Abstract
Objective
To use functional MRI (fMRI) to investigate whether hippocampal activation during a memory task can predict cognitive decline in individuals with mild cognitive impairment (MCI).
Methods
25 older individuals with MCI performed a visual scene encoding task during fMRI scanning, and were followed clinically for at least 4 years after scanning. A hypothesis driven analysis of fMRI data was performed. First, fMRI data were analysed at the group level to identify the regions of the hippocampal formation that were engaged by this memory task. Parameter estimates of each subject's memory related hippocampal activation (% signal change) were extracted and were analysed with a linear regression model to determine whether hippocampal activation predicted the degree or rate of cognitive decline, as measured by change in Clinical Dementia Rating Sum-of-Boxes (CDR-SB).
Results
Over 5.9 (1.2) years of follow-up after scanning, subjects varied widely in degree and rate of cognitive decline (change in CDR-SB ranged from 0 to 6, and the rate ranged from 0 to 1 CDR-SB unit/year). Greater hippocampal activation predicted greater degree and rate of subsequent cognitive decline (p<0.05). This finding was present even after controlling for baseline degree of impairment (CDR-SB), age, education and hippocampal volume, as well as gender and apolipoprotein E status. In addition, an exploratory whole brain analysis produced convergent results, demonstrating that the hippocampal formation was the only brain region where activation predicted cognitive decline.
Conclusions
In individuals with MCI, greater memory task related hippocampal activation is predictive of a greater degree and rate of cognitive decline subsequent to scanning. fMRI may provide a physiological imaging biomarker useful for identifying the subgroup of MCI individuals at highest risk of cognitive decline for potential inclusion in disease modifying clinical trials.
In groups of individuals with mild cognitive impairment (MCI), smaller hippocampal volume measured with structural MRI, which correlates with greater neurofibrillary tangle burden,1 is predictive of subsequent progression to a clinical diagnosis of Alzheimer's disease (AD).2-5 Although synaptic abnormalities are present in MCI6 and physiological dysfunction of memory circuits may be present early in the course of AD prior to substantial neuropathology,7-9 little is known about whether in vivo measures of medial temporal lobe (MTL) function in MCI can predict subsequent cognitive decline.
In a functional MRI (fMRI) study, we previously reported that greater MTL activation was present in a subgroup of individuals with MCI who demonstrated cognitive decline 2.5 years after scanning, compared with a subgroup that remained clinically stable.10 Given that MCI encompasses a range of impairment, however, the use of dichotomous outcomes tells only part of the story: if at baseline an individual is closer to the outcome of interest, relatively little change is required to cross the threshold (eg, to dementia). For other individuals with milder impairment at baseline, a greater degree of change in cognition would be required to meet a dementia outcome. It is not yet known whether abnormalities of brain function detected with fMRI can be used to predict the degree or rate of cognitive decline. Furthermore, a variety of factors have been shown to be predictors of cognitive decline within MCI, including baseline level of clinical impairment,11 age and education,12 as well as hippocampal volume. It is not yet known whether measures of MTL function can predict cognitive decline in MCI after controlling for these other important factors. Finally, since our previous investigation employed an a priori region of interest based approach, it is unclear whether there are brain regions outside the MTL where activation may be predictive of cognitive decline. An improved understanding of the ability of functional brain activation measures to predict the degree of cognitive decline in MCI is the first step towards building multivariate models that could be applied to identify MCI subgroups at highest risk for the greatest degree of imminent cognitive decline.
To address these questions, we analysed fMRI data from individuals spanning a range of impairment along the MCI spectrum who were followed with annual longitudinal clinical evaluations for at least 4 years after fMRI scanning. Given our previous findings, we predicted that greater hippocampal activation would be associated with a greater degree of cognitive decline, even after controlling for baseline degree of clinical impairment, age, education and hippocampal volume. For this study, we focused specifically on the prediction of worsening of cognitive symptoms in daily life, as graded by the Clinical Dementia Rating Sum-of-Boxes (CDR-SB), a measure with potential relevance for clinical trials. As it would be desirable to apply fMRI to detect functional abnormalities very early in the course of AD, long before dementia, we focused on predicting the degree of cognitive decline, as measured ordinally using the CDR-SB, rather than a dichotomised diagnostic outcome, such as dementia. We also analysed data with respect to overall rate of decline, as measured by the estimated change in CDR-SB per year.
