Abstract
In source leaves of resistant tobacco, oxidative burst and subsequent formation of hypersensitive lesions after infection with Phytophthora nicotianae was prevented by inhibition of glucose-6-phosphate dehydrogenase (G6PDH) or NADPH oxidases. This observation indicated that plant defense could benefit from improved NADPH availability due to increased G6PDH activity in the cytosol. A plastidic isoform of the G6PDH-encoding gene, G6PD, displaying high NADPH tolerance was engineered for cytosolic expression (cP2), and introduced into a susceptible cultivar. After infection, transgenic (previously susceptible) lines overexpressing cP2 showed early oxidative bursts, callose deposition, and changes in metabolic parameters. These responses resulted in timely formation of hypersensitive lesions similar to resistant plants, although their extent varied considerably between different transgenic lines. Additional RNAi suppression of endogenous cytosolic G6PD isoforms resulted in highly uniform defense responses and also enhanced drought tolerance and flowering. Cytosolic G6PDH seems to be a crucial factor for the outcome of plant defense responses; thus, representing an important target for modulation of stress resistance. Because isoenzyme replacement of G6PDH in the cytosol was beneficial under various kinds of cues, we propose this strategy as a tool to enhance stress tolerance in general.
Keywords: oxidative burst, source-to-sink transition, NADPH availability, pathogen resistance, tobacco
Plants are under continuous threat of being challenged by pathogenic microorganisms, which try to exploit them as a source of carbohydrates and other assimilates. Timely recognition of invading microorganisms and rapid and effective induction of defense responses are presently considered the main difference between pathogen-resistant and susceptible plant lines (1). So-called compatible interactions between pathogens and plants result if the pathogen can overcome plant defense barriers and establish disease symptoms. During an incompatible interaction, a wealth of defense mechanisms [e.g., generation of reactive oxygen species (ROS), synthesis of pathogenesis-related (PR) proteins, cell-wall fortification, and the hypersensitive reaction (HR)] can efficiently limit pathogen growth. The HR, which includes ROS formation and programmed cell death (PCD), represents the most efficient mechanism of plant defense. ROS were previously proposed to orchestrate the establishment of plant defense responses (2, 3). NADPH oxidases at the plasma membrane are considered the main source of extracellular ROS formation during defense, the so-called oxidative burst. Down-regulation or elimination of NADPH oxidase leads to suppression of pathogen-induced oxidative bursts and HR (4).
The oxidative burst, an evolutionarily conserved mechanism, is part of the innate immune response, and (besides triggering plant PCD) it is thought to contribute to direct killing of invading microorganisms. It also helps to mount an invasion barrier through radical-mediated polymerization of cell-wall components, which is later followed by formation of hypersensitive lesions (2). Importantly, NADPH oxidase activity (and resulting ROS production) relies on rapid regeneration of NADPH in the cytosol, which requires an enhanced carbohydrate metabolism.
Defense reactions are always associated with increased demand for reducing equivalents and carbon skeletons. However, in photoautotrophic plant tissues, the metabolic situation is not well suited for defense. Due to rapid export, soluble sugar levels are fairly low and carbohydrate-consuming pathways important for defense, such as glycolysis, respiration, oxidative pentose-phosphate pathway (OPPP), and shikimate pathway are tuned down. Nonetheless, photosynthetically active source leaves of resistant plants can exhibit strong defense reactions, including HR. We have previously shown for a resistant tobacco cultivar that metabolic source-to-sink shifts accompany early defense reactions in source leaves (5, 6), and that sink situations stimulate de novo synthesis of cytosolic glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49) in leaf discs (7), suggesting a role for this enzyme during plant defense.
G6PDH is present in the cytosol of all eukaryotic cells, and regulates NADPH provision via the OPPP. Defective yeast mutants were found to be susceptible to oxidative stress (8), and a role for G6PDH in supplying NADPH for free-radical scavenging has also been observed in stressed mammalian cells (9). In higher plants, the OPPP is a major source of reduction power (NADPH) required for anabolic biosyntheses and assimilatory processes in the cytosol, as well as in plastids, providing key intermediates for the shikimate pathway and nucleic acid biosynthesis (10, 11). Because in higher plants only the oxidative branch of the OPPP seems to be present in the cytosol (12), produced C5-sugar phosphates have to be exchanged with plastids via a pentose-phosphate translocator (13). Plastidic G6PDH isoenzymes fall into 2 classes: P1 enzymes are characterized by reductive inactivation (to avoid futile interactions with the Calvin cycle in chloroplasts), whereas P2 enzymes display relaxed redox regulation, but enhanced tolerance toward NADPH (14). The latter is needed in the stroma during dark phases to sustain ferredoxin-dependent reactions, especially in heterotrophic plastids (14, 15). In this context, enhanced NADPH tolerance means that the inhibition constant Ki[NADPH] is higher than the Km of the enzyme for NADP (14, 16). In contrast, cytosolic G6PDH isoenzymes show similar Ki[NADPH] and Km[NADP] values, which means they are inhibited earlier by rising NADPH levels (17).
