Abstract
Neuropathic pain, a highly debilitating pain condition that commonly occurs after nerve damage, is a reflection of the aberrant excitability of dorsal horn neurons. This pathologically altered neurotransmission requires a communication with spinal microglia activated by nerve injury. However, how normal resting microglia become activated remains unknown. Here we show that in naive animals spinal microglia express a receptor for the cytokine IFN-γ (IFN-γR) in a cell-type-specific manner and that stimulating this receptor converts microglia into activated cells and produces a long-lasting pain hypersensitivity evoked by innocuous stimuli (tactile allodynia, a hallmark symptom of neuropathic pain). Conversely, ablating IFN-γR severely impairs nerve injury-evoked microglia activation and tactile allodynia without affecting microglia in the contralateral dorsal horn or basal pain sensitivity. We also find that IFN-γ-stimulated spinal microglia show up-regulation of Lyn tyrosine kinase and purinergic P2X4 receptor, crucial events for neuropathic pain, and genetic approaches provide evidence linking these events to IFN-γR-dependent microglial and behavioral alterations. These results suggest that IFN-γR is a key element in the molecular machinery through which resting spinal microglia transform into an activated state that drives neuropathic pain.
Keywords: allodynia, cytokine, glia, Lyn tyrosine kinase, purinergic receptor
Neuropathic pain is a chronic pain condition that occurs after nerve damage, such as that induced by bone compression in cancer, diabetes, infection, autoimmune disease, or physical injury (1). One troublesome hallmark symptom of neuropathic pain is pain hypersensitivity to normally innocuous stimuli, a phenomenon known as “tactile allodynia,” which is refractory to currently available treatments such as nonsteroidal anti-inflammatories and opioids (2, 3). Accumulating evidence from diverse animal models of neuropathic pain suggests that neuropathic pain might involve aberrant excitability of the nervous system, notably at the levels of the primary sensory ganglia and the dorsal horn of the spinal cord, resulting from multiple functional and anatomical alterations following peripheral nerve injury (3, 4). Although it long was thought that these alterations occur mainly in neurons, emerging lines of evidence show that they also occur in spinal microglia, a group of immune cells (5–9). After injury to peripheral nerves, microglia in the normal state (traditionally called “resting” microglia) in the spinal dorsal horn are converted to an activated state through a series of cellular and molecular changes. Activated spinal microglia show hypertrophied soma, thickened and retracted processes, and increased proliferation activity (6, 9, 10). Furthermore, these microglia induce or enhance expression of various genes including neurotransmitter receptors (11) (e.g., P2X4R, a subtype of ATP-gated cation channels (12)) and intracellular signaling kinases (e.g., mitogen-activated protein kinases (13–16) and Lyn tyrosine kinase (17)). By responding to extracellular stimuli such as ATP, the activated microglia evoke various cellular responses such as production and release of bioactive factors, including cytokines and neurotrophic factors (18, 19). Importantly, pharmacological, molecular, and genetic manipulations of the function or expression of these microglial molecules substantially influence nerve injury-induced pain behaviors (12–16, 20–22) and the hyperexcitability of the dorsal horn pain pathway (23, 24). Therefore, spinal microglia activated after nerve injury critically contribute to the pathologically enhanced pain processing in the dorsal horn that underlies neuropathic pain (5–9). Understanding how resting spinal microglia are transformed into activated cells after nerve injury may be an important step in unraveling the pathogenesis of neuropathic pain, but the mechanism remains unclear.
In the present study, we investigated this issue, focusing on the proinflammatory cytokine IFN-γ. IFN-γ is among the biologically active signaling molecules that have been reported to activate primary cultured microglial cells (25). A recent study has indicated that IFN-γ levels are increased in the spinal cord after nerve injury (20), leading to speculation that IFN-γ has a role in neuropathic pain. However, there is no direct evidence indicating that IFN-γ signaling contributes to microglia activation in the dorsal horn and tactile allodynia under neuropathic pain conditions. Here, we report that the receptor for IFN-γ (IFN-γR), which is constitutively expressed in normal resting microglia in the dorsal horn, is a key component in the molecular machinery through which peripheral nerve injury converts spinal microglia from the resting state to the activated state that underlies the pathogenesis of neuropathic pain.
Results
Stimulating IFN-γRs in Spinal Microglia Under Normal Conditions Induces Activation of Microglia and Long-Lasting Allodynia.
