Abstract
Influenza prophylaxis would benefit from a simple method to administer influenza vaccine into skin without the need for hypodermic needles. In this study, solid metal microneedle arrays (MNs) were investigated as a system for cutaneous vaccine delivery using influenza virus antigen. The MNs with 5 monument-shaped microneedles per array were produced and coated with inactivated influenza virus A/PR/8/34 (IIV). As much as 10 μg of viral proteins could be coated onto an array of 5 microneedles, and the coated IIV was delivered into skin at high efficiency within minutes. The coated MNs were used to immunize mice in comparison with conventional intramuscular injection at the same dose. Analysis of immune responses showed that a single immunization with IIV-coated MNs induced strong antibody responses against influenza virus, with significant levels of hemagglutination inhibition activities (>1:40), which were comparable to those induced by conventional intramuscular immunization. Moreover, mice immunized by a single dose of IIV coated on MNs were effectively protected against lethal challenge by a high dose of mouse-adapted influenza virus A/PR/8/34. These results show that MNs are highly effective as a simple method of vaccine delivery to elicit protective immune responses against virus infection.
Keywords: immunity, intradermal, antibody, infection
Influenza virus is a major cause of serious respiratory illness. Typically, the virus causes seasonal epidemics of disease that lead to about 40,000 deaths and 200,000 hospitalizations in the United States (1) and up to 1.5 million deaths worldwide (2). Inactivated influenza virus (IIV) vaccines have been extensively used and shown to confer effective protection against influenza virus infection (3). However, despite the high efficacy of current seasonal influenza vaccines and increasing awareness of their benefits to public health, vaccination coverage is still incomplete (4). This has been attributed to misinformation about influenza vaccination and also in part to needle phobia of persons who fear pain in association with the conventional vaccination approach by intramuscular (IM) injection (5, 6). Thus, novel vaccine delivery systems that avoid hypodermic needles may significantly improve vaccination coverage and provide better control of seasonal influenza epidemics.
Alternate routes of vaccination against influenza virus have been investigated. A recently introduced attenuated influenza vaccine is administered by nasal spray (7), but it is only approved for use in healthy and nonpregnant recipients between 2 and 49 years old (4). Also, intranasal delivery of inactivated influenza vaccines using an adjuvant and multiple immunizations was reported to be more effective in eliciting heterosubtypic immune responses against influenza virus infection (8–10). Immunization via the skin has received heightened attention, in large part because of the abundance of resident Langerhans and dermal dendritic cells, which are powerful antigen-presenting cells (11). Cutaneous immunization has been shown to elicit a broad range of immune responses, including humoral, cellular, and mucosal responses (12). The highly successful campaign that resulted in worldwide eradication of smallpox was based on vaccine administration using a dual-pronged microfork that pierced the skin to deposit a liquid vaccine formulation (13). Recent results in support of skin-based influenza vaccination showed that intradermal (ID) administration of influenza vaccine elicited immunogenicity that was commensurate with IM injection (14–16). Further, the effectiveness of ID immunization in patients older than 60 years has also been demonstrated (17), which is extremely important because 90% of influenza-related deaths in the United States occur in this population (18). However, application of the classical ID injection method (the Mantoux technique) is limited by the requirement for highly trained personnel (19).
In recent years, microneedles (MNs) have been fabricated by leveraging tools from the microelectronics industry and investigated as devices to facilitate ID delivery (20, 21). As shown through in vitro, animal, and recent human trials, arrays of these micrometer-scale needles can be prepared as patches coated with a drug for simple administration to the skin in a painless manner (22, 23). In this study, we investigate the delivery of IIV using vaccine-coated MNs. Our results show that the vaccine can be efficiently coated onto MNs and rapidly delivered into the skin to vaccinate animals. We further compared immune responses for coated MNs to those induced by conventional IM injection, and we determined the ability of vaccine delivery using coated MNs to protect against challenge with a high dose of pathogenic influenza virus.
Results
MN Delivery of BSA Induces Strong Antibody Responses.
