Abstract
Lead is stored in the skeleton and can serve as an endogenous source for many years. Lead may influence the risk of fracture, through direct effects on bone strength or indirectly by disturbing neuromuscular function and increasing the risk of falls. The objective of this analysis is to test the hypothesis that women with higher blood lead levels experience higher rates of falls and fracture. This was a prospective cohort study of 533 women 65–87 yr of age enrolled in the Study of Osteoporotic Fractures at two U.S. research centers (Baltimore, MD; Monongahela Valley, PA) from 1986 to 1988. Blood lead levels (in μg/dl) were measured in 1990–1991 by atomic absorption spectrophotometry and classified as “low” (≤3; lower 15th percentile, referent); “medium” (4–7); or “high” (≥8; upper 15th percentile). Total hip BMD was measured by DXA twice, 3.55 yr apart. Information on falls was collected every 4 mo for 4 yr. Incident nonspine fractures were identified and confirmed over 10 yr. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% CI of fracture. Generalized estimating equations were used to calculate the incident rate ratio of falls (95% CI). The mean blood lead level was 5.3 ± 2.3 (SD) μg/dl (range, 1–21 μg/dl). Baseline BMD was 7% lower in total hip and 5% lower in femoral neck in the highest compared with lowest blood lead group (p < 0.02). Hip bone loss tended to be greater in the high lead group, but differences were not significant. In multivariable adjusted models, women with high blood lead levels had an increased risk of nonspine fracture (HR = 2.50; 95% CI = 1.25, 5.03; p trend = 0.016) and higher risk of falls (incident rate ratio = 1.62; 95% CI = 1.07, 2.45; p trend = 0.014) compared with women with lowest lead level. Blood lead levels are associated with an increased risk of falls and fractures, extending the negative health consequences of lead to include osteoporotic fractures.
Key words: environmental lead, blood lead, fractures, falls
INTRODUCTION
The skeleton is the major repository for lead, sequestering up to 95% of lead, and can serve as an endogenous source for many years after exposure.(1) Lead is contained in the mineral matrix of bone in close association with calcium, sharing many physical and chemical characteristics and intracellular pathways.(2,3) Lead can compete with calcium in intestinal absorption and deposition into bone. Analogous to calcium, lead absorption from the intestine is regulated by PTH, 1,25-dihydroxyvitamin D, and other factors.(4) Lead may exert both direct (osteoblast and osteoclast function) and indirect (kidney dysfunction) actions on bone turnover.(5)
Lead may be mobilized from the skeleton during conditions of high bone turnover, such as pregnancy, lactation, menopause, and aging.(4) The U.S. population-based NHANES II dataset (n = 20,322), compiled between 1976 and 1980, showed that there was a highly significant increase in both whole blood and calculated plasma lead after menopause independent of age,(4) providing evidence that bone lead is mobilized into blood during conditions of bone demineralization. Menopausal women had adjusted median blood lead levels that were 25% higher than those of premenopausal women. Current use of hormone replacement therapy was associated with significantly lower adjusted median blood lead levels.(6)
Elevated blood lead levels may have a direct causative role in the pathogenesis of osteoporosis.(3,7) Animal studies have shown an association between increased bone lead concentration and decreased mechanical strength.(8–12) In animal models, lead inhibits axial bone development and decreases bone mass because of enhanced resorption.(9) An inverse relationship has also been documented between elevated blood lead levels and skeletal development, chest circumference, and stature in children.(13–15) Lead exposure has been reported to accelerate bone maturation by inhibiting the effects of PTH-related peptide.(16) Accelerated maturation of bone may ultimately result in a lower peak BMD, thus predisposing to osteoporosis in later life.(16) A hypothetical “lead-exposed female” may never reach her peak BMD, and thus, be at risk for fracture.