For elicited tobacco suspension culture cells, Pugin et al. (18) showed that activation of glycolysis and the OPPP provides NADPH for the oxidative burst. Based on this evidence and our own work, we demonstrate by this study that G6PDH activity in the cytosol is a key determinant during early pathogen defense, drought stress, and plant development, due to controlling metabolic flux through the oxidative branch of the OPPP.
Results
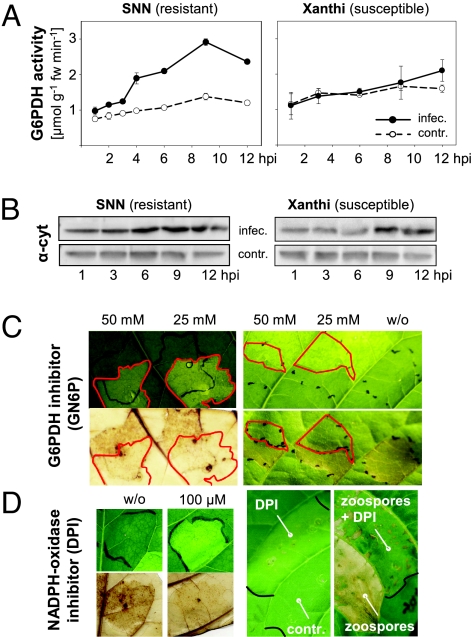
After Pathogen Infection, G6PDH Activity Rises in Leaves of a Resistant, but Not of a Susceptible, Tobacco Cultivar.
To check whether G6PDH activity could be a key determinant for establishing plant defense, zoospores of Phytophthora nicotianae were infiltrated into a resistant and a susceptible tobacco cultivar. Significant 2- to 3-fold rises in G6PDH activity compared with water-infiltrated controls were recorded for the resistant cultivar Nicotiana tabacum var. Samsun NN (SNN) from 4 h postinfiltration (hpi) on. Leaves of the susceptible Xanthi reacted much slower, reaching only a 25% increase compared with water-infiltrated controls at later time points (10–12 hpi; Fig. 1A). Immunoblot analyses with various G6PDH antisera (14, 19) demonstrated that these defense-induced rises in G6PDH activity mainly result from up-regulated expression of cytosolic isoforms, encoded by G6PD (Fig. 1B), probably due to sensing elevated soluble sugar levels (7), known to accompany source-to-sink transitions of infected tobacco source leaves (5, 6).
Fig. 1.
G6PDH activity is essential for the oxidative burst. (A) After infection with P. nicotianae, early rises of G6PDH can be recorded in tobacco source leaves of the resistant SNN, but not of the susceptible Xanthi. Data are shown as means ± SE. (B) Immunoblot with antibodies to cytosolic G6PDH (α-cyt) (14, 19) after infection and in water-infiltrated controls. (C) Different concentrations of glucosamine 6-phosphate (GN6P, infiltrated areas marked red) impede ROS formation [visualized by brown staining due to 3,3′-diaminobenzidine (DAB) polymerization in the presence of H2O2; 6 hpi] and hypersensitive lesions (24 hpi). GN6P is a competitive inhibitor of G6PDH (20). (D) When treated with 100 μM diphenyleneiodonium chloride (DPI), a well-known inhibitor of plant NAD(P)H oxidases (18), infection with P. nicotianae induced neither ROS formation (6 hpi) nor hypersensitive lesions (48 hpi). fw, fresh weight; w/o, without.
Inhibition of Either G6PDH or NADPH Oxidase Activity Interferes with ROS Production and Hypersensitive Lesion Formation in a Resistant Tobacco Cultivar.
G6PDH is known to regulate NADPH provision via the oxidative branch of the OPPP. Therefore, we tested whether G6PDH-dependent NADPH provision in the cytosol might be critical for the formation of hypersensitive lesions, the final result of successful HR in resistant plants (see pathway schematic representation of Fig. 2A). Coinfiltration of the competitive G6PDH inhibitor glucosamine 6-phosphate (GN6P) (20) prevented both formation of ROS [brown staining due to 3,3′-diaminobenzidine (DAB) polymerization in the presence of H2O2 (Fig. 1C Left) and hypersensitive lesions (Fig. 1C Right)]. Also, coinfiltration of diphenyleneiodonium chloride (DPI), an inhibitor of NAD(P)H oxidases (18), interfered with defense-induced oxidative burst and HR (Fig. 1D). This observation pointed to plasma membrane NADPH oxidases as the main source of ROS production, important for the formation of hypersensitive lesions in tobacco leaves after infection with P. nicotianae. Also, these results were strong support for pursuing a strategy to enhance NADPH provision in the cytosol of a susceptible tobacco cultivar: Xanthi plants were engineered for ectopic (cytosolic) expression of a heterologous plastidic G6PD isoform with superior kinetic characteristics (P2-type, Ki[NADPH] > Km[NADP]; Fig. S1, and pathway schematic representation of Fig. 2A) to support oxidative bursts at the plasma membrane.