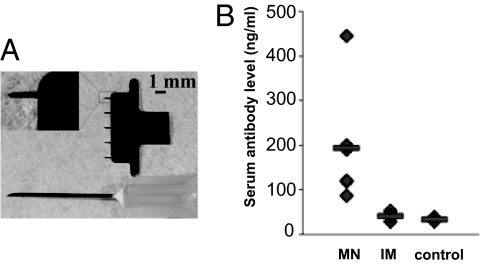
The type of MN used in this study is shown in Fig. 1A in comparison with a 27-gauge hypodermic needle. We observed that BSA can be efficiently coated onto these solid metal MNs at about 10 μg of total protein per 5-needle array (i.e., 2 μg of total protein per needle). To test whether MN delivery of this protein antigen can efficiently induce immune responses, we used BSA-coated MN to immunize mice and compared the results with IM injection. As shown in Fig. 1B, antibody responses induced by IM injection of BSA were minimal and only slightly above background. In contrast, immunization with the same dose of BSA-coated MNs induced significantly higher levels of antibody response against BSA in all mice compared with IM injection, despite the observed variance within the group after a single immunization (Student's t test, P < 0.05). These results show that MN delivery of a soluble protein antigen is significantly more effective than IM injection for eliciting immune responses, which is consistent with previous observations using ovalbumin as a model antigen (22).
Fig. 1.
MN design and delivery of a soluble protein antigen. (A) An MN is shown next to a 27-gauge hypodermic needle. (Inset) A single MN at higher magnification. (B) Immunization by BSA-coated MNs induced higher levels of antibody responses than IM injection. MNs were produced and coated with BSA as described in Materials and Methods. Mice (groups of 6) were immunized with MNs coated with 10 μg of BSA or by IM injection of 10 μg of BSA. A control group of mice received IM injection of PBS. On day 14 after immunization, blood samples were collected. Antibody responses against BSA were determined by ELISA and expressed as the amount of BSA-specific antibody in 1 mL of serum samples (ng/mL). Data are presented as the mean ± SD.
IIV Vaccine Is Efficiently Coated onto Solid Metal MNs and Delivered to Mice upon Immunization.
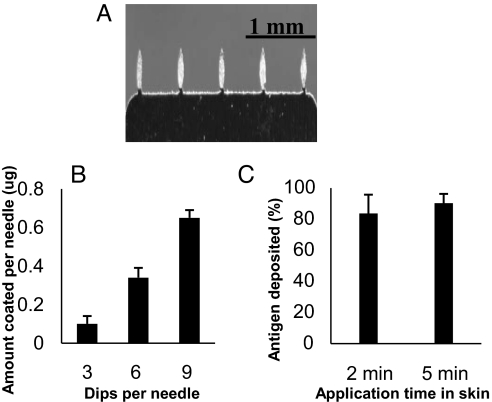
To investigate coating and delivery of IIV vaccines by MNs, purified influenza virus A/PR/8/34 was inactivated and concentrated to 5 mg/mL by microfiltration. Coating was performed by repeatedly dipping MNs into a reservoir containing concentrated IIV formulated with a surfactant to facilitate uniform wetting of the microneedle surface and a viscosity enhancer to increase coating thickness (see Materials and Methods). An IIV-coated MN array is shown in Fig. 2A. As shown in Fig. 2B, the amount of IIV coated onto MNs increased with the number of dipping steps during the coating process (Student's t test, P < 0.01), and vaccine was efficiently coated onto MNs at levels that reached 3.3 ± 0.2 μg per array of 5 needles (i.e., 0.65 ± 0.04 μg per needle). A 2-fold increase in IIV concentration led to coating of 9.8 ± 0.5 μg of vaccine per array of 5 needles.
Fig. 2.
Coating of MN with IIV and release of vaccine into mouse skin. (A) MNs coated with IIV. (B) Dependence of the amount of IIV coated onto MNs on the number of dips during the coating process. Coating was carried out by dipping the MNs into a coating solution containing 5 mg/mL IIV as described in Materials and Methods. MNs were coated by repeating the dip-coating process 3, 6, or 9 times, and the amount of protein coated was determined after air drying. (C) Delivery rate of IIV vaccines into mouse skin in vivo by coated MNs. Immediately after removal from the skin, the MNs were immersed into 1 mL of PBS, and the amount of residual HA protein was determined by a quantitative ELISA as described in Materials and Methods. The percent delivery was calculated as (HA on unused MN − HA on MN after insertion) / (HA on unused MN). Data are presented as the mean ± SD for n = 3 independent experiments.
To investigate the delivery rate of IIV vaccines, MNs were inserted into mouse skin after anesthesia and held in place for 2 or 5 min. After removal from the skin, the MNs were immersed in PBS, and the residual amount of hemagglutinin (HA) left on MNs was determined by a quantitative ELISA. Insertion for 2 min led to delivery of 83% ± 7% of coated IIV to mouse skin, whereas insertion for 5 min resulted in delivery of 90% ± 5% of the antigen (Fig. 2C). These results show that IIV vaccines can be effectively coated onto MNs, and the coated vaccines can be rapidly delivered into the skin after an insertion period of just a few minutes.
