Abstract
The production, consumption, and exploitation of common resources ranging from extracellular products in microorganisms to global issues of climate change refer to public goods interactions. Individuals can cooperate and sustain common resources at some cost or defect and exploit the resources without contributing. This generates a conflict of interest, which characterizes social dilemmas: Individual selection favors defectors, but for the community, it is best if everybody cooperates. Traditional models of public goods do not take into account that benefits of the common resource enable cooperators to maintain higher population densities. This leads to a natural feedback between population dynamics and interaction group sizes as captured by “ecological public goods.” Here, we show that the spatial evolutionary dynamics of ecological public goods in “selection-diffusion” systems promotes cooperation based on different types of pattern formation processes. In spatial settings, individuals can migrate (diffuse) to populate new territories. Slow diffusion of cooperators fosters aggregation in highly productive patches (activation), whereas fast diffusion enables defectors to readily locate and exploit these patches (inhibition). These antagonistic forces promote coexistence of cooperators and defectors in static or dynamic patterns, including spatial chaos of ever-changing configurations. The local environment of cooperators and defectors is shaped by the production or consumption of common resources. Hence, diffusion-induced self-organization into spatial patterns not only enhances cooperation but also provides simple mechanisms for the spontaneous generation of habitat diversity, which denotes a crucial determinant of the viability of ecological systems.
Keywords: cooperation, evolutionary game theory, pattern formation, population dynamics
Spontaneous and complex pattern formation represents a common principle in physical, chemical, and biological systems ranging from hydrodynamical phenomena such as the ripples in the sand, to interacting chemical fronts (1), and the coloration of plants and animals such as the spots of the jaguar (2, 3), or vegetation patterns in arid ecosystems (4). Mathematical models of pattern formation use either cellular automata (5) or differential equations (6). Here, we show that similar types of patterns emerge in evolutionary settings of social dilemmas (7, 8). Social dilemmas are characterized by a conflict of interest between individuals and the group. Such conflicts arise in humans (9) and microorganisms alike (10–12). In antibiotic resistance, for example, bacteria secrete an enzyme that prevents cell wall degradation (11, 13, 14). Synthesizing the enzyme is costly to the bacterium, and releasing the enzyme creates a public good by protecting not only the bacterium itself but also the surrounding bacteria. Thus, enzyme production represents an act of cooperation that is prone to exploitation by mutant strains, which synthesize fewer or no enzymes. Such situations are captured by public-goods games (7, 15).
In a typical public-goods experiment, N individuals have the opportunity to cooperate and invest a fixed amount, c, into a common pool or to defect and invest nothing. The total investment is multiplied by a factor, r > 1, and distributed equally among all participants—irrespective of whether they have invested or not. Thus, every invested unit returns rc/N units to the investor (as well as to all other participants). Consequently, for r < N, rational players withhold their investments—but if all participants reason in this manner, the group foregoes the benefits of the public good, and no one receives anything. In contrast, had everybody cooperated, they would have been better off with a return of (r − 1)c. Conversely, if r > N rational players invest in the public good (7) because, even with only a single investor, the return from the public goods exceeds the investment (rc/N > c).
In populations containing a fraction, u, of cooperators and a fraction, v = 1 − u, of defectors, the evolutionary dynamics can be described by the replicator equation (16): u̇ = u(1 − u)(gC − gD). The fitness of cooperators and defectors, gC, gD, is determined by their performance in public-goods interactions in groups of size N that are randomly formed according to binomial sampling. The average number of cooperators among the N − 1 interaction partners is u(N − 1). Therefore, the average payoff of defectors is gD = u(N − 1)rc/N, and of cooperators it is gC = gD + rc/N − c. Thus, rc/N − c measures the effective cost of cooperation. For r < N, defectors win. For r > N, cooperators win. However, the replicator dynamics neglects the fact that cooperator populations have a higher productivity than defector populations. This should be reflected in the natural assumption that cooperators are capable of maintaining higher population densities than defectors.