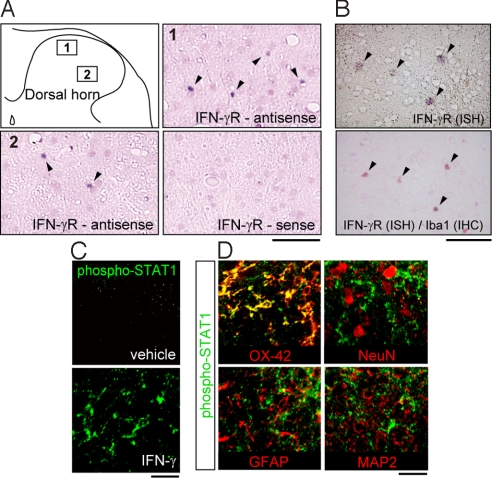
We first investigated expression of IFN-γRs by in situ hybridization for IFN-γR mRNA on sections of the fifth lumbar (L5) dorsal spinal cord of naive rats. Signals for IFN-γR mRNA were detected readily in the dorsal horn (Fig. 1A, intense violet dots indicated by arrowheads); these signals were not observed in sections hybridized with a corresponding sense probe (Fig. 1A). Similar results were obtained with another set of cRNA probes for IFN-γR (data not shown). To identify the type of cells expressing IFN-γRs, we performed in situ hybridization combined with immunohistochemistry for ionized calcium-binding adapter molecule-1 (Iba1), a marker of microglia, and showed that the IFN-γR mRNA signals were restricted to cells labeled with Iba1 (arrowheads in Fig. 1B). In addition, IFN-γR protein also was detected in homogenates from the spinal cord of naive rats and microglial cells in culture in Western blot analysis (data not shown). To determine whether IFN-γRs are expressed as functional receptors in spinal microglia, we spinally administered recombinant IFN-γ to naive rats and immunohistochemically examined the level of activated signal transducer and activator of transcription 1 (phospho-STAT1), a molecule downstream of IFN-γR (26). At 15 min after intrathecal IFN-γ administration (1,000 U), immunofluorescence of phospho-STAT1 was increased in the dorsal horn (Fig. 1C). Consistent with microglia-restricted localization of IFN-γR, STAT1 phosphorylation evoked by IFN-γ also occurred specifically in cells that were double-labeled with OX-42, a microglial marker, but not in cells labeled with glial fibrillary acidic protein (GFAP; an astrocyte marker) or with the neuronal markers microtubule-associated protein 2 (MAP2) or neuronal nuclei (NeuN) (Fig. 1D). STAT1 phosphorylation also was induced in cultured spinal microglial cells stimulated directly with IFN-γ (data not shown). These findings suggest that under normal conditions IFN-γRs are expressed as functional receptors in resting microglia in the dorsal horn.
Fig. 1.
IFN-γRs in the spinal cord are expressed in microglia under normal conditions. (A) In situ hybridization analysis of the IFN-γ receptor (IFN-γR) mRNA in the dorsal horns of normal rats (arrowheads indicate IFNγR mRNA signals.) (Scale bar, 80 μm.) (B) IFN-γR mRNA signals overlapped with immunoreactivity for Iba1 (arrowheads). IHC, immunohistochemistry; ISH, in situ hybridization. (Scale bar, 80 μm.) (C) Phospho-STAT1 immunofluorescence 15 min after IFN-γ injection. (Scale bar, 20 μm.) (D) Double immunofluorescence labeling for phospho-STAT1 (green) and cell-type markers (OX-42, a marker of microglia; GFAP, a marker of astrocytes; MAP2 and NeuN, markers of neurons). (Scale bar, 20 μm.)
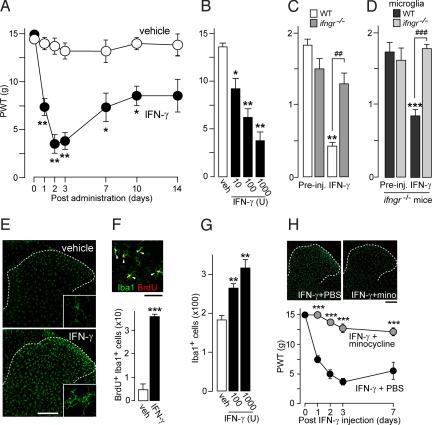
To examine the in vivo responses evoked by IFN-γ, we spinally administered IFN-γ to naive rats and subsequently used a behavioral assay for tactile allodynia. We found that a single intrathecal administration of IFN-γ (1,000 U) produced marked and long-lasting tactile allodynia: the paw withdrawal threshold (PWT) to mechanical stimulation applied to the hindpaw progressively decreased over the first 2 days, peaking between days 2 and 3 (P < 0.01), and the decreased PWT persisted for at least 10 days after the administration (P < 0.05; Fig. 2A). The IFN-γ-induced allodynia was dose dependent (10 U: P < 0.05; 100 and 1,000 U: P < 0.01 on day 3; Fig. 2B). Although a similar allodynic behavior was produced in wild-type C57BL/6J mice injected intrathecally with IFN-γ (10 U, P < 0.01; Fig. 2C), IFN-γR-deficient (ifngr−/−) mice failed to produce this response. Interestingly, we found that ifngr−/− mice that had been intrathecally infused with primary cultured microglia taken from wild-type C57BL/6J mice showed a decrease in the PWT after IFN-γ administration, but there was no change in threshold in ifngr−/− mice infused with ifngr−/− microglia (Fig. 2D). This finding suggests that IFNγ-induced allodynia impaired in ifngr−/− mice is rescued by infusing microglia expressing IFN-γR. In addition, the PWT was not affected by infusion of microglia from either wild-type C57BL/6J or ifngr−/− mice alone (data not shown). These results indicate that stimulating IFN-γRs in spinal microglia produces persistent tactile allodynia in otherwise naive animals.
