cooperates with premeal insulin release and other cephalic responses to optimize lipid use after ingestion of a fat-rich meal (8).
The ability to remember important contextual information about food sources, including their exact location and safety of access, is clearly advantageous to animals foraging in the wild; and it has been shown that rats and magpies possess such ability (9, 10). It is also well established that stress hormones activated by emotional arousal enhance memories of cues associated with the arousal (11, 12). Such findings suggest that hormonal and neural signals elicited by feeding might also enhance the consolidation of recent experiences. To test this idea, in the present study we examined whether the fat-induced satiety factor OEA influences memory consolidation. Our results provide evidence that OEA enhances memory consolidation by activating noradrenergic transmission in the basolateral complex of the amygdala (BLA), a neuromodulatory mechanism that is critically implicated in the consolidation of emotional memories.
Results
OEA Enhances Memory Consolidation.
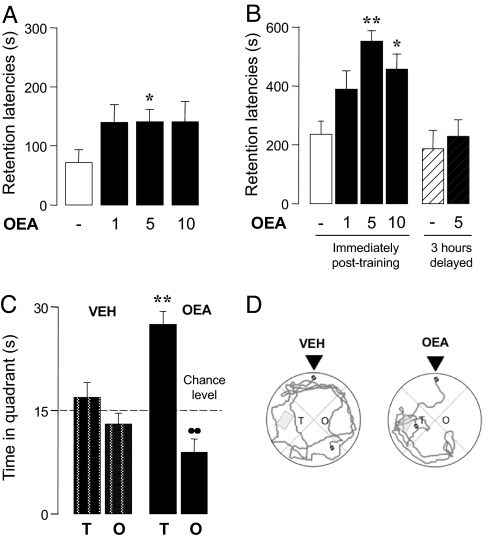
We first examined whether OEA administration affects rats' retention of inhibitory avoidance training. Pretraining injections of OEA (1–10 mg·kg−1, i.p., i.p.) did not change entrance latencies during training (H3 = 0.686, non-significant; data not shown), but caused a statistically detectable, albeit moderate improvement in 24 hours retention performance (Fig. 1A). A marked dose-dependent increase in retention was also observed when OEA was administered immediately after training, a procedure that eliminates potential sensory-motor effects influencing acquisition of the task (Fig. 1B). Memory enhancement was not induced by OEA 3h after training (Fig. 1B), providing evidence that the compound strengthens consolidation of recent memory traces (12). A further experiment assessed the effect of post-training OEA administration on 48 hours' retention performance of rats in the Morris water maze task. All experimental subjects were trained to swim to a hidden platform during training (F5,22 = 17.003, P < 0.0001, data not shown). Post-training OEA injections (5 mg·kg−1, i.p.) increased the time rats spent in proximity of the platform location on the retention test compared with vehicle injections (Fig. 1C). Figure 1D illustrates representative swim paths taken during probe trials by rats treated with vehicle or OEA. The findings indicate that OEA enhances memory consolidation in two cognitive tasks that involve contextual and spatial memory.
Fig. 1.
OEA enhances memory consolidation in the inhibitory avoidance (A, B) and water maze (C, D) tasks. (A, B) Performance in the 24-hour retention test (mean ± SEM) of (A) Wistar rats given pretraining injections of OEA (mg·kg−1, i.p., H3 = 7.871, P = 0.049, n = 12), and (B) Sprague-Dawley rats given post-training injections of OEA either immediately (open and filled bars, H3 = 15.023, P = 0.0009, n = 11–15) or 3 hours after training (striped bars). Post hoc comparisons: *, P < 0.05 and **, P < 0.01 vs. vehicle. (C) Time (mean ± SEM) spent during the 1-minute probe trial (48 hours after training) in target (T) and opposite (O) quadrants by rats injected with vehicle (open bars) or OEA (5 mg·kg−1, i.p., filled bars) immediately after training. Two-way ANOVA showed a significant treatment × quadrant interaction (F1,44 = 15.146, P = 0.0003, n = 11–13). **, P < 0.01 within quadrants; ●●, P < 0.01 within treatment. (D) Representative swimming paths of animals treated with vehicle or OEA (5 mg·kg−1, i.p.) immediately after training. Arrows indicate starting positions.
Role of Peripheral Afferents.
