Abstract
Aim: To describe the tomographic findings of a case of myopic traction maculopathy using Spectral Domain Optical Coherence Tomography (SD-OCT) and present the results of its surgical intervention.
Design: Observational case report and review of the literature.
Methods: A 61-year-old male with metamorphopsia was examined clinically and with the use of SD-OCT. The diagnosis of myopic traction maculopathy was made. The patient underwent pars plana vitrectomy with removal of the vitreomacular adhesions, the epiretinal and the internal limiting membrane.
Results: Visual acuity increased by two Snellen lines, metamorphopsia disappeared, macular morphology was improved and myopic traction maculopathy was resolved.
Conclusions: Imaging with SD-OCT is capable of documentation and measurement of the early stages of myopic traction maculopathy. Moreover, vitrectomy was beneficial for the visual and anatomic outcome of the patient.
Keywords: myopic traction maculopathy, spectral domain optical coherence tomography
Myopia is a common optical aberration with increasing prevalence around the world. High myopia (>-6D) is a major cause of legal blindness in many developed countries1. In a study of visual disability in the United States, the National Eye Institute (1976) found myopia to be the fifth most frequent cause of impaired vision, the eighth most frequent cause of severe visual impairment, and the seventh most frequent cause of legal blindness2.
High myopia is associated with progressive anteroposterior elongation of the eye. As a result of that, various complications may develop, such as changes involving the sclera, retina, choroid, and optic nerve head2. The greater the myopia, the more likely are complications that can threaten vision. Ageing was also found an important factor in the development of maculopathy. Shin et al noted that the incidence of cases of myopia with maculopathy increased with the age3.
Clinically, the funduscopic changes associated with high myopia include straightened and stretched vessels, temporal peripapillary atrophic crescent, tilting of the optic disc, posterior staphyloma, lacquer cracks in the Bruch's membrane, geographic areas of retinal pigment epithelium and choroid atrophy, subretinal haemorrhage, and choroidal neovascularisation. With time and progression, traction and tension phenomena are observed. Maculopathy is the most common cause of visual loss in high myopic patients4.
The impact of myopic retinopathy on visual impairment is important because it is often bilateral and irreversible, and it frequently affects individuals during their productive years. However, the early stages of such damage are barely detectable by common diagnostic tools and are apparent only by using the optical coherence tomography (OCT)5,6. The use of OCT in large case series of high myopia has recently demonstrated that unsuspected posterior retinal anomalies affect up to one third of these eyes5,7. Panozzo and Mercanti7,8 used for the first time the term "tractional myopic maculopathy" to refer to these pathologies, in a retrospective study on 218 eyes in which epiretinal traction was found in 46% and retinal damages in 34%. Until 2004, many high myopic patients who exhibited central visual reduction without choroidal neovascularisation or any other cause that explained it were diagnosed with idiopathic myopic choroidosis.
Spectral domain OCT (SD-OCT) is a variant of the Stratus OCT. It creates higher speed and resolution scans of the retina compared to the previous technologies. More scans can be acquired to create three-dimensional images of retinal structures. These data cubes can be precisely registered with each other and with the fundus images. The clinician can view individual B-scans from the cube, or view all the slices together in an animated, "flythrough" sequence. Images have twice the axial resolution and are scanned in 1/75th of the time compared to the Stratus OCT. Improved resolution visualizes vitreoretinal features in greater detail. Higher scanning speed can be used to either shorten examination time or to increase the extent and detail of the retinal images.
The purpose of this study was to present a case with myopic traction maculopathy studied with SD-OCT, its associated complications, the treatment and the visual outcome.