Fig. 2.
Intrathecal delivery of IFN-γ to normal animals induces long-lasting tactile allodynia and activation of spinal microglia. (A) PWT after a single intrathecal administration of recombinant IFN-γ (1,000 U; n = 6) or vehicle (PBS; n = 6) to naive rats (*, P < 0.05; **, P < 0.01). (B) Dose-dependent tactile allodynia on day 3 (n = 5–6; *, P < 0.05; **, P < 0.01). (C) PWT in wild-type C57BL/6J (n = 6) and IFN-γR-deficient (ifngr−/−) mice (n = 6) 3 days after IFN-γ (10 U) administration (**, P < 0.01 vs. before injection; ##, P < 0.01 vs. wild-type C57BL/6J mice injected with IFN-γ). (D) Effects of intrathecal infusion of wild-type (C57BL/6J) and ifngr−/− primary cultured microglia to ifngr−/− mice on IFN-γ (10 U)-induced change in PWT (wild-type microglia group, n = 5; ifngr−/− microglia group, n = 6; ***, P < 0.0001 vs. before injection; ###, P < 0.0001 vs. wild-type C57BL/6J microglia with IFN-γ injection). (E) Immunofluorescence of Iba1 in the dorsal horn 3 days after administration of IFN-γ (1,000 U). (Scale bar, 200 μm.) Insets are images at high magnification (45 μm wide). (F) (Upper) Double immunofluorescence labeling of Iba1 (green) with BrdU (red) in the dorsal horn 1 day after administration of IFN-γ (arrowheads indicate cells positive to Iba1 and BrdU). (Scale bar, 80 μm.) (Lower) The number of BrdU+Iba1+ cells in the dorsal horn of rats (n = 3; ***, P < 0.001). (G) The number of Iba1+ microglia in the dorsal horn of vehicle- and IFN-γ-treated rats (**, P < 0.01). (H) Effect of minocycline (40 mg/kg, i.p.) on IFN-γ-induced microglia activation. Photographs show immunofluorescence of Iba1 on day 3 (scale bar, 200 μm) and tactile allodynia (n = 5; ***, P < 0.001 vs. IFN-γ/PBS group). Data are mean ± SEM (A–D, F, G, and H).
These results prompted us to investigate the status of microglia in the dorsal horn after IFN-γR stimulation, and we performed immunohistochemical analyses on sections of L5 dorsal spinal cord. On day 3 after IFN-γ administration, microglial cells in the dorsal horn had enhanced Iba1 labeling, hypertrophic cell bodies, and thickened and shortened processes (Fig. 2E). By contrast, immunofluorescence of ED-1 (a macrophage marker) was not enhanced by IFN-γ, and neither the morphology nor the number of ED-1+ cells was changed (SI Text and Fig. S1). In addition, only a very few circulating ED-1+ macrophages were labeled by intravenously injected PKH26-PCL (Fig. S2), an inert fluorescent dye that selectively labels cells with phagocytic capabilities (27), suggesting that intrathecal administration of IFN-γ does not cause macrophage infiltration. Next, to test whether IFN-γ-stimulated microglia undergo proliferation, we visualized proliferating cells by administering i.p. a single dose of BrdU, a marker of the S-phase of the cell cycle. Intrathecal administration of IFN-γ drastically increased the number of BrdU+Iba1+ cells in the dorsal horn on day 1 (P < 0.001; Fig. 2F). In addition, we observed a few BrdU+Iba1− cells in the dorsal horn, the number of which was not altered by IFN-γ (vehicle: 3.0 ± 1.5 cells; IFN-γ: 4.7 ± 2.2 cells). The proliferation of spinal microglia is strongly supported by our further immunohistochemical analyses demonstrating that the number of OX-42+ microglia positive for Ki-67, a nuclear protein expressed in all phases of the cell cycle except the resting phase, also was increased in the dorsal horn of rats to which IFN-γ was administered (Fig. S3A). In addition, there were very few Ki-67+ED-1+ cells in the dorsal horn (Fig. S3A). By counting microglial cells within the dorsal horn on day 3, we observed that the total number of Iba1+ microglia increased in rats treated with IFN-γ compared with rats treated with vehicle (P < 0.01; Fig. 2G). IFN-γ did not increase the number of OX-42+ P2Y12R− cells in the dorsal horn, and almost all OX-42+ cells are double-labeled with an antibody of P2Y12 purinoceptor (P2Y12R) (Fig. S4), a G protein-coupled receptor that is expressed in microglia but not in macrophages (29, 30). These changes in the morphology and number of microglia in rats treated with IFN-γ are consistent with immunohistochemical criteria for activated microglia in vivo (28) and are observed in the dorsal horn after nerve injury (6).
