Abstract
We studied the development of chemosensitivity during the neonatal period in rat Nucleus tractus solitarii (NTS) neurons. We determined the percentage of neurons activated by hypercapnia (15% CO2) and assessed the magnitude of the response by calculating the chemosensitivity index (CI). There were no differences in the percentage of neurons that were inhibited (9%) or activated (44.8%) by hypercapnia or in the magnitude of the activated response (CI 164±4.9%) in NTS neurons from neonatal rats of all ages. To assess the degree of intrinsic chemosensitivity in these neurons we used chemical synaptic block medium and the gap junction blocker carbenoxolone. Chemical synaptic block medium slightly decreased basal firing rate but did not affect the percentage of NTS neurons that responded to hypercapnia at any neonatal age. However, in neonates aged <P10, but not in older neonates, chemical synaptic block medium increased CI. Carbenoxolone did not significantly alter the number of NTS neurons activated by hypercapnia in neonatal rats of any age. In summary, the response of NTS neurons from neonatal rats appears to be intrinsic and largely unchanged throughout early development. In young neonates (<P10) chemical synaptic input reduces the magnitude of the firing rate response to hypercapnia, but otherwise neither chemical synaptic input nor gap junctions significantly alter the percentage of NTS neurons that respond to hypercapnia or the magnitude of that response.
1. Introduction
Central chemosensitive neurons in the brainstem respond to changes in the levels of CO2 and are major contributors to ventilatory control (Nattie, 1999). Studies have confirmed their presence in various sites in the brainstem including the locus coeruleus (LC), the Nucleus tractus solitarii (NTS), the medullary raphé, and the retrotrapezoid nucleus (RTN) (Dean et al., 1990; Coates et al., 1993; Nattie, 1999; Nattie, 2001; Richerson et al., 2001; Filosa et al., 2002; Mulkey et al. 2004; Ritucci et al. 2005). The ventilatory response to inspired CO2 in rats appears to undergo a postnatal development before reaching an adult ventilatory response (Abu-Shaweesh et al., 1999; Serra et al., 2001; Stunden et al., 2001; Wickström et al., 2002; Putnam et al. 2005; Davis et al., 2006). The exact form of this developmental change is not clear. We have shown that this postnatal developmental pattern of CO2 chemosensitivity may be triphasic, with a strong ventilatory response to hypercapnia at postnatal day 1 (P1—the first day after birth), decreasing to a minimum at approximately P8, and then increasing to adult levels by day P14 (Stunden et al., 2001). Others report a major increase in the response of ventilation to inspired hypercapnia starting on about day P14 (Davis et al., 2006). Regardless of the pattern, there is clearly a change in the ventilatory response to inspired hypercapnia throughout the early neonatal period of development in rats, especially when comparing rats younger than P10 with those P10 and older.
One possible explanation for this developmental pattern of the ventilatory response to hypercapnia is that the development of the neuronal sensors of hypercapnia may parallel the development of the ventilatory response to hypercapnia (Putnam et al., 2005). To date, the development of chemosensitivity has been studied in neurons from 3 brainstem regions, the medullary raphé (Wang and Richerson, 1999), the LC (Stunden et al., 2001) and the RTN (Ritucci et al., 2005). Wang and Richerson (1999) found that neurons from the medullary raphé region of the brainstem had a higher percentage of neurons that respond to hypercapnia and a larger magnitude of the firing rate response to hypercapnia in rats > P12 than in raphé neurons from younger rats, suggesting a maturation of the neuronal chemosensitive response during the first 2 weeks of life. In contrast, Stunden et al. (2001) examined the firing rate response to hypercapnia of neurons in the LC in brain slices from rats ranging in age from P1 to P16 and found at most a slight decline in the magnitude of hypercapnia-induced increased firing rate with age. Ritucci et al. (2005) reported that the percentage of RTN neurons that responded to hypercapnia and the magnitude of their firing rate response were not different in neurons in brain slices from rats younger than P10 compared to neurons from rats P10 and older. These data indicate that the response to hypercapnia is nearly fully developed at birth and does not change during the first 2 weeks of life in LC and RTN neurons.
The NTS has been suggested to be an important site of ventilatory control since ventilation is altered when the NTS is focally acidified (Coates et al., 1993; Nattie, 2001) and it contains CO2-sensitive neurons (Dean et al., 1989; Dean et al., 1990; Huang et al., 1997; Mulkey et al., 2004). However, the developmental response to hypercapnia in neurons from the NTS has not been explored. It is known that there are a number of changes in NTS neurons during early development, including morphological (Vincent and Tell, 1999) and electrophysiological (Rao et al., 1997; Vincent and Tell, 1997; Kawai and Senba, 2000) changes. Further, in no area has there been a detailed analysis of the role of synaptic input or gap junctions in the response of neurons to hypercapnia, although it has been shown that CO2-sensitive neurons from the NTS are preferentially gap junction-coupled (Huang et al. 1997).
The goal of this study, therefore, was to investigate the development of neuronal CO2 chemosensitivity in the NTS during the first three weeks of postnatal life. Both the percentage of neurons that respond and the magnitude of their response to hypercapnia were compared in NTS neurons in brainstem slices from neonatal rats in 3 different age groups (from P1 to P19). The intrinsic sensitivity of NTS neurons to hypercapnia was also studied using chemical synaptic block solution and the gap junction blocker carbenoxolone. We found that inhibition of gap junctions has no effect on either the percentage of NTS neurons that respond to hypercapnia or on the magnitude of those responses. Chemical synaptic input reduces the magnitude of the hypercapnic response of NTS neurons from young neonates (<P10) but does not affect the magnitude in older neonates (≥P10) and has no effect on the percentage of NTS neurons that respond to hypercapnia in neonates of any age. Thus, it appears that during the neonatal period, the hypercapnic response of NTS neurons is largely intrinsic and unchanged.
Preliminary reports of these findings have previously been published (Conrad et al., 2004, 2005).
2. Methods
2.1. Slice preparation
Medullary slices were prepared from neonatal Sprague-Dawley rats of postnatal (P) ages P1–19 (day P1 is the first day after the birth of the rats), as previously described (Dean et al. 1990; Ritucci et al. 1996; Dean et al., 1997; Ritucci et al., 1997). Rats aged P1–P13 were anesthetized by hypothermia and rapidly decapitated. Rats aged P14–P19 were anesthetized by CO2 and then rapidly decapitated. The brainstem was subsequently removed and placed onto a vibratome (Pelco Vibratome 1000). Transverse brainstem slices (300μm thick) containing the NTS were cut into artificial cerebrospinal fluid (aCSF) at 4–6°C. Slices were incubated at room temperature in aCSF equilibrated with 95% O2- 5% CO2 (pH ~ 7.45) until needed. All procedures involving the use of animals were in agreement with the Wright State University Animal Care and Use Committee guidelines and were approved by the committee. Wright State University is accredited by AAALAC and is covered by NIH Assurance (no. A3632-01).
