Abstract
Despite the recent identification of the transcriptional regulatory circuitry involving SOX2, NANOG, and OCT-4, the intracellular signaling networks that control pluripotency of human embryonic stem cells (hESCs) remain largely undefined. Here, we demonstrate an essential role for the serine/threonine protein kinase mammalian target of rapamycin (mTOR) in regulating hESC long-term undifferentiated growth. Inhibition of mTOR impairs pluripotency, prevents cell proliferation, and enhances mesoderm and endoderm activities in hESCs. At the molecular level, mTOR integrates signals from extrinsic pluripotency-supporting factors and represses the transcriptional activities of a subset of developmental and growth-inhibitory genes, as revealed by genome-wide microarray analyses. Repression of the developmental genes by mTOR is necessary for the maintenance of hESC pluripotency. These results uncover a novel signaling mechanism by which mTOR controls fate decisions in hESCs. Our findings may contribute to effective strategies for tissue repair and regeneration.
Keywords: differentiation, pluripotency, OCT-4, long-term undifferentiated growth
Human embryonic stem cells (ESCs) from blastocyst-stage early human embryos have 2 remarkable characteristics: long-term self-renewal and the potential to develop into multiple cell types (known as “pluripotency”) (1, 2). They represent a theoretically inexhaustible source of precursor cells to treat diseases and injuries and an invaluable research tool (3, 4). Before these potentials can be realized, obstacles such as the control of cell-fate decisions and cell cultivation must be overcome. This requires an improved understanding of signaling pathways that govern hESC long-term undifferentiated growth and directed differentiation.
hESC long-term self-renewal suggests cell proliferation with continuing repressed differentiation. Several factors that control this complex process are known. Extrinsic factors from the feeder cells, such as mouse embryonic fibroblasts (MEFs) or their conditioned medium (CM), are necessary for long-term undifferentiated growth of hESCs (1, 5). Growth factors such as basic FGF (bFGF) and TGF-β are capable of activating the transcriptional activity of pluripotency-regulating transcription factors, including OCT-4, NANOG, and SOX2 (1, 6–9). These regulators function in concert to maintain pluripotency in ESCs by positively controlling target genes necessary for the ESC identity and contributing to repression of lineage-specification factors (ref. 10 and references therein).
However, the intracellular signaling pathways that mediate long-term undifferentiated growth of hESCs are largely unknown. Furthermore, knowledge accumulated on murine embryonic stem cells (mESCs) cannot automatically be extrapolated to hESCs. For example, leukemia inhibitory factor (LIF) can substitute for feeder cells to maintain pluripotency in mESCs, but not in hESCs (11). Instead, bFGF can replace feeder cells to support hESC pluripotency, but only at significantly higher concentrations than those used with the feeder cells (6). Distinction between mESCs and hESCs may be attributed to differences in species divergence and/or temporal origins during development (12). Therefore, the understanding of the signaling networks underlying hESC pluripotency requires rigorous studies relying primarily on the hESC model. Here, we identified mTOR as a critical pluripotency-maintaining molecule in hESCs and uncovered an mTOR-dependent signaling mechanism for maintaining pluripotency and suppressing differentiation.
Results
mTOR Stabilizes OCT-4, SOX2, and NANOG.
To identify key intracellular signaling components of pluripotency in hESCs, we screened specific kinase inhibitors to assess their effects on the undifferentiated hESC growth. We used 2 hESC cell lines with distinct genotypes [WA01 (H1), XY; WA09 (H9), XX] (1) to determine whether the kinase pathways are conserved among different hESCs. To eliminate indirect effects on hESCs we used feeder-free culture conditions (5). Treatment of H9 or H1 cells with rapamycin, a bacterial macrolide and a highly specific inhibitor of mTOR, markedly impaired the pluripotency of both cell lines (Fig. 1 A–C and supporting information (SI) Fig. S1 A–E). mTOR is a serine/threonine kinase and master regulator of cell growth, proliferation, and differentiation. It senses nutrient availability and energy levels (13). Loss of Frap1 (which encodes mTOR) function causes early embryonic lethality in mice and suggests a role in development (14, 15).
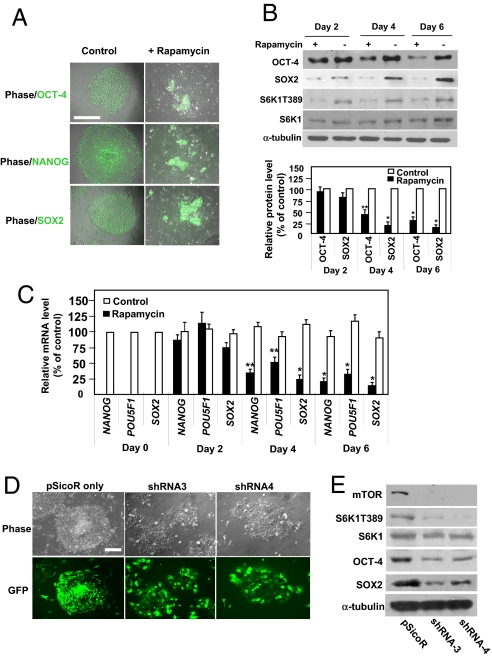
Fig. 1.