Induction of Antibody Responses by MN Delivery of IIV Vaccines.
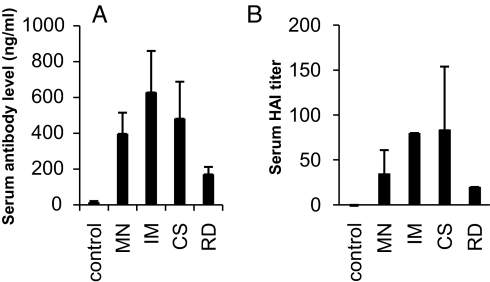
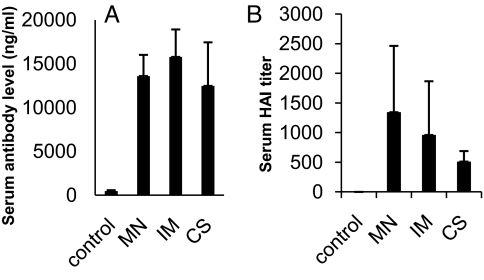
The immune responses induced by MN delivery were evaluated in comparison with those obtained by IM injection (Fig. 3). Sera were collected on day 14 after a single immunization and analyzed for antibody responses against the HA protein and their hemagglutination inhibition (HAI) titers against influenza virus. As shown in Fig. 3A, antibody responses induced by MN delivery of IIV were statistically indistinguishable from those observed for IM injection (Student's t test, P = 0.08). However, MN delivery differs from IM delivery in a number of ways. In addition to the different route of administration, MN delivery involves suspension of vaccine in a coating solution containing surfactant and viscosity enhancer. To assess the effect of this coating formulation, IIV suspended in coating solution was injected IM [coating solution group (CS)] and found to induce the same antibody responses as the MN and IM groups (P > 0.1). Another difference is that IIV is dried onto MNs, which could affect vaccine immunogenicity. To assess the effect of drying, IIV was first coated onto MN, then dissolved off in vitro and injected IM into mice [i.e., redissolved group (RD)]. This was found to induce lower antibody levels compared with the other groups (P < 0.05). This result suggests that although the MN coating process reduced IIV immunogenicity, there was a compensatory enhancement because of increased IIV immunogenicity using the ID route of administration. We further analyzed HAI titers of sera obtained by using the different immunization approaches. As shown in Fig. 3B, HAI activity was detected in all vaccinated groups, and the levels were in direct correlation with the levels of antibody response against HA as detected by ELISA.
Fig. 3.
Comparison of antibody responses induced in mice after immunization by coated MNs or IM injection of IIV vaccines. Five groups of mice (6 per group) were used in the immunization study. Group 1 mice (control) received immunization by MNs coated with 10 μg of BSA. Group 2 mice (MN) received immunization by MNs coated with 10 μg of IIV (3 arrays were used per mouse, each coated with 3.3 μg of virus). Group 3 mice (IM) received IM injection of 10 μg of IIV dissolved in PBS. Group 4 mice (CS) received IM injection of 10 μg of IIV dissolved in MN-coating solution. Group 5 mice (RD) received 10 μg of virus that was redissolved from coated MNs in PBS. Sera were collected on day 14 after immunization and analyzed for antibody responses against influenza virus. (A) Antibody responses against the influenza HA protein. The levels of antibody responses against HA were determined by ELISA using purified HA proteins as coating antigens and are expressed as the amount of HA-specific antibodies in 1 mL of serum samples (ng/mL). (B) HAI activity of sera from immunized mice. The HAI activity was determined as described in Materials and Methods and is expressed as the highest dilution that resulted in complete inhibition of hemagglutination (HAI titer). Data are presented as the mean ± SD.