In older adults, data are sparse. In NHANES III, there was a significant inverse association between blood lead levels and BMD.(6) Factors related to bone turnover were significant predictors of blood lead level.(6) In a study of former workers from a lead smelting factory, there was a significant inverse association between spine BMD and blood lead levels in women.(17) In contrast, a recent study of 112 postmenopausal women reported higher lumbar spine BMD with higher tibia lead levels. There was no relationship between BMD and blood lead or patella lead levels.(18)
In addition to direct effects on bone, lead exposure could predispose to fractures because of increased risk of falls. In adults, environmental lead exposure had been associated with cognitive decline,(19) essential tremors,(20,21) slower response time,(22,23) sensorimotor delay,(24) decreased motor speed and muscle strength,(25,26) depression and mood changes,(25,27) coordination,(22) impaired balance,(20,21) and decreased visual acuity.(28) Compromise in these complex neuromuscular, neurological, and sensory characteristics may disturb coordination(29) and predispose to falls.(30)
In this study, we examined the association between blood lead levels and BMD, changes in BMD, falls, and fractures in a subset of 533 women enrolled in the Study of Osteoporotic Fractures (SOF). We hypothesized that women with higher blood lead concentrations will have a greater risk of falls and fractures.
MATERIALS AND METHODS
Study population
The SOF is a longitudinal cohort study that enrolled 9704 white women from 1986 to 1988 using population-based listings in Baltimore, MD; Minneapolis, MN; Portland, OR; and the Monongahela Valley near Pittsburgh, PA. To be eligible to participate, women had to be ≥65 yr of age and ambulatory.
An ancillary study of blood lead levels was conducted in 1990–1991 in a consecutive sample of 533 white women 65–87 yr of age enrolled in SOF at either the University of Pittsburgh (Monongahela Valley near Pittsburgh) or University of Maryland (Baltimore) clinics. Initially, we examined the correlates of blood lead(22) and the association of blood lead to cognitive function.(31) We found a relationship between higher blood lead and worse cognitive function as measured by the Part B Trailmaking Test, but this association was confined to the rural (Monongahela Valley) clinic.(22,31) In this paper, we extend the lead study to outcomes of BMD, rates of decline in BMD, and incident fractures and falls. The protocol and consent forms were approved by the institutional review boards at the participating institutions. All women provided written informed consent.
Blood lead measurements
A 5.0-ml sample of whole blood was drawn into vacutainer tubes (BD Vacutainer Systems, Rutherford, NJ, USA). Blood samples were analyzed at the Clinical Chemistry Laboratory of the University of Maryland, certified for the analysis of lead in blood by the Occupational Safety and Health Administration and Centers for Disease Control and Prevention, and documents a lower limit of detection for lead of 1 μg/dl. Blood lead was determined by graphite furnace atomic absorption spectrometry (AAS model 5100; HGA with Zeeman Effect Background Correction; Perkin Elmer, Norwalk, CT, USA). To study intra-laboratory variability in both the measurement of lead and the stability of lead over 1 yr, additional blood samples were collected from a random sample of participants in SOF.(22) Two tubes of blood were drawn from each of the 100 randomly selected women (50 women from each clinic) during a later clinic visit. The laboratory was blind with respect to the second blood sample and therefore the intra-laboratory variability in both the measurement of lead and the stability of lead over time was determined. The intraclass correlation coefficient for the duplicates was 0.88. Mean values of 4.76 (range, 1–13 μg/dl) and 4.67 μg/dl (range, 1–12 μg/dl) were obtained for the first and second determinations, respectively.
BMD
BMD of total hip and femoral neck were measured at the second (1988–1990) and at the fourth examination (1993–1994) by DXA using Hologic QDR 1000 scanners (Bedford, MA, USA) an average of 3.55 yr apart. Calcaneal BMD was measured at the baseline visit (1986–1988) and at the fourth visit (1993–1994), with OsteoAnalyzers (Siemens-Osteon, Wahiawa, HI, USA) using single-photon absorptiometry at the baseline examination and single X-ray absorptiometry (Osteoanalyzer; Dove Medical Systems) at the fourth examination (mean follow-up = 5.7 ± 0.33 yr). Annual percent decline in BMD was estimated for the femoral neck, total hip, and calcaneus. A random sample of scans was reviewed by the UCSF Coordinating Center. To assess longitudinal performance of the scanners, a spine phantom was scanned daily, and a hip phantom was scanned once per week at each clinic. Details of these measurement methods and densitometry quality control procedures have been published.(32,33) The mean CV for the femoral neck between centers was 1.2% for two research staff who visited all centers. The intraindividual region-specific correlation coefficients between hip measurements at the second and fourth examinations (3.55 ± 0.29 [SD] yr between examinations) were 0.96 (total hip) and 0.94 (femoral neck). The in vivo mean CV for the calcaneus was 1.2% between centers; the intraindividual correlation coefficient between calcaneus measurement at baseline and fourth examinations was 0.94%.