Case report
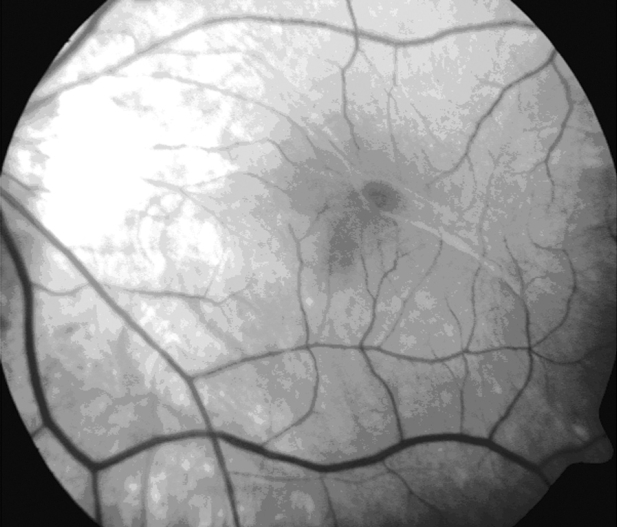
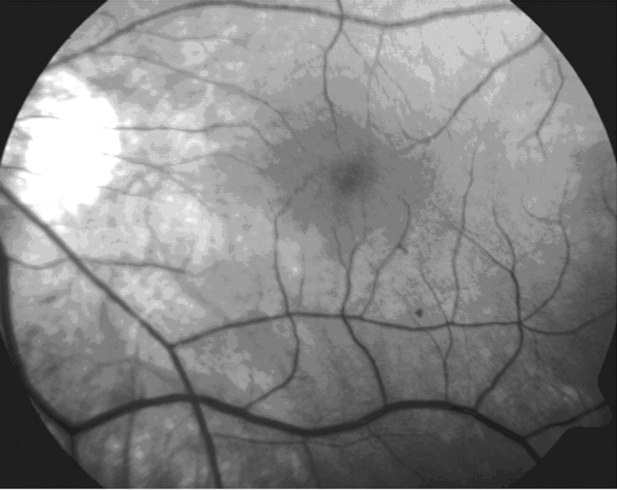
A 61–year–old man presented to the consulting room with the complaint of metamorphopsia in the left eye. Visual acuity examination was performed on both eyes and yielded 8/10 -5.25 in the right eye and 5/10 -5.25 in the left eye. Amsler test was found positive in the left eye. Anterior chamber examination was unremarkable for both eyes. Dilated fundus examination and fundus photography revealed vitreomacular traction outside the fovea and an epiretinal membrane (ERM) in the left eye (Figure 1).
Figure 1: Fundus photography. The focal area of vitreomacular traction in myopic traction maculopathy is usually located outside the fovea.
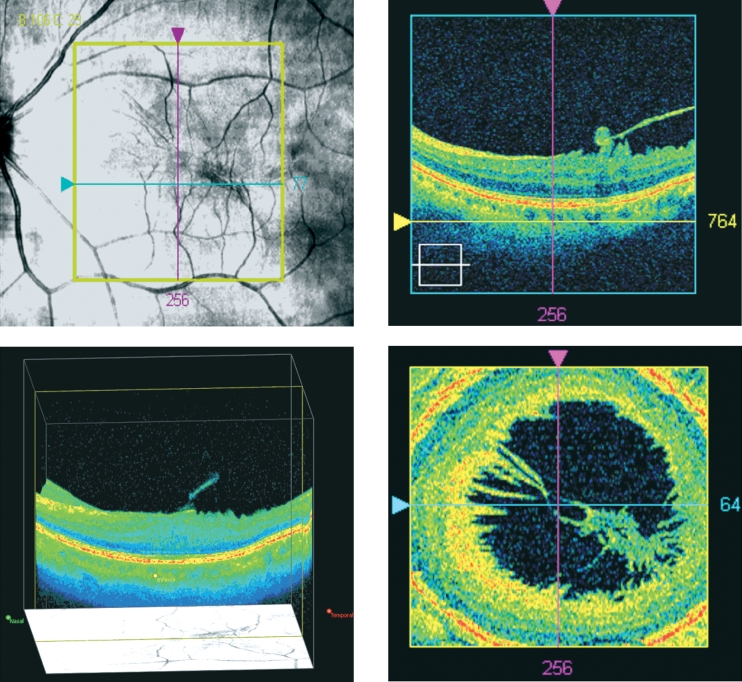
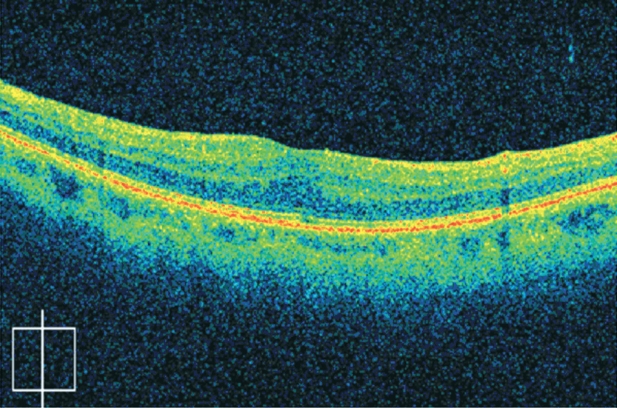
SD-OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Inc.) demonstrated partial detachment of the posterior hyaloid in the left eye with continued attachment at the fovea, abnormal foveal contour and thickness, as well as an ERM. SD-OCT is a relatively new optical coherence tomography device that allows a three dimensional structural imaging of the retina; its advantages over the classical time domain OCT, include precise point-by-point registration of the entire posterior pole of the eye and reproducibility of each scan. With the use of SD-OCT the precise location of pathology was identified and three dimensional images of the macular area were obtained, revealing the vitreoretinal traction and the retinal folding as a result of the tangential traction of the ERM (Figure 2). Based on these SD-OCT findings, the diagnosis of myopic traction maculopathy was made.