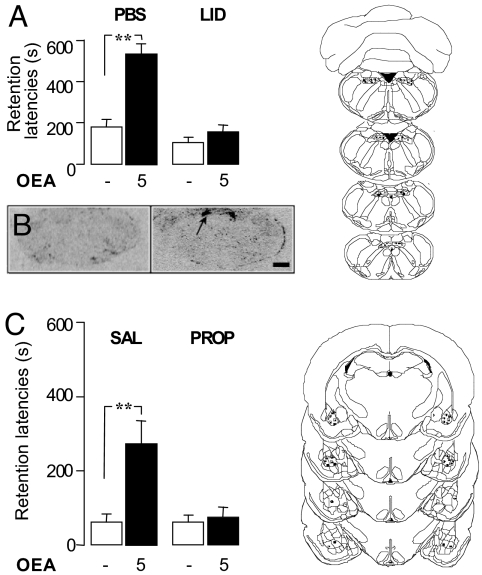
OEA induces satiety through a peripheral mechanism that requires the sensory vagus nerve (1). To test whether vagal afferents also contribute to the cognitive effects of OEA, we reversibly blocked neurotransmission in the NTS, the primary relay site of the afferent vagus in the brain, with the local anesthetic lidocaine. The lidocaine infusion prevented the memory-enhancing effects of post-training systemic injections of OEA (5 mg·kg−1, i.p.) in the inhibitory avoidance task (Fig. 2A). By contrast, vehicle infusion into the NTS (Fig. 2A) or lidocaine infusion into the cerebellum (CB) (U10–11 = 22.500, P = 0.0186; data not shown) did not alter the effect of OEA. These findings suggest that OEA strengthens memory consolidation by recruiting afferent autonomic signals that travel to the forebrain through the NTS. Consistent with this view, systemically administered OEA (10 mg·kg−1, i.p.) did not enter the brain [supporting information (SI) Fig. S1], but elicited a marked increase in c-fos mRNA expression in the NTS (Fig. 2B) (1).
Fig. 2.
Neural pathways underlying the memory enhancing effects of OEA in the inhibitory avoidance task. (A) Performance (mean ± SEM) in the 48-hour retention test of Sprague-Dawley rats given post-training systemic injections of OEA (5 mg·kg−1, i.p.) and intracerebral injection of phosphate-buffered saline (PBS, 0.5 μl) or lidocaine (2%, 0.5 μl) into the nucleus tractus solitarii (NTS). (B) Expression of c-fos mRNA in the NTS of rats given systemic injections of OEA (10 mg·kg−1, i.p.); arrow indicates NTS location; bar, 100 mm (C) Performance of rats given post-training systemic injections of OEA (5 mg·kg−1, i.p.) and intracerebral injection of saline (0.2 μl) or propranolol (0.5 μg in 0.2 μl) into the basolateral complex of the amygdala (BLA). Kruskal-Wallis ANOVA showed a significant effect (A) H3 = 19.049, P = 0.0003, n = 10–11 and (C) H3 = 13.866, P = 0.031, n = 10–12, for NTS and BLA, respectively. **, P < 0.01 compared with corresponding control group. Diagrams on the right show rat brain coronal sections (24) demonstrating injection sites randomly selected among rats included in the final analysis (● vehicle; ■ OEA).
Role of Noradrenergic Activity in the BLA.
Emotionally salient experiences activate noradrenergic projections from the NTS to the BLA, which play a crucial role in transforming such experiences into stable memories via BLA projections to other brain regions (13). These findings suggest that blocking noradrenergic transmission in the BLA might also prevent the memory modulation induced by OEA. In support of this implication, infusions of the β-adrenergic antagonist propranolol, but not its vehicle, into the BLA blocked the memory-enhancing effects of post-training administration of OEA in the inhibitory avoidance test (Fig. 2C). These findings suggest that, similarly to adrenal catecholamines and other arousal-activated neurohormones (11, 15) OEA enhances memory consolidation by increasing noradrenergic activity in the BLA. This similarity raised the question as to whether OEA might influence cognition by eliciting a non-selective state of arousal. This is unlikely, however, because in rats OEA administration (1–10 mg·kg−1, i.p.) (i) did not affect behavior in a novel open field (Fig. S2); (ii) did not evoke anxiety-like responses in the elevated plus-maze (Fig. S3); and (iii) did not increase plasma glucose and corticosterone levels (1, 14).