Other measurements
Potential confounders of the association between blood lead and outcomes of interest were identified based on previous lead(22,31) and BMD and fracture analyses(34–37): sociodemographic factors (age, study site/clinic), education in years, smoking history, alcohol consumption, medication use (including hormone therapy [HT], calcium and vitamin D supplements, central nervous system [CNS] active medications [benzodiazepines, antidepressants, anticonvulsants, and narcotics], health status compared with others, history of fractures after age 50, and falls over the previous year). Height and weight were obtained using a Harpenden stadiometer (Holtain, Crymych, UK) and a standard balance beam, respectively, and body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
Cognitive function was tested with a modified form of Mini-Mental (MMSE) status examination,(38,39) a global measurement of cognitive function, with components for orientation, concentration, language, praxis, and immediate and delayed memory. The modified MMSE score ranges from 0 to 26, with higher scores representing better cognitive functioning.(40) The Trails B is a timed test that measures attention, sequencing, visual scanning, and executive function. A faster time for completion (in seconds) represents better cognitive functioning.(41)
Neuromuscular assessment was comprised of muscle strength, balance, gait, coordination, and reaction time. Grip strength was recorded as the average of the right and left sides using an adjustable hand grip dynameter (Preston Grip Dynameter; Takei Kiki Kogyo, Tokyo, Japan).(42) The chair stand test determined whether the participant could stand up five times from a chair without using the arms.(43) Static balance was measured as the ability to hold tandem stands with eyes open for 10 s.(44) Gait speed was assessed as average of two trials of walking speed at usual pace (m/s).
The groove pegboard test is a measure of manual dexterity and the speed and accuracy of hand-eye coordination. Time to insert and remove pegs in a board with the dominant hand was recorded in seconds. Reaction time was tested on the foot and hand of the dominant side with a two clock response and movement time device (Lafayette Instrument) that measures the speed of reactions and the speed of movement of hands and feet on the dominant side in 1/1000 of a second.(45) Hand and foot reaction time were highly related (r = 0.83). Corrected vision was measured as visual acuity 20/40 or worse (both eyes) on Bailey-Lovie acuity targets.(46,47)
Vibration threshold was measured with a Vibratron-II (Sensortek) to quantify the ability to detect vibratory stimuli at right big toe. The Vibration threshold was calculated as the average of the vibration settings of the five errors and five lowest scores.(43) Light touch discrimination was measured twice on both big toes using a Von-Frey type esthesiometer probe. The thinnest filament that was identified correctly was recorded both times.(43) An increase in these performance measures indicates a decline in function.
Fracture/falls ascertainment
After the initial enrollment visit, all women were contacted by either postcard or telephone every 4 mo to determine whether any fractures or falls had occurred in the preceding 4 mo. After >10 yr, follow-up remains >95% complete. Women who reported a fracture were interviewed by telephone about the circumstances under which the fracture occurred. Fractures occurring from major trauma (e.g., motor vehicle crash) were excluded. All fractures were confirmed by radiographic report or review of the X-rays. Data were prospectively collected on the number of falls for 4 yr after baseline visit. On each postcard, participants were asked if they had fallen in the past 4 mo and, if so, how many times. Participants were contacted by phone when postcards were not returned.