METHODS
Participants
Subjects in this study were drawn from participants in a longitudinal study examining preclinical predictors of AD.10 11 Participants in the longitudinal study were recruited from the community and were required (based on inclusion criteria) to be non-demented; free of significant underlying medical, neurological or psychiatric illness (based on a comprehensive clinical evaluation13 and standard laboratory tests); and to have a CDR rating of normal (CDR = 0) or mildly impaired (CDR = 0.5).14 Individuals with evidence of major vascular risk factors (eg, atrial fibrillation, insulin dependent diabetes, cerebral infarcts, etc) were excluded. All but seven of the participants were 65 years of age or older at the time of enrolment.
At baseline, the study procedures included a medical evaluation (ie, medical history and physical examination, ECG and standard laboratory tests), a semi-structured interview, neuropsychological testing, and MRI and single photon emission computed tomography scans. Subjects were followed annually, as described below. All subjects and their informants provided informed consent at the time of enrolment in accordance with the guidelines of the Human Research Committee of the Massachusetts General Hospital (Boston, Massachusetts, USA).
Assessment and grading of clinical status
A semi-structured evaluation was administered annually to quantify the degree of clinical impairment. The evaluation, which was used to generate an overall CDR rating and CDR-SB, was based on the initial subject protocol used in the development of the CDR scale.14 15 It includes a set of questions regarding cognitive function in daily life, asked of the subject and an informant (eg, family member, friend), and a standardised neurological, psychiatric and mental status evaluation of the subject.
Diagnostic assessment
A consensus diagnosis was assigned to participants who developed significant cognitive and functional impairment, incorporating clinical history, medical records, laboratory evaluation and neuroimaging studies.16 17 Individuals with dementia were classified as AD or another diagnostic entity (eg, frontotemporal dementia, multi-infarct dementia, etc), according to standard clinical research criteria.17-19
To capture the broadest range of MCI, non-demented individuals with CDR 0.5 were operationally defined as MCI as we and others have done previously.10 20-22 To be included in the present study, participants had to meet the following criteria for MCI, in that they had: (1) a memory complaint corroborated by an informant, (2) normal general cognitive function, (3) normal activities of daily living, (4) an overall CDR rating of 0.5 with at least 0.5 in the memory subcategory and (5) no dementia. Given our interest in including individuals at the very mild end of the MCI spectrum, as in previous studies,10 20 22 we did not require that subjects perform below specific cut-offs on psychometric testing. In addition, to be included in this analysis, subjects must have been clinically followed for at least 4 years after fMRI scanning.
Clinical outcome measures
For this study, a semi-quantitative (ordinal) clinical measure of the degree of cognitive decline was derived by subtracting the CDR-SB score based on the clinical evaluation that occurred closest to the date of the scan from the most recently available CDR-SB score based on our annual clinical evaluation. This primary outcome measure represents worsening of cognitive symptoms in daily life. In this study, as we were interested in grading the degree of cognitive decline, we used this difference score rather than a binary indicator of the presence or absence of decline. We also evaluated the approximate rate of decline by dividing the change in CDR-SB by the number of years of follow-up.
Imaging parameters
Subjects were scanned on one of two 1.5 T scanners: a General Electric (GE) Signa (Advanced NMR Systems, Wilmington, Massachusetts, USA) scanner or a Siemens Sonata (Siemens Medical Systems, Erlangen, Germany). We have previously reported no systematic effect of scanner type in these data.10
During a single imaging session, T1 weighted anatomical images (GE SPGR sequence: TR/TE 35/5 ms, field of view 240, FA 45°, 124 coronal slices, thickness 1.5 mm, matrix 256×256; Siemens MP-RAGE sequence: TR/TI/TE 2730/1000/3 ms, field of view 256; FA 7°, 128 sagittal slices, thickness 1.33 mm, matrix, 192×256) and blood oxygen level dependent functional data (GE asymmetric spin echo sequence: TR/TE 2500/70 ms, FA 90°, 20 slices, 7 mm thick with 1 mm gap, voxel dimensions 3.125 mm2; Siemens gradient echo T2* sequence: TR/TE 2500/40 ms; FA 90°, 29 slices, 5 mm thick with 1 mm gap, voxel dimensions 3.125 mm2) were acquired. Functional data were acquired in an oblique coronal orientation, perpendicular to the anterior-posterior commissure line, to maximise in-place resolution in the hippocampus. Each functional run lasted 4 min and 15 s (102 time points). The first four time points of each run were acquired for T1 stabilisation and discarded before analysis.