2.2. Solutions
The artificial cerebral spinal fluid (aCSF) contained (in mM) 5.0 KCl, 124 NaCl, 1.3 MgSO4, 26 NaHCO3, 1.24 KH2PO4, 10 glucose, and 2.4 CaCl2. Gap junction block solutions were made from aCSF to which 100 μM carbenoxolone was added, which has been shown to block gap junctions (Dean et al. 2001). Synaptic block aCSF contained (in mM) 5.0 KCl, 119 NaCl, 11.4 MgSO4, 26 NaHCO3, 1.24 KH2PO4, 10 glucose, and 0.2 CaCl2 which has previously been shown to essentially eliminate chemical synaptic activity (Dean et al. 1990). Normocapnic solutions were equilibrated with 95% O2-5% CO2, pH ~7.45 while hypercapnic solutions were equilibrated with 85% O2-15% CO2, pH ~6.8–6.9 (all at 37°C).
Whole cell patch pipette filling solution consisted of (in mM) 130 K+- gluconate, 10 KCl, 10 HEPES, 0.4 EGTA, 1 MgCl2, 0.3 GTP, and 2 ATP. This reduced EGTA and no Ca2+ solution reduces washout of the electrical response to hypercapnia (Filosa and Putnam, 2003). Perforated patch pipette filling solution consisted of (in mM) 130 CH4O3S, 130 KOH, 20 KCl, 5 HEPES, and 1 EGTA (Rae et al., 1991). This solution filled the tip of the electrode while the rest of the electrode was backfilled with the same solution containing 240mg/mL of amphotericin B diluted in filling solution from an amphotericin B stock solution in DMSO (60mg/mL). Whole cell and perforated patch solutions were adjusted to a pH of ~ 7.35 (at 35°C) with KOH.
2.3. Recording chamber
At the time of the experiment, slices were placed in a superfusion chamber and held with a nylon grid. In large 150 ml syringes, the normocapnic solution was equilibrated with 95% O2-5% CO2, while the hypercapnic solution was equilibrated with 85% O2-15% CO2. The syringes were placed in a water bath maintained at approximately 40°C. Solution was gravity fed from the syringes through a bubble trap and a thermoelectric Peltier assembly to the superfusion chamber through stainless steel tubing (outer diameter 1.6mm, 0.5mm inner diameter) at a rate of approximately 3–5 ml/min. The temperature of the solutions within the superfusion chamber was maintained at approximately 35 ± 1°C.
2.4. Electrophysiological recordings
Whole cell and perforated patch recordings were used in this study. All electrodes were made with borosilicate glass (outer diameter 1.5mm, inner diameter 1.12mm). The tip resistance of electrodes was ~5 MΩ. Electrodes were held and maneuvered into place by a micro-manipulator (Narishige MX-1). Pipette filling solutions (see solutions above) correlated with the respective patch recording technique. NTS neurons were visualized with an upright microscope (Nikon Optiphot-2) rigged for IR-video microscopy using an X40 water-immersion objective (Hoffmann Modulation Contrast optics; N.A. 0.55, 3.0-mm working distance). Current was injected during each experiment by an amplifier (Axopatch 1D) controlled by a timer (Winston A-65). Whole cell patch experiments began once a giga-ohm patch was sealed, action potentials were approximately 60mV high, and a stable Vm baseline and firing rate (~1Hz) were established. Perforated patch recordings began once action potentials were larger than 10 mV and a stable baseline-firing rate was established. The data from a neuron were used only if the initial firing rate was between 0.2 and 4 Hz, to prevent extremely low or high values from affecting the determination of chemosensitivity index (see below). This resulted in the exclusion of at most 10% of the neurons studied regardless of age. In each case, only 1 neuron per slice was used. For perforated patch experiments, at total of 112 slices from 75 neonatal rats were used (~1.5 slice/rat) and for whole cell experiments, a total of 120 slices from 73 neonatal rats were used (~1.6 slice/rat). It is possible that our percentages of activated neurons could be skewed by this approach, if one type of NTS neuron (say hypercapnia-activated) were more easily patched than non-chemosensitive or inhibited neurons. However, the percentages of activated NTS neurons that we report here (40–50%) are similar with both perforated and whole cell patch techniques, and agree with previously reported values for NTS neurons in neonates using perforated patch techniques (Huang et al., 1997) and in adults using either whole cell patch techniques (Nichols et al. 2008a; 2008b) or sharp-tipped microelectrodes (Mulkey et al., 2003).
2.5. Data analysis
Membrane potential and firing rate were recorded on a personal computer using Axoscope pClamp 8 interface and software (Axon Instruments, Inc.). The firing rates during normocapnia and hypercapnia were collected by a window discriminator (FHC) using 10 second bins to define the integrated firing rate in Hz (action potentials/second). The firing rates were then averaged using Excel (Microsoft Office 97). Individual neurons were categorized as activated, non-sensitive, or inhibited. Under hypercapnic conditions, neurons reaching a firing rate of more than 20% above the control firing rate under normocapnic conditions were considered activated, neurons that did not alter their control firing rate by more than 20% were considered nonchemosensitive, and neurons that decreased their firing rate by more than 20% of the control firing rate were considered inhibited. While we recognize that the 20% change is an arbitrary value, we used it for comparison with the same criterion used in previous studies (Mulkey et al., 2003). Regardless of whether we used perforated or whole cell patch techniques, at most only 5–6% of the neurons designated as activated had values slightly above the 20% criterion, while all other activated neurons increased their firing rate by over 40%. NTS neurons were further categorized into age groups based on the age of the rat from which the slice was taken: P1–P4, P5–P9, and P10–P19. In some cases, to maintain large sample sizes, the cells were divided into 2 age groups based on the age of the rat from which the slice was taken: < P10 and ≥ P10. The magnitude of the firing rate response to hypercapnia was quantified using the chemosensitivity index (CI), calculated using the equation from Wang and Richerson (1999):
where FRhc is the firing rate in hypercapnic solution (15% CO2), FRnc is the firing rate in normocapnic solution (5% CO2), pH5 is the solution pH equilibrated with 5% CO2 and pH15 is the solution pH equilibrated with 15% CO2.
2.6. Statistics
The numbers of activated, non-sensitive, and inhibited neurons from each age group were compared using contingency tables with Fisher’s exact tests. Chemosensitivity index values were compared using ANOVA with multiple paired comparisons being done using Tukey pairwise comparisons. The percentage of neurons that were activated or inhibited with carbenoxolone were compared using Fisher’s exact tests and the mean values for CI from experiments using synaptic block and carbenoxolone were compared using paired t tests. All tests were considered significantly different with a P < 0.05.