Inhibition or depletion of mTOR disrupts pluripotency of hESCs. (A) Immunofluorescence studies of OCT-4, SOX2, and NANOG proteins (green) in H9 cells with and without rapamycin (100 nM, 6 d). Fluorescence and phase-contrast images were merged. Untreated cells formed compact colonies with homogenous expression of OCT-4, SOX2, and NANOG. Treated cells spread out and lost OCT-4, SOX2, and NANOG immunofluorescence. Cells treated with vehicle (DMSO) exhibited no defects (data not shown). Quantification of colonies is shown in Fig. S1 B and C. (Scale bar, 500 μm.) (B) Western blots of OCT-4, SOX2, threonine-phosphorylated S6K1 (at residue 389: S6K1T389), and total S6K1 in cells with or without 100 nM rapamycin. α-tubulin was a loading control. (Top) A typical blot. (Bottom) Quantification of 6 separate experiments. y axis represents relative intensities (measured with Image J) with values normalized to the signal (= 100%) without rapamycin. Each bar represents mean ± SEM (error bars). Student t tests compared data between experimental groups. Asterisks indicate that the value for cells treated with rapamycin differs statistically from the control (*, P < 0.001; **, P < 0.01). Similar levels of mTOR protein were detected among these conditions (data not shown). (C) mRNA levels in untreated and rapamycin-treated cells (100 nM, for 2, 4, and 6 days), assessed by real-time PCR. Results from 5 separate experiments are shown as means ± SEM. ACTB (β-actin) was an internal control. All values were normalized to the level (= 100%) of mRNA in control cells on day 0. Asterisks indicate that the value for cells treated with rapamycin differs statistically from the control (*, P < 0.001; **, P < 0.01). (D) Phase-contrast and fluorescent images of H9 cells with or without mTOR depletion. Cells were infected with lentivirus and thereafter sorted against GFP fluorescence to enrich the infected cell population. Cells were sorted on day 5 after 2 rounds of infection, and sorted cells were cultured for 4 days and used directly for analysis. (Scale bar, 500 μm.) (E) Western blot of mTOR, S6K1T389, S6K1, SOX2, and OCT-4 in cells with or without mTOR depletion. Infection of cells with shRNA3 led to 85, 73, and 65% reductions in the levels of mTOR, SOX2, and OCT-4 protein, respectively, while shRNA4 led to 83, 63, and 59% reductions. mTOR depletion also caused a reduction in the level of S6K1T389 protein. α-tubulin was a loading control. A typical experiment from 4 separate experiments is shown.
Rapamycin treatment disrupted the compact morphology of hESC colonies. Cells began to flatten 2 days after treatment and were nearly completely spread out after 6 days (Figs. 1A and S1 A–C). These morphological changes are often correlated with loss of pluripotency (1). We assessed OCT-4, NANOG, and SOX2 expression to determine the pluripotent state of the cells. Immunofluorescence and Western blot analysis revealed a marked reduction in OCT-4, NANOG, and SOX2 expression 6 days after treatment (Fig. 1 A and B and Fig. S1 B and C). Rapamycin indeed inhibited mTOR activity, as indicated by decrease in threonine-389 phosphorylation of the ribosomal subunit S6 kinase 1 (S6K1), a downstream target of mTOR widely used to monitor mTOR activity (Fig. 1B). In contrast, rapamycin did not induce cell death: the percentage of apoptotic cells in treated cells (2–3%) was similar to control levels (approximately 3%). Lower levels of OCT-4, NANOG, and SOX2 appeared to be caused by decreased transcription: rapamycin sharply reduced mRNA levels in H9 or H1 cells (Fig. 1C and data not shown). Furthermore, in time-course experiments, down-regulation of NANOG and SOX2 transcription and expression began 2 days after rapamycin treatment and preceded down-regulation of POU5F1 (which encodes OCT-4) (Fig. 1 B and C). These results suggest that mTOR activity is required for transcriptional regulation of SOX2, NANOG, and POU5F1. Alternatively, because OCT-4, SOX2, and NANOG may collaborate to regulate ESC pluripotency, which could be destabilized by changing in the abundance of one protein (10), down-regulation of POU5F1 may indirectly result from SOX2 and/or NANOG down-regulation. In either scenario, our results demonstrate that mTOR is required to stabilize the regulatory circuitry in hESCs.
In contrast, OCT-4 and SOX2 expression in mESCs appeared less sensitive to mTOR inhibition. Rapamycin treatment of 2 mESC lines (E14Tg2a.4 and W4/129S6) markedly reduced the size of cell colonies (Fig. S1F), suggesting an inhibitory effect on cell proliferation. However, this treatment failed to perturb the compact morphology of mESC colonies or decrease OCT-4 and SOX2 protein expression (Fig. S1 F and G). The critical role of mTOR in controlling mESC proliferation was demonstrated earlier by experiments with Frap1 deletion mutants (15). We do not know whether the differential effects of rapamycin on mESCs and hESCs are due to their differences in species, origin of development or other undefined characteristics.