Stimuli and cognitive task
While being scanned, subjects performed a scene encoding task described in detail in Dickerson and colleagues.10 The task consisted of three conditions that were alternated in blocks during each scanning run: (1) fixation: subjects viewed a white fixation cross-hair on a black background; (2) novel: subjects viewed 12 novel scenes per block and were asked to try to remember them; (3) repeated: subjects viewed four scenes, previously viewed during a practice trial, repeated in the same order, three times each per block. Each of six scanning runs consisted of the following blocks: fixation (6 s), novel (36 s), fixation (24 s), repeated (36 s), fixation (24 s), novel (36 s), fixation (24 s), repeated (36 s), fixation (6 s). Before these scanning runs, subjects underwent a practice run that was not scanned to assure that they could see the stimuli clearly, and to familiarise them with the scenes that would later be used in the “repeated” condition. The visual scenes, presented for 3 s each, consisted of 148 complex colour pictures (four repeated scenes, 144 novel scenes) obtained from a commercial collection of digitised photographs (Corel Corporation, Dallas, Texas, USA) and were presented using a standard fMRI projection system. Approximately 20 min after scanning, subjects performed a recognition memory test of the novel scenes.
fMRI data analysis
Data were preprocessed using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK) for Matlab (The Mathworks, Inc, Natick, Massachusetts, USA). Functional data were realigned using INRIAlign, a motion correction algorithm unbiased by local signal changes.23 24 The data were then normalised to the standard SPM2 EPI template and resampled into 3 mm isotropic resolution in MNI space. Data were then smoothed using a 5 mm Gaussian kernel. A high pass filter of 120 s was used to remove low frequency signal (eg, drifts across run). The data were modelled with a fixed response (box-car) and convolved with the canonical haemodynamic response function.
To identify memory related brain regions functionally engaged by the task, SPM2 activation maps of the novel condition versus the repeated condition were generated for each subject. To test for the difference between novel versus repeated conditions across the group, the subject level SPM2 maps were entered into a random effects model. Clusters of activation were considered significant if the voxels within the cluster survived a threshold of p<0.001 uncorrected.
To test the main hypothesis that the magnitude of hippocampal activation could predict the degree of subsequent cognitive decline, a mask was first created of the areas within the hippocampal formation that were engaged by this task at the group level in the novel versus repeated contrast (fig 1). The purpose of this mask was to functionally localise the specific areas within the hippocampal formation that responded to the paradigm. As this functional region of interest (ROI) was generated at the group level, it was unbiased with respect to the within group variables of interest in this study. The beta values (parameter estimates of per cent signal change) were then extracted from each subject's novel versus repeated contrast image, after applying this functional ROI mask, and entered into a linear regression model in SPSS as the independent variable. The goal of this model was to predict change in CDR-SB (the dependent variable). This procedure of extracting beta values from functional masks has been performed in other studies to run regression analyses.25 26 To test the hypothesis that hippocampal activation would predict subsequent decline even after controlling for other variables thought to be predictors of decline, the regression model was also run after several covariates were entered. These variables included age at time of scan, years of education, amount of time between scan and final CDR-SB measurement, CDR-SB at time of scan (baseline clinical impairment) and hippocampal volume at the time of the scan. Functional activation was considered to be a significant predictor in these regression models if its significance in the model survived a threshold of p<0.05.
Figure 1.