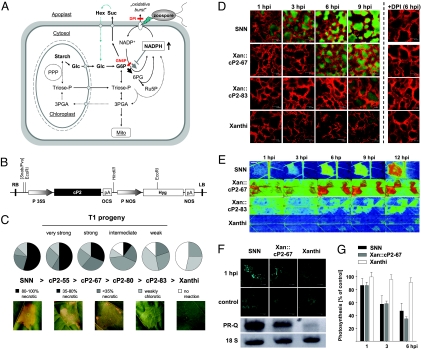
Fig. 2.
Overexpression of a kinetically superior G6PDH in the cytosol enhances pathogen resistance. (A) Pathway schematic representation of the consequences of expressing a plastidic G6PD isoform of P2-type from Arabidopsis in the cytosol of the susceptible tobacco cultivar. GN6P and DPI blocks are indicated in red, endogenous cytosolic G6PDH is shown as gray, and the engineered cP2 isoform as black arrow. Hex, hexoses; Suc, sucrose; Glc, glucose; G6P, glucose 6-phosphate; Mito, Mitochondrion; 3PGA, 3-phosphoglycerate; 6PG, 6-phosphogluconate; Ru5P, ribulose 5-phosphate; Triose-P, triose phosphates. For further explanation, see main text. (B) Expression cassette of binary vector used for the generation of Xanthi::cP2 lines. (C) Evaluation of lesions formed in leaves of different Xanthi::cP2 lines (T1 progeny) versus resistant SNN and susceptible Xanthi WT plants. In the various Xanthi::cP2 lines, resistance increased, as indicated by the extent of lesions formed within infected areas. Based on the examples given below the pie charts, cP2 lines were categorized “weak” to “very strong.” The Xanthi::cP2 lines displayed enhanced resistance against P. nicotianae compared with Xanthi WT plants, but the degree of resistance differed considerably between weak to very strong lines. (D) Representative confocal laser scanning microscopy images of defense-induced ROS release by mesophyll cells at the infection sites (dichlorofluorescein fluorescence in green, indicating the formation of ROS; chlorophyll fluorescence in red). (E) Representative chlorophyll-a fluorescence images of defense-induced changes in dark metabolism. Accelerated induction of photosynthesis after a dark period is characteristic for high levels of OPPP intermediates (5, 38). Low values (blue) indicate slow, high values (green to red) indicate fast induction; for color scale, see Fig. 3E. Photosynthesis induction was accelerated in infection zones of the resistant SNN as well as in Xan::cP2–67, indicating increase in metabolites of the OPPP. Also, Xanthi::cP2 transformants displayed fast photosynthetic induction in whole leaves per se (green background), which is indicative of a higher permanent activation of the OPPP (Fig. 3D). (F) Defense-induced formation of callose (bright blue fluorescence), a well-known plant defense reaction (39), and transcript accumulation of PR protein Q (PR-Q), occurred in SNN and strongly responding Xanthi::cP2 lines. Initially, callose distinctively appeared at the cell-to-cell contacts, which shuts down sucrose export routes and supports source-to-sink shifts by retaining carbohydrates within infected cells (5, 6). Total RNA was isolated from infected source leaf areas (6 hpi). 18S cDNA served as loading control. (G) Early stomata-dependent inhibition of photosynthesis is enhanced in infected leaves of resistant SNN and Xan::cP2-67 compared with susceptible Xanthi WT. Data are calculated from chlorophyll-a fluorescence images (2% O2; Fig. S3) and are shown as means ± SD. O2-sensitive inhibition of photosynthetic electron transport indicates inhibition of photosynthetic CO2 fixation as a result of stomata closure (directing overall flux toward photorespiration) (5). Because stomata closure is known to involve ROS (40), this effect could be due to improved ROS formation in the transformants.
Expression of a Plastidic G6PD Isoform in the Cytosol of a Susceptible Tobacco Cultivar Enhances Defense Reactions.
For permanent overexpression of a kinetically superior G6PD isoform in the cytosol of the susceptible tobacco cultivar Xanthi (Fig. 2A), we chose a plastidic P2 isoform (14) from Arabidopsis that is highly induced by nitrogen starvation in roots (21), and displays a higher Ki[NADPH] compared with Km[NADP] value (16). Kinetic parameters of the modified enzyme without signal peptide were tested in a G6PDH-deficient Escherichia coli strain (22) and confirmed the Ki > Km characteristics of P2-type G6PDH enzymes. Absolute values (Ki[NADPH] 60 μM > Km[NADP] 24 μM) differed from the enzyme with C-terminal strep tag (Ki[NADPH] 22 μM > Km[NADP] 17 μM) (16), and most importantly, from reduced dithiothreitol (DTTred)-resistant (cytosolic) G6PDH activity (17) extracted from Xanthi and SNN source leaves (Ki ≈ Km), showing 2-fold higher affinity for G6P and 2-fold lower affinity for NADP (Fig. S1).