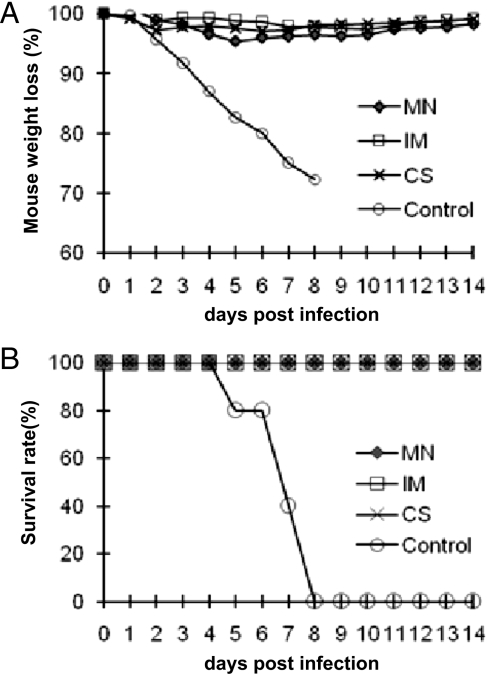
A Single MN Immunization with IIV Vaccine Protects Against Lethal Influenza Virus Challenge.
The results presented above show that MN delivery of IIV induced strong antibody responses with significant HAI titers after a single immunization. Based on these results, we further tested whether these mice would be protected against lethal challenge by influenza virus. At 4 weeks after immunization, mice were challenged with 100× LD50 of mouse-adapted influenza virus A/PR/8/34. As shown in Fig. 4A, although all of the control group mice succumbed to challenge and were killed between days 5 and 8 after challenge, all mice immunized by IIV-coated MNs survived the challenge, as did those immunized by IM injection. Further, as shown in Fig. 4B, no significant differences in weight loss were observed after challenge for mice that were vaccinated by IIV-coated MNs or by IM injection of IIV.
Fig. 4.
Protection of immunized mice against a high-dose lethal challenge. At 4 weeks after immunization, mice were challenged by intranasal instillation of 100× LD50 of mouse-adapted influenza virus A/PR/8/34. Mice were monitored daily after challenge and were killed when found to display severe signs of illness or loss of more than 25% body weight in accordance with Institutional Animal Care and Use Committee guidelines. Groups of 6 mice were immunized by MNs coated with 10 μg of BSA (control), MNs coated with 10 μg of IIV (MN), IM injection of 10 μg of IIV dissolved in PBS (IM), or IM injection of 10 μg of IIV dissolved in coating solution (CS). (A) Survival rate of each group of mice after challenge. (B) Percentage change of body weight in each group of mice after challenge.
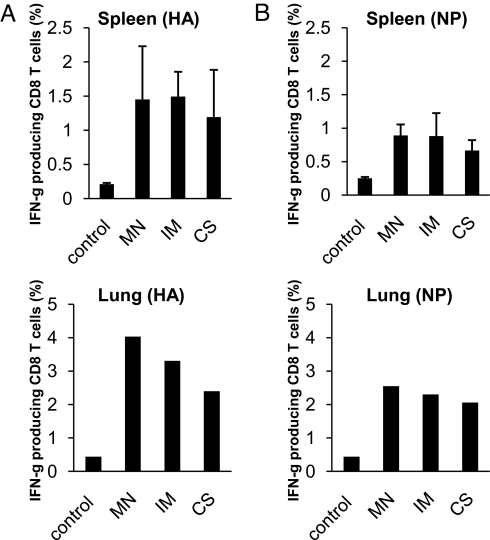
Mice that survived the challenge were killed on day 14 after infection, and the levels of antibody and T cell responses induced after challenge were determined for comparison. As shown in Fig. 5, antibody responses against the HA protein as well as HAI titers were boosted in all vaccinated groups to similar levels. CD8+ T cell responses against viral antigens are more effectively induced by antigens produced during virus infection, and their levels may reflect the levels of virus replication after challenge. Therefore, we also collected spleens and lungs at the time of sacrifice to prepare lymphocytes and analyze CD8+ T cell responses against known epitopes in the influenza virus HA and nucleoprotein (NP). As shown in Fig. 6, the levels of IFN-γ-producing CD8+ T cells stimulated by peptides corresponding to epitopes in the influenza virus HA (Fig. 6A) or NP (Fig. 6B) proteins were similar in both splenocytes and lung lymphocytes between IIV-coated MN delivery and IM injection. These results further demonstrate that immunization by IIV-coated MNs results in similar postchallenge parameters when compared with those induced by conventional IM injection of the same vaccine dose.
Fig. 5.
Comparison of antibody responses induced in mice after challenge. On day 14 after challenge, blood samples were collected and analyzed for antibody responses against influenza virus. (A) Antibody responses against the influenza HA protein. (B) HAI activity of sera from challenged mice. Data are presented as the mean ± SD for n = 6 mice per group.
Fig. 6.