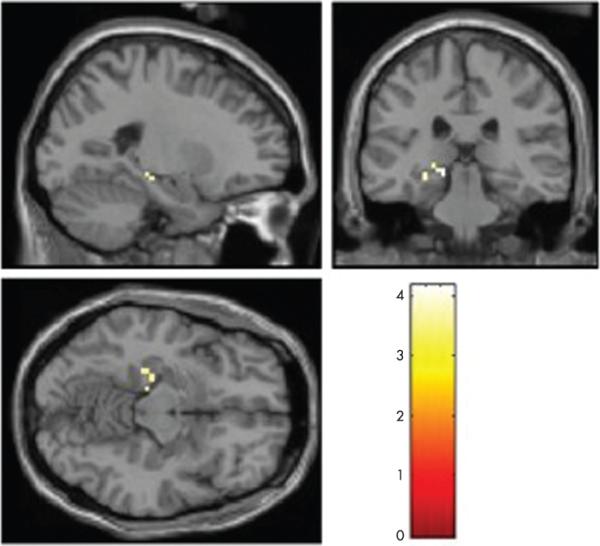
Hippocampal region from which percentage signal change was extracted in hypothesis driven analysis. Recruitment of a portion of the hippocampal formation during performance of this task is thought to occur, at least in part, because of the learning of new information. This activated portion of the hippocampal formation (as identified using the novel versus repeated contrast) was used as a functional region of interest for the present study to investigate whether hippocampal activation predicts cognitive decline. Left side of the brain is shown on the left of the image.
For exploratory purposes, a whole brain SPM analysis was also performed. To test whether activation differences between the novel versus repeated conditions in other areas of the brain could predict subsequent cognitive decline, subject level whole brain SPM2 maps of the novel versus repeated contrast were entered into a simple regression analysis with change in CDR-SB as the covariate. Clusters were considered significant at p<0.001 uncorrected.
Structural MRI analysis
For the purposes of measuring hippocampal volume, T1 structural data were processed through a semi-automated anatomical reconstruction and labelling procedure,27-29 the output of which provides labels indicating which voxels are part of the hippocampal formation (using Freesurfer software, http://surfer.nmr.mgh.harvard.edu/). Hippocampal labels were visualised using the Freesurfer tkmedit tool in multiple planes, and were edited primarily in the coronal orientation to conform to our laboratory's anatomical protocol.2 10 22 30 The volumes of these labels were extracted and divided by total intracranial volume to adjust for head size differences between subjects.
RESULTS
Clinical status of subjects
Twenty-five subjects met the inclusion criteria for this study, in that they met criteria for MCI, had undergone an fMRI scan of sufficient quality for this analysis and had been followed longitudinally for at least 4 years after the scan. At the baseline visit, all subjects were non-demented with an overall CDR of 0.5. CDR-SB scores ranged from 0.5 to 3.5 (at least 0.5 in the memory subcategory). Over an average of 5.9 (1.2) years of follow-up, the degree of cognitive decline in these participants varied from no change to a CDR-SB change of 6.0. Fourteen of the 25 subjects were diagnosed with probable AD. Subject demographics and clinical measures are shown in table 1.
Table 1.
Clinical characteristics and demographics of the subjects
| Variable | Mean (SD) (range) |
|---|---|
| Age (y) | 73.2 (5.3) (65−86) |
| Education (y) | 15.8 (3.1) (12−21) |
| Sex (M/F) | 14/11 |
| MMSE (baseline) | 29.4 (0.71) (27−30) |
| CDR-SB (baseline) | 1.66 (0.33) (0.5−3.5) |
| Follow-up (y) | 5.93 (1.15) (4−8) |
| Change in CDR-SB | 2.22 (1.79) (0.0−6) |
| Rate of change in CDR-SB/year | 0.39 (0.32) (0.0−1.0) |
CDR-SB, Clinical Dementia Rating Sum-of-Boxes; MMSE, Mini-Mental State Examination.
Hippocampal region engaged by this fMRI task
Areas demonstrating greater activation for novel than repeated scenes at the group level included a number of regions activated by similar tasks,26 31 32 including the bilateral occipital, bilateral fusiform and ventral temporal, bilateral parahippocampal cortices, and the left hippocampal formation (voxel threshold of p<0.001 uncorrected and cluster threshold of p<0.05 correcting for multiple comparisons across the whole brain). As seen in fig 1, results from a one sample t test showed significantly greater activation for novel scenes than repeated scenes in a region within the left hippocampal formation (peak MNI coordinates −15, −30, −6). These voxels were used to create the hippocampal functional ROI for the a priori hypothesis driven analysis described next.