3. Results
3.1 Basic properties of NTS neurons
NTS neurons were spontaneously firing which made determination of initial Vm problematic. In general, initial Vm for all neurons, regardless of technique used, was between −40 and −60 mV (e.g. see Fig. 1). The initial spontaneous firing rate also did not differ among the techniques used or the age of the animal from which the slice was taken, and averaged 1.29 ± 0.092 Hz (n = 232).
FIGURE 1.
A hypercapnia-activated (A) and a hypercapnia–inhibited (B) NTS neuron. (A) Whole cell recording of a hypercapnia-activated NTS neuron in control medium (equilibrated with 5% CO2), experimental medium (equilibrated with 15% CO2), and then back to control medium. The top panel shows action potentials while the bottom panel shows integrated firing rate (action potentials/s, collected in 10 s bins) in Hz. As shown, when the neuron is exposed to hypercapnia, the membrane potential depolarizes and the integrated firing rate increases. Upon return to normocapnic medium, the responses reverse. (B) Whole cell recording of a hypercapnia-inhibited NTS neuron in control medium (equilibrated with 5% CO2), experimental medium (equilibrated with 15% CO2), and then back to control medium. The top panel shows action potentials while the bottom panel shows the integrated firing rate (action potentials/s, collected in 10 s bins) in Hz. As shown, when the neuron is exposed to hypercapnia, the membrane potential hyperpolarizes slightly and the integrated firing rate decreases. These effects are reversible upon re-exposure to normocapnic medium.
3.2. Percentage of chemosensitive NTS neurons as a function of age
Three different types of NTS neurons could be distinguished based on their response to hypercapnia. Neurons that were activated showed an increase in integrated firing rate by more than 20% when switched from aCSF equilibrated with 5% to one equilibrated with 15% CO2 (Fig. 1A). This increased firing rate was reversible upon return to normocapnic (5% CO2) solution (Fig. 1A). Other NTS neurons had their firing rate inhibited by hypercapnia, with integrated firing rate decreasing reversibly by over 20% when exposed to 15% CO2 solutions (Fig. 1B). Finally, in many NTS neurons, the integrated firing rate was unaffected (i.e. it did not change by more than 20%) upon exposure to hypercapnic (15% CO2) solution. Based on their firing rate response to hypercapnic solution, NTS neurons were assigned to one of three groups: activated, inhibited or non-sensitive.
We used two electrophysiological recording techniques to collect data from NTS neurons of neonatal rats: perforated patch and whole cell recordings. We initially used perforated patch techniques to avoid any effects of washout of the electrical response to hypercapnia when using whole cell pipettes (Dean and Reddy, 1995; Richerson, 1995). The results from these studies are shown in Figure 2A. We found that 44–51% of the neurons were activated by hypercapnia in each of three age groups (P0–P4, P5–P9, and P10–P19, see Fig. 2A). A similar percentage of neurons (41–50%) were non-sensitive in each of the three age groups. A small percentage of NTS neurons (6–12%) in each age group were inhibited by hypercapnia. No significant differences (P = 0.83) in the percentages of each type of neuron were found among the three age groups, suggesting no change in the percentage of chemosensitive NTS neurons during neonatal development.
FIGURE 2.
Summary of results of the firing rate responses of NTS neurons to hypercapnia measured in neonatal rats of different ages. (A) Responses measured with perforated patch techniques. (B) Responses measured with whole cell techniques. (C) Combined responses measured with perforate patch and with whole cell techniques. Neurons were divided into one of three age groups based on the age of the rat from which the slice was taken (P0–P4, P5–P9, or P10–P19). The percentage of non-sensitive (white bars), activated (gray bars) and inhibited (black bars) NTS neurons in each age group is shown. The total number (N) of neurons in each group is given in the bar. For each type of neuron, its percentages in the three age groups were compared using a contingency table with Fisher’s exact test.
The results of recordings collected using the whole cell patch technique are shown in Figure 2B. We used a modification of the whole cell pipette filling solution (reducing EGTA and eliminating Ca2+) which was shown to prevent washout of the electrical response to hypercapnia in LC neurons (Filosa and Putnam, 2003). This technique thus allowed the use of whole cell electrodes to study the chemosensitive response of NTS neurons. We found that 41–44% of NTS neurons were activated and 8–12% were inhibited upon exposure to hypercapnia in all neonatal age groups (Fig. 2B) and these proportions were similar to values found with perforated patch pipettes (compare Figs. 2A and B). No significant differences (P=0.99) in the percentages of neurons were found among the age groups when measured with whole cell patch electrodes. Thus, once again we see no developmental changes in the percentage of chemosensitive NTS neurons. Further, the similarity of these data to those collected with perforated patch pipettes shows that our technique prevented washout of the chemosensitive response in NTS neurons.
To best characterize the development of chemosensitivity in NTS neurons, we combined the results from perforated and whole cell patch studies into one group. The percentages of non-sensitive, activated and inhibited neurons in each neonatal age group are shown in Figure 2C. Once again, we see that the percentage of hypercapnia-activated neurons does not vary significantly with age (P=0.94) comprising 43–47% of NTS neurons regardless of age (from P0–P19). The percentage of hypercapnia-inhibited NTS neurons also did not differ significantly with age, comprising about 8–12% of NTS neurons (Fig. 2C).