We next tested the effect of minocycline, which inhibits microglia activation in vivo (31), on IFN-γ-induced tactile allodynia. Minocycline (40 mg/kg) suppressed the decrease in the PWT at all time points of testing (P < 0.001; Fig. 2H) as well as the activation of microglia in the dorsal horn on day 3 after intrathecal administration of IFN-γ (Fig. 2H). Together, these findings indicate that stimulation of spinal IFN-γRs expressed selectively in microglia under normal conditions causes tactile allodynia by directly activating spinal microglia.
Lack of IFN-γR Impairs Activation of Microglia and Tactile Allodynia After Nerve Injury.
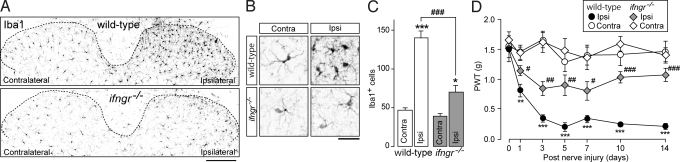
Next, to investigate the role of IFN-γR in microglia activation and tactile allodynia in the dorsal horn under neuropathic pain conditions, we injured the L5 spinal nerve of wild-type C57BL/6J and ifngr−/− mice, an animal model of neuropathic pain. Consistent with our previous studies (17, 32), in wild-type C57BL/6J mice, a marked activation of microglia was observed on the ipsilateral side of the dorsal horn 14 days after nerve injury, as indicated by alterations in Iba1 immunofluorescence (Fig. 3A), morphology (Fig. 3B), and number (P < 0.001, Fig. 3C). However, these alterations in activated microglia were severely impaired in ifngr−/− mice with nerve injury, (Fig. 3 A and B), and the number of Iba1+ cells was much lower than in wild-type C57BL/6J mice (P < 0.001; Fig. 3C). The number of microglia in the contralateral dorsal horn was similar in these 2 genotypes (Fig. 3C). Behaviorally, wild-type C57BL/6J mice showed a marked decrease in PWT after nerve injury (day 1: P < 0.01; days 3–14: P < 0.001; Fig. 3D). By contrast, the nerve injury-induced allodynic behavior was strikingly attenuated in ifngr−/− mice at all time points of testing (days 1 and 7: P < 0.05; days 3 and 5: P < 0.01; days 10 and 14: P < 0.001; Fig. 3D). The loss of IFN-γR did not change either basal mechanical sensitivity or the PWT of the contralateral hindpaw after nerve injury (Fig. 3D). Nor did IFN-γR deficiency affect motor behaviors in the rotarod test (time on rotarod for wild-type C57BL/6J: 55.4 ± 4.6 sec; for ifngr−/−: 53.2 ± 6.8 sec). These findings indicate that IFN-γR-mediated signaling is required for switching spinal microglia to the activated phenotype in the spinal dorsal horn after nerve injury and for producing the subsequent tactile allodynia.
Fig. 3.
Lack of IFN-γR impairs activation of microglia and tactile allodynia after nerve injury. (A) Photographs show immunofluorescence of Iba1 in the L5 dorsal spinal cord of wild-type C57BL/6J and ifngr−/− mice 14 days after nerve injury. (Scale bar, 150 μm.) (B) Morphological changes in the spinal microglia in the spinal dorsal horn of wild-type C57BL/6J or ifngr−/− mice. (Scale bar, 20 μm.) (C) The change in the number of Iba1-positive microglia cells in the dorsal horn of wild-type C57BL/6J (n = 5) and ifngr−/− (n = 5) mice 14 days after nerve injury (*, P < 0.05; ***, P < 0.001 vs. the contralateral side; ###, P < 0.001 vs. the ipsilateral side in wild-type C57BL/6J mice). (D) PWT of wild-type C57BL/6J (n = 7) and ifngr−/− mice (n = 7) before and after nerve injury (**, P < 0.01; ***, P < 0.001 vs. the contralateral side of wild-type C57BL/6J mice; #, P < 0.05; ##, P < 0.01; ###P < 0.001 vs. the ipsilateral side of wild-type C57BL/6J mice). Data are mean ± SEM (C and D).
Lyn Tyrosine Kinase Is a Critical Intermediary in the IFN-γR-Dependent Activation of Spinal Microglia.