Relationship of hippocampal activation to future cognitive decline
To test the hypothesis that hippocampal activation would predict subsequent cognitive decline, we first extracted beta values from the novel versus repeated contrast image for each subject within the hippocampal functional ROI mask. These values represent parameter estimates of the magnitude of differential activation between the two conditions (novel > repeated). The extracted hippocampal beta values were entered as an independent variable into a linear regression model in SPSS to predict change in CDR-SB. As can be seen from fig 2, greater hippocampal activation predicted a greater degree of cognitive decline (β = 0.45, p = 0.02, r2 = 0.21). To further assess whether this relationship would be present after controlling for other variables (ie, baseline CDR-SB, age, education, gender, APOE status, scanner type, number of years of follow-up, hippocampal volume), we performed second linear regression with these variables entered as covariates. Again, the main effect of hippocampal activation was still found to be a significant predictor of change in CDR-SB (β = 0.62, p = 0.01). Although all of the covariates were forced into this second model, the only one that contributed significantly was the presence of an APOE4 ε4 allele (β = 0.57, p = 0.002), with a trend for hippocampal volume (β = −0.47, p = 0.07; p values of all other variables >0.35).
Figure 2.
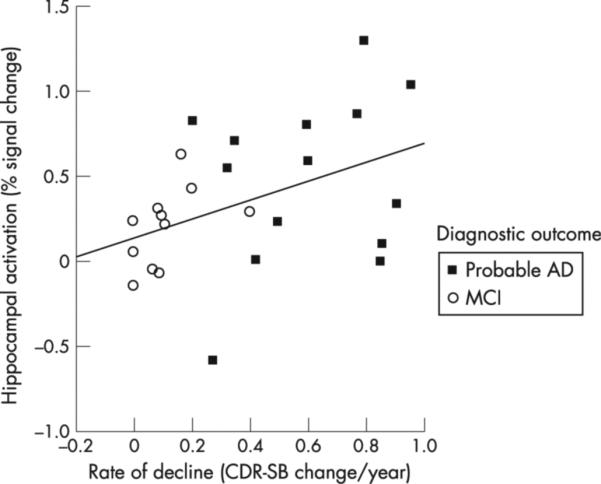
Greater hippocampal activation at the time of functional MRI (MRI) scanning predicts greater cognitive decline over the ensuing ∼6 years. Scatterplot shows, on the y axis, parameter estimates (representing per cent signal change) of differential hippocampal activation in novel versus repeated contrast, extracted from the hippocampal region of interest shown in fig 1. The x axis shows estimated rate of change in Clinical Dementia Rating Sum-of-Boxes (CDR SB) score per year over ∼6 years after fMRI scan in participants who remained classified as having mild cognitive impairment (MCI) and in those who were diagnosed with probable Alzheimer's disease (AD) during the follow-up interval.
We also performed separate regression models as above, but focusing on the rate of cognitive decline (change in CDR-SB/time) as the dependent variable. In a simple linear regression model, greater hippocampal activation predicted a greater rate of cognitive decline (β = 0.43, p = 0.03, r2 = 0.19). In a multivariate linear regression model controlling for the variables listed above, hippocampal activation was also a significant predictor of rate of change in CDR-SB (β = 0.57, p = 0.03). Although all of the covariates were forced into this second model, the only one that contributed significantly was the presence of an APOE4 ε4 allele (β = 0.51, p = 0.008; p values of all other variables >0.16).
Exploratory whole brain analysis
To explore whether activation differences between novel and repeated conditions in other areas of the brain predicted subsequent decline, a voxel-wise whole brain SPM2 correlation analysis was performed using the subjects’ novel versus repeated contrast maps regressed against change in CDR-SB. At p<0.001 uncorrected, the only voxels showing significant correlations were located in the left hippocampal formation (MNI coordinates −18, −24, −9, Z = 3.23, p<0.001, cluster size = 2 voxels) (fig 3). An analysis was also run exploring the data for inverse correlations (regions of lesser activity in the novel versus repeated contrast—relative deactivations—relating to the greater degree of decline), and no clusters were identified.