From Xanthi leaf discs transformed with agrobacteria carrying the binary cP2 construct (Fig. 2B), primary transformants (T0) were regenerated and selected according to cP2-signal strength on Northern and Western blottings (Fig. S2 A and B). Defense responses after infection with P. nicotianae were evaluated among hygromycin-resistant T1 individuals of different cP2 lines and compared with those of Xanthi and SNN WT plants. All Xanthi::cP2 lines showed increased lesion formation compared with Xanthi WT and were categorized into weak to very strong responding lines (Fig. 2C). However, cP2 plants grew slower than WT, although this habit did not correlate well with extent of lesion formation or differences in cP2-protein levels. Nevertheless, within individual lines, responses were stable up to the T3 progeny (Fig. S2C).
Enhanced resistance was also reflected by further defense-associated parameters, as shown, for example, for cP2-67 and cP2-83 (Fig. 2 D–F). Compared with susceptible Xanthi, defense-induced oxidative burst was significantly increased in all Xanthi::cP2 lines (especially in strong responders like cP2-67), and could be mostly prevented by coinfiltration of DPI (NADPH oxidase inhibitor; Fig. 2D). Also, defense-induced accumulation of OPPP metabolites in the dark was recorded by imaging photosynthesis induction (deduced from chlorophyll-a fluorescence), which is based on changes in metabolite pools shared between the OPPP and the Calvin cycle. This technique is a robust indicator for the activity of the OPPP in either sink leaves or on induction of sink-type metabolism during defense (for further explanation, see Fig. 2E and Fig. 3D) (5, 6). Now, interestingly, this behavior also occurred in the Xanthi background, and was accompanied by callose deposition (Fig. 2F), leading to obstruction of sucrose export and decline in photosynthesis, partially due to stomata closure (Fig. 2G; Fig. S3). In summary, these changes effectively alter carbon partitioning in favor of metabolic source-to-sink transitions within infected leaf areas (5, 6). Additional evidence for an altered pathogen response in cP2 plants is the accumulation of transcripts encoding PR-Q (5), which now also occurred in the Xanthi background, comparable with the resistant SNN (Fig. 2F).
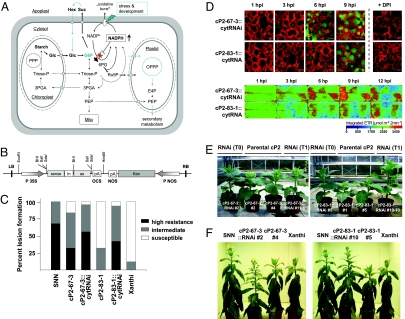
Fig. 3.
G6PDH isoenzyme replacement enhances pathogen resistance, abiotic stress tolerance, and development. (A) Schematic representation of the isoenzyme-replacement strategy for elimination of endogenous cytosolic G6PDH activity in the cP2 lines by an RNAi approach. Also depicted are possible benefits for secondary metabolism. Note that NADPH oxidases are activated by transient calcium elevation in the cytosol (41). Hence, NADPH will be consumed only in response to signals (biotic and abiotic cues), which prevents interference with normal physiology and development. PEP, phosphoenolpyruvate; E4P, erythrose 4-phosphate. (B) Expression cassette of the binary cytG6PD-RNAi construct. (C) Disease evaluation of parental cP2 lines versus the corresponding RNAi transformants after pathogen challenge compared with SNN and Xanthi WT. High resistance, 80–100% lesions per area (lpa); intermediate resistance, 35–80% lpa; susceptible response, 0–10% lpa at infiltration site. (D) Representative images of defense-induced ROS release and subsequent changes in dark metabolism. After isoenzyme replacement, descendents of formerly weak line Xan::cP2–83 react much stronger. The same trend was observed for the T1 progeny (compare Fig. S6). For further explanations to A and D, see legends of Fig. 2 and Fig. S5. (E and F) G6PDH isoenzyme replacement revokes growth differences displayed by the parental cP2 lines (E), and enhances abiotic stress tolerance (5 days without watering) (F). Reduced water loss from excised leaf discs further supports enhanced drought tolerance of the cP2 transformants. Average water loss (6 h after detachment, n = 3) was 55% ± 3% for SNN, 67% ± 2% for Xanthi WT, and 50% ± 5% for Xan::cP2-83–1::cytRNAi #10. By feeding abscissic acid (ABA) to excised leaves via the petiole, we excluded that observed drought tolerance is a result of compromised stomatal regulation. Besides ABA-mediated stoma regulation (40), ROS are probably also involved in drought sensing and ABA synthesis (32).