Model and Results
Ecological public goods allow the population density u + v to fluctuate (17). This extension establishes a link between ecological and evolutionary dynamics. Variable population densities not only introduce feedbacks between reproductive success and carrying capacity but also affect the effective group size, S, in public-goods interactions. With u + v ≤ 1, the densities u, v can be interpreted in terms of probabilities when attempting to form interaction groups with N participants [see supporting information (SI) Text]. In particular, each binomial sampling trial fails with probability w = 1 − u − v, such that the average interaction group size is reduced to S = (u + v)N. For r < N, this results in an interesting feedback, which enables cooperators and defectors to coexist: If population densities are high, interaction groups are large (S > r) and defectors increase, but this reduces the return from the public good and results in a decline of the population density. Decreasing population densities lead to smaller interaction groups until eventually S < r holds, and cooperators thrive. This restores high population densities, and the cycle continues. Similar feedback mechanisms have been discussed in the complementary context of competitive interactions (18).
Unstructured Populations.
The performance of cooperators and defectors in ecological public goods, fC, fD (see SI Text), depends not only on the composition of the interaction groups but also on their size. Moreover, increasing population density exerts competitive pressure on the rates of reproduction, and the evolutionary dynamics become:
Cooperators and defectors have an equal and constant per capita death rate, d, and a per capita birth rate of w(fC + b) and w(fD + b), which decreases for increasing population densities. The baseline birthrate, b, ensures fC + b ≥ 0 because fC can become negative in the limit v → 1, which is not meaningful (17).
Hereafter, we assume that the death rate exceeds the baseline birth rate (d > b), such that defectors cannot survive in the absence of cooperators. However, cooperators and defectors can coexist at an equilibrium, Q, or exhibit rich population dynamics depending on r (17). In particular, Q changes stability through a Hopf bifurcation at rHopf. For r > rHopf coexistence in Q is stable but the basin of attraction limited. For unfavorable initial configurations, the returns from the public good are insufficient to offset the death rate, d, and the population goes extinct: At low densities, too few public-goods interactions occur, or, if defectors abound, exploitation irrecoverably diminishes the public resource. Generally, for r < rHopf, the equilibrium Q is unstable, and the population cannot survive (17).
Spatial Dynamics.
To consider effects of spatial extension on the dynamics of ecological public goods, we introduce diffusive migration in 2 dimensions (19):
The functions u, v denote the density of cooperators and defectors at location (x,y) and time t. As before, w = 1 − u − v determines the negative feedback between population density and birth rates. The diffusion constants DC and DD specify the migration rates for cooperators and defectors and ∇2 denotes the diffusion operator. This continuous spatial extension reveals a fascinating world of dynamical pattern formation in ecological public goods [see Fig. 1, Fig. S1, Movie S1, Movie S2, Movie S3, and Movie S4, and interactive online tutorials Hauert C (2009) Virtuallabs: Interactive tutorials on evolutionary game theory, www.univie.ac.at/virtuallabs].
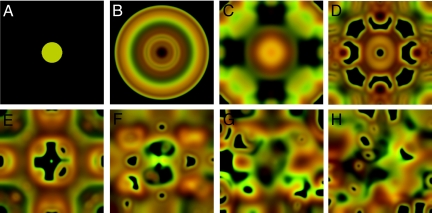
Fig. 1.
Chaotic pattern formation in spatial ecological public goods. A sequence of snapshots A–H demonstrates the spatial density distribution of cooperators (green) and defectors (red) over time (see Movie S1). The symmetry of the initial configuration A should be preserved in a deterministic system, but after some time it breaks down and disappears because of limitations of the numerical integration of Eq. 2. The exponential amplification of arbitrarily small disturbances characterizes chaotic systems. The initial configuration is a vacant L × L square (L = 400) with no flux boundaries and a homogeneous disk with radius L/10 in the center, where cooperators and defectors coexist at equal density (udisk = vdisk = 0.1 and u = v = 0 elsewhere). The parameters of the ecological public goods are N = 8, d = 1.2, b = 1, r = 2.34, c = 1. The multiplication factor r lies slightly below the Hopf bifurcation rHopf = 2.3658, such that the fixed point Q is unstable, and in the absence of space, the population disappears. Diffusion of defectors is twice that of cooperators (DC = 1, DD = 2). The color brightness indicates the density of cooperators (green) and defectors (red). The snapshots are taken at times t = 0 (A), 1,200 (B), 1,800 (C), 2,000 (D), 2,200 (E), 2,600 (F), 2,800 (G), 4,000 (H). The numerical integration uses a spatial grid with dx = 0.8 and step size dt = 0.01.