Figure 3.
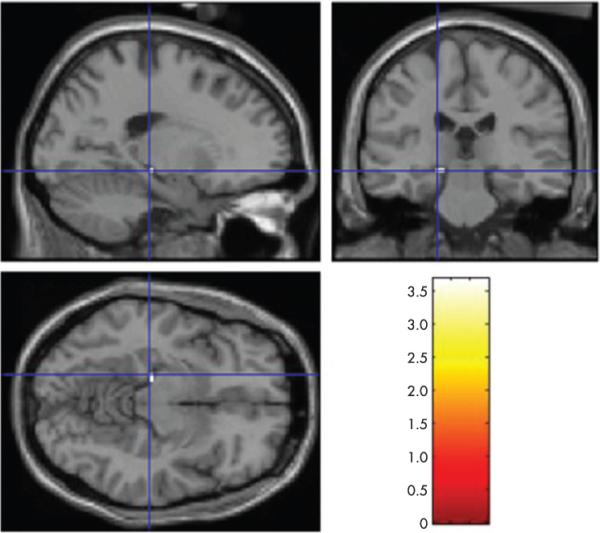
Results of exploratory whole brain analysis, showing brain regions where functional activation predicts cognitive decline. Exploratory whole brain analysis is convergent with a priori analysis, indicating that the hippocampal formation is the only place where activation predicts cognitive decline (p<0.001).
DISCUSSION
This study provides evidence that an fMRI measure of hippocampal activation represents a quantitative imaging biomarker that predicts the degree to which individuals with MCI will demonstrate future cognitive decline, as well as the approximate rate of decline over the ensuing 6 years. Hippocampal activation is predictive of decline even after controlling for the degree of cognitive impairment at the time of scanning, age, education, gender, APOE status and hippocampal volume. As some individuals who declined were ultimately diagnosed clinically with probable AD, this finding suggests that relatively greater physiological activity of the hippocampal formation during memory task performance is present early in the course of prodromal AD, when symptoms are clinically detectable but mild enough that individuals are not considered to be demented.
Within this sample of MCI subjects, because relatively greater hippocampal activation precedes a relatively steeper slope of cognitive decline, we hypothesise that hippocampal hyperactivation may represent an attempted compensatory response to accumulating neurodegenerative pathology,10 20 22 such as synaptic dysfunction.9 Regardless of the mechanism, however, the observation that this physiological imaging biomarker predicts not only the likelihood of decline but also the relative degree and rate of decline suggests that it has utility for identifying individuals with MCI at highest risk of imminent cognitive decline, which would be valuable for a variety of types of studies, including clinical trials of putative disease modifying therapeutic agents.
The individuals with MCI in this study were volunteers from the community who participated in our longitudinal study of memory and aging. Based on a detailed clinical evaluation at the time of scanning, all of the individuals in this study were judged clinically to have been exhibiting mild symptoms of cognitive impairment in daily life, representing a change from their own previous baseline (CDR rating 0.5). Specific neuropsychological performance cut-off scores were not used to make this determination, but memory performance in this group, on the whole, was below the performance of participants in our sample who are considered clinically normal (CDR rating 0). In our cohort and other cohorts, mildly impaired groups defined on this basis are at greater risk of future cognitive decline than groups of normal individuals.11 16 33-35
As our approach to assessment enables a semi-quantitative (ordinal) grading of the degree of impairment within MCI,11 this can also be applied to grade the degree of worsening of cognitive symptoms over time. The use of change in CDR-SB as an outcome measure enables progression of cognitive symptoms to be graded, regardless of whether individuals “cross the threshold” to dementia. Recent data demonstrating that AD neuropathology correlates with subtle memory impairment in otherwise normal individuals has led some investigators to call for more widespread use of methods to grade subtle cognitive change rather than only conversion to a more impaired diagnostic category.36-38 In this study, hippocampal activation predicted the degree and rate of cognitive decline, even after controlling for other relevant measures (age, baseline degree of impairment, education, gender, APOE status and hippocampal volume).