Isoenzyme Replacement Is Superior to Overexpression of a P2-Type G6PD Isoform in the Cytosol of a Susceptible Tobacco Cultivar.
To limit contribution of endogenous cytosolic G6PDH activity (modified pathway schematic representation of Fig. 3A) and thereby prove that the effects observed in the susceptible tobacco cultivar are due to ectopic (cytosolic) expression of a kinetically different G6PD isoform, we additionally transformed 2 cP2 lines (strong cP2-67-3 and weak cP2-83-1) with a binary RNAi construct specifically targeting cytosolic G6PD isoforms of Xanthi (Fig. 3B). RNAi-mediated suppression was confirmed by G6PDH activity tests in the presence of DTTred, which is known to inhibit plastidic isoenzymes (Fig. S1) (22, 23), and immunoblot analyses with isoform-specific G6PDH antisera (14, 19). Although cP2-protein levels were reduced compared with those detected in the 2 parental lines (Fig. S4), both isoenzyme-replaced Xanthi lines showed similar extents of lesion formation after infection (Fig. 3C). Notably, the responses were even stronger than those of the parental cP2 lines, and more similar to the resistant tobacco cultivar SNN. Thus, enhanced resistance displayed by the isoenzyme-replaced lines is independent of cP2 protein levels, underscoring that enzyme quality and not quantity is important for the observed effects. Time-resolved analyses of defense-induced ROS production and dark metabolism (Fig. 3D and Fig. S5) showed that cP2-83-1::cytRNAi (formerly weak) and cP2-67-3::cytRNAi (formerly strong) Xanthi lines exhibit highly uniform, strong defense responses. Stability of the effects was confirmed through side-by-side analysis of enzyme-replaced T0 and T1 plants for defense-induced ROS release (Fig. S6). Similar defense responses were observed after application of elicitors such as necrosis-inducing Phytophthora protein 1 (NPP1) and cell-free zoospore filtrate (5).
There is strong evidence for permanently elevated OPPP intermediates in source leaves of the Xanthi transformants (Fig. S5). G6PDH isoenzyme replacement revoked the growth retardation observed to different extents within the cP2 lineages (Fig. 3E) and enhanced drought tolerance (Fig. 3F) and flowering (Fig. S7), compared with Xanthi WT and the 2 parental cP2 lines. These results demonstrate that channeling flux through a single but crucial metabolic step improves stress tolerance and influences developmental processes in plants.
Discussion
Activity assays and inhibitor studies (Fig. 1) indicated that G6PDH activity is essential for the oxidative burst, and could, therefore, determine the outcome of plant–pathogen interactions. Thus, we engineered a susceptible tobacco cultivar for constitutive overexpression of a kinetically superior G6PDH in the cytosol, which should result in improved NADPH provision for pathogen-activated NADPH oxidases. The resulting Xanthi::cP2 lines displayed enhanced resistance against the oomycete P. nicotianae compared with Xanthi WT, but the degree of resistance differed considerably between weak and very strong lines (Fig. 2 and Fig. S2). This finding was confirmed by detailed and time-resolved analyses of further parameters, known to accompany plant defense responses. Oxidative burst, defense-related metabolic source-to-sink transitions, induced expression of a PR protein, as well as callose deposition and early stomata closure, known to precede the onset of hypersensitive lesion formation in the resistant SNN (5, 6), were now also detected in the Xanthi background (Fig. 2). There is increasing evidence that constitutive overexpression of enzymes associated with “normal” metabolism can have critical roles for the induction of plant defense against pathogens (24). Genes encoding proteins that confer enhanced resistance to host plants have been designated enzymatic resistance (eR) genes. For example, expression of peroxisomal glyoxylate aminotransferase in a susceptible melon cultivar led to higher resistance (25). In this context, ectopic expression of a P2-type G6PD isoform in the cytosol can be considered another example for a successful eR gene.
The obtained data are in accordance with immediate and sustained oxidative bursts of plasma membrane NADPH oxidase after activation, which relies on effective NADPH regeneration in the cytosol. The pivotal role of G6PDH in eR became obvious by yet another transgenic approach. As independent proof that the observed effects result from introduction of a kinetically different enzyme into a susceptible cultivar, a weak line and a strong line of the Xanthi::cP2 transformants were supertransformed with a binary RNAi construct to additionally eliminate endogenous cytosolic G6PDH activity. This isoenzyme replacement resulted in highly uniform defense responses of the supertransformants, independent of the parental line (Fig. 3), which was even more astonishing, considering that the RNAi approach also reduced cP2 protein levels (Fig. S4) and is probably due to low selectivity of the targeted sequence region. Through this unintended cP2 reduction, the benefit of isoenzyme replacement, with respect to altering development and stress responses, becomes even more obvious. In addition to improved resistance, isoenzyme replacement revoked the growth retardation observed among several cP2 lines, conferred enhanced drought tolerance, and accelerated the onset of flowering (Fig. 3F and Fig. S7). These examples demonstrate the potential of isoenzyme replacement compared with eR resulting from mere overexpression of heterologous enzymes in plants. There, enhanced resistance was mostly associated with reduced growth, lower yield, or other disadvantages. One example is overexpression of yeast invertase in the apoplast of tobacco plants, which led to high virus resistance but also spontaneous lesion formation in source leaves (26). As a new approach, isoenzyme replacement (combined with moderate expression) intends to optimize metabolic channeling, as shown in this work for the oxidative branch of the OPPP.