PPAR-α Activation Mediates the Cognitive Effects of OEA.
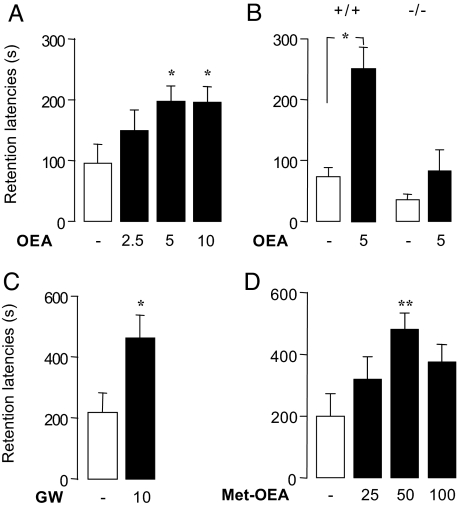
Endogenous OEA produces satiety by activating PPAR-α nuclear receptors (4) in the small intestine (5). To determine whether the memory-enhancing properties of OEA also depend on PPAR-α, we assessed the cognitive effects of this compound in PPAR-α−/− mice (4). Pretraining OEA injections (2.5–10 mg·kg−1, i.p.) dose-dependently improved the retention performance of wild-type C57Bl6J mice in the inhibitory avoidance task (Figs. 3A, 3B), without affecting entrance latencies during training (H3 = 2.211, NS, data not shown). This effect was strikingly absent in PPAR-α−/− mice (Fig. 3B), which also did not respond to OEA during training (H3 = 1.281, NS, data not shown). We next examined, in rats, the effects of two structurally distinct PPAR-α agonists, GW7647 (4) (10 mg·kg−1, i.p.) and (R)-1′-methyl-oleoylethanolamide (3) (25–100 mg·kg−1, p.o.), on performance in the inhibitory avoidance task. Post-training administration of either agent markedly improved performance, compared with vehicle (Figs. 3C, 3D). The effect of GW7647 was comparable to that of the same i.p. dose of OEA, which is consistent with the similar agonist potencies of these two compounds at PPAR-α (4). Because OEA can also activate transient receptor potential vanilloid–1 (TRPV-1) channels (15), we tested the effects of the TRPV1 antagonist capsazepine on OEA-induced memory enhancement. Capsazepine administration (10 mg·kg−1, i.p.) did not affect the response to OEA in the rat inhibitory avoidance task, indicating that TRPV1 receptors are not involved in such response (Fig. S4). Together, the results suggest that PPAR-α activation is both necessary and sufficient for OEA to enhance memory consolidation.
Fig. 3.
PPAR-α mediates the memory-enhancing effects of OEA in the inhibitory avoidance task. Pretraining OEA administration (2.5–10 mg·kg−1, i.p.) (A) improves 24-hour retention (mean ± SEM) in wild-type C57BL/6J mice (H3 = 8.214, P = 0.042, n = 11–13), but (B) has no effect on PPAR-α−/− mice (H3 = 8.921, P = 0.03, n = 8). (C,D) Post-training administration of PPAR-α agonists improves 24-hour retention in Sprague-Dawley rats: (C) GW7647 (GW: 10 mg·kg−1, i.p., n = 9–10) and (D) (R)-1′-methyl-oleoylethanolamide (met-OEA; 25–100 mg·kg−1, oral, n = 11–12). *, P < 0.05, **, P < 0.01 compared with control.
Discussion
Emotional arousal facilitates the consolidation of memory traces, an adaptive phenomenon that is primarily mediated by secretion of the adrenal stress hormones, epinephrine and cortisol, into the bloodstream (11, 12). Circulating epinephrine does not enter the brain and is thought to initiate its memory-enhancing effects by activating β-adrenergic receptors located on sensory terminals of the vagus nerve. The afferent signal generated by epinephrine projects to the NTS in the brainstem, where it stimulates noradrenergic neurons that activate the BLA and other forebrain structures (13). Norepinephrine release in the BLA is particularly critical for mediating the effects of peripheral epinephrine on memory consolidation; indeed, infusions of β-adrenergic receptor antagonists into the BLA block such effects, whereas infusions of β-adrenergic receptor agonists mimic them (12, 14, 15).