3.3. Magnitude of the response of chemosensitive NTS neurons as a function of age
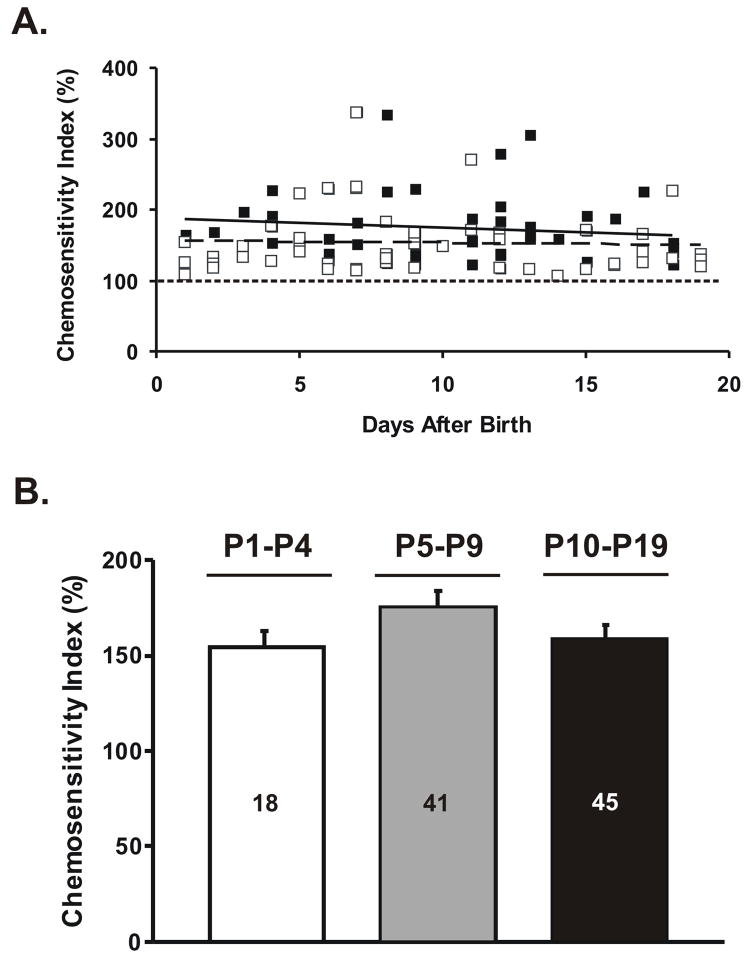
A chemosensitivity index (CI) value was calculated for each neuron that was activated or inhibited by hypercapnia. These values were used to compare the magnitude of the hypercapnic response in neurons from rats of different age. CI values from perforated patch recordings were: P0–P4 = 134 ± 5.7% (n=11), P5–P9 = 168 ± 12.8% (n=20), and P10–P19 = 149 ± 8.6% (n=21), none of which were significantly different (P=0.10). CI values from the whole cell recordings were somewhat higher and were: P0–P4 = 184 ± 9.5% (n=7), P5–P9 = 176 ± 13.9% (n=21), and P10–P19 = 171 ± 9.6% (n=24), none of which were significantly different (P=0.25). To assure that pooling these data by age did not obscure short term developmental changes in CI, we made a scatter plot of the CI values vs. age measured with perforated patch and whole cell techniques (Fig. 3A). There was no significant relationship between age and CI for either technique and no obvious short term differences (Fig. 3A). Pooling all data across age for each technique yielded CI values of 153 ± 6.3% (n=52) using perforated patch and 175 ± 7.2% (n=52) using whole cell recordings. CI measured with whole cell recordings were significantly (P=0.025) higher than CI values measured with perforated patch techniques, although we have no explanation for this modest difference.
FIGURE 3.
The magnitude of the response of hypercapnia-activated NTS neurons, measured as the chemosensitivity index (CI), as a function of age. (A) A scatter plot of all CI data vs. the age of the rat from which the slice was taken measured with the perforated patch technique (open squares) or with the whole cell technique (filled squares). The dotted line is the least squares regression line fit to the perforated patch data (CI = −0.26(age) + 156; r2 = 0.001) and the solid line is the least squares regression line fit to the whole cell data (CI = −1.35(age) + 188; r2 = 0.012). In neither case is there a significant relationship between CI and the age of the neonatal rat. (B) The combined CI values for both perforate patch and whole cell techniques pooled into three age groups. The total number (N) of neurons in each group is given in the bar. The height of each bar represents the mean CI for that age group and the error bars represent 1 SEM. Once again there was no significant relationship between CI and the age of the neonatal rat.
We combined CI values across techniques into a single data set for each age group (Fig. 3B). There were no statistical differences (P=0.15) among the CI values across the neonatal age groups (Fig. 3B). These data indicate that the magnitude of the chemosensitive response of activated NTS neurons also does not vary with age throughout neonatal development.
There are far fewer CO2-inhibited neurons but based on the few at each age group, the CI does not appear to change with age but is between about 53 and 72%. The overall average CI of inhibited neurons measured with perforated patch recordings is 56 ± 5.8% (n=9) and with whole cell recordings is 68.8 ± 4.5% (n=12). These values are not statistically different (P=0.1). Thus, it appears that CO2 inhibited neurons are fully developed at birth and do not show appreciable development throughout the neonatal period.
3.4. Effect of synaptic block medium on the development of the hypercapnia response of NTS neurons
We were concerned that some fraction of the NTS neurons that responded to hypercapnia with an increased firing rate may not be intrinsically chemosensitive, but driven through chemical synaptic input from another neuron within the slice that was chemosensitive. To test this, we studied the response of NTS neurons to hypercapnia in the presence of synaptic block medium to eliminate all chemical synaptic input. The perforated patch technique was used for these experiments. NTS neurons were studied from two groups, based on the age of the rat from which the slice was taken: < P10 and ≥P10. In each experiment, a neuron was first shown to be chemosensitive based on its response to hypercapnia in medium without synaptic block (firing rate increased in response to hypercapnia), and the response to hypercapnia was again measured in the same neuron, this time in synaptic block medium. The results of such an experiment are shown in Figure 4. In this case, an NTS neuron whose firing rate was reversibly activated by hypercapnia in control conditions was also reversibly activated by hypercapnia in synaptic block medium (Fig. 4). In all, 5 hypercapnia-activated neurons from young neonates (<P10) and 5 from older neonates (≥P10) were studied, and in every case, these neurons were also activated by hypercapnia in synaptic block medium, suggesting that nearly all, if not all, of the neurons we find to be activated by hypercapnia are intrinsically activated, and not dependent on chemical synaptic input for that activation.
FIGURE 4.
The integrated firing rate of a hypercapnia-activated NTS neuron in control and synaptic block medium. Note that the neuron showed a reversible increase in firing rate in response to hypercapnia (15% CO2) in control medium and had a similar reversible increase in firing rate in response to hypercapnia in synaptic block medium. Note also the lower initial spontaneous firing rate in the presence of synaptic block medium. This figure is representative of 10 similar neurons, 5 from rats aged <P10 and 5 from rats aged ≥P10.
Note that the initial spontaneous firing rate tended to be lower in synaptic block medium than in control medium (Fig. 4). This was observed in most of the neurons studied. In neurons from the younger neonates (<P10), the initial firing rate decreased from 1.42 ± 0.31 Hz in control medium to 0.66 ± 0.26 Hz (n=5) in synaptic block medium and in neurons from older neonates (≥P10), it decreased from 1.44 ± 0.49 to 0.61 ± 0.16 Hz (n=5) (neither difference is significant by paired t test, P=0.1). These data suggest that the tonic synaptic input to NTS neurons in slices on balance tends to be excitatory.
We also quantified, by calculating CI, the magnitude of the hypercapnic response of NTS neurons in the presence vs. the absence of chemical synaptic block medium. The results of this analysis are shown in Figure 5. The CI values for each medium were not statistically different between the two age groups (P=0.4). However, in young neonates (<P10), the CI value in synaptic block medium (218 ± 30.2%) was significantly higher (P=0.025, paired t test) than the CI value in normal medium (159 ± 24.3%) (Fig. 5). In older neonates (≥P10) the CI values were not significantly different (P=0.1) between normal and synaptic block medium, 177 ± 30.0% vs. 226 ± 26.3%, respectively (Fig. 5). Thus, chemical synaptic input decreases the magnitude of the firing rate response to hypercapnia in NTS neurons from neonates younger than P10 but does not affect CI in NTS neurons from neonates aged P10 or older.