Statistical analyses
Analyses were performed by categorizing the study participants into three groups of blood lead corresponding to the upper and lower 15th percentiles of the distribution of blood lead. Thus, the three groups were low (≤3 μg/dl, median = 3 μg/dl; n = 122); medium (4–7 μg/dl, median = 5 μg/dl; n = 332); and high (≥8 μg/dl, median = 9 μg/dl n = 79). This categorization was determined a priori based on our previous study of blood lead and cognitive function.(22)
The χ2 test was used to compare baseline characteristics for categorical variables by blood lead status; ANOVA was used for continuous variables. Two-tailed p values were used for all tests at 5% statistical significance. We plotted the cumulative incidence of fracture in the three groups by Kaplan-Meier survival curves. We used Cox proportional hazard regression analysis to estimate the hazard ratio (HR) of fracture and 95% CIs across the three blood lead groups. The relative risk (RR) of falls was computed using Poisson regression models with generalized estimating equations (GEEs) to adjust SE for correlated data points at 4-mo intervals. A Huber White Sandwich estimator of variance was used with GEE to construct valid standard errors. The incident rate ratio (IRR) was computed as fall rate (average number of falls per 4 mo) in a specific category of blood lead compared with the lowest (referent) category. In multivariate models, we simultaneously analyzed blood lead levels and other potential risk factors. A forward stepwise selection process was used to add or remove potential covariates from the multivariate regression models (exit criteria: p ≥ 0.15) until a final model of significant variables (p < 0.05) was selected. We initially adjusted for age and clinic. In multivariate fracture models, we adjusted for age, clinic, education, BMI, alcohol use, baseline total hip BMD, prior fracture, health status, falls in past year, smoking, estrogen use, and calcium and vitamin D use. The initial multivariable model for falls included age, clinic, education, BMI, alcohol use, health status, smoking, falls in past year, CNS active medications, estrogen use, and calcium and vitamin D use. To test whether these associations were mediated by muscle strength, neuromuscular function, balance sensory, or cognitive function, we adjusted for grip strength, lower limb reaction time, fine motor coordination, visual acuity, balance, vibration and touch sensitivity, chair stands, walking speed, and Trial B. In secondary analysis, we determined the HR of fracture and falls per SD increase in blood lead. The proportionality assumptions of the Cox models were confirmed with Schoenfeld residuals. Data were analyzed with Stata (edition 9; StataCorp, College Station, TX, USA).
RESULTS
The mean blood lead was 5.3 ± 2.3 (SD) μg/dl (range, 1–21 μg/dl). Women in the highest blood lead category (≥8 μg/dl) had lived more years after menopause, were less likely to use estrogen, and were more likely to smoke and drink alcohol and less likely to take vitamin D supplements (Table 1). Blood lead levels were significantly lower in women who reported current use of estrogen (4.6 μg/dl) compared with past users (4.8 μg/dl) or nonusers (5.3 μg/dl; p < 0.05). Cognitive function, health status, muscle strength, balance, gait speed, vibration or touch sensitivity, reaction time, fine motor coordination, or visual acuity did not differ across blood lead categories. Women with highest blood lead had lower mean baseline total hip (7%; p < 0.02) and femoral neck (5%; p < 0.03) BMD compared with women with lowest blood lead category (Table 1). Baseline calcaneal BMD did not differ across the three groups.
Table 1.
Baseline Characteristics of Women in SOF Across Blood Lead Level
| Low | Medium | High | p | |
| Level (μg/dl) | ≤3 | 4–7 | ≥8 | |
| N = 533 | N = 122 | N = 332 | N = 79 | |
| Age [yr; mean (SD)] | 70.3 (4.3) | 70.4 (4.4) | 71 (4.5) | 0.411 |