Spatial patterns unfold if defectors diffuse (migrate) faster than cooperators, DD ≥ DC. Consequently, the dominant effect of spatial dynamics is not cooperators outrunning defectors but, instead, the defectors' relentless search of productive patches. Slow migration facilitates aggregation of cooperators, whereas fast migration supports defectors to readily locate cooperator patches, but it also impedes their ability to exploit one particular patch.
The equilibrium Q of Eq. 1 translates into a trivial solution of Eq. 2 in the form of a spatially homogeneous strategy distribution with densities according to Q. However, if Q is stable, this does not necessarily imply the stability of the corresponding homogeneous state. In the vicinity of Q, Eq. 2 takes on the form of an activator–inhibitor system. Any deviation from the equilibrium is amplified by cooperators (activators) but suppressed by defectors (inhibitors). For DD > DC, these antagonistic forces may give rise to the formation of complex patterns (Turing instability) (3, 6) (see Fig. 2A). Any small local disturbance propagates through the system and induces stable heterogeneous strategy distributions (see SI Text, Fig. S2, and Movie S3). Local disturbances may give rise to rearrangements of the patterns but then quickly relax into another qualitatively indistinguishable distribution of cooperators and defectors.
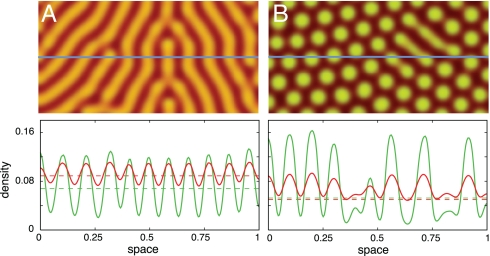
Fig. 2.
Typical stationary patterns for diffusion-induced instability (Turing patterns) (A) and diffusion induced coexistence (B) on an L × L square (no flux boundaries). The density variation of cooperators (green) and defectors (red) is shown along a cross-section (solid blue line). (A) In the absence of space, cooperators and defectors coexist for suitable initial configurations (r = 2.4 > rHopf = 2.3658). Diffusion destabilizes the spatially homogeneous state and induces stable and static heterogeneous strategy distributions, where individuals spontaneously aggregate in spots or striped patterns. (B) In the absence of space, the population goes extinct (r = 2.24 < rHopf). Diffusion stabilizes persistence of the population and coexistence of cooperators and defectors by inducing heterogeneous strategy distributions. In both scenarios, the parameters of the ecological public goods are n = 8, c = 1, d = 1.2, b = 1, DC = 1, DD = 10, with an initial configuration where densities are randomly drawn in [0, 0.1]. Numerical integration is performed on a spatial grid with L = 283, dx = 1.4, and a step size of dt = 0.1. The brightness of the colors indicates the strategy densities (Upper) and the dashed horizontal lines mark the densities at Q (Lower).
Conversely, if Q is unstable, then spatial extension and migration often prevents extinction and stabilizes coexistence of cooperators and defectors either in static spots and stripes similar to Turing patterns (see Fig. 2B) or in chaotic dynamics of ever-changing patterns (see Fig. 1 and Movie S1, Movie S2, and Movie S4). In general, a homogeneous population near Q exhibits periodic density oscillations of increasing amplitude that eventually result in extinction. However, any small local disturbance can propagate through space and trigger stationary, heterogeneous strategy distributions (see Fig. 2B). The pattern formation is again driven by the opposing forces of cooperators (activators) and defectors (inhibitors). However, the activator–inhibitor system develops in the vicinity of an unstable fixed point (see SI Text, Fig. S3, and Movie S4), which could be termed “diffusion-induced coexistence” in contrast to the classical “diffusion-induced instability” of Turing patterns. Also note that, whereas Turing patterns rely on substantial differences in the diffusion constants of activators and inhibitors (see SI Text and Fig. S1), this does not apply to diffusion-induced coexistence, where dynamic patterns emerge even for DD = DC (see Fig. 3).