The high selectivity of rapamycin toward mTOR has been documented (16). Nevertheless, to further confirm the specificity of rapamycin we used lentivirus-mediated infection to stably express small hairpin RNAs (shRNAs) to deplete mTOR in hESCs. We used a modified pSicoR lentiviral vector, which allows expression of shRNAs for RNA interference and of GFP in hESCs as a marker [17; SI Text]. Two shRNA sequences effectively depleted mTOR in HEK293 cells and the pluripotent human embryonal carcinoma NTera-2 cells (18) (Fig. S1 J–L, and data not shown). Because of the relatively low infection efficiency of hESCs (GFP-positive cells < 50%), we used GFP-based cell sorting to enrich shRNA-containing cells. pSicoR empty vector was a negative control (Fig. 1D). Similar to rapamycin treatment, depletion of mTOR in H9 cells induced morphological changes and down-regulation of OCT-4 and SOX2, confirming that the effect of rapamycin was caused by mTOR inhibition (Fig. 1 D and E). Consistently, both mTOR depletion and rapamycin treatment sharply reduced OCT-4 and SOX2 expression in NTera-2 cells (Fig. S1L). Notably, mTOR depletion in H9 cells markedly increased apoptosis (approximately 20%) over cells infected with vector alone (5–10%). Because rapamycin treatment failed to increase apoptosis, the additional effect in mTOR-depleted H9 cells may result from disruption of rapamycin-insensitive function of mTOR (19).
mTOR Suppresses Endoderm and Mesoderm Activities.
Disruption of OCT-4, SOX2 and NANOG often leads to ESC differentiation (10). To ask whether mTOR inhibition also caused hESCs to differentiate, we assessed expression of markers for 3 germ layers [i.e., SOX1 and NEUROD1 for ectoderm, Brachyury (T gene) and MESP1 for mesoderm, and GATA4, GATA6, and SOX17 for endoderm] and trophoectoderm (CGB7) in cells cultured with or without rapamycin. Rapamycin treatment of H9 hESCs cultivated under feeder-free conditions was already capable of triggering differentiation activities, as shown by increased expression of several differentiation markers, which, interestingly, were those for endoderm and mesoderm lineages (Fig. 2A). By contrast, markers for ectoderm and trophoectoderm were down-regulated (Fig. 2A).
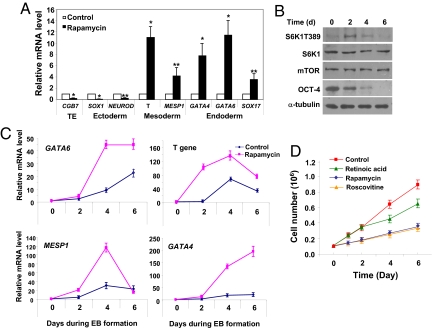
Fig. 2.
mTOR inhibition induces endoderm and mesoderm activities and impairs cell proliferation. (A) mRNA levels in untreated and rapamycin-treated cells (100 nM, 6 days), assessed by real-time PCR. Results from 4 separate experiments are shown as means ± SEM. ACTB (β-actin) was an internal control. All values were normalized to the level (= 1) of mRNA in untreated cells. Asterisks indicate that the value for cells treated with rapamycin differs statistically from the control (*, P < 0.001; **, P < 0.01). TE denotes trophoectoderm. (B) Western blot of S6K1T389, S6K1, mTOR, and OCT-4 in H9 cells during EB formation. The level of S6K1T389 elevated transiently while OCT-4 and SOX2 proteins (data not shown) exhibited a gradual decrease. α-tubulin was a loading control. A typical experiment from 4 separate experiments is shown. (C) mRNA levels in control EBs or in rapamycin-treated EBs assessed by real-time PCR. Four separate experiments were conducted, and quantification of 4 replicates of a typical experiment is shown. Each bar represents the mean ± SEM (error bars). All values were normalized to the level (= 1) of mRNA in the control cells on day 0. Rapamycin treatment enhanced and/or prolonged the expression of GATA4, GATA6, MESP1, T gene, and SOX17 (data not shown). ACTB (β-actin) was used as an internal control. (D) H9 cells (0.1 × 106) were treated or not treated with rapamycin (100 nM), retinoid acid (10 μM), or roscovotine (25 μM) for 1, 2, 4, and 6 days. After treatment, cells were dissociated with trypsin and counted with a hemocytometer. Quantification of 4 separate experiments is shown. Each represents the mean ± SEM (error bars).
The effect of mTOR inhibition on hESC differentiation was further evaluated with the embryoid body (EB) formation assay commonly used to examine the differentiation potential of hESCs (1). H9 cells were induced to form EBs with or without the presence of rapamycin. We observed a moderate up-regulation of mTOR activity after EB induction (Fig. 2B). Treatment with rapamycin caused a reduction in the size of EBs after 6 days (approximately 23%, data not shown). A time-course analysis revealed that during EB formation, expression of differentiation markers, albeit with distinct dynamic patterns, was consistently up-regulated in untreated control EBs (Figs. 2C and S2A). Rapamycin treatment enhanced and/or prolonged the expression of endoderm and mesoderm markers (Fig. 2C). The same treatment prevented the up-regulation of NEUROD1 and CGB7 but exerted few effects on SOX1 (Fig. S2A). These results further point to a role for mTOR in suppressing mesoderm and endoderm activities in hESCs.