The present results reveal striking mechanistic similarities between the memory-enhancing actions of epinephrine and those of OEA. Like epinephrine (13), OEA increases memory consolidation by eliciting an autonomic signal that reaches the forebrain through the NTS and results in the noradrenergic activation of neurons in the BLA Accordingly, infusions of the local anesthetic lidocaine into the NTS or the β-adrenergic antagonist propranolol into the BLA prevent the memory-enhancing effects of both epinephrine (12) and OEA (present study). The two hormones are markedly different, however, with respect to the physiological context in which they operate. Epinephrine is released from the adrenal gland during arousal and stress, whereas OEA is produced by small-intestinal enterocytes in response to the arrival of dietary fat (8). This suggests that salient stimuli of diverse modalities—nutritional as well as emotional—converge on the same neuromodulatory system in the brain to facilitate memory consolidation.
Despite these mechanistic commonalities, epinephrine and OEA have profoundly different consequences on behavior. Epinephrine administration can produce a behavioral state characterized by heightened anxiety and incidence of panic attacks (16). By contrast, the present study shows that, even at doses that maximally inhibit food intake and enhance memory, OEA does not change rats' behavior in a novel open field and does evoke anxiety-like responses in the elevated plus-maze. Moreover, OEA does not increase plasma glucose levels, a typical effect of adrenergic activation, and does not evoke corticosterone release (1, 14). This profile distinguishes OEA not only from epinephrine but also from gut-derived peptides such as cholecystokinin and psychostimulant agents such as amphetamine, the appetite-suppressing and cognition-enhancing actions of which are associated with increased arousal and anxiety (17). Thus, peripheral signals of satiety and arousal, which are conveyed to the brain through the autonomic nervous system and the NTS, may broadly diverge within the forebrain to activate distinct sets of neural substrates. In the case of OEA, such substrates appear to include the BLA, which may be involved in mediating the memory enhancing effects of OEA (present findings) along with the paraventricular and supraoptic nuclei in the hypothalamus, which may be responsible for the anorexic actions of this compound (1).
The evidence from the present studies that post-training administration of OEA enhanced memory of training in a water-maze, a task that assesses memory for spatial context, as well as inhibitory avoidance, an aversively motivated task for which memory of context is an essential component, strongly suggests that OEA may have a general memory-modulatory influence that is not restricted to any specific kinds of experiences associated with its endogenous release. Prior studies of the effects of adrenergic receptor agonists indicate that such treatments enhance memory of a wide variety of types of training experiences (11, 14). Such findings, as well as the present findings, thus support the view that endogenously released modulators of memory consolidation act independently of the kinds of information associated with their activation.
Previous studies have shown that OEA is a high-affinity PPAR-α agonist (4) and that activation of this nuclear receptor accounts for most pharmacological actions of OEA—including prolongation of satiety (3, 4), stimulation of lipolysis (18), and decrease in body weight gain (4). The present results show that PPAR-α is also responsible for the memory-enhancing effects of OEA, because such effects are mimicked by two distinct PPAR-α agonists, are absent in mutant PPAR-α−/− mice, and are not affected by the TRPV-1 antagonist capsazepine. Moreover, although PPAR-α is expressed in the central nervous system (19), the fact that OEA does not cross the blood–brain barrier indicates that the compound acts on PPAR-α located in peripheral tissues. PPAR-α is a key regulator of lipid metabolism and is thought to serve important functions in the absorption, storage, and use of dietary fat (20–22). Our findings broaden the functional reach of this nuclear receptor to include a previously unsuspected role in the regulation of memory, and raise questions concerning the precise cellular localization of PPAR-α involved in the cognitition-enhancing effects to OEA and the target genes responsible for such effects.
In conclusion, the ability of OEA to improve memory consolidation underscores the importance of metabolic peripherally acting signals in the regulation of higher brain function (23). Such ability further suggests that pharmacological strategies aimed at mimicking or amplifying OEA signaling—including PPAR-α agonists or inhibitors of OEA degrading enzymes—might offer new opportunities for therapeutic intervention in cognitive disorders.
Materials and Methods
Animals.