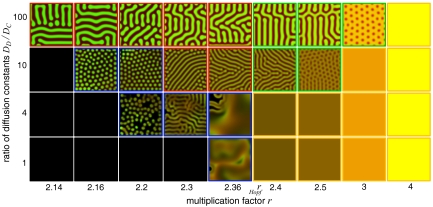
Fig. 3.
Diversity of spatial distributions in terms of the ratio of the diffusion of defectors to cooperators DD/DC and the multiplication factor r in spatial ecological public-goods games. The brightness of the colors indicates the density of cooperators (green) and defectors (red). In the absence of space, the population survives for r > rHopf. If cooperator diffusion exceeds defector diffusion, DD/DC ≤ 1, the dynamics is barely affected by space, except for a small chaotic region (blue frame) near rHopf. The dynamics becomes much richer for DD/DC > 1. For r < rHopf, the chaotic regime increases (blue frame) and is replaced by diffusion induced coexistence patterns for high DD/DC (red frame). For r > rHopf, the homogeneous spatial distributions (orange frame) are replaced by diffusion-induced instability (Turing patterns; green frame) for high DD/DC. For very large r, all patterns disappear. The parameters are N = 8, c = 1, d = 1.2, b = 1, DC = 1, L = 283, dx = 1.4, dt = 0.1 (rHopf = 2.3658) and an initial configuration with random cooperator and defector densities in [0, 0.1]. For a detailed phase plane diagram and other initial configurations see SI Text and Figs. S1 and S5.
Discussion
In ecology, Turing patterns occur in spatial predator–prey models (20). Particularly rich dynamics develop in the vicinity of Turing–Hopf bifurcations (3, 21), as in the present case. Turing instabilities also occur in replicator–diffusion systems, but require at least 3 different strategic types (22). Two types are sufficient, however, if population densities can vary across space (23). In ecological public goods, cooperators and defectors suffice to produce rich spatiotemporal dynamics not only in the form of Turing patterns but also through diffusion-induced coexistence or deterministic chaos. Spatial dimensions generally support cooperation (24), and here, this holds in 3 different ways. First, the ratio of cooperators versus defectors is altered in favor of cooperators, which leads to an increase of the overall population density (see Fig. 4). Second, the robustness with respect to variations in the initial configuration is improved such that coexistence is much more easily achieved in spatial settings (see SI Text and Figs. S4–S6). Finally, and most importantly, spatial pattern formation based on diffusion-induced coexistence substantially extends the parameter range for the persistence of cooperators to settings where, otherwise, defectors would drive the population to extinction (see Figs. 3 and 4 for r < rHopf).
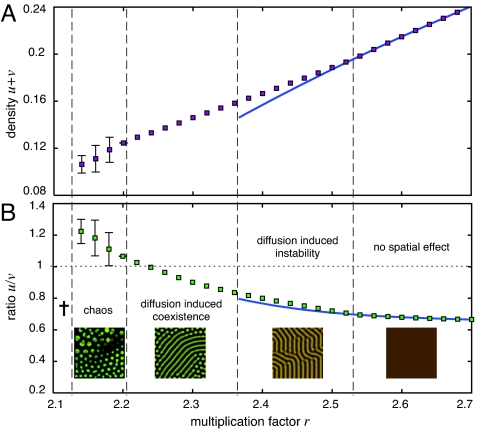
Fig. 4.
Average global population density (A) and ratio of cooperators to defectors (B) as a function of the multiplication factor r in ecological public-goods interactions. The nonspatial stable equilibrium (solid lines; requires r > rHopf) is shown together with numerical results for the stationary spatial distributions (dots). The chaotic regime depicts the average and standard deviation of the time series from t = 5,000 to 10,000 with dt = 0.1, dx = 1.4 and L = 283. For small r, the population goes extinct, but for increasing r, the population persists and exhibits chaotic dynamics that change into quasistatic and static patterns emerging through diffusion-induced coexistence (r < rHopf). For r > rHopf, static patterns are triggered by diffusion-induced instability (Turing patterns) and relax into spatially homogeneous coexistence for high r. Diffusion supports cooperation by significantly increasing the persistence region of the population and in the chaotic regime, cooperator densities even exceed those of defectors. Snapshots illustrate typical patterns emerging in the different dynamical regimes. The brightness of the colors indicates the density of cooperators (green) and defectors (red). The parameters for the ecological public goods game are N = 8, c = 1, d = 1.2, b = 1, DC = 1, DD = 10 such that rHopf = 2.3658.