Experiments with the EB formation assay provided additional evidence that mTOR inhibition impairs pluripotency of hESCs and induces them to commit to endoderm and mesoderm activities. We treated H9 cells with rapamycin for 6 days under feeder-free conditions and then induced them to form EBs in the absence of rapamycin (Fig. S2B). The purpose of this manipulation was to assess whether the cells, after rapamycin treatment, still retained the potential to differentiate into all lineages. In control cells without rapamycin pretreatment, SOX1, NEUROD1, and CGB7 were rapidly up-regulated after induction of EBs (Fig. S2B). By contrast, their expression was markedly reduced in cells with rapamycin pretreatment (Fig. S2B, denoted as Day 0; a similar experiment was shown in Fig. 2A) and remained significantly lower than that in control cells after EB induction, thus indicating that rapamycin-pretreated cells were unable to properly differentiate along the ectoderm lineages. Control cells expectedly exhibited drastic up-regulation of mesoderm and endoderm markers after EB induction (Fig. S2B). In rapamycin-pretreated cells their expression was already significantly higher than that in control cells before EB induction (Fig. S2B) and remained at high levels during EB formation (Fig. S2B). Rapamycin pretreatment also reduced the number (to 31% of control) and the sizes of EBs (Fig. S2 C and D). Significant cell death (>25%) was observed for rapamycin-pretreated cells when they were induced to form EBs for more than 5 days; the expression of differentiation markers was thus not assessed beyond 5 days. The underlying mechanism for increased cell death is unknown.
mTOR Is Required for hESC Proliferation.
A remarkable feature of hESC long-term self-renewal is the cells' ability to proliferate almost indefinitely under proper conditions (1, 5). This ability was compromised in hESCs with mTOR inhibition. Rapamycin treatment markedly reduced proliferation of H9 cells (Fig. 2D). The effect was observed within 24 h after rapamycin addition. In contrast, all-trans retinoic acid, an inducer of hESC differentiation, showed inhibition only 3–4 days after treatment, which may result indirectly from cell differentiation. To determine if inhibiting proliferation alters pluripotency, we tested roscovitine, an inhibitor of cyclin-dependent kinases (Cdks) and cell-cycle progression. As expected, roscovitine caused rapid and marked growth inhibition of H9 cells, similar to rapamycin (Fig. 2D). However, it failed to induce differentiation morphology or to down-regulate OCT-4 and SOX2 (Fig. S2 E and F). This agrees with accumulating evidence, suggesting inhibition of hESC proliferation per se cannot disrupt hESC pluripotency (20, 21). Because rapamycin's effect was rapid, we inferred mTOR is necessary not only for repression of differentiation but also for proliferation in hESCs.
Modulation of mTOR Activity by Extrinsic Factors.
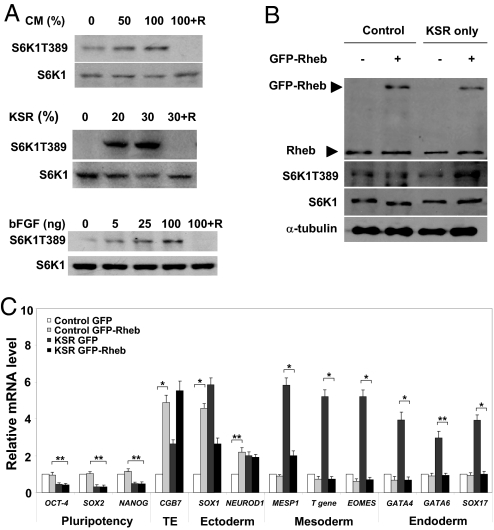
To determine how mTOR supports hESC long-term self-renewal, we first assessed whether mTOR could be activated by extrinsic cues that maintain hESC pluripotency. Under feeder-free conditions, the extracellular factors include mainly mouse embryonic fibroblast-conditioned medium (MEF-CM), bFGF, and a chemically defined serum replacement (denoted as knockout serum replacement: KSR) (5). Removal of any of these from hESC culture medium disrupts pluripotency; KSR seems the most critical component (5, 6, 22). Accordingly, we assessed whether MEF-CM, bFGF, or KSR controls mTOR activity. Treating H9 cells with each component alone triggered S6K1 phosphorylation, with KSR being the most potent stimulus (Fig. 3A and Fig. S3 A–C), thereby consistent with their effects on pluripotency (22). While lower doses of bFGF (5–10 ng) typically used in conjunction with MEF-CM in feeder-free cultures (5) triggered S6K1 phosphorylation, higher doses (50–200 ng) capable of replacing MEF-CM to maintain hESC pluripotency (6) further increased S6K1 phosphorylation. Thus, mTOR can be activated by key extrinsic pluripotency-supporting factors in hESCs.