FIGURE 5.
Chemosensitivity index (CI) values for NTS neurons in control medium (CON) and synaptic block (SNB) medium. Note that CI was significantly (P=0.025; paired t test) higher in SNB than in control medium in neurons from young neonates (<P10) and was higher, but not significantly so, in SNB than in control medium in neurons from older neonates (≥P10). The height of each bar represents the mean CI and the error bar represents 1 SEM. Each bar represents the results from 5 neurons and in each age group, the same 5 neurons were used to collect values in both normal and synaptic block medium.
3.5. Effect of carbenoxolone on the development of the hypercapnia response of NTS neurons
The effects of blockage of gap junctions by carbenoxolone on both the magnitude and the percentage of the response of NTS neurons to hypercapnia were studied. It has previously been shown that chemosensitive NTS neurons are preferentially coupled with gap junctions (Dean et al. 1997; Huang et al. 1997) but it is not clear what the role of gap junction coupling is in chemosensitivity (Dean et al., 2002). The response of an NTS neuron to hypercapnia was first determined in aCSF. It was then exposed to carbenoxolone and its response to hypercapnia tested again. Carbenoxolone did not significantly alter the percentage of NTS neurons that were activated or inhibited by hypercapnia either in rats aged <P10 (P=0.08; contingency table with Fisher’s exact test) or in rats aged P10 or older (P=1.0) (Fig. 6). These data suggest that gap junction coupling does not play a major role in the intrinsic response to hypercapnia of NTS neurons, although there is a trend for fewer activated neurons in the younger neonates (<P10) (Fig. 6). Gap junctions also do not appear to play a role in the magnitude of the response of chemosensitive NTS neurons to hypercapnia. We examined a sub-group of 6 neurons from rats aged <P10 and 12 neurons from rats aged ≥P10. For each neuron we had both a control response to hypercapnia and one in carbenoxolone so we could compare the responses with a paired t test. Carbenoxolone did not have a significant effect (P=0.35) on the CI in response to hypercapnia in neurons from neonatal rats of all ages (Fig. 7). Further, carbenoxolone did not appear to alter the magnitude of the CI in NTS neurons that are inhibited by hypercapnia. In rats age <P10, the CI of inhibited NTS neurons was 68 ± 11% and 70 ± 7% (n=8) in the absence and presence of carbenoxolone, respectively, and in rats age ≥P10, the CI of inhibited NTS neurons was 68 ± 5% and 52 ± 11% (n=5) in the absence and presence of carbenoxolone, respectively.
FIGURE 6.
The effect of carbenoxolone on the percentage of neurons that responded to hypercapnia in neurons from young (P1–P9) (A) and older (P10–P19) (B) neonatal rats. (A) The percentage of NTS neurons that did not change firing rate (white bars), that were activated by (gray bars) and that were inhibited by (black bars) hypercapnia is shown for young (P1–P9) neonatal rats in the absence (CONTROL) and the presence (CARBENOXOLONE) of 100 μM carbenoxolone. Although there was a slight decrease in the number of NTS neurons activated by hypercapnia in the presence of carbenoxolone it was not significant (P=0.08; contingency table with Fisher’s exact test). (B) The percentage of NTS neurons that did not change firing rate (white bars), that were activated by (gray bars) and that were inhibited by (black bars) hypercapnia is shown for older (P10-P19) neonatal rats in the absence (CONTROL) and the presence (CARBENOXOLONE) of 100 μM carbenoxolone. Note that the percentage of neurons that responded to hypercapnia was unaffected by carbenoxolone in these older neonates. In both figures, the total number (N) of neurons in each group is given in the bar.
FIGURE 7.
Chemosensitivity index (CI) values for NTS neurons in control medium (CON) and in the presence of 100 μM carbenoxolone (CARB) containing medium. Note that CI was the same in neurons from young (<P10) vs. older (≥P10) neonates and was unaffected by carbenoxolone. The height of each bar represents the mean CI and the error bar represents 1 SEM. The total number (N) of neurons in each group is given in the bar.
4. Discussion
The main finding of this study is that throughout neonatal development both the percentage of neurons that respond to hypercapnia and the magnitude of their response is relatively constant. Interestingly, gap junctions do not appear to alter the chemosensitive response in NTS neurons from neonates of any age. Chemical synaptic input appears to reduce the magnitude of the chemosensitive response of NTS neurons from young (<P10) but not older (≥P10) neonates, and has no effect on the percentage of NTS neurons that respond to hypercapnia. Our findings suggest that the chemosensitive response of NTS neurons is largely intrinsic and nearly fully developed at birth.
4.1. Perforated patch vs. whole cell recordings
In this study we used both perforated patch and whole cell recording techniques. It has previously been reported that the use of whole cell recordings results in washout of the electrical response to hypercapnia in neurons from the raphé (Richerson, 1995), the LC (Filosa and Putnam, 2003), and especially the NTS (Dean and Reddy, 1995; Mulkey et al., 2004). We have previously shown that this washout can be largely prevented in LC neurons by lowering the EGTA and eliminating the added Ca2+ in the whole cell pipette filling solution (Filosa and Putnam, 2003). In the current study, we found a similar percentage of chemosensitive NTS neurons using either whole cell or perforated patch techniques (Fig. 2), indicating that the modified pipette filling solution prevents washout of the chemosensitive response in NTS neurons. Thus, whole cell patch techniques can be used to study the chemosensitive response of NTS neurons as well as LC neurons.
4.2. Synaptic input to NTS neurons
The nature of the neural network in the NTS is not fully understood but it is likely that NTS neurons receive both inhibitory and excitatory inputs (Kawai and Senba, 2000). While we do not know what the balance is in vivo, the fact that chemical synaptic block medium tended to decrease firing rate of NTS neurons in our slices suggests that excitatory chemical synaptic input may be somewhat stronger in NTS neurons from neonates (e.g. Fig. 4). A reduction in tonic firing of NTS neurons from adult rats in chemical synaptic block medium has also been observed (Dean et al., 1990), suggesting a balance favoring excitatory input to NTS neurons from rats of all ages. However, recent work from our laboratory found a marked increase in firing rate with chemical synaptic block medium in adult rats (Nichols et al., 2008a) suggesting that the tonic excitatory input to NTS neurons in neonates may become tonic inhibitory input in adults. Interestingly, the earlier study of adult NTS neurons in chemical synaptic block medium (Dean et al., 1990) was done in transected slices, leaving only the dorsal portion of the medullary slice, whereas in our recent study (Nichols et al., 2008a) we used the intact medullary slice, as in the current study. This difference in slice preparation may account for why Dean et al. (1990) found that chemical synaptic block medium resulted in decreased firing while Nichols et al. (2008a) found that it resulted in increased firing in adult NTS neurons. If this is the case, it suggests that inhibitory chemical synaptic input to adult NTS neurons may derive from ventral medullary neurons that would normally lie within the slice but were removed in the transected, dorsal medullary slice preparation of Dean et al. (1990).