Spatial games are traditionally based on 2 distinct types of discrete models that differ significantly from our approach. One type considers single individuals located in each site of a lattice or general network (25–28). Interactions are limited to a local neighborhood, and individuals are sedentary. Note that even small migration rates would severely challenge cooperation in these models. The other type of model considers metapopulations that consist of patches that are arranged on a lattice. Patches are linked to neighboring patches through migrating individuals (29–33). In the case of predator–prey interactions, this corresponds to a system of coupled oscillators that can exhibit highly complex dynamics (29–31). Our model represents a continuum limit of this second type of models, applied to the problem of cooperation.
The formulation in terms of continuous space based on partial differential equations (PDE) allows for a deeper understanding of the relevant mechanisms that drive the spontaneous generation of spatial heterogeneity and temporal fluctuations, which are ultimately responsible for supporting cooperation. Additionally, PDEs allow one to derive macroscopic quantities such as the relevant scale as well as the threshold for the onset of patterns emerging through Turing instabilities—features that are only empirically accessible in lattice models.
In our model, the local variability of the population density is a crucial feature combined with differences in migration rates of cooperators and defectors. The feedback between population density and interaction group size introduces a coupling of ecological and evolutionary dynamics, which enables cooperators to survive in well-mixed models (17). Fixing population densities at constant levels eliminates cooperation in both well-mixed and continuous spatial settings. In contrast to lattice models, continuous spatial extension alone is not enough to avert the extinction of cooperators, because diffusion prevents spatial segregation of cooperators and defectors, and hence, cooperators are unable to prevent exploitation by forming clusters. It is not merely the spatial dimensions but the emerging spatial heterogeneity that enables cooperators to persist at higher densities, or even more importantly, to survive under conditions that would inevitably result in the demise of the population otherwise.
Increased migration rates of defectors are motivated by the fact that defectors deplete common resources (or are unable to sustain them), and this causes them to move elsewhere. Conversely, migration rates of cooperators should be lower to enable them to take advantage of the locally sustained common resource.
In the context of antibiotic resistance, our results suggest that in spatial settings, susceptible free-riding strains (defectors) with high motility may actually increase the chances of survival as well as the density of the enzyme-producing, resistant strains (cooperators) and thereby aggravate the challenges for medical treatments. An interesting twist is introduced in microbial populations where migration rates or cell motility itself is linked to public-goods interactions. Examples include collective lubricant production in Paenibacillus to move across hard surfaces (34), the creation of an extracellular fibril matrix in Myxococcus to allow for cooperative swarming (12), or the formation of biofilms in Pseudomonas to gain competitive advantages in accessing limiting resources such as oxygen (10, 35). Particularly intriguing pattern formations are observed in Escherichia coli (36, 37). The chemotactic response to excreted aspartate amplifies spatial heterogeneity and enables cells to efficiently exploit available nutrients at the cost of producing the chemical signal. In the wake of the swarming cells, aggregates of sessile cells form. Cell aggregation may alleviate oxidative stress. Hence, in response to 2 common resources, nutrients and oxygen, E. coli strains diversify into 2 types with different motility (37).
Spatial heterogeneity supports coexistence (29, 30). In ecology, the most prominent example of pattern formation refers to the emergence of spatial vegetation patterns, which are usually associated with the risk of impending desertification (4, 38) because they indicate the ecosystems' bistability (39). Actually, the patterns stabilize the vegetated state, but environmental changes (absence of precipitation, overgrazing) may induce catastrophic shifts toward the desert state. In ecological public goods, similarly sharp transitions to extinction or homogeneous states are triggered by extreme external changes affecting the yield of the public resource.
Individuals consuming common resources, such as in Hardin's Tragedy of the commons (8), or producing common resources may alter and shape their environment in an enduring manner. This is particularly evident in microbial systems involving extracellular products such as in antibiotic resistance (11, 13, 14), biofilms (10), or swarming (12) and represent crucial determinants of microbial ecology (40). Spatial ecological public goods model concurrent spontaneous habitat diversification and species coexistence and hence suggest a mechanism to promote biodiversity (30, 41).