Fig. 3.
mTOR integrates extrinsic pluripotency-supporting signals. (A) Western blot of S6K1T389 in H9 stimulated with MEF-CM, KSR, or bFGF with or without rapamycin. Cells were cultured under feeder-free conditions for 4 days; starved for 24 h in DMEM/F12 basal medium in the absence of KSR, bFGF, or MEF-CM; stimulated or not stimulated with MEF-CM (50 or 100%), KSR (20 and 30%), or bFGF (5, 25, and 100 ng) (all in DMEM/F12) for 30 min; lysed; and subjected to SDS/PAGE. Cells were pretreated with rapamycin (100 nM) for 30 min before stimulation. (Top) A typical blot is shown; quantification of blots from 4 separate experiments is shown in Fig. S3. Total S6K1 was used for equal loading in the different lanes. Note hESCs are typically cultured in 100% MEF-CM, 20% KSR, and 5 ng bFGF. (B) Western blots of Rheb and S6K1T389 in GFP or GFP-Rheb H9 overexpressing cells, cultivated in control medium (i.e., with MEF-CM, bFGF, and KSR; denoted as control) or in medium without CM and bFGF (denoted as KSR only) for 6 days. Endogenous Rheb protein (21 kDa) and GFP-Rheb (approximately 48 kDa) were detected. Overexpression of GFP-Rheb increased the S6K1T389 levels approximatle 2-fold. Total S6K1 and α-tubulin were loading controls. A typical experiment from 4 separate experiments is shown. (C) mRNA levels in H9 cells expressing GFP or GFP-Rheb assessed by real-time PCR. Both types of cells were cultured in control medium (denoted as control) or in medium without CM and bFGF (denoted as KSR) for 6 days. Four separate experiments were conducted, and quantification of 4 replicates of a typical experiment is shown. Each bar represents the mean ± SEM (error bars). ACTB (β-actin) was an internal control. All values were normalized to the level (= 1) of mRNA in the GFP-expressing cells. Pairs of treatments showing a significant difference are marked (*, P < 0.001; **, P < 0.01).
Effects of Constitutive Activation of mTOR.
It has been well established that withdrawal of MEF-CM and/or bFGF causes hESCs to lose pluripotency and differentiate (5, 6). Because both factors activate mTOR (Fig. 3A), we asked whether mTOR activation could retain pluripotency and/or suppress differentiation when MEF-CM and bFGF were removed from the feeder-free medium. H9 cells expressing a GFP-tagged Rheb protein (a GTPase upstream of mTOR) moderately increased phosphorylated S6K1 levels over those expressing GFP alone (Fig. 3B). Under self-renewal conditions (i.e., with bFGF and MEF-CM), activation of mTOR elevated the mRNA levels of SOX1, NEUROD1, and CGB7 (Fig. 3C; compare control GFP cells with control GFP-Rheb cells), in agreement with the finding that mTOR inhibition down-regulated the same markers (Fig. 2A). Expression of pluripotency, mesoderm and endoderm markers was insensitive to mTOR activation and remained largely unaltered under the same conditions (Fig. 3C). As expected, withdrawal of bFGF and MEF-CM reduced expression of pluripotency genes and meanwhile caused differentiation genes to up-regulate (Fig. 3C; compare control GFP cells with KSR GFP cells). Activation of mTOR effectively repressed up-regulation of T gene, MESP1, GATA4, GATA6, EOMES, and SOX17 (Fig. 3C; compare KSR GFP cells with KSR GFP-Rheb cells), again suggesting a role for mTOR in suppressing mesoderm and endoderm activities in hESCs. The failure of mTOR activation to rescue the pluripotency markers (Fig. 3C) may be attributed to the relative small elevation of mTOR activity and/or higher responsiveness of differentiation genes to mTOR signaling.
Downstream Signals of mTOR in hESCs.
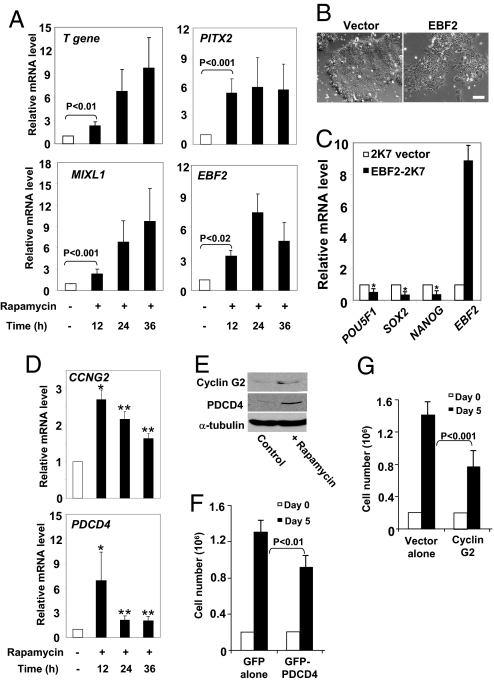
To dissect the signaling mechanism by which mTOR regulates long-term undifferentiated growth in hESCs, we used Affymetrix-based whole genome microarrays to compare global gene-expression profiles of hESCs with and without rapamycin. Focusing on early times (12, 24, and 36 h) after mTOR inhibition, we sought to identify downstream targets that mediate mTOR's pro-proliferation and anti-differentiation roles. Microarray analyses identified approximately 100 genes that are differentially expressed in response to rapamycin. Expression of 19 genes was consistently altered more than 2.5-fold from 12 to 36 h (Fig. S4A). Remarkably, mTOR inhibition resulted in a rapid, robust, and continuous up-regulation of several differentiation-promoting transcription factors, including Mix1 homeobox-like 1 (MIXL1), T gene, early B-cell factor 2 (EBF2), and the homeobox protein PITX2. These changes in gene expression were further verified by real-time PCR analysis (Fig. 4A). Interestingly, MIXL1, T gene, and PITX2 have established roles in mesoderm and endoderm development (23–25), and the involvement of MIXL1 and PITX2 in human development was implied by a rapid up-regulation of gene expression during EB formation of hESCs (Fig. S4B). mTOR's suppression of mesoderm and endoderm differentiating activities was further suggested by modest but consistent up-regulation of EOMES (Fig. S4C), a gene required for mesoderm differentiation (26). Interestingly, neither the mRNA nor protein levels of OCT-4, SOX2, and NANOG were altered 36 h after rapamycin treatment, when the transcriptional activity of MIXL1, T gene, PITX2, and EBF2 was already up-regulated (Figs. 1 B and C and 4A).