| Education [yr; mean (SD)] | 12 (3) | 12.5 (3) | 12.6 (3) | 0.360 |
| Years since menopause [mean (SD)] | 21 (8) | 23 (7) | 25 (8) | 0.017 |
| Age at menopause [yr; mean (SD)] | 49 (6) | 48 (6) | 46 (6) | 0.011 |
| Ever breastfed [n (%)] | 66 (24) | 181 (66) | 29 (11) | 0.014 |
| Current estrogen use [n (%)] | 22 (18) | 33 (10) | 4 (5) | 0.030 |
| Body mass index [kg/m2; mean (SD)] | 27 (5) | 27 (5) | 26 (4) | 0.125 |
| Current smoker [n (%)] | 7 (11) | 39 (59) | 20 (30) | <0.001 |
| Alcohol [drinks/wk; mean (SD)] | 1 (3) | 2 (4) | 3 (6) | 0.002 |
| Calcium intake [mg/d; mean (SD)] | 674 (357) | 654 (399) | 653 (513) | 0.886 |
| Vitamin D supplement [n (%)] | 50 (42) | 114 (35) | 18 (23) | 0.052 |
| No of years Vit D use [mean (SD)] | 13 (15) | 10 (11) | 5 (7) | 0.019 |
| Self reported health [n (%) excellent/good] | 99 (81) | 282 (85) | 66 (83) | 0.493 |
| Any fracture after age 50 [n (%)] | 40 (33) | 109 (33) | 22 (28) | 0.683 |
| Fallen in past year (≥1) [n (%)] | 29 (24) | 75 (23) | 20 (25) | 0.873 |
| CNS medication use [n (%)] | 31 (25) | 52 (16) | 14 (18) | 0.058 |
| Cognitive function | ||||
| m MMSE [mean (SD)] | 25 (1.6) | 25 (1.4) | 25 (1) | 0.185 |
| Trails B [s; mean (SD] | 116 (36) | 121 (39) | 126 (44) | 0.192 |
| Muscle strength | ||||
| Average grip strength both sides [kg; mean (SD)] | 20.6 (3.8) | 20.9 (4.2) | 20.7 (4.1) | 0.756 |
| Use of arm for chair stand five times [n (%)] | 1 (11) | 8 (89) | 0 (0) | 0.230 |
| Balance: poor /very poor [n (%)] | 40 (23) | 111 (63) | 25 (14) | 0.953 |
| Gait: walk speed [m/s; mean (SD)] | 1.01 (0.2) | 1.04 (0.2) | 1.03 (0.2) | 0.483 |
| Somatosensory discrimination | ||||
| Light touch sensitivity [mm; mean (SD)]* | 4.1 (0.5) | 4.1 (0.4) | 4.0 (0.4) | 0.752 |
| Vibration threshold [units; mean (SD)]* | 21 (19) | 22 (18) | 23 (24) | 0.837 |
| Reaction time (number of seconds) | ||||
| Hand [mean (SD)]* | 0.63 (0.1) | 0.65 (0.2) | 0.65 (0.2) | 0.723 |
| Foot [mean (SD)]* | 0.64 (0.1) | 0.66 (0.2) | 0.66 (0.2) | 0.499 |
| Coordination | ||||
| Groove peg board [number of seconds; mean (SD)]* | 91 (27) | 93 (30) | 95 (25) | 0.679 |
| Vision acuity 20/40 or worse both eyes [n (%)] | 13 (15) | 57 (66) | 48 (7) | 0.100 |
| BMD (g/cm2) | ||||
| Total hip [mean (SD)] | 0.77 (0.13) | 0.76 (0.13) | 0.72 (0.12) | 0.021 |
| Femoral neck [mean (SD)] | 0.65 (0.11) | 0.66 (0.12) | 0.62 (0.09) | 0.033 |
| Calcaneus [mean (SD)] | 0.41 (0.09) | 0.42 (0.09) | 0.39 (0.09) | 0.145 |
* An increase in these performance measures indicates a decline in function.
Change in BMD
Women with highest blood lead experienced a greater rate of decline in hip BMD (Table 2), but the association was not statistically significant. The annualized rate of decline in calcaneal BMD was higher for women in the higher blood lead category compared with those in lower categories in age- and multivariable-adjusted models.
Table 2.
Age and Multivariate-Adjusted Annualized Percentage Rate of Bone Loss Across Blood Lead Levels
| BMD decline (%/yr) |
Blood lead level
|
p trend | |||||
| Low (≤3 μg/dl) (n = 122) | Medium (4–7 μg/dl) (n = 332) | High (≥8 μg/dl) (n = 79) | |||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Total hip | |||||||
| Age, clinic adjusted | −0.31 | −0.61, −0.01 | −0.46 | −0.64, −0.28 | −0.81 | −1.21, −0.41 | 0.14 |
| MV adjusted* | −0.34 | −0.61, −0.06 | −0.41 | −0.58, −0.26 | −0.63 | −1.00, −0.25 | 0.50 |
| Femoral neck | |||||||
| Age, clinic adjusted | −0.11 | −0.50, −0.27 | −0.57 | −0.80, −0.34 | −0.76 | −1.27, −0.25 | 0.07 |
| MV adjusted* | −0.21 | −0.60, 0.18 | −0.59 | −0.82 −0.36 | −0.59 | −1.12, −0.05 | 0.25 |
| Calcaneus | |||||||
| Age, clinic adjusted | −1.02 | −1.31, −0.73 | −1.50 | −1.68, −1.32 | −1.65 | −2.04, −1.26 | 0.01 |
| MV adjusted* | −1.01 | −1.27, −0.74 | −1.41 | −1.57, −1.24 | −1.49 | −1.86, −1.10 | 0.03 |
* The multivariate model included the following: age, clinic, BMI, weight change between visit 2 and visit 4, smoking, chair stands, fracture history, estrogen use, and baseline BMD.