Figure 2: Preoperative images from the SD-OCT showing myopic traction maculopathy with epiretinal and vitreoretinal traction. Visual acuity is 5/10. A: Precise location of pathology and placement of the scan. B: Three dimensional image of the macular area in cubic form. C: Cross section of the macular area. Partial detachment of the posterior hyaloid with continued attachment at the fovea. D: Sagittal section in front of the internal limiting membrane indicating the vitreoretinal traction in the macular area.
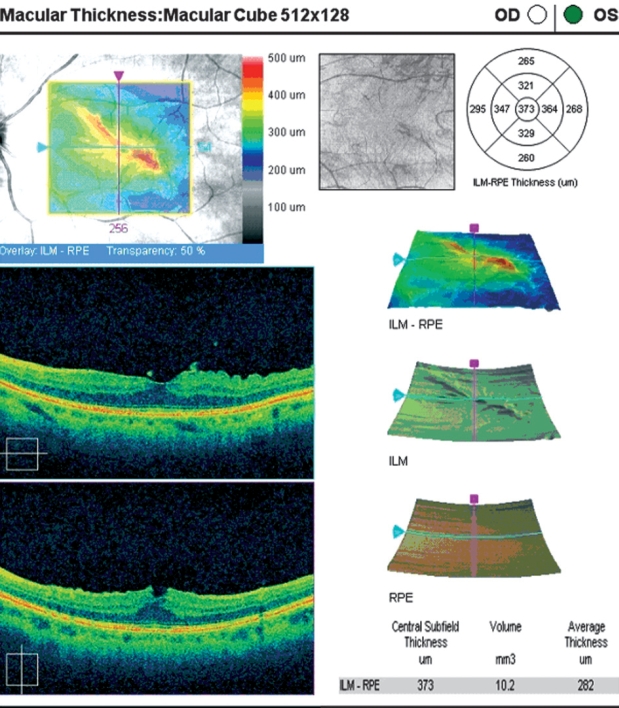
The thickness values of the central five subfields showed a marked increase in retinal thickness corresponding to the thickness map (according to the Age Related Eye Disease Study (AREDS) subfields (Age- Related Eye Disease Study Group, 2005). Central foveal thickness was 373 µm using the standard OCT macular mapping software (Figure 3).
Figure 3: Preoperative image of the macular report of the SD-OCT. Horizontal and vertical crosshair line images indicate the retinal folding in the macular area.
The decision for vitrectomy was made, to release the vitreomacular traction and remove the ERM. A standard 25-gauge 3-port pars plana vitrectomy was performed under local anaesthesia. The central vitreous core was first removed, paying particular attention to visualizing any vitreoretinal adhesions (focal or diffuse). Vitrectomy was then extended to the midperiphery. Brilliant blue G (Membran Blue, D.O.R.C.) was used for better visualization of the ERM and the internal limiting membrane (ILM). Focal or diffuse vitreoretinal adhesions were removed, as well as the epiretinal membrane. The ILM was finally removed in the macular area.
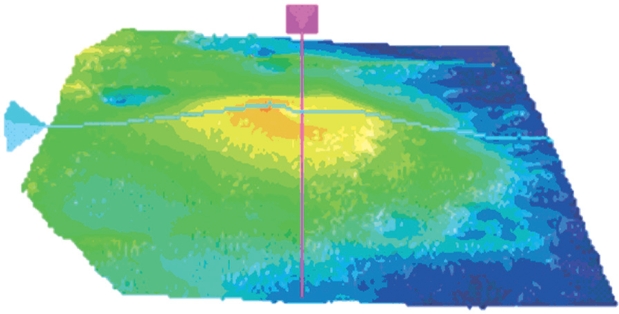
One month after surgery, the patients vision has increased to 7/10 in the left eye and the Amsler test was negative. The release of the vitreomacular traction led to a stable anatomical recovery and visual improvement. Retinal thickness has decreased (Figure 4) and traction was no longer visible, neither in the fundus photography (Figure 5) nor in SD-OCT imaging (Figure 6).
Figure 4: Postoperative three-dimensional macular thickness map. One month after surgery, retinal thickening is reduced and traction is no longer visible. Visual acuity has increased to 7/10.
Figure 5: Post-operative fundus photography. Vitreomacular traction is released and imaging of the macula is normal.
Figure 6: Vertical cross sectional image of the macular area one month after pars plana vitrectomy. The epiretinal and vitreomacular traction is released and the anatomical structure is normal. The macular morphology with retinal microfolds is improved and myopic traction maculopathy is completely resolved.