The clarity of the obtained results was somewhat surprising, because plant cells possess an alternative glycolytic step via nonreversible d-glyceraldehyde-3-phosphate dehydrogenase (GAPN) that could also contribute to NADPH supply in the cytosol. GAPN was originally proposed to mediate indirect export of NADPH from chloroplasts during photosynthesis (27) via the triose-P/3-PGA shuttle (see pathway schematic representations). Thus, defense-induced activation of glycolysis (18) could be an additional source of NADPH for the oxidative burst in plants. However, in heterotrophic cells, activity of GAPN is regulated by phosphorylation and binding to 14-3-3 proteins (28), suggesting that these mechanisms might also limit the contribution of the enzyme to NADPH supply in the cytosol during defense-induced source-to-sink transitions.
Lately, NADH kinase (29) and external mitochondrial NADPH dehydrogenase (30) have also been suggested to contribute to cytosolic NADPH levels. However, one has to keep in mind that any increase in the activity of these enzymes affects the ratio between NADH and NADPH in the cytosol, and, thus, inevitably disturbs the balance between catabolic (largely NADH-dependent) and anabolic (largely NADPH-dependent) pathways. This switch might make sense in particular situations, for example, during prolonged stress, when metabolism shifts completely to synthesizing defense compounds (e.g., phytoalexins, lignin precursors, etc.). Therefore, it is unlikely that those enzymes can replace G6PDH.
In summary, isoenzyme replacement in the cytosol has convincingly shown that G6PDH is a key determinant for the provision of reducing equivalents to NADPH oxidases at the plasma membrane during early oxidative bursts. From this metabolic modification, obviously both biotic (defense reactions) and abiotic (drought) stress responses seem to benefit. Additional suppression of endogenous cytosolic G6PDH activity not only eliminates competition (modified pathway schematic representation of Fig. 3A) but probably also interferes with transcriptional up-regulation of this activity that is triggered by rising soluble sugar levels (7). In the resistant SNN, increased G6PDH activity (Fig. 1 A and B) is most likely also induced by an early infection-dependent accumulation of soluble sugars (5, 6). Clearly, the susceptible Xanthi is able to recognize the pathogen, because basic cytosolic G6PDH levels also rise, but infection-induced timely sugar accumulation and G6PDH increase to high levels (Fig. 1A) are absent. In the Xanthi transformants, the missing sugar feedback is probably overcome through replacement of sugar-regulated cytosolic G6PD isoforms by a permanently (but moderately) expressed P2-type isoform (for further explanation, see Fig. S5). On elicitation, immediate flux through reduced levels of the kinetically different isoenzyme appears to be equivalent or even superior to up-regulation of endogenous cytosolic G6PDH activity in the resistant tobacco SNN (Fig. 1A) during defense-induced source-to-sink transitions (5). Whether stable reduction of cytosolic G6PDH activity would conversely render a resistant cultivar susceptible to pathogen attack remains unstudied so far.
Especially interesting for agronomic applications are the developmental and abiotic stress aspects of this pathway modification. Among the enzyme-replaced Xanthi lines, flowering was accelerated, inflorescences enlarged (Fig. S7), and drought resistance improved (Fig. 3F). There is abundant evidence that NADPH-driven ROS formation has pivotal roles in cellular growth and development (31). Obviously, ROS-dependent ABA signaling during water stress in leaves (32) also profits from enhanced NADPH supply in the cytosol (Fig. 3F). Whether the described G6PD isoenzyme replacement alters developmental processes and abiotic stress resistance in general needs to be determined in more detail and with a larger set of plants.
By converting a susceptible into a resistant-like tobacco cultivar, this study demonstrates that fine adjustment of critical steps in primary metabolism can drastically improve resistance to biotic and abiotic stress. Because the approach seems to be beneficial under various kinds of cues, enzyme replacement might also improve stress responses in other plants or systems.
Methods
Cloning Strategies.
For a detailed description, see SI Methods.
Tobacco Transformation.
Transgenic tobacco plants were generated as described previously (33, 34, 35).
RNA Analyses.
Northern blot analyses was conducted as described previously (5, 14, 19, 35).
Protein Analyses.