Comparison of CD8+ T cell responses induced in mice after challenge. On day 14 after challenge, surviving mice were killed, and spleens and lungs were collected. After preparation, splenocytes and lung lymphocytes were stimulated with peptides corresponding to known CD8+ T cell epitopes in HA or NP proteins for 6 h in the presence of Brefeldin A, and then they were stained for cell surface CD8 and CD4 molecules as well as intracellular IFN-γ protein, followed by flow cytometry analysis. Shown is the percentage of IFN-γ-producing CD8+ T cells in splenocytes (from each individual mouse) and lung lymphocytes (pooled for each group) stimulated by a peptide corresponding to a known epitope in (A) HA or (B) NP. The controls were obtained by stimulating splenocytes or lung lymphocytes with an irrelevant peptide corresponding to an epitope in the HIV Gag protein. Data are presented as the mean ± SD for n = 6 mice per group.
Discussion
The skin serves not only as a physical barrier of the body, but also as an important part of the immune system. With abundant professional antigen-presenting cells, such as Langerhans cells and resident dermal dendritic cells, delivery of antigens to the skin has been shown to induce potent immune responses (11, 12). Although the potency of ID immunization has been recognized for decades, in practice this approach is only used with rabies and bacillus Calmette–Guérin vaccines because of the difficulty associated with the traditional ID injection technique (24, 25). Our results show that whole-IIV vaccine can be efficiently coated onto MNs and rapidly delivered into mouse skin with high efficiency. Moreover, a single immunization with IIV vaccine delivered by this approach induced strong antibody responses with significant HAI activities, and it conferred effective protection against a high-dose lethal challenge. These results provide support for further development of ID vaccination enabled by MNs.
Although ID vaccination is advantageous, it has been impractical given the difficulties of the conventional Mantoux technique of ID injection. We propose that vaccine-coated MNs offer an enabling technology to facilitate ID vaccination because of the ease of MN administration. Previous work with hollow MNs and small hypodermic needles has demonstrated vaccine delivery, but this requires expert insertion into the skin and attachment to a syringe or other infusion device (15, 26, 27). Uncoated, solid MNs have been used to pierce or scrape skin to increase its permeability, but this typically involves a cumbersome 2-step process to pretreat the skin and then apply a topical patch for delivery into the skin, which often leaves most of the active component on the skin surface (28, 29). Coated MNs have been used previously to deliver ovalbumin and to facilitate ID electroporation of a DNA plasmid (22, 30). In the present study, we have shown that a vaccine consisting of large lipid-enveloped IIV particles can be coated on MNs and is efficiently delivered to mouse skin within minutes to generate a robust immune response. This is significant because ID delivery using MNs is expected to be advantageous for influenza vaccine administration. In particular, coated MNs are differentiated from hypodermic needles and other MN approaches in that (i) they directly lend themselves to a patch-like format for simple, rapid administration, possibly by patients themselves, and (ii) they introduce vaccine in a dry state that does not require reconstitution, which further simplifies administration and may increase vaccine stability. Overall, the present results demonstrate that coated MNs offer a simple method for ID vaccine delivery that represents a significant advance over previous approaches.
In evaluating immune responses, we observed that delivery of BSA by coated MNs induced significantly higher levels of antibody responses compared with IM injection of the same antigen dose. This demonstrates the superior immunogenicity achieved by ID delivery of soluble protein antigens using coated MNs, consistent with previous results using ovalbumin (22). In the present studies with a particulate viral antigen, MN delivery of IIV vaccine induced significant levels of antibody responses that exhibited strong HAI activity. Of note, IIV vaccine dry-coated and dissolved off MNs and then delivered by IM injection (the RD group) had reduced antibody levels compared with the MN group. Thus, similar to soluble proteins, such as ovalbumin and BSA, MN delivery of particulate IIV vaccines was also more effective than IM injection when the vaccines were similarly processed. On the other hand, MN- and IM-vaccinated groups had similar levels of antibody responses against HA. It is possible that the immunogenicity of particulate IIV vaccines may be more sensitive to effects of the coating process compared with soluble protein antigens, and optimization of the coating formulation and process should further improve the efficacy of IIV vaccines delivered by this approach.
MN immunization conferred complete protection against lethal challenge by a high dose of homologous mouse-adapted influenza virus, with no visible signs of disease and little weight loss after infection, similar to the result with IM injection of unprocessed vaccine. Further, both antibody responses and CD8+ T cell responses in the lung and spleen were at similar levels between these groups after challenge. These results provide evidence that the immune responses induced by MN delivery of influenza vaccines into the skin are as effective as those induced by conventional IM injection in protection against influenza virus infection.