Discussion
The posterior pole of highly myopic eyes is an environment in which chorioretinal stretching and atrophy from marked scleral concavity and staphyloma are often associated with persistent vitreoretinal adhesions causing macular traction. The formation of macular hole and posterior retinal detachment are well-known advanced consequences of this unstable condition5,9.
The important role of vitreous traction is confirmed by the good results obtained by vitrectomy in cases of myopic foveal retinoschisis10.
Panozzo and Mercanti7,8 in a study of 24 eyes with myopic traction maculopathy reported that twenty-three (95.8%) of 24 eyes treated with vitrectomy had complete and stable resolution of myopic traction maculopathy after a mean of 4.4 months. At OCT examination before surgery, all 24 eyes had foveal retinal swelling with separation between retinal layers, combined with foveal detachment in 5 eyes. In their study, mean visual improvement was 2.5 Snellen lines. Their conclusion was that vitrectomy without fluid/gas exchange leads to stable resolution of myopic traction maculopathy and good visual improvement.
This large retrospective case series of 24 highly myopic eyes with myopic traction maculopathy demonstrates that vitrectomy with release of vitreoretinal traction leads to stable anatomical recovery and visual improvement. Previously published smaller case series10,11 and case reports have reported similar results.
The excellent and stable anatomical results after surgical release of traction demonstrate that this is the main cause of myopic traction maculopathy6,12.
In our study, the release of vitreofoveal attachment with restoration of smooth foveal contour shown by SDOCT, correlated with resolution of symptoms. Traction from the posterior hyaloid attachment was causing visual distortion in the patient, as well as the anatomical distortion of the foveal contour seen with SD-OCT. This finding demonstrates that vitreomacular attachment may cause metamorphopsia. The improvement in visual acuity by two Snellen lines is similar to that described in literature in cases with high myopia that underwent surgical treatment7,8.
After the pars plana vitrectomy and the removal of the vitreoretinal adhesions and the ERM, the ILM was also removed. As demonstrated by Kwok et al11 ILM peeling is probably not essential in eyes with myopic traction maculopathy, but the removal of the ILM remains an effective method for ascertaining the absence of any residual traction on the retinal surface.
Concluding, this case demonstrates that imaging with SD-OCT is capable of documentation and measurement of the early stages of myopic traction maculopathy. Moreover, it seems reasonable to consider surgery in eyes where this condition involves the macular area and is judged to be damaging or jeopardizing visual function.
References
- 1.Saw SM, Katz J, Schein OD, et al. Epidemiology of myopia. Epidemiol Rev. 1996;18:175–187. doi: 10.1093/oxfordjournals.epirev.a017924. [DOI] [PubMed] [Google Scholar]
- 2.Curtin BJ, editor. The myopias: basic science and clinical management. Philadelphia: Harper and Row; 1985. pp. 7–10. [Google Scholar]
- 3.Shih YF, Ho TC, Hsiao CK, Lin LLK. Visual outcomes for high myopic patients with or without myopic maculopathy: a 10 year follow up study. Br J Ophthalmol. 2006;90:546–550. doi: 10.1136/bjo.2005.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanski JJ. Ch. 17, Degenerative myopia. In: Kanski JJ, editor. Clinical Ophthalmology, a systematic approach. 6th eds. Philadelphia: Elsevier; 2007. pp. 654–655. [Google Scholar]
- 5.Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128:472–476. doi: 10.1016/s0002-9394(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou N, Massin P, Haouchine B, Erginay A, Gaudric A. Macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2002;133:794–800. doi: 10.1016/s0002-9394(02)01394-6. [DOI] [PubMed] [Google Scholar]
- 7.Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122:1455–1460. doi: 10.1001/archopht.122.10.1455. [DOI] [PubMed] [Google Scholar]
- 8.Panozzo G, Mercanti A. Vitrectomy for Myopic Traction Maculopathy. Arch Ophthalmol. 2007;125:767–772. doi: 10.1001/archopht.125.6.767. [DOI] [PubMed] [Google Scholar]
- 9.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol. 2002;134:645–660. doi: 10.1016/s0002-9394(02)01883-4. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Kishi S. Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology. 2003;110:1702–1707. doi: 10.1016/S0161-6420(03)00714-0. [DOI] [PubMed] [Google Scholar]
- 11.Kwok AKH, Lai TYY, Yip WWK. Vitrectomy and gas tamponade without internal limiting membrane peeling for myopic foveoschisis. Br J Ophthalmol. 2005;89:1180–1183. doi: 10.1136/bjo.2005.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiba J, Konno S, Yoshida A. Retinal detachment associated with a macular hole in severely myopic eyes. Am J Ophthalmol. 1999;128:654–655. doi: 10.1016/s0002-9394(99)00240-8. [DOI] [PubMed] [Google Scholar]