The procedures met National Institutes of Health guidelines for the care and use of laboratory animals, those of the Italian Ministry of Health (D.L. 116/92), and were approved by the local Institutional Animal Care and Use Committees. Wistar rats and C57BL/6J mice were purchased from Harlan (Italy); Sprague-Dawley rats were purchased from Charles River Laboratories (USA); PPAR-α−/− mice and their wild-type controls were purchased from Taconic (USA). Pellet food and tap water were available ad libitum.
Chemicals.
OEA and (R)-1′-methyl-oleoylethanolamide were synthesized as described (3). All other compounds were purchased from Sigma (USA).
Surgery.
Rats (280–320 g) were anesthetized with sodium pentobarbital (50 mg·kg−1, i.p.) and given atropine sulfate (0.1 mg·kg−1, i.p.) to maintain respiration. The skull was positioned in a stereotaxic frame (Kopf Instruments), and two stainless-steel guide cannulae (15 mm; 23 gauge) were implanted 2 mm above the NTS, the CB, or the BLA (coordinates, NTS: AP −13.3, ML ± 1.0, DV −5.6; CB: AP −13.3, ML ± 1.0, DV −4.0; BLA: AP −2.8 mm, ML ± 5.0 mm, DV −6.5 mm) (24). The cannulae were affixed to the skull with two anchoring screws and dental cement. Stylets (15-mm-long pins) were inserted into each cannula to maintain patency. Rats were allowed to recover 7 days before training. Cannula placements were verified by post mortem histology as described in the SI Text, Materials and Methods section and Fig. S5.
Inhibitory Avoidance Task.
The rats were handled 1 minute per day for 3 days before training on an inhibitory avoidance task. The apparatus was divided into two compartments, separated by a sliding door. The starting compartment was made of white plastic and was well lit; the shock compartment was made of dark, electrifiable metal plates and was not illuminated. Training and testing were conducted as previously described (25). Briefly, each animal was placed in the starting compartment of the apparatus and allowed to enter the dark compartment. After the animal stepped completely into the dark compartment, the door was closed and a single, inescapable foot shock was delivered. The animal was removed from the shock compartment 15 seconds after shock termination and returned to its home cage. On the retention test, each animal was placed in the starting compartment and the latency to re-enter the dark compartment was recorded and used as the measure of retention. Longer latencies were interpreted as indicating better retention. Extensive prior evidence indicates that avoidance of the shock area is indicative of specific memory of the place where shock had been received (26). Details on the infusion and drug administration procedures may be found in the SI Text, Materials and Methods section.
Water Maze Task.
The rats were handled 1 minute per day for 3 days before training on the water maze task. The water maze was a circular tank, 1.83 m in diameter and 0.58 m in height, filled with water (26 °C) to a depth of 20 cm. A transparent Perspex platform (20 × 25 cm) was submerged 2.5 cm below the surface of the water in the northwest quadrant of the maze during training and could not be seen by the rats. The maze was located in a room containing several visual cues. We used a slightly modified procedure of one previously described (27). Briefly, the rats were given a training session of six trials. On each trial, the animal was placed in the tank facing the wall at one of the six designated start positions and allowed to escape onto the hidden platform. If an animal failed to find the platform within 60 seconds, it was manually guided to the platform. The rat was allowed to remain on the platform for 15 seconds and was then placed into a holding cage for 25 seconds until the start of the next trial. The time each animal spent to reach the platform was recorded as the escape latency. OEA (5 mg·kg−1, i.p.) or its vehicle was administered to Sprague-Dawley rats immediately after the sixth training trial. The rats were returned to the water maze 48 hours later for a retention test consisting of a 60-second probe trial (in which the platform was removed). The parameter measured from the probe trial was the time spent in the quadrant containing the platform during training.
Open-Field and Elevated Plus Maze Tests.
Open-field and elevated-plus maze experiments were conducted as previously described (28, 29). Details on the protocols may be found in the SI Text, Materials and Methods section.
OEA Measurements.
OEA (10 mg kg−1, i.p.) or vehicle (PEG/Tween80/saline, 5:5:90) was administered to Wistar rats, and the animals were killed at various times after injections. Tissues were removed and quickly frozen in liquid N2. Lipids were extracted and was OEA quantified by liquid chromatography/mass spectrometry (2).
In Situ Hybridization.
Rats were habituated to handling and injection procedures for 5 days before the experiment. On day 6, OEA (10 mg kg−1, i.p.) or vehicle was administered and rats were killed 60 minutes later by decapitation under anesthesia. In situ hybridization was performed using [35S]-labeled antisense cRNA riboprobe for c-Fos (1).