Despite this effect of chemical synaptic blockade on tonic firing properties, chemical synaptic input does not appear to be required for the chemosensitive response of most NTS neurons. All 10 neurons that increased their integrated firing rate in response to hypercapnia in the absence of chemical synaptic block medium also did so in the presence of chemical synaptic block medium (e.g. Fig. 4). In younger (<P10) neonates, chemical synaptic input reduced, but did not eliminate the magnitude of the response, determined as CI, of NTS neurons to hypercapnia (Fig. 5). This effect of chemical synaptic block medium increasing the magnitude of the response of chemosensitive NTS neurons the hypercapnia in young neonates may be due to an increased input resistance of these neurons with synaptic block, rendering them more excitable. However, chemical synaptic input had no significant effect on the CI of NTS neurons from older (≥P10) neonates (Fig. 5). These findings indicate that virtually all, if not all, chemosensitive NTS neurons are intrinsically chemosensitive with respect to chemical synaptic input. The lack of a need for chemical synaptic input for the chemosensitive response of brainstem neurons has previously been reported for neurons from the medullary raphé (Richerson, 1995), from the LC (Kawai et al., 1996), and from both neonate (Huang et al., 1997) and adult (Dean et al., 1990) NTS neurons. In neonates, Huang et al. (1997) found that chemical synaptic block medium only reduced by a small amount the percentage of hypercapnia-activated NTS neurons, and in adults 90% of the neurons that were activated by hypercapnia also were activated by hypercapnia in chemical synaptic block medium (Dean et al., 1990), supporting our findings that nearly all chemosensitive NTS neurons remain chemosensitive in the absence of chemical synaptic input.
4.3. Role of gap junctions in the chemosensitive response of NTS neurons
Our findings show that gap junctions do not appear to play a major role in the chemosensitive response of NTS neurons from neonatal rats. This is based on the use of carbenoxolone, which is a widely used gap junction blocker (Dean et al., 2001; Parisian et al., 2004; Rekling and Feldman, 1997) that has additional effects unrelated to gap junction coupling. It has been shown to inhibit a key enzyme in glucocorticoid metabolism (Zhang et al., 2006), to uncouple oxidative phosphorylation (Pivato et al., 2006), to inhibit voltage-gated Ca2+ channels (Vessey et al., 2004) as well as other non-specific effects (Rouach et al., 2003). However, chemosensitive neurons from the NTS have been shown to be extensively anatomically coupled with evidence of electrical coupling (Dean et al., 1997; Huang et al., 1997). Moreover, neurons in the NTS contain the proteins that constitute the gap junctions, connexins Cx26 and Cx32 (Solomon et al., 2001) and Cx36 (Solomon, 2003). Connexin expression has a complex developmental pattern that varies with the specific connexin (Dermietzel et al., 1989; Belliveau and Naus, 1995; Solomon et al., 2001). The potential role of gap junctions in chemosensitive neurons has been discussed but is not clear (Dean et al., 2001; Dean et al., 2002; Solomon and Dean, 2002), although focal inhibition of gap junctions in NTS neurons has been shown to reduce the ventilatory response to inspired CO2 in young (<10 weeks), but not old (>10 weeks) rats (Parisian et al., 2004).
Our data suggest that gap junctions do not play a role in the expression of chemosensitivity in neurons within the NTS. We did observe a trend for a decreased percentage of NTS neurons that are activated by hypercapnia when gap junctions are blocked in young (<P10) neonates (Fig. 6), although this difference was not quite significant (P=0.08). It may be that gap junctions couple intrinsically chemosensitive neurons with non-chemosensitive neurons to amplify the chemosensitive response in the NTS of very young rats, but such an effect would have to be confirmed by additional experiments. We have recently observed that gap junction coupling increases the responsiveness of locus coeruleus neurons from neonatal rats (Hartzler et al., 2007). Our data also strongly suggest that once an NTS neuron becomes intrinsically chemosensitive, gap junctions do not contribute to or alter the cell signaling machinery that allow that neuron to respond to hypercapnia since gap junction inhibition does not alter the magnitude of the response of chemosensitive NTS neurons from neonates to hypercapnia (Fig. 7).
4.4. Development of the chemosensitive response in NTS neurons
We found that the magnitude of the response of an NTS neuron to hypercapnia was fully developed at birth and did not change throughout neonatal development (Fig. 3). This magnitude could be decreased by chemical synaptic input in young neonates but this effect was lost in neonates ≥P10. This suggests that once a neuron has developed the ability to respond to hypercapnia, the cellular signaling machinery does not appear to undergo further major developmental changes. The percentage of NTS neurons that are intrinsically activated by hypercapnia may be somewhat lower up to about P10 (26%) but in neonates ≥P10 the percentage of intrinsically chemosensitive neurons is between 40–50% and remain at that level into adulthood (Dean et al. 1990; Nichols et al., 2008b). This value is somewhat higher than percentages previously reported (30–40%) (Dean et al., 1990; Huang et al., 1997) and may be due to our taking our slices from the caudal-most regions of the NTS, which appear to have a larger responsiveness to hypercapnia than more rostral regions of the NTS (Nattie and Li, 2002). Interestingly, the somewhat smaller percentage of intrinsically CO2 activated neurons in the NTS from young neonates is not seen if gap junctions are not inhibited. With intact gap junctions, nearly 50% of the neurons are activated by CO2 in NTS neurons from rats <P10 (Fig. 2) suggesting that gap junctions could compensate in some way for fewer intrinsically chemosensitive neurons early in development.
Hypercapnia-inhibited neurons were a rather small percentage of NTS neurons neonatally (about 10%). This value is less than previous reports of about 25–30% of NTS neurons being inhibited by hypercapnia in more rostral slices from neonatal rats (Huang et al., 1997). These CO2-inhibited neurons appear to be intrinsically chemosensitive and the magnitude of their inhibition by CO2 does not change throughout the neonatal development period. It is not clear what role these CO2 inhibited NTS neurons play in ventilatory control.