Immunoblot analyses were conducted as described previously (14, 19); blots were probed with various G6PDH antisera, developed by using the ECL Advance chemiluminescence detection kit (Amersham), and documented digitally (GeneGnome; Syngene).
Determination of G6PDH Activity.
Assays were performed according to ref. 36, in a robot-based platform at the Max Planck Institute for Molecular Plant Physiology, Golm, Germany. For further details, see Fig. S1.
Leaf-Infiltration Assays.
Source leaves of 6- to 10-week-old tobacco plants were infiltrated with a zoospore suspension of P. nicotianae (Isolate 1828; Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany). Cultivation of plants and pathogens and inoculations were done as described previously (5).
Microscopic Analyses.
ROS production was checked with DAB staining or 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes). ROS-dependent oxidation of nonfluorescent H2DCFDA to highly fluorescent DCF (488-nm excitation and 510- to 535-nm detection) is proportional to the ROS produced (37). Callose was stained with aniline blue. For detailed description, see refs. 5 and 6.
Chlorophyll-a Fluorescence Imaging.
Photosynthetic electron transport, gas exchange, and defense-induced stomata closure were determined as described previously (5).
Evaluation of Lesion Formation.
The extent of hypersensitive lesions formed at least 50 infiltration sites infected with P. nicotianae on 5 plants were evaluated after 2 days. Values are the means of 3 or more independent rounds of infections.
Supplementary Material
Acknowledgments.
We thank O. Becker, K. Fischer, I. Schmitz-Thom, and K. Topp for excellent technical assistance and H. E. Neuhaus and J. Tjaden for stimulating discussions. E. Ostendorf assisted with cloning, and P. Bones and Y. Gibon with measurements. This work was partly supported by Deutsche Forschungsgemeinschaft Grant SCHA 541/8-3.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812902106/DCSupplemental.
References
- 1.Yang Y, Shah J, Klessig DF. Signal perception and transduction in plant defense responses. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]
- 2.Torres MA, Jones JD, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E. The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot. 2008;59:501–520. doi: 10.1093/jxb/erm239. [DOI] [PubMed] [Google Scholar]
- 4.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Cur Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Scharte J, Schön H, Weis E. Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae. Plant Cell Environ. 2005;28:1421–1435. [Google Scholar]
- 6.Essmann J, et al. RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol. 2008;147:1288–1299. doi: 10.1104/pp.108.121418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauschild R, von Schaewen A. Differential regulation of glucose-6-phosphate dehydrogenase activity in potato (Solanum tuberosum L. ) Plant Physiol. 2003;133:47–62. doi: 10.1104/pp.103.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juhnke H, Krems B, Kotter P, Entian KD. Mutants that show increased sensibility to hydrogen peroxide reveal an important role for the pentose phosphate pathway in protection of yeast against oxidative stress. Mol Gen Genet. 1996;252:454–464. doi: 10.1007/BF02173011. [DOI] [PubMed] [Google Scholar]
- 9.Salvemini F, et al. Enhanced glutathione levels and oxidoresistance mediated by increased glucose 6-phosphate dehydrogenase. J Biol Chem. 1999;274:2750–2757. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- 10.Neuhaus HE, Emes MJ. Non-photosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- 11.Krüger NJ, von Schaewen A. The oxidative pentose phosphate pathway: Structure and organization. Curr Opin Plant Biol. 2003;6:236–246. doi: 10.1016/s1369-5266(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 12.Schnarrenberger C, Flechner A, Martin W. Enzymatic evidence for a complete oxidative pentose phosphate pathway in chloroplasts and an incomplete pathway in the cytosol of spinach leaves. Plant Physiol. 1995;108:609–614. doi: 10.1104/pp.108.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eicks M, Maurino V, Knappe S, Flügge UI, Fischer K. The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiol. 2002;128:512–522. doi: 10.1104/pp.010576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendt UK, Wenderoth I, Tegeler A, von Schaewen A. Molecular characterization of a novel glucose-6-phosphate dehydrogenase from potato (Solanum tuberosum L. ) Plant J. 2000;23:723–733. doi: 10.1046/j.1365-313x.2000.00840.x. [DOI] [PubMed] [Google Scholar]
- 15.Bowsher CG, Boulton EL, Rose J, Nayagam S, Emes MJ. Reductant for glutamate synthase is generated by the oxidative pentose phosphate pathway in non-photosynthetic root plastids. Plant J. 1992;2:893–898. [Google Scholar]
- 16.Wakao S, Benning C. Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. 2005;41:243–256. doi: 10.1111/j.1365-313X.2004.02293.x. [DOI] [PubMed] [Google Scholar]
- 17.Fickenscher K, Scheibe R. Purification and properties of the cytoplasmic glucose-6-phosphate dehydrogenase from pea leaves. Arch Biochem Biophys. 1986;247:393–402. doi: 10.1016/0003-9861(86)90598-9. [DOI] [PubMed] [Google Scholar]