We also investigated antibody responses to other viral proteins, and the results showed that antibodies induced by immunization with coated MNs or IM injection recognized similar viral proteins (Fig. S1). However, antibodies induced by IM injection were found to exhibit enhanced reactivity to internal viral proteins, such as NP and M1, compared with antibodies induced by coated MNs. The ability of coated MNs to elicit strong responses to HA, the main target of neutralizing antibodies, is of particular interest for vaccination against influenza virus infection. We further compared antibody responses against a heterologous influenza virus strain (A/WSN, H1N1), which differs from the A/PR8 strain by about 8% in HA sequence. The results showed that antibodies against the A/WSN strain were detected in all vaccinated mice but at low levels (Fig. S2). Immunization by IM injection induced significantly higher levels of antibody responses against the heterologous A/WSN virus than coated MNs, probably because of the enhanced responses against internal viral proteins, such as NP and M1 proteins, as shown in Fig. S1. In support of this, no significant HAI activity against the A/WSN strain was detected in any group of mice. Therefore, although delivery of IIV vaccines by the coated MN technology is as efficacious as conventional IM injection, further development is needed to enhance the breadth of protection against infection by a heterologous influenza virus.
A number of alternative strategies to deliver vaccines into the skin are under investigation, including liquid and particulate jet injection, electroporation, microseeding, and topical application (31). Recently, hollow microneedles have been evaluated to inject liquid IIV vaccines into the skin and have been shown to induce immune responses comparable to IM injection in clinical studies (15, 17, 27). However, such approaches still require the use of syringes by clinical personnel, and the vaccine dose may be limited by the reduced volume used in intradermal injection. In comparison with these approaches, a coated MN offers several potential advantages. First, it is painless and does not look like a hypodermic needle and syringe to patients (32). This should reduce fear of needles and thus increase vaccine coverage. Second, it is easy to use, without the need for an additional device or power supply, and the coated vaccines are rapidly delivered into the skin. Thus, administering a vaccine by coated MNs should require only minimally trained personnel or possible self-administration by patients. Vaccine patches can be prepared with an adhesive to facilitate sticking to the skin for the few minutes needed for vaccine dissolution from the MNs (23). Combined with an expected manufacturing cost in mass production similar to hypodermic needles (21), this should significantly reduce the cost and increase the speed of vaccination. Third, coated MNs are very small in size compared with hypodermic needles, and thus should reduce the storage and shipping volume as well as the amount of sharp and biohazardous waste in clinics. Vaccines for potential H5N1 influenza pandemic strains may require significantly larger doses compared with seasonal influenza vaccines (33). By using coated MNs, a larger vaccine dose can be achieved by increasing the array size to include more needles per array. In conclusion, we propose that further improvement of coated MNs through optimization of coating formulation and patch design can lead to the development of a vaccine delivery technology that is efficacious, easy to use, cost-effective, and widely acceptable to the public.
Materials and Methods
Influenza Virus Vaccines.
Influenza virus A/Puerto Rico/8/34 (H1N1) was grown in chicken eggs, purified by centrifugation through a sucrose gradient, and then inactivated by formalin (0.04%), followed by dialysis to remove excess formalin. Inactivated virus was resuspended in PBS and stored at −80 °C until use (34).
MN Fabrication and Antigen Coating.
MNs were fabricated from stainless steel sheets (Trinity Brand Industries, SS 304, 75 μm thick; McMaster–Carr) and then treated by electropolishing using methods described previously (23). MNs used in this study were prepared as individual rows of 5 needles, as shown in Fig. 1A. The needle has the geometry of a pointed tip on an elongated shaft that is 500 μm in length, 50 mm in thickness, and 200 mm in width at the base.