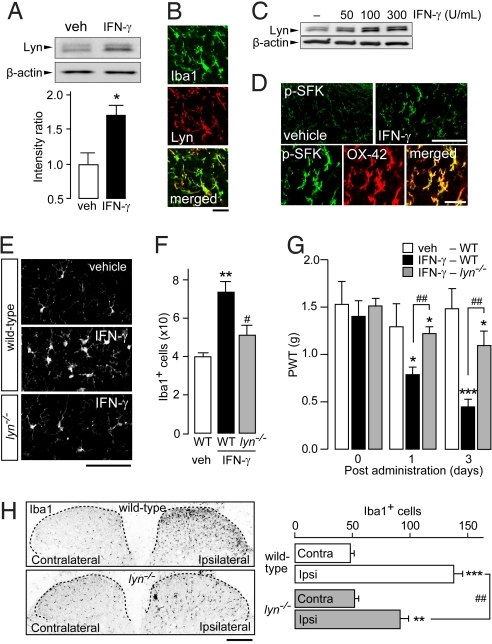
To elucidate the molecular mechanism by which IFN-γ activates spinal microglia, we examined the role of Src-family kinases (SFKs), which have been implicated in cellular responses including the proliferation of many types of cells (33). Among 5 major SFKs (Src, Fyn, Lck, Yes, and Lyn) known to be expressed in the CNS (34), expression of Lyn in spinal microglia, which is essential for tactile allodynia after nerve injury, is up-regulated and activated by nerve injury (17). In the spinal cord of animals administered IFN-γ intrathecally, expression of Lyn protein was increased 1 day after the administration (P < 0.05; Fig. 4A), and this increased expression was observed selectively in microglia labeled by Iba1 (Fig. 4B). When cultured microglial cells were treated with IFN-γ, the up-regulation of Lyn expression also was observed in a dose-dependent manner (Fig. 4C). We also found that immunofluorescence for the active form of SFKs, including Lyn, that were autophosphorylated in the kinase domain (phospho-SFK) increased exclusively in microglia labeled by OX-42 in the dorsal horn after intrathecal administration of IFN-γ (Fig. 4D), suggesting that Lyn kinase may become activated in spinal microglia stimulated by IFN-γ. To determine the in vivo role of Lyn kinase, we spinally administered IFN-γ to wild-type C57BL/6J and Lyn-knockout (lyn−/−) mice. In contrast to the changes in the morphology (Fig. 4E) and number of microglia (P < 0.01; Fig. 4F) in the dorsal horn of wild-type C57BL/6J mice following IFN-γ administration, dorsal horn microglia lacking Lyn showed less activated morphology (Fig. 4E) and a smaller increase in number (P < 0.05; Fig. 4F). The loss of Lyn also blunted the decrease in PWT following intrathecal administration of IFN-γ (P < 0.01; Fig. 4G). Moreover, the nerve injury-induced increase in the number of microglial cells in the ipsilateral dorsal horn was lower in lyn−/− mice than in wild-type C57BL/6J mice (P < 0.001; Fig. 4H). These results indicate that Lyn tyrosine kinase is a critical intermediary in the activation of microglia caused by IFN-γ administration and nerve injury.
Fig. 4.
Lyn is a crucial kinase mediating both IFN-γ- and nerve injury-induced activation of spinal microglia. (A) Western blot analysis of Lyn in spinal cord homogenates from rats treated intrathecally with IFN-γ (1,000 U). The relative values of Lyn protein were normalized to β-actin protein (n = 3; *, P < 0.05). (B) Double immunofluorescence labeling for Iba1 (green) and Lyn (red) in the dorsal horn of IFN-γ-treated rats (merged: yellow). (Scale bar, 30 μm.) (C) Lyn and β-actin proteins in a whole-cell lysate from primary cultured microglial cells treated with IFN-γ (50–300 U/ml) for 24 h. (D) Immunofluorescence of phospho-SFK (green) and double immunofluorescence labeling with OX-42 (red) in the dorsal horn of IFN-γ-treated rats (merged: yellow). (Scale bars, 80 μm [Upper] and 30 μm [Lower]). (E) Iba1 immunofluorescence in the dorsal horn of wild-type C57BL/6J and Lyn-deficient (lyn−/−) mice 3 days after intrathecal administration of IFN-γ (10 U). (Scale bar, 80 μm.) (F) The number of Iba1+ microglia in the dorsal horn [C57BL/6J: n = 4 (vehicle); n = 6 (IFN-γ); lyn−/−: n = 6 (vehicle), n = 5 (IFN-γ); **, P < 0.01 vs. vehicle-treated wild-type C57BL/6J mice; ##, P < 0.01 vs. IFN-γ-treated wild-type C57BL/6J mice). (G) PWT after intrathecal administration of IFN-γ in wild-type C57BL/6J (n = 6) and lyn−/− (n = 5) mice (**, P < 0.01; ***, P < 0.001 vs. before IFN-γ injection; ##, P < 0.01 vs. IFN-γ-treated wild-type C57BL/6J mice). (H) Photographs show Iba1 immunofluorescence in the dorsal horn of C57BL/6J or lyn−/− mice 10 days after nerve injury and the number of Iba1+ microglia in the dorsal horns (n = 5; **, P < 0.01; ***, P < 0.001 vs. contralateral side in wild-type C57BL/6J mice; ##, P < 0.01 vs. ipsilateral side in wild-type C57BL/6J mice). (Scale bar, 80 μm.) Data are mean ± SEM (A, F, G, and H).