Without taking account of intrinsically chemosensitive neurons, our findings suggest that the responses of hypercapnia-activated NTS neurons are fully developed at birth and do not change, at least with respect to the percentage that respond to hypercapnia and the magnitude of that response, throughout neonatal development. These results are similar to the developmental response of chemosensitivity in LC neurons, where nearly all neurons are hypercapnia-activated and have a similar magnitude of response from rats aged P0 through adult (Stunden et al., 2001). The finding of a full chemosensitive response at birth that does not change with development has also been suggested for RTN neurons. About 40–45% of these neurons are activated by hypercapnia (Mulkey et al., 2004; Ritucci et al., 2005) and neither this percentage nor the magnitude of this response varies in rats younger than P10 compared with rats older than P10 (Ritucci et al., 2005). These studies were done without determining the development of intrinsic chemosensitive neurons. In the NTS, it appears that intrinsic chemosensitivity is nearly fully developed at birth, although the percentage of hypercapnia-activated neurons may increase somewhat and the magnitude of the response may be modified by chemical synaptic input during the first 10 days of life. By day P10, however, intrinsic chemosensitivity is clearly fully developed in the NTS. The development of intrinsically chemosensitive LC neurons is far more complex than the pattern reported by Stunden et al. (2001), involving changes in both percentage and magnitude of hypercapnia responses of LC neurons throughout neonatal development (Hartzler et al., 2007). Unfortunately at this time we do not understand the respiratory control network sufficiently to understand how to relate individual chemosensitive neuronal responses to output from an entire nucleus. For instance, does it matter more to the overall NTS output to the respiratory network whether all responding neurons are intrinsically chemosensitive or does it only matter what percentage are coupled together and thereby respond to hypercapnia? In addition, despite the same percentage of NTS neurons responding to hypercapnia at all neonatal ages, if the total number of NTS neurons increases with development, there could be a greater CO2-induced output from the NTS despite having a constant percentage of chemosensitive neurons. These are questions that will need to be addressed experimentally before we can relate the development of chemosensitive neurons within a given region to the development of ventilatory responses to inspired CO2 in the intact organism.
4.5. Perspective
The ventilatory response to inspired CO2 in rats has been found to show a complex developmental pattern, although there is still some controversy regarding the exact nature of that pattern. We have previously shown a triphasic developmental pattern. At birth, there is a substantial ventilatory response to hypercapnia that wanes to reach a nadir with very little ventilatory increase to hypercapnia after the first week of life (P7–P10) (Stunden et al., 2001). The ventilatory response to hypercapnia increases again and reaches a level similar to adult levels by about P14 (Stunden et al., 2001). A similar pattern, albeit with a smaller ventilatory response to CO2 during the initial few days has been shown by Serra et al. (2001). This pattern of a substantial hypercapnic ventilatory response during the first few postnatal days is also consistent with the substantial increase in respiratory rate induced by hypercapnia in very young rats (Wickström et al., 2002). In contrast, recent studies have been unable to replicate the triphasic pattern and find instead a low hypercapnic ventilatory response up until about day P14 (Davis et al., 2006). After P14, the hypercapnic ventilatory response increases substantially, suggesting a fundamental change in central chemoreception in the third postnatal week of life in rats. Thus, while the initial large ventilatory response to hypercapnia early in life is in question, all studies agree on an increase in ventilatory sensitivity to hypercapnia around day P14. This is somewhat consistent with an increase in the percentage of intrinsically CO2 sensitive NTS neurons reported in the current study. However, based on the findings of the current study and others (Wang and Richerson, 1999; Stunden et al., 2001; Ritucci et al., 2005), there appears to be a poor correlation between the development of putative central chemosensitive neurons and organismic ventilatory control. Medullary raphé neurons and intrinsically chemosensitive NTS neurons have a developmental pattern that correlates somewhat with the increase (after P14) in the ventilatory response to hypercapnia, but chemosensitive neurons from the LC and RTN, and from the NTS region in general (with gap junctions intact) do not.
One possible explanation for this apparent poor correlation between the development of chemosensitive neurons and the hypercapnic ventilatory response during the neonatal period is that while chemosensitive neurons from many of these regions appear to be well developed at birth, their contribution to ventilatory control may require the development of the neural network, including growth of neuronal projections and synapse (Putnam et al., 2005). Evaluation of this possibility must await measurements of the development of the network properties associated with ventilatory control. Another possibility is that the hypercapnic ventilatory response of neonates may be largely mediated by the peripheral chemoreceptors, i.e. the glomus cells of the carotid bodies, which have been shown to be CO2 sensitive (Gonzalez et al., 1994). However, the CO2 sensitivity of these cells does not appear to change during early development (Bamford et al., 1999) and thus it appears unlikely that these cells are responsible for the pattern of chemoreception during early rat development. It is also possible that some other chemosensitive cells, located either peripherally or centrally, that have not been previously studied, may account for this early developmental pattern of the hypercapnic ventilatory response. In this regard, a recent study on CO2 sensitivity of adrenal medulla cells is of interest (Muñoz-Cabello et al., 2005). Adrenal medulla cells were shown to be highly responsive to hypercapnia when taken from neonatal rats and this response leads to increased secretion of catecholamines, which could stimulate ventilation. The response of adrenal medulla cells to hypercapnia gradually declined throughout postnatal maturation (Muñoz-Cabello et al., 2005). Thus, the chemosensitivity of these adrenal medulla cells seems to fit best with an early (<P10) hypercapnic ventilatory response (Stunden et al., 2001) but does not fit with an increase in this response after P10.
In summary, we have shown that the magnitude of the response of hypercapnia-activated and hypercapnia-inhibited NTS neurons are nearly fully developed at birth and do not change appreciably throughout the neonatal period. The percentage of hypercapnia-activated and –inhibited NTS neurons also does not change throughout neonatal development. However, the percentage of NTS neurons that are intrinsically activated by hypercapnia may increase somewhat from birth up to P10. The developmental pattern of neuronal chemosensitivity in NTS neurons and those from other chemosensitive areas does not appear to strongly match the developmental pattern of the hypercapnic ventilatory response seen in neonatal rats. This disparity suggests that either other chemosensitive sites are involved in ventilatory control early in development or that the developmental pattern of the respiratory neural network is important in determining the development of the organismic ventilatory pattern to inspired CO2.