Statistical Analyses.
Inhibitory avoidance data were subjected to nonparametric analysis. Kruskal-Wallis analysis of variance was applied to evaluate the main effect of treatment, and the Mann-Whitney U test was used to assess the significance of differences between treatment groups using the Bonferroni's correction for multiple comparisons (post hoc comparisons). Water maze training data were analyzed with a one-way analysis of variance (ANOVA) with the six acquisition trials as repeated measure. Quadrant search times on the probe trial were analyzed with a two-way ANOVA. Tukey's post hoc test was performed on the treatment × time interaction to determine the source of detected significances.
Supplementary Material
Acknowledgments.
We thank Fariba Oveisi and Daniela Valeri for discussion and help with experiments. The contribution of the Agilent Technologies/UCI Analytical Discovery Facility, Center for Drug Discovery is gratefully acknowledged. This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK073955 to D.P.) and Agilent Foundation (to D.P.); National Institute on Mental Health (MH 12526 to J.L.M.); and Ministero Istruzione Università e Ricerca (PRIN 2007 to V.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903038106/DCSupplemental.
References
- 1.Rodríguez de Fonseca F, Navarro M, Gómez R, Escuredo L, Nava F. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- 2.Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton TR. Pharmacological characterization of hydrolysis-resistant analogs of oleoylethanolamide with potent anorexiant properties. J Pharmacol Exp Ther. 2006;318:563–570. doi: 10.1124/jpet.106.105221. [DOI] [PubMed] [Google Scholar]
- 4.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 5.Fu J, Kim J, Oveisi F, Astarita G, Piomelli D. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R45–R50. doi: 10.1152/ajpregu.00126.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Chen M, Georgeson KE, Harmon CM. Mechanism of oleoylethanolamide on fatty acid uptake in small intestine after food intake and body weight reduction. Am J Physiol Regul Integr Comp Physiol. 2007;292:R235–R241. doi: 10.1152/ajpregu.00270.2006. [DOI] [PubMed] [Google Scholar]
- 7.Oveisi F, Gaetani S, Eng KT, Piomelli D. Oleoylethanolamide inhibits food intake in free-feeding rats after oral administration. Pharmacol Res. 2004;49:461–466. doi: 10.1016/j.phrs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts WA, Feeney MC, Macpherson K, Petter M, McMillan N. Episodic-like memory in rats: Is it based on when or how long ago? Science. 2008;320:113–115. doi: 10.1126/science.1152709. [DOI] [PubMed] [Google Scholar]
- 10.Zinkivskay A, Nazir F, Smulders TV. What-where-when memory in magpies (Pica pica) Anim Cogn. 2009;12:119–125. doi: 10.1007/s10071-008-0176-x. [DOI] [PubMed] [Google Scholar]
- 11.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 12.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 13.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 14.González-Yanes C, Serrano A, Bermúdez-Silva FJ, Hernández-Dominguez M, Páez-Ochoa MA. Oleylethanolamide impairs glucose tolerance and inhibits insulin-stimulated glucose uptake in rat adipocytes through p38 and JNK MAPK pathways. Am J Physiol Endocrinol Metab. 2005;289:E923–E929. doi: 10.1152/ajpendo.00555.2004. [DOI] [PubMed] [Google Scholar]
- 15.Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- 16.Veltman JA, Gaillard AW. Physiological workload reactions to increasing levels of task difficulty. Ergonomics. 1998;41:656–669. doi: 10.1080/001401398186829. [DOI] [PubMed] [Google Scholar]
- 17.Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol. 2007;7:570–574. doi: 10.1016/j.coph.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzmán M, Lo Verme J, Fu J, Oveisi F, Blázquez C. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha) J Biol Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- 19.Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 21.Bookout AL, et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR-alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 25.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang KC. Memory Consolidation. Washington, DC: American Psychological Association; 2001. pp. 165–183. [Google Scholar]
- 27.Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proc Natl Acad Sci USA. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laviola G, Renna G, Bignami G, Cuomo V. Ontogenetic and pharmacological dissociation of various components of locomotor activity and habituation in the rat. Int J Dev Neurosci. 1988;6:431–438. doi: 10.1016/0736-5748(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 29.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.