The limitations of this study include the relatively small sample size and the specific characteristics of the participants that reduce generalisability. These individuals, along with a relative or close friend (informant), volunteer from the community for a memory study that involves annual assessments. As such, they may be more attuned than the average person to subtle changes in memory function in daily life. In addition, this sample is relatively well educated. Some of the findings in this sample may relate to education, cognitive reserve or similar factors, which we have not directly investigated.
As disease modifying therapies for AD enter clinical trials, it will be valuable to develop and test biomarkers that can identify non-demented individuals in whom AD neuropathology may be present (eg, with imaging tracers such as amyloid-positron emission tomography) along with biomarkers that can predict a high risk for imminent cognitive decline.39 Biomarkers that can predict which individuals are likely to experience the greatest degree or rate of cognitive decline may enable these individuals to be targeted more aggressively for clinical trials of agents to try to slow or prevent this disabling progression of symptoms.
Acknowledgements
This study was supported by grants from the NIA (PO1-AG04953; K23-AG22509), the NINDS (K23-NS02189), the NCRR (P41-RR14075), the Beeson Scholars in Aging Program (American Federation of Aging Research) and the Mental Illness and Neuroscience Discovery (MIND) Institute.
We thank the staff of the MGH Gerontology Research Unit for assistance with subject recruitment and evaluation, Mary Hyde for data management, and Mary Foley and Larry White for assistance with MRI data collection. We express special appreciation to our subjects for their valuable contributions, without which this study would not have been possible.
Footnotes
Competing interests: BCD has received research support from Pfizer and Janssen. RAS has received research support from Eli Lilly, Glaxo Smith Kline and Forest Laboratories, support for clinical trials from Wyeth/Elan and Neurochem and lecture honoraria from Janssen, Novartis, Forest Laboratories and Pfizer.
Ethics approval: Ethics approval was obtained.
REFERENCES
- 1.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–7. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 2001;22:747–54. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–9. [PubMed] [Google Scholar]
- 5.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–85. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 6.Scheff SW, Price DA, Schmitt FA, et al. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63:1155–62. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- 8.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 9.Stern EA, Bacskai BJ, Hickey GA, et al. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–40. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly E, Zaitchik D, Copeland M, et al. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–80. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 12.Kryscio RJ, Schmitt FA, Salazar JC, et al. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66:828–32. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 13.Copeland MP, Daly E, Hines V, et al. Psychiatric symptomatology and prodromal Alzheimer's disease. Alzheimer Dis Assoc Disord. 2003;17:1–8. doi: 10.1097/00002093-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–14. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 15.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 16.Albert MS, Moss MB, Tanzi R, et al. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–9. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 19.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 20.Celone K, Calhoun V, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: An independent component analysis. J Neurosci. 2006;26:10222–31. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–7. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–22. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- 24.Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–84. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- 25.Gutchess AH, Welsh RC, Hedden T, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- 26.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 30.Goncharova II, Dickerson BC, Stoub TR, et al. MRI of human entorhinal cortex: a reliable protocol for volumetric measurement. Neurobiol Aging. 2001;22:737–45. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 31.Sperling RA, Bates JF, Cocchiarella AJ, et al. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14:129–39. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern CE, Corkin S, Gonzalez RG, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:8660–5. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64:862–71. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 34.Dickerson BC, Sperling RA, Hyman BT, et al. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1443–50. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storandt M, Grant EA, Miller JP, et al. Progression in mild cognitive impairment (MCI) and PreMCI: A comparison of diagnostic criteria. Neurology. 2006;67:467–73. [Google Scholar]
- 36.Barnes LL, Schneider JA, Boyle PA, et al. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–5. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 38.Lippa CF, Morris JC. Alzheimer neuropathology in nondemented aging: keeping mind over matter. Neurology. 2006;66:1801–2. doi: 10.1212/01.wnl.0000234879.82633.f3. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson BC, Sperling RA. Neuroimaging biomarkers for clinical trials of disease-modifying therapies in Alzheimer's disease. Neurotherapeutics. 2005;2:348–60. doi: 10.1602/neurorx.2.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]