Supplementary Material
Acknowledgments.
J.Y.W. acknowledges support from Japanese Science Foundation Grant JSPS.KAKENHI 18-09976. M.A.N. and C.H. acknowledge support from the John Templeton Foundation and the National Science Foundation/National Institutes of Health (NIH) joint program in mathematical biology (NIH Grant R01GM078986). C.H. acknowledges support from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812644106/DCSupplemental.
References
- 1.Lee KJ, McCormick WD, Ouyang Q, Swinney HL. Patterns formation by interacting chemical fronts. Science. 1993;261:192–194. doi: 10.1126/science.261.5118.192. [DOI] [PubMed] [Google Scholar]
- 2.Gell-Mann M. The Quark and the Jaguar: Adventures in the Simple and the Complex. New York: Freeman; 1994. [Google Scholar]
- 3.Pearson JE. Complex patterns in a simple system. Science. 1993;261:189–192. doi: 10.1126/science.261.5118.189. [DOI] [PubMed] [Google Scholar]
- 4.Rietkerk M, Dekker SC, de Ruiter PC, van de Koppel J. Self-organized patchiness and catastrophic shifts in ecosystems. Science. 2004;305:1926–1929. doi: 10.1126/science.1101867. [DOI] [PubMed] [Google Scholar]
- 5.Wolfram S, editor. Theory and Applications of Cellular Automata. Singapore: World Scientific; 1986. [Google Scholar]
- 6.Turing AM. The chemical basis of morphogenesis. Philos Trans R Soc London Ser B. 1952;237:37–72. [Google Scholar]
- 7.Hauert C, Michor F, Nowak M, Doebeli M. Synergy and discounting of cooperation in social dilemmas. J Theor Biol. 2006;239:195–202. doi: 10.1016/j.jtbi.2005.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- 9.Milinski M, Semmann D, Krambeck HJ, Marotzke M. Stabilizing the earth's climate is not a losing game: Supporting evidence from public goods experiments. Proc Natl Acad Sci USA. 2006;103:3994–3998. doi: 10.1073/pnas.0504902103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rainey PB, Rainey K. Evolution of cooperation and conflict in experimental bacterial populations. Nature. 2003;425:72–74. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- 11.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 12.Velicer GJ, Yu YTN. Evolution of novel cooperative swarming in the bacterium Myxococcus xanthus. Nature. 2003;425:75–78. doi: 10.1038/nature01908. [DOI] [PubMed] [Google Scholar]
- 13.Lenski RE, Hattingh SE. Coexistence of two competitors on one resource and one inhibitor: A chemostat model based on bacteria and antibiotics. J Theor Biol. 1986;122:83–93. doi: 10.1016/s0022-5193(86)80226-0. [DOI] [PubMed] [Google Scholar]
- 14.Dugatkin LA, Perlin M, Lucas JS, Atlas R. Group-beneficial traits, frequency-dependent selection and genotypic diversity: An antibiotic resistance paradigm. Proc R Soc London Ser B. 2005;272:79–83. doi: 10.1098/rspb.2004.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagel JH, Roth AE, editors. The Handbook of Experimental Economics. Princeton: Princeton Univ Press; 1995. [Google Scholar]
- 16.Hofbauer J, Sigmund K. Evolutionary Games and Population Dynamics. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 17.Hauert C, Wakano JY, Doebeli M. Ecological public goods games: Cooperation and bifurcation. Theor Pop Biol. 2008;73:257–263. doi: 10.1016/j.tpb.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rankin DJ. Resolving the tragedy of the commons: The feedback between intraspecific conflict and population density. J Evol Biol. 2007;20:173–180. doi: 10.1111/j.1420-9101.2006.01211.x. [DOI] [PubMed] [Google Scholar]
- 19.Wakano JY. A mathematical analysis on public goods games in the continuous space. Math Bio Sci. 2006;201:72–89. doi: 10.1016/j.mbs.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Mimura M, Murray J. On a diffusive prey-predator model which exhibits patchiness. J Theor Biol. 1978;75:249–262. doi: 10.1016/0022-5193(78)90332-6. [DOI] [PubMed] [Google Scholar]