- 18.Pugin A, et al. Early events induced by the elicitor cryptogein in tobacco cells: Involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Schaewen A, Langenkamper G, Graeve K, Wenderoth I, Scheibe R. Molecular characterization of the plastidic glucose-6-phosphate dehydrogenase from potato in comparison to its cytosolic counterpart. Plant Physiol. 1995;109:1327–1335. doi: 10.1104/pp.109.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser BL, Brown DH. Purification and properties of D-glucose 6-phosphate dehydrogenase. J Biol Chem. 1955;216:67–79. [PubMed] [Google Scholar]
- 21.Wang R, Okamoto M, Xing X, Crawford NM. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003;132:556–567. doi: 10.1104/pp.103.021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenderoth I, Scheibe R, von Schaewen A. Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J Biol Chem. 1997;272:26985–26990. doi: 10.1074/jbc.272.43.26985. [DOI] [PubMed] [Google Scholar]
- 23.Graeve K, von Schaewen A, Scheibe R. Purification, characterization, and cDNA sequence of glucose-6-phosphate dehydrogenase from potato (Solanum tuberosum L. ) Plant J. 1994;5:353–361. doi: 10.1111/j.1365-313x.1994.00353.x. [DOI] [PubMed] [Google Scholar]
- 24.Eckardt NA. Aminotransferases confer “enzymatic resistance” to downy mildew in melon. Plant Cell. 2004;16:1–3. [Google Scholar]
- 25.Taler D, Galperin M, Benjamin I, Cohen Y, Kenigsbuch D. Plant eR genes that encode photorespiratory enzymes confer resistance against disease. Plant Cell. 2004;16:172–184. doi: 10.1105/tpc.016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbers K, Meuwly P, Frommer W, Metraux J, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: Possible hexose sensing in the secretory pathway. Plant Cell. 1996;8:793–803. doi: 10.1105/tpc.8.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly GJ, Gibbs K. Non-reversible D-glyceraldehyde 3-phosphate dehydrogenase of plant tissues. Plant Physiol. 1973;52:111–118. doi: 10.1104/pp.52.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bustos DM, Iglesias AA. Phosphorylated non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from heterotrophic cells of wheat interacts with 14-3-3 proteins. Plant Physiol. 2003;133:2081–2088. doi: 10.1104/pp.103.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chai MF, et al. NADK3, a novel cytoplasmic source of NADPH, is required under conditions of oxidative stress and modulates abscisic acid responses in Arabidopsis. Plant J. 2006;47:665–674. doi: 10.1111/j.1365-313X.2006.02816.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu YJ, et al. The mitochondrial external NADPH dehydrogenase modulates the leaf NADPH/NADP+ ratio in transgenic Nicotiana sylvestris. Plant Cell Physiol. 2008;49:251–263. doi: 10.1093/pcp/pcn001. [DOI] [PubMed] [Google Scholar]
- 31.Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiol. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z, Chen G, Zhang C. Interaction between reactive oxygen species and nitric oxide in drought-induced abscisic acid synthesis in root tips of wheat seedlings. Aust J Plant Physiol. 2001;28:1055–1061. [Google Scholar]
- 33.Voelker T, Sturm A, Chrispeels MJ. Differences in expression between two seed lectin alleles obtained from normal and lectin-deficient beans are maintained in transgenic tobacco. EMBO J. 1987;6:3571–3577. doi: 10.1002/j.1460-2075.1987.tb02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Schaewen A, Stitt M, Schmidt R, Willmitzer L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate, inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990;9:3033–3044. doi: 10.1002/j.1460-2075.1990.tb07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenderoth I, von Schaewen A. Isolation and characterization of plant N-acetyl glucosaminyltransferase I (GntI) cDNA sequences. Functional analyses in the Arabidopsis cgl mutant and in antisense plants. Plant Physiol. 2000;123:1097–1108. doi: 10.1104/pp.123.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibon Y, et al. Adjustment of diurnal starch turnover to short days: Depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004;39:847–862. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 37.Rat P, et al. Micro-titration fluorimetric assays on living cells (MiFALC tests): New tools for screening in cell pharmacotoxicology. In: Van Zutphen LFM, Balls M, editors. Animal Alternatives, Welfare, and Ethics. Paris: Elsevier; 1997. pp. 813–825. [Google Scholar]
- 38.Meng Q, et al. Sink-source transition in tobacco leaves visualized using chlorophyll fluorescence imaging. New Phytol. 2001;151:585–595. doi: 10.1046/j.0028-646x.2001.00224.x. [DOI] [PubMed] [Google Scholar]
- 39.Maor R, Shirasu K. The arms race continues: Battle strategies between plants and fungal pathogens. Curr Opin Microbiol. 2005;8:399–404. doi: 10.1016/j.mib.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Pei ZM, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 41.Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell. 2002;14:2627–2641. doi: 10.1105/tpc.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.