P2X4 Receptors Up-Regulated in Microglia Are Required for IFN-γ-Induced Allodynia.
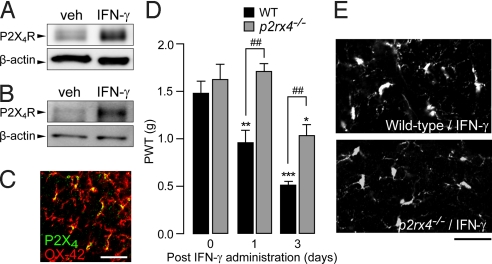
Following nerve injury, activated spinal microglia up-regulate expression of P2X4 receptors (P2X4Rs), a principal subtype of ATP-gated ion channels crucial for neuropathic pain (12, 23). We therefore determined whether IFN-γR-induced allodynia involves P2X4Rs. Applying IFN-γ directly to primary cultured microglial cells increased the level of P2X4R protein (Fig. 5A). Furthermore, intrathecal administration of IFN-γ increased the expression of P2X4R protein in the spinal cord of rats (Fig. 5B). In the dorsal horn, cells showing P2X4R immunofluorescence were double-labeled with OX-42 (Fig. 5C). Using P2X4R-deficient mice (p2rx4−/−), we found that the marked decrease in PWT induced by intrathecal administration of IFN-γ in wild-type C57BL/6J mice was significantly attenuated in p2rx4−/− mice (P < 0.01; Fig. 5D). In contrast to the PWT, IFN-γ-induced activation of microglia in the dorsal horn was similar in the 2 genotypes (Fig. 5E). This finding is consistent with our previous findings demonstrating that an antisense knockdown of P2X4R in the spinal cord fails to affect microglia activation (12). These results indicate that IFN-γR-mediated tactile allodynia depends on microglial P2X4R.
Fig. 5.
IFN-γ-stimulated microglia show up-regulated expression of P2X4 receptor, which is required for tactile allodynia. (A, B) Western blot analysis of P2X4 receptor (P2X4R) and β-actin proteins in a whole-cell lysate from cultured microglial cells treated with IFN-γ and vehicle for 24 h (A) and in homogenates from the spinal cord of rats 1 day after intrathecal administration of IFN-γ (1,000 U) or vehicle (B). (C) Double immunofluorescence labeling for P2X4R (green) and OX-42 (red) in the L5 dorsal spinal cord of IFN-γ-treated rats (merged: yellow). (Scale bar, 30 μm.) (D) PWT after intrathecal administration of IFN-γ (10 U) in wild-type C57BL/6J (n = 6) or P2X4R-deficient (p2rx4−/−) mice (n = 5). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. before IFN-γ injection; ##, P < 0.01 vs. IFN-γ-treated wild-type C57BL/6J mice. (E) Photographs show immunofluorescence of Iba1 in the dorsal horn of wild-type C57BL/6J or p2rx4−/− mice 3 days after intrathecal IFN-γ injection. (Scale bar, 40 μm.) Data are mean ± SEM.
Discussion
Growing evidence has revealed several microglial molecules involved in neuropathic pain (5–9). Although the expression levels or activities of these molecules are up-regulated in activated spinal microglia after nerve injury, they remain at low levels in resting microglia under normal conditions (9). By contrast, we show that in otherwise naive animals, spinal microglia express IFN-γRs in a cell-type-specific manner and that acute stimulation of these receptors alone induces a conversion of these cells into the activated state and produces long-lasting tactile allodynia. Our genetic approach revealed that IFN-γR deficiency results in a marked attenuation in spinal microglia activation and tactile allodynia in a model of neuropathic pain in which it was reported that IFN-γ levels were increased in the spinal cord (20). The attenuated microglia activation observed in IFN-γR-deficient mice seems to be in stark contrast to the phenotype observed in mice lacking P2Y12R. P2Y12R is among the molecules expressed in microglia under normal conditions (30) and is implicated in neuropathic pain (32, 35); however, the loss of P2Y12R fails to affect nerve injury-induced morphological and numerical alterations of spinal microglia (32). Therefore, these results suggest that the IFN-γ/IFN-γR system is critical in transforming resting spinal microglia into the activated state and thereby linking them to tactile allodynia. This possibility is supported further by our evidence demonstrating that, in addition to cellular alterations, stimulation of resting spinal microglia by IFN-γ causes changes in their molecular profile (increased expression of Iba1, Lyn, and P2X4R), changes that also occur in animal models of neuropathic pain (6–8, 12, 17). However, the fact that activation of spinal microglia after nerve injury was not completely eliminated in IFN-γR-deficient mice suggests that IFN-γR-mediated signaling, although important, is not the only mechanism underlying microglia activation and that there may be independent and/or cooperative mechanisms involving other signals (8).