Coating of MNs with BSA was carried out by dip coating as described previously (35). Coating of MNs with IIV, a particulate antigen, was carried out by using a similar dip-coating process with slight modifications and a specially formulated coating solution. Briefly, the vaccine was first concentrated to 10 mg/mL by microfiltration using 300-kDa cutoff filters (Vivaspin 500; Sartorius Stedim Biotech) and then suspended in a final coating solution composed of 1% (wt/vol) carboxymethylcellulose sodium salt (low viscosity, US Pharmacopoeia grade; Carbo-Mer), 0.5% (wt/vol) Lutrol F-68 NF (BASF Bioresearch), and 5 mg/mL vaccine. The coating-solution reservoir consisted of 2 laminated parts: the “bottom plate” and the “cover plate,” both of which were made of polymethylmethacrylate (McMaster–Carr). The cover plate had 7 holes: 5 holes (400-μm diameter) drilled into it in the same positions as the row of microneedles and 2 larger holes (1.5-mm diameter) at either end of a feeding channel. Coating solution (40 μL) was filled into the channel, and coating was performed manually, with the dipping process monitored by a video camera attached to a microscope (SZX16; Olympus America). MNs were dipped 9 times, with a delay of 30 s between dips to allow for partial drying. Control over the length of the microneedle shaft to be coated was exercised manually by using an X-micropositioner (Velmex). To measure the amount of vaccine coated per row of MNs, the coating on 3 rows from each batch of coated MNs was dissolved into 200 μL of PBS. The protein concentration eluted in the solution was determined by a BCA protein assay (Pierce Biotechnology), and the amount of vaccine antigen coated onto each row of MNs was calculated.
Immunization, Blood Sample Collection, and Challenge of Mice.
Female BALB/c mice (6–8 weeks old) were obtained from Charles River Laboratories and housed at the Emory University animal facility in microisolator cages. For immunization by IM injection, 10 μg of inactivated influenza virus dissolved in 100 μL of PBS was injected into both side mouse quadriceps (50 μL per side). For immunization by coated MNs, the hair on the back of the mouse skin was removed 3 days before vaccination by application of depilatory cream (Nair) and gentle washing with 70% alcohol. Under anesthesia by isofluorane inhalation, the mouse back skin was lightly stretched by hand, and coated MNs were pressed into the skin and held in position for 5 min. On day 14 after immunization, blood samples were collected by retroorbital bleeding, heat-inactivated, and stored at −80 °C until further analysis.
Live influenza virus challenge was conducted in a BSL2+ animal facility. Four weeks after vaccination, mice were challenged under light anesthesia by intranasal instillation with 100× LD50 of mouse-adapted influenza virus A/PR/8/34 diluted in 50 μL of PBS. After challenge, mice were monitored for weight loss and signs of illness on a daily basis. Mice that exhibited severe signs of illness and significant weight loss (>25%) were killed in accordance with Institutional Animal Care and Use Committee guidelines. At the end of the challenge study, mice that survived the challenge were killed to collect blood samples as well as spleens and lungs for analysis of antibody and T cell responses.
ELISA.
Influenza HA-specific antibodies were measured in individual mouse sera by an ELISA following established protocols (36). Purified His-tagged HA proteins were used as coating antigens, and a standard curve was constructed by coating each ELISA plate with serial 2-fold dilutions of purified mouse IgG with known concentration. The concentrations of influenza HA-specific antibodies in serum samples were calculated by using the obtained standard curves and expressed as the amount of HA-specific antibody in 1 mL of serum samples (ng/mL).
HAI Assay.
The HAI assay was performed following established protocols (37). Briefly, mouse sera were heat-inactivated at 56 °C for 1 h and then treated with receptor-destroying enzyme (Kenka Seiken) at 37 °C overnight according to the manufacturer's instructions. After treatment, 25-μL aliquots of 2-fold serially diluted serum samples were incubated with 25 μL of virus containing 4 HA units of influenza virus A/PR/8/34 at 37 °C for 1 h, followed by incubation with 50 μL of 0.5% chicken red blood cells (LAMPIRE Biological Laboratories) at 25 °C for 45 min. The HAI titer was defined as the reciprocal of the highest serum dilution that inhibited hemagglutination.
Intracellular Cytokine Staining and Flow Cytometry.
Mice that survived the challenge were killed on day 14 after challenge to prepare splenocytes following established protocols (38). At the time of sacrifice, lungs were first perfused with 5 mL of PBS plus heparin (5 μg/mL), and then lung tissues were collected for preparation of lymphocytes. Lung lymphocytes (pooled for each group) were also prepared by treatment of lung tissues with 100 U/mL collagenase IV (Worthington Biochemical Corporation) for 1 h at 37 °C under vigorous shaking, followed by pelleting and purification through a discontinuous Ficoll gradient. Lymphocytes were stimulated with a peptide corresponding to a CD8+ T cell epitope for the influenza virus A/PR/8/34 HA or NP proteins (IYSTVASSL and TYQRTRALV, respectively, synthesized at the Emory Microchemical Facility, Atlanta, GA) or an irrelevant peptide corresponding to a segment in the HIV Gag protein (AMQMLKETI, negative control) at 10 μg/mL for 6 h in presence of 10 μg/mL Brefeldin A (Sigma), and CD8+ T cell responses were determined by intracellular cytokine staining and analyzed by flow cytometry on a BD FACSCalibur with CELLQuest software (Becton Dickinson) as described previously (38).