Acknowledgments
We would like to thank Phyllis Douglas for technical assistance. This work was supported by National Institutes of Health Grant HL-56683 (RWP and JBD). NLN was supported by the Biomedical Sciences Ph.D. Program at Wright State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Shaweesh J, Dreshaj I, Thomas A, Haxhiu M, Strohl K, Martin R. Changes in respiratory timing induced by hypercapnia in maturing rats. J Appl Physiol. 1999;87:484–490. doi: 10.1152/jappl.1999.87.2.484. [DOI] [PubMed] [Google Scholar]
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am J Physiol. 1999;276:L875–L884. doi: 10.1152/ajplung.1999.276.5.L875. [DOI] [PubMed] [Google Scholar]
- Belliveau DJ, Naus CC. Cellular localization of gap junction mRNAs in developing rat brain. Develop Neurosci. 1995;17:81–96. doi: 10.1159/000111277. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brainstem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Conrad SC, Mulkey DK, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitive neurons in the NTS. FASEB J. 2004;18:A337. [Google Scholar]
- Conrad SC, Mulkey DK, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarius (NTS) FASEB J. 2005;19:A649. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Dean JB, Ballantyne D, Cardone DL, Erlichman JS, Solomon IC. Role of gap junctions in CO2 chemoreception and respiratory control. Am J Physiol. 2002;283:L665–L670. doi: 10.1152/ajplung.00142.2002. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Milhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dean JB, Huang RQ, Erlichman JS, Southard TL, Hellard DT. Cell-cell coupling occurs in dorsal medullary neurons after minimizing anatomical- coupling artifacts. Neuroscience. 1997;80:21–40. doi: 10.1016/s0306-4522(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Dean JB, Kinkade EA, Putnam RW. Cell-cell coupling in CO2/H+-excited neurons in brainstem slices. Resp Physiol. 2001;129:83–100. doi: 10.1016/s0034-5687(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Milhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Dean JB, Reddy RB. Effects of intracellular dialysis on CO2/H+ chemosensitivity in brainstem neurons. In: Trouth CO, Millis RM, Kiwull-Schöne HF, Schläfke ME, editors. Ventral Brainstem Mechanisms and Control of Respiration and Blood Pressure. Dekker; New York: 1995. pp. 453–461. [Google Scholar]
- Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MV, Spray DC, Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci USA. 1989;86:10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol. 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol. 2003;284:C124–C155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. Developmental changes in the chemosensitive response in locus coeruleus neurons from neonatal rats. Soc Neurosci Abstr. 2007 Program No. 297.8. [Google Scholar]
- Huang RQ, Erlichman JS, Dean JB. Cell-cell coupling between CO2 – excited neurons in the dorsal medulla oblongata. Neuroscience. 1997;80:41–57. doi: 10.1016/s0306-4522(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Senba E. Postnatal differentiation of local networks in the nucleus of the tractus solitarius. Neuroscience. 2000;100:109–114. doi: 10.1016/s0306-4522(00)00257-8. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Henderson RA, Putnam RW, Dean JA. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+-sensitive neurons in rat brain stem slices. J Appl Physiol. 2003;95:910–921. doi: 10.1152/japplphysiol.00864.2002. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Henderson RA, Ritucci NA, Putnam RW, Dean JB. Oxidative stress decreases pHi and Na+/H+ exchange and increases excitability of solitary complex neurons from rat brain slices. Am J Physiol. 2004;286:C940–C951. doi: 10.1152/ajpcell.00323.2003. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cabello AM, Toledo-Aral JJ, López-Barneo J, Echevarría M. Rat adrenal chromaffin cells are neonatal CO2 sensors. J Neurosci. 2005;25:6631–6640. doi: 10.1523/JNEUROSCI.1139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE. CO2, brainstem chemoreceptors, and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. The characterization of the chemosensitive response of individual nucleus tractus solitarius (NTS) neurons from adult rats. Am J Physiol. 2008a doi: 10.1152/ajpregu.90769.2008. IN REVISION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Wilkinson KA, Powell FL, Putnam RW. Chronic hypoxia (CHx) suppresses the chemosensitive response of individual nucleus tractus solitarius (NTS) neurons from adult rats. FASEB J. 2008b;221:1172.1. [Google Scholar]
- Parisian K, Wages P, Smith A, Jarosz J, Hewitt A, Leiter JC, Erlichman JS. Ventilatory effects of gap junction blockade in the NTS in awake rats. Respir Physiol Neurobiol. 2004;142:127–143. doi: 10.1016/j.resp.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Pivato LS, Constantin RP, Ishii-Iwamoto EL, Kelmer-Bracht AM, Yamamoto NS, Constantin J, Bracht A. Metabolic effects of carbenoxolone in rat liver. J Biochem Molec Toxicol. 2006;20:230–240. doi: 10.1002/jbt.20139. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir Physiol Neurobiol. 2005;149:165–179. doi: 10.1016/j.resp.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Meth. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rao H, Jean A, Kessler JP. Postnatal ontogeny of glutamate receptors in the rat nucleus tractus solitarii and ventrolateral medulla. J Auton Nerv Syst. 1997;65:25–32. doi: 10.1016/s0165-1838(97)00031-3. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Bidirectional electrical coupling between inspiratory motoneurons in the newborn mouse nucleus ambiguus. J Neurophysiol. 1997;78:3508–3510. doi: 10.1152/jn.1997.78.6.3508. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Dean JB, Putnam RW. Intracellular pH response to hypercapnia in neurons from chemosensitive areas of the medulla. Am J Physiol. 1997;273:R433–R441. doi: 10.1152/ajpregu.1997.273.1.R433. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Dean JB, Putnam RW. A fluorescence technique to measure intracellular pH of single neurons in brainstem slices. J Neurosci Methods. 1996;68:149–163. doi: 10.1016/0165-0270(96)00051-9. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from retrotrapezoid nucleus. Am J Physiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Segal M, Koulakoff A, Giaume C, Avignone E. Carbenoxolone blockade of neuronal network activity in culture is not mediated by an action on gap junctions. J Physiol. 2003;553:729–745. doi: 10.1113/jphysiol.2003.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A, Forster HV, Brozoski D, Hedin N, Franciosi R. Effects of carotid body denervation in newborn and adult rats. J Appl Physiol. 2001;91:1298–1306. doi: 10.1152/jappl.2001.91.3.1298. [DOI] [PubMed] [Google Scholar]
- Solomon IC. Connexin36 distribution in putative CO2-chemosensitive brainstem regions in rat. Respir Physiol Neurobiol. 2003;139:1–20. doi: 10.1016/j.resp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Dean JB. Gap junctions in CO2-chemoreception and respiratory control. Respir Physiol Neurobiol. 2002;131:155–173. doi: 10.1016/s1569-9048(02)00090-3. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Halat TJ, El-Maghrabi MR, O’Neal MH., III Localization of connexin26 and connexin32 in putative CO2-chemosensitive brainstem regions in rat. Respir Physiol. 2001;129:101–121. doi: 10.1016/s0034-5687(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly MEM, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Vincent A, Tell F. Postnatal changes in electrophysiological properties of rat nucleus tractus solitarii neurons. Eur J Neurosci. 1997;9:1612–1624. doi: 10.1111/j.1460-9568.1997.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Vincent A, Tell F. Postnatal development of rat nucleus tractus solitarius neurons: morphological and electrophysiological evidence. Neuroscience. 1999;93:293–305. doi: 10.1016/s0306-4522(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphé neurons. Neuroscience. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Wickström R, Hokfelt T, Lagercrantz H. Development of CO2 – response in the early newborn period in rat. Respir Physiol Neurobiol. 2002;132:145–158. doi: 10.1016/s1569-9048(02)00076-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Kang YM, Yu Y, Wei SG, Schmidt TJ, Johnson AK, Felder RB. 11beta-hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation. Hypertension. 2006;48:127–133. doi: 10.1161/01.HYP.0000224296.96235.dd. [DOI] [PubMed] [Google Scholar]