Among numerous genes whose expression levels have been reported to be altered in microglia stimulated by IFN-γ (36), the present study identified Lyn tyrosine kinase to be up-regulated by IFN-γ and required for IFN-γR-dependent microglia activation. This kinase was up-regulated exclusively in microglia in a model of neuropathic pain (17) and was implicated in various cellular events in microglia (37–40). Previous work using Lyn-deficient mice also showed attenuated microglia activation evoked by β-amyloid peptide in the brain (39), supporting our notion that Lyn is critically involved in the molecular machinery underlying microglia activation. Consistent with the impaired neuropathic allodynia in mice lacking Lyn (17), these mice also were resistant to IFN-γ-induced tactile allodynia. We previously have indicated that Lyn kinase in microglia also controls the expression of P2X4R (17), a receptor that is crucial for tactile allodynia (6–8, 12, 23, 41–43). Interestingly, we further found that IFN-γ-stimulated spinal microglia allowed up-regulated expression of P2X4R and that the deletion of P2X4R blunted tactile allodynia, suggesting that IFN-γR-dependent tactile allodynia involves the P2X4R. As shown previously (23), activation of P2X4R up-regulated in activated spinal microglia may lead to the release of bioactive factors such as BDNF, which causes hyperexcitability of dorsal horn neurons by reducing inhibition and converting GABAA receptor-mediated inhibition to excitation (23) and, in turn, produces tactile allodynia (12, 23). It is of particular interest that intrathecal delivery of IFN-γ to normal rats enhances the excitability of dorsal horn neurons evoked by innocuous stimulation in vivo (44, 45), possibly involving a reduction of GABAergic inhibitory control (45). Although such effects of IFN-γ have been considered a neuronally mediated phenomenon (44, 46, 47), our present findings indicating that spinal microglia are stimulated directly by IFN-γ administered spinally and contribute to IFN-γR-dependent pain hypersensitivity, together with recent evidence (6–9, 12, 23), suggest a mechanism in which spinal microglia are key intermediaries for the modulation of spinal pain processing by IFN-γ.
Our present study demonstrates that IFN-γR is a key element in the molecular machinery through which resting spinal microglia transform into the activated state that underlies the pathogenesis of neuropathic pain. On the other hand, the loss of IFN-γR did not change microglia morphology and number under normal conditions, despite the expression of IFN-γR in resting microglia. The lack of a phenotype in resting microglia also has been reported in the brains of IFN-γ-deficient mice (48). It thus is conceivable that this receptor may not affect the development and localization of microglia in the spinal cord and may not be activated under normal conditions. The lack of obvious activation of STAT1 in the normal spinal cord supports this notion. Together, these findings suggest that the attenuating effects of IFN-γR deficiency on the activation of spinal microglia in a model of neuropathic pain may be caused by the activation of this receptor by IFN-γ, which is elevated in the spinal cord after nerve injury (20). Because the loss of IFN-γR did not change basal pain sensitivity, our results also suggest that interfering with IFN-γR-mediated signaling in spinal microglia may be a novel approach to treating neuropathic pain without affecting physiological acute pain.
Materials and Methods
Detailed methods are presented in SI Methods.
Behavioral Studies.
All experimental procedures were performed under the guidelines of Kyushu University. The PWT was measured using calibrated von Frey filaments in Wistar rats or mice (ifngr−/− [B6.129S7-Ifngr1xtm1Agt/J, The Jackson Laboratory], lyn−/− (49), p2rx4−/− (50), and their background wild-type control, C57BL/6J). The 3 knockout mouse lines were backcrossed to C57BL/6J mice for more than 10 generations. Minocycline was administered i.p. once a day from day 0 to day 7.
In Situ Hybridization.
A digoxigenin-labeled antisense probe for the rat IFN-γR (NM_053783, sequence position 997-1713) was used.
Immunohistochemistry.
Transverse L5 spinal cord sections (30 μm) were cut and processed for immunohistochemistry as described previously (12, 17). To visualize proliferating cells, BrdU (75 mg/kg, i.p.) was injected 22 h after intrathecal administration of IFN-γ; 2 h later, BrdU-treated rats were fixed by paraformaldehyde.
Microglial Culture.
Rat primary cultured microglia were prepared in accordance with a method described previously (12, 51).
Western Blotting.