Fig. 4.
mTOR represses expression of growth inhibitory and developmental genes in hESCs. (A) mRNA levels in untreated control cells (white bars) and in cells treated with rapamycin (12, 24, and 36 h; black bars) assessed by real-time PCR. Quantification of 4 separate experiments is shown. Each bar represents the mean ± SEM (error bars). All values were normalized to the level (= 1) of mRNA in the absence of rapamycin at respective time points. Student t tests compared data between experimental groups. Pairs of treatments showing a significant difference are marked. ACTB (β-actin) was used as internal control. (B) Phase-contrast images of H9 cells with or without EBF2 overexpression. Cells were infected with lentivirus containing the 2K7/Neo empty vector or EBF2 and were evaluated 7 days after infection. (Scale bar, 500 μm.) (C) mRNA levels in 2K7/Neo cells and in cells expressing EBF2 assessed by real-time PCR. Each bar represents the mean ± SEM (error bars). All values were normalized to the level (= 1) of mRNA in the 2K7/Neo cells. Asterisk indicates that the value for EBF2 overexpression differs statistically from untreated cells (*, P < 0.001). (D) mRNA levels of CCNG2 and PDCD4 in untreated control cells (white bars) and in cells treated with rapamycin (12, 24, and 36 h; black bars) assessed by real-time PCR. Quantification of 4 separate experiments is shown. Each bar represents the mean ± SEM (error bars). All values were normalized to the level (= 1) of mRNA without rapamycin at respective time points. Asterisks indicate that the value for cells treated with rapamycin differs statistically from the control (*, P < 0.001; **, P < 0.01). (E) Western blot of Cyclin G2 and PDCD4 in H9 cells treated with rapamycin (100 nM, 24 h). Rapamycin induced levels of Cyclin G2 and PDCD4 to increase by 110% and 160%, respectively. α-tubulin was a loading control. A typical experiment from 4 separate experiments is shown. (F) H9 cells (0.2 × 106) expressing GFP or GFP-PDCD4 were plated on day 0, cultured for 5 days, dissociated with trypsin, and counted with a hemocytometer. Quantification of 4 separate experiments is shown. Each represents the mean ± SEM (error bars). Similar experiments were conducted in (G), where growth of H9 cells containing the 2K7/Neo vector or Cyclin G2 was evaluated. Pairs of treatments showing a significant difference are marked. Overexpression of Cyclin G2 exerted no effects on the pluripotency markers (data not shown).
The up-regulation of the developmental genes appeared sufficient to disrupt pluripotency. Ectopic expression of EBF2 impaired the compact morphology of hESCs and markedly down-regulated the expression of SOX2, NANOG, and POU5F1 (Fig. 4 B and C). Overexpression of MIXL1 also induced modest down-regulation of SOX2 and NANOG (Fig. S4D). Therefore, these results suggest that repression of developmental genes is necessary for the stability of the OCT-4/NANOG/SOX2 regulatory circuitry.
Microarray experiments also revealed that several components of the Wnt signaling pathway, including the extracellular ligand Wnt3 and intracellular molecules DACT1 and FRAT1, were up-regulated after treatment (Fig. S4A). These results were verified by real-time PCR analysis (Fig. S4E). We then determined if Wnt pathway activity was enhanced by mTOR inhibition. Indeed, rapamycin rapidly increased luciferase activity in H9 cells transiently transfected with the Top-flash reporter gene (Fig. S4F), which assesses Wnt/β-catenin-mediated transcriptional activation (27).
The Wnt signaling pathway is essential in embryogenesis, but whether it is necessary for hESC pluripotency remains controversial. Recent evidence suggests that long-term activation of the Wnt pathway in hESCs promotes differentiation (28, 29). Because mTOR inhibition rapidly activates the Wnt pathway, we assessed the effects of short-term activation. Stimulation of H9 cells with Wnt3a expectedly enhanced the top-flash reporter activity (Fig. S4F) and rapidly up-regulated expression of MIXL1, T gene, PITX2, and EBF2 (Fig. S4G). There were no effects on POU5F1, NANOG, and SOX2 (data not shown). Because Wnt3a stimulation mirrored the effect of rapamycin on the developmental genes (albeit not to the same extent), we inferred that mTOR represses the transcriptional activity of MIXL1, T gene, PITX2, and EBF2 partially by suppressing the Wnt signaling pathway in hESCs.