- 21.Baurmann M, Gross T, Feudel U. Instabilities in spatially extended predator–prey systems: Spatio-temporal patterns in the neighborhood of Turing–Hopf bifurcations. J Theor Biol. 2007;245:220–229. doi: 10.1016/j.jtbi.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Vickers GT, Hutson VCL, Budd CJ. Spatial patterns in population conflicts. J Math Biol. 1993;31:411–430. [Google Scholar]
- 23.Cressman R, Vickers GT. Spatial and density effects in evolutionary game theory. J Theor Biol. 1997;184:359–369. doi: 10.1006/jtbi.1996.0251. [DOI] [PubMed] [Google Scholar]
- 24.Doebeli M, Hauert C. Models of cooperation based on the prisoner's dilemma and the snowdrift game. Ecol Lett. 2005;8:748–766. [Google Scholar]
- 25.Nowak MA, May RM. Evolutionary games and spatial chaos. Nature. 1992;359:826–829. [Google Scholar]
- 26.Ohtsuki H, Hauert C, Lieberman E, Nowak MA. A simple rule for the evolution of cooperation on graphs and social networks. Nature. 2006;441:502–505. doi: 10.1038/nature04605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabó G, Fáth G. Evolutionary games on graphs. Phys Rep. 2007;446:97–216. [Google Scholar]
- 28.Taylor PD, Day T, Wild G. Evolution of cooperation in a finite homogeneous graph. Nature. 2007;447:469–472. doi: 10.1038/nature05784. [DOI] [PubMed] [Google Scholar]
- 29.Hassell MP, Comins HN, May RM. Spatial structure and chaos in insect population dynamics. Nature. 1991;353:255–258. [Google Scholar]
- 30.Hassell MP, Comins HN, May RM. Species coexistence and self-organizing spatial dynamics. Nature. 1994;370:290–292. [Google Scholar]
- 31.Jansen VAA, Sigmund K. Shaken not stirred: On permanence in ecological communities. Theor Pop Biol. 1998;54:195–201. doi: 10.1006/tpbi.1998.1384. [DOI] [PubMed] [Google Scholar]
- 32.Alizon S, Taylor P. Empty sites can promote altruistic behavior. Evolution (Lawrence, Kans) 2008;62:1335–1344. doi: 10.1111/j.1558-5646.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 33.Sella G, Lachmann M. On the dynamic persistence of cooperation: How lower individual fitness induces higher survivability. J Theor Biol. 2000;206:465–485. doi: 10.1006/jtbi.2000.2130. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Jacob E, Levine H. Self-engineering capabilities of bacteria. J R Soc Interface. 2006;3:197–214. doi: 10.1098/rsif.2005.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xavier JB, Foster KR. Cooperation and conflict in microbial biofilms. Proc Natl Acad Sci USA. 2007;104:876–881. doi: 10.1073/pnas.0607651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budrene EO, Berg HC. Complex patterns formed by motile cells of Escherichia coli. Nature. 1991;349:630–633. doi: 10.1038/349630a0. [DOI] [PubMed] [Google Scholar]
- 37.Budrene EO, Berg HC. Dynamics of formation of symmetrical patterns by chemotactic bacteria. Nature. 1995;376:49–53. doi: 10.1038/376049a0. [DOI] [PubMed] [Google Scholar]
- 38.Scanlon TM, Caylor KK, Levin SA, Rodriguez-Iturbe I. Positive feedbacks promote power-law clustering of Kalahari vegetation. Nature. 2007;449:209–212. doi: 10.1038/nature06060. [DOI] [PubMed] [Google Scholar]
- 39.von Hardenberg J, Meron E, Shachak M, Zarmi Y. Diversity of vegetation patterns and desertification. Phys Rev Lett. 2001;87:198101. doi: 10.1103/PhysRevLett.87.198101. [DOI] [PubMed] [Google Scholar]
- 40.Prosser JI, et al. The role of ecological theory in microbial ecology. Nat Rev Microbiol. 2007;5:384–392. doi: 10.1038/nrmicro1643. [DOI] [PubMed] [Google Scholar]
- 41.Tilman D, Reich PB, Knops JMH. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.