Western blot analyses of Lyn and P2X4R expression in the membrane fractions from spinal cord segments L4-L6 and in whole-cell lysates of cultured microglial cells were performed in accordance with methods described previously (17).
Statistics.
Statistical analyses of the results were made with Student's t test, Student's paired t test, or 1-way ANOVA with a post hoc test (Dunnett's multiple comparison test).
Supplementary Material
Acknowledgments.
We thank Dr. Tadashi Yamamoto and Dr. Joji Ando of the University of Tokyo for providing Lyn- and P2X4R-knockout mice, respectively. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M.T. and K.I.). T.M. is a research fellow of the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810420106/DCSupplemental.
References
- 1.Baron R. Mechanisms of disease: Neuropathic pain—a clinical perspective. Nature Clinical Practice Neurology. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ, Mannion RJ. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 3.Scholz J, Woolf CJ. Can we conquer pain? Nature Neuroscience. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 4.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 5.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 6.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nature Reviews Neuroscience. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 8.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nature Neuroscience. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 9.Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain? Neuron Glia Biology. 2007;3:255–268. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 13.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 Mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Obata K, et al. Roles of extracellular signal-regulated protein kinases 5 in spinal microglia and primary sensory neurons for neuropathic pain. J Neurochem. 2007;102:1569–1584. doi: 10.1111/j.1471-4159.2007.04656.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda M, et al. Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 18.Inoue K. The function of microglia through purinergic receptors: Neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Farber K, Kettenmann H. Purinergic signaling and microglia. Pflugers Arch. 2006;452:615–621. doi: 10.1007/s00424-006-0064-7. [DOI] [PubMed] [Google Scholar]
- 20.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. European Journal of Pain (London, England) 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Griffin RS, et al. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci. 2007;27:8699–8708. doi: 10.1523/JNEUROSCI.2018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 24.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Molecular Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 26.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: A paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 27.Maus U, et al. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. American Journal of Physiology. Lung, Cellular, and Molecular Physiology. 2001;280:L58–68. doi: 10.1152/ajplung.2001.280.1.L58. [DOI] [PubMed] [Google Scholar]
- 28.Raivich G, et al. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Research Brain Research Reviews. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki Y, et al. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- 30.Haynes SE, et al. The P2Y(12) receptor regulates microglial activation by extracellular nucleotides. Nature Neuroscience. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 31.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 32.Tozaki-Saitoh H, et al. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 34.Salter MW, Kalia LV. Src kinases: A hub for NMDA receptor regulation. Nature Reviews Neuroscience. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi K, et al. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock RB, et al. Transcriptional response of human microglial cells to interferon-gamma. Genes and Immunity. 2005;6:712–719. doi: 10.1038/sj.gene.6364246. [DOI] [PubMed] [Google Scholar]
- 37.Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan J, Town T, Mullan M. CD45 inhibits CD40L-induced microglial activation via negative regulation of the Src/p44/42 MAPK pathway. J Biol Chem. 2000;275:37224–37231. doi: 10.1074/jbc.M002006200. [DOI] [PubMed] [Google Scholar]
- 39.Moore KJ, et al. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 40.Hooper C, Taylor DL, Pocock JM. Pure albumin is a potent trigger of calcium signalling and proliferation in microglia but not macrophages or astrocytes. J Neurochem. 2005;92:1363–1376. doi: 10.1111/j.1471-4159.2005.02982.x. [DOI] [PubMed] [Google Scholar]
- 41.Nasu-Tada K, Koizumi S, Tsuda M, Kunifusa E, Inoue K. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X(4), a key molecule for mechanical allodynia. Glia. 2006;53:769–775. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- 42.Tsuda M, et al. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152:495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 44.Vikman KS, Siddall PJ, Duggan AW. Increased responsiveness of rat dorsal horn neurons in vivo following prolonged intrathecal exposure to interferon-gamma. Neuroscience. 2005;135:969–977. doi: 10.1016/j.neuroscience.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 45.Vikman KS, Duggan AW, Siddall PJ. Interferon-gamma induced disruption of GABAergic inhibition in the spinal dorsal horn in vivo. Pain. 2007;133:18–28. doi: 10.1016/j.pain.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Robertson B, et al. Interferon-gamma receptors in nociceptive pathways: Role in neuropathic pain-related behaviour. NeuroReport. 1997;8:1311–1316. doi: 10.1097/00001756-199703240-00050. [DOI] [PubMed] [Google Scholar]
- 47.Vikman KS, Hill RH, Backstrom E, Robertson B, Kristensson K. Interferon-gamma induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. Pain. 2003;106:241–251. doi: 10.1016/S0304-3959(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 48.Mount MP, et al. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27:3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishizumi H, et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in Lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto K, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima K, et al. Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem. 1992;58:1401–1408. doi: 10.1111/j.1471-4159.1992.tb11356.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.