Using microarray experiments, we also identified potential growth-regulatory genes controlled by mTOR in hESCs. In response to rapamycin treatment, H9 cells up-regulated gene expression of CCNG2 (which encodes Cyclin G2) and programmed cell death 4 (PDCD4), both of which inhibit cell proliferation (30, 31). The increase in mRNA levels, verified by real-time PCR (Fig. 4D), appeared to cause an increase in protein expression 24 h after treatment (Fig. 4E). To study their roles, we overexpressed Cyclin G2 and PDCD4 in H9 cells and assessed their effects on cell proliferation and pluripotency. Overexpression of Cyclin G2 and PDCD4 markedly reduced cell growth (to 53% and 68% of control, respectively) but failed to affect cell morphology or reduce OCT-4 and SOX2 expression (Figs. 4 F and G and S4 H and I, and data not shown). Thus, Cyclin G2 and PDCD4 are downstream targets of mTOR in hESCs and regulate cell proliferation, but not pluripotency.
Discussion
In conclusion, we identified a central role of the protein kinase mTOR in regulating long-term undifferentiated growth in hESCs. In this scenario (Fig. 5), mTOR responds to extrinsic factors and integrates and transmits signals to promote cell proliferation and suppress differentiation activities in hESCs. Using genome-wide microarray analysis, we identified the downstream targets of mTOR in hESCs that contribute to the dual functions of mTOR in controlling long-term hESC self-renewal. Our results suggest that suppression of both Cyclin G2 and PDCD4 expression by mTOR plays a role in promoting hESC proliferation, while inhibition of the Wnt signaling pathway and the suppression of a subset of differentiation-promoting transcription factors are essential for maintaining the undifferentiated state of hESCs. Repression of the differentiation genes (MILXL1, T gene, PITX2 and EBF2) and the Wnt signaling pathway by mTOR has not been documented. Expression of Cyclin G2 and PDCD4 has been shown to be negatively regulated by mTOR signaling at the mRNA or the protein level (32, 33), but this regulation has not been reported in ESCs. Thus, our results have unveiled a novel signaling mechanism involved in the fate determination of hESCs.
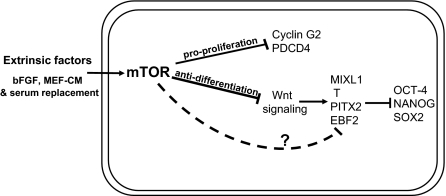
Fig. 5.
mTOR supports hESC long-term self-renewal by promoting cell proliferation and repressing differentiation: A model. mTOR responds to extrinsic factors such as bFGF, MEF-CM, and KSR, integrates and transmits pluripotency-supporting signals. Activation of mTOR inhibits the Wnt signaling pathway, which combines with unidentified mechanisms (dotted line; question mark) to repress the transcriptional activity of MIXL1, Brachyury (T gene), EBF2 and PITX2 in hESCs. Repression of the developmental genes is necessary for the stability of the OCT-4/SOX2/NANOG circuitry. mTOR also suppresses growth inhibitory molecules including Cyclin G2 and PDCD4 and thus promotes cell proliferation.
Our studies also demonstrate an interesting example that modulation of a single protein kinase can drastically alter the fate of hESCs. When cells are cultivated under self-renewal conditions, mTOR inhibition suffices to disrupt pluripotency and triggers mesoderm and endoderm activities. This was demonstrated by experiments with known markers for pluripotency (OCT-4, SOX2, and NANOG) and endoderm and mesoderm (T gene, MESP1, MIXL1, PITX2, EOMES, GATA4, GATA6, and SOX17). Some of the genes respond rapidly to mTOR inhibition, play a role in destabilizing the pluripotency circuitry and probably cause more profound endoderm and mesoderm activities gradually. Conversely, constitutive activation of mTOR effectively prevents endoderm and mesoderm activities induced by the removal of MEF-CM and bFGF. mTOR inhibition impairs the cells' differentiation potential to ectoderm, as revealed by EB formation assay, probably because they already begin to commit to the endoderm and mesoderm lineages. The ability of mTOR to suppress endoderm and mesoderm is not limited to cells under self-renewal conditions: during EB formation, when hESCs undergo spontaneous multilineage differentiation, mTOR inhibition further enhances and/or prolongs the expression of mesoderm- and endoderm-specific markers. These findings may prove valuable for directing hESC differentiation and thus may contribute to effective strategies for tissue repair and regeneration.
mTOR has a well established role in regulating ribosomal biogenesis and protein translation. By using microarray analysis, we identified in hESCs signaling molecules controlled transcriptionally by mTOR pathway. A recent study demonstrated that a hierarchy of translational regulators, including mTOR, its downstream effectors 4EBP1, and the RNA-binding proteins DAZL and GRSF1, control global and selective protein synthesis during differentiation of mESCs (34). However, functional dissection of mTOR-dependent translational control in hESC fate determination awaits future experiments.