Supplementary Material
Acknowledgments.
We thank Ms. Erin-Joi Collins for excellent assistance with manuscript preparation and Dr. Mark Allen for the use of laser fabrication facilities. This work was supported in part by National Institutes of Health Grants AI074579 and EB006369 and carried out in part at the Center for Drug Design, Development, and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology.
Footnotes
Conflict of interest statement: M.R.P. and H.S.G. are inventors on patents on microneedles, some of which have been licensed. M.R.P. also serves as a consultant or advisory board member for companies working on microneedles. Because the microneedle system reported here is different from microneedle products under development, the information in this manuscript is only indirectly related to those products.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812652106/DCSupplemental.
References
- 1.Dushoff J, Plotkin JB, Viboud C, Earn DJ, Simonsen L. Mortality due to influenza in the United States–an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163:181–187. doi: 10.1093/aje/kwj024. [DOI] [PubMed] [Google Scholar]
- 2.Osterholm MT. Preparing for the next pandemic. N Engl J Med. 2005;352:1839–1842. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- 3.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;2:CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Fiore AE, et al. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 5.Smedley J, et al. Influenza immunisation: attitudes and beliefs of UK healthcare workers. Occup Environ Med. 2007;64:223–227. doi: 10.1136/oem.2005.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canning HS, Phillips J, Allsup S. Health care worker beliefs about influenza vaccine and reasons for non-vaccination–a cross-sectional survey. J Clin Nurs. 2005;14:922–925. doi: 10.1111/j.1365-2702.2005.01190.x. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy MW, Kockler DR. Trivalent intranasal influenza vaccine, live. Ann Pharmacother. 2004;38:2086–2093. doi: 10.1345/aph.1E191. [DOI] [PubMed] [Google Scholar]
- 8.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003;21:3212–3218. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 10.Wee JL, et al. Pulmonary delivery of ISCOMATRIX influenza vaccine induces both systemic and mucosal immunity with antigen dose sparing. Mucosal Immunol. 2008;1:489–496. doi: 10.1038/mi.2008.59. [DOI] [PubMed] [Google Scholar]
- 11.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat Rev. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glenn GM, et al. Transcutaneous immunization and immunostimulant strategies: Capitalizing on the immunocompetence of the skin. Expert Rev Vaccines. 2003;2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 13.Baxby D. Smallpox vaccination techniques; from knives and forks to needles and pins. Vaccine. 2002;20:2140–2149. doi: 10.1016/s0264-410x(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 14.Belshe RB, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 15.Belshe RB, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–6763. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 17.Holland D, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 18.Thompson WW, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. J Am Med Assoc. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 19.Howard A, Mercer P, Nataraj HC, Kang BC. Bevel-down superior to bevel-up in intradermal skin testing. Ann Allergy Asthma Immunol. 1997;78:594–596. doi: 10.1016/s1081-1206(10)63222-x. [DOI] [PubMed] [Google Scholar]
- 20.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. In: Compans RW, Orenstein WA, editors. Current Topics in Microbiology & Immunology: Vaccines for Pandemic Influenza. Vol 333. Berlin: Springer; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widera G, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies. 1996 http://libdoc.who.int/hql1996/WHO.EMC.ZOO.96.6.pdf.
- 25.World Health Organization. BCG vaccine. WHO position paper. Wkly Epidemiol Rec. 2004;79:27–38. [PubMed] [Google Scholar]
- 26.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14:375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Damme P, et al. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 28.Wermeling DP, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci USA. 2008;105:2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikszta JA, et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 30.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitragotri S. Immunization without needles. Nat Rev. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 32.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 34.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74:4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, et al. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology. 2009;383:12–21. doi: 10.1016/j.virol.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge J, et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J Virol. 2007;81:150–158. doi: 10.1128/JVI.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye L, et al. Surface stability and immunogenicity of the human immunodeficiency virus envelope glycoprotein: role of the cytoplasmic domain. J Virol. 2004;78:13409–13419. doi: 10.1128/JVI.78.24.13409-13419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.