We show that mTOR inhibition markedly decreases OCT-4, SOX2 and NANOG protein expression. The reduction of proteins correlates with down-regulation of their mRNAs. We do not know if mTOR inhibition affects the promoter activities of the genes or (and) impairs their mRNA stability. In addition, we cannot rule out the possibility that mTOR inhibition renders the proteins less stable. Interestingly, the mRNA and protein levels of OCT-4, SOX2, and NANOG were unaltered 36 h after rapamycin treatment, when the transcriptional activity of MIXL1, T gene, PITX2, and EBF2 was already up-regulated. These results raise the possibility that mTOR inhibits the transcriptional activity of MIXL1, T gene, PITX2, and EBF2 independently of the OCT-4/SOX2/NANOG circuitry. Alternatively, mTOR inhibition could cause rapid perturbations of NANOG, SOX2, and OCT-4 functions (e.g., via posttranslational modifications) before reduction of their protein levels, which would subsequently lead to the differentiation activities. More complete understanding of the detailed molecular mechanisms underlying the functional interactions among mTOR, its downstream developmental genes, and the OCT-4/SOX2/NANOG circuitry awaits future experimentation.
Materials and Methods
Cell Culture.
Federally registered hESC lines H1 and H9 were purchased from WiCell Research Institute and routinely maintained under feeder conditions as described in reference (1). The culture medium consists of DMEM/F12 with 20% knockout serum replacement (KSR), 1 mM glutamine, 1% non-essential amino acid, 0.1 mM β-mercaptoethanol, and 4 ng/ml bFGF (Invitrogen) (1). For feeder-free cultures, cells are cultured on plates coated with Matrigel (BD Biosciences) in the presence of conditioned medium from MEFs, which replaces the MEF feeders (5). The MEF-CM was generated as described (5). The experiments described in this study were conducted with H9 and H1 cells between passage 30 and 60. HEK293T cells and NTera-2 cells were purchased from American Type Culture Collection (ATCC) and were cultured according to ATCC recommendations. W4/129S6 and E14Tg2a.4 mESC lines were from Taconic and Mutant Mouse Regional Resource Centers (MMRRC), respectively. Their culture conditions are available in the SI Text.
Additional experimental procedures and associated references are available in the SI Text. Information for antibodies and PCR primers was described in Tables S1 and S2, respectively.
Supplementary Material
Acknowledgments.
We thank Dr. David Suter for providing the 2K7/Neo lentiviral vector, Dr. Linzhao Cheng for providing the Top-flash reporter construct, Dr. Jenny Drnevich for analyzing the microarray data, and members of the Wang lab for helpful discussions. Support was provided by grants from National Institutes of Health Grant GM-83812 to F.W., the Illinois Regenerative Medicine Institute Grant 2006–05516 to F.W. and T.T., and the Research Board of the University of Illinois and the National Natural Science Foundation of China Grant 30728022 to F.W. and E.D.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901854106/DCSupplemental.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Silva J, Smith A. Capturing pluripotency. Cell. 2008;132:532–536. doi: 10.1016/j.cell.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Xu C, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 6.Levenstein ME, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells (Dayton) 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu RH, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 8.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig TE, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 10.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daheron L, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells (Dayton) 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 12.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 13.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Gangloff YG, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bain J, et al. The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventura A, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews PW, et al. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest. 1984;50:147–162. [PubMed] [Google Scholar]
- 19.Inoki K, Guan KL. Complexity of the TOR signaling network. Trends Cell Biol. 2006;16:206–212. doi: 10.1016/j.tcb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Bendall SC, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 21.Andang M, et al. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 22.Ding V, Choo AB, Oh SK. Deciphering the importance of 3 key media components in human embryonic stem cell cultures. Biotechnol Lett. 2006;28:491–495. doi: 10.1007/s10529-006-0005-8. [DOI] [PubMed] [Google Scholar]
- 23.Faucourt M, Houliston E, Besnardeau L, Kimelman D, Lepage T. The pitx2 homeobox protein is required early for endoderm formation and nodal signaling. Dev Biol. 2001;229:287–306. doi: 10.1006/dbio.2000.9950. [DOI] [PubMed] [Google Scholar]
- 24.Hart AH, et al. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- 25.Rashbass P, Cooke LA, Herrmann BG, Beddington RS. A cell autonomous function of Brachyury in T/T embryonic stem cell chimaeras. Nature. 1991;353:348–351. doi: 10.1038/353348a0. [DOI] [PubMed] [Google Scholar]
- 26.Russ AP, et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- 27.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 28.Bakre MM, et al. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J Biol Chem. 2007;282:31703–31712. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- 29.Woll PS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65:6034–6041. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- 31.Bennin DA, et al. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B' subunits in active complexes and induces nuclear aberrations and a G1/S phase cell cycle arrest. J Biol Chem. 2002;277:27449–27467. doi: 10.1074/jbc.M111693200. [DOI] [PubMed] [Google Scholar]
- 32.Dorrello NV, et al. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 33.Le XF, et al. Roles of human epidermal growth factor receptor 2, c-jun NH2-terminal kinase, phosphoinositide 3-kinase, and p70 S6 kinase pathways in regulation of cyclin G2 expression in human breast cancer cells. Mol Cancer Ther. 2007;6:2843–2857. doi: 10.1158/1535-7163.MCT-07-0109. [DOI] [PubMed] [Google Scholar]
- 34.Sampath P, et al. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;2:448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.