Fractures
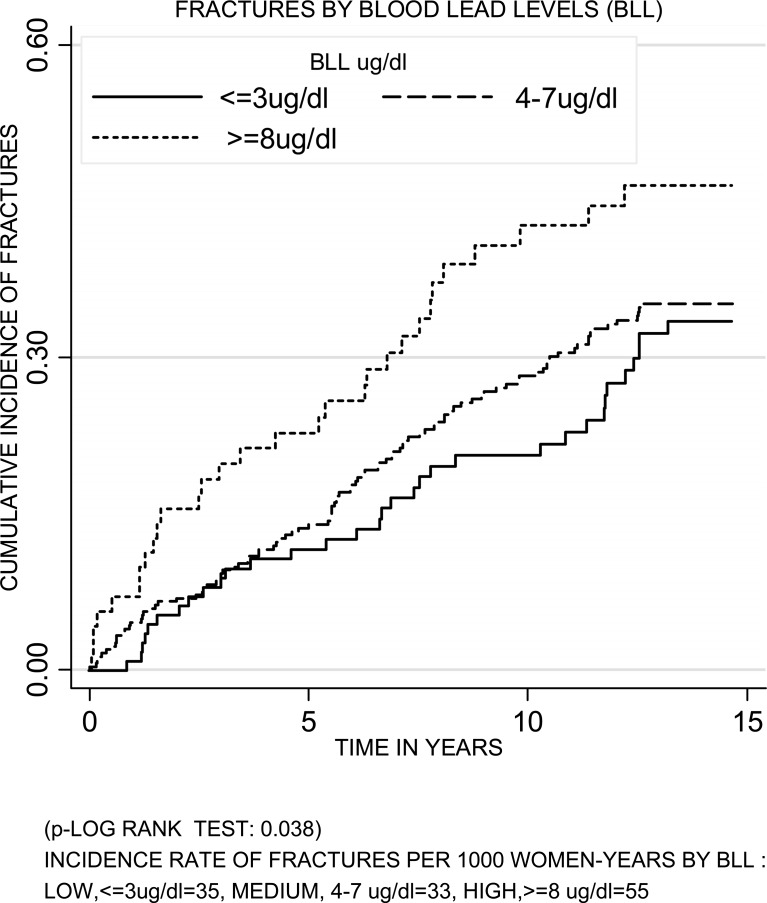
After an average follow-up of 10.5 yr, 163 (31%) women reported an incident fracture. The cumulative incidence of fracture increased over time and differed by blood lead. Women with the highest lead had the highest cumulative incidence of fractures (log-rank test = 0.038; Fig. 1). In age- and clinic-adjusted models, women with the highest blood lead had an increased risk of fracture (HR = 1.61; 95% CI = 0.97–2.66; Table 3). In multivariable-adjusted models, women with the highest blood lead had a significant increased risk of fracture in comparison with women with the lowest blood lead (HR = 1.94; 95% CI = 1.13, 3.32; p trend = 0.024). Further adjustment for neurological and neuromuscular variables increased the HR (HR = 2.50, 95% CI = 1.25, 5.03; p trend = 0.016). Women in the medium blood lead category were not at an increased risk of fracture. Using blood lead as a continuous variable in multivariable-adjusted models, the RH of fracture per SD increase in blood lead was 1.17 (95% CI = 1.00, 1.37; p = 0.039).
FIG. 1.
Cumulative incidence of fractures in women in SOF across blood lead levels.
Table 3.
Age and Multivariate-Adjusted HRs (95% CIs) of Incident Fractures and Incidence Rate Ratios of Falls by Blood Lead Levels
|
Blood lead levels (μg/dl)
|
||||||
| Low (≤3) (N = 122) | Medium (4–7) (N = 332) | High (≥8) (N = 79) | ||||
| HR of incident nonspine fracture | Referent | HR | 95% CI | HR | 95% CI | ptrend |
| Age, clinic adjusted | 1.0 | 1.10 | 0.74–1.64 | 1.61 | 0.97–2.66 | 0.080 |
| MV adjusted* | 1.0 | 1.16 | 0.76–1.75 | 1.94 | 1.13–3.32 | 0.024 |
| MV adjusted† | 1.0 | 1.13 | 0.65–1.95 | 2.50 | 1.25–5.03 | 0.016 |
| Incidence rate ratio (RR) of falls | Referent | IRR | 95% CI | IRR | 95% CI | ptrend |
| Age, clinic adjusted | 1.0 | 1.14 | 0.87–1.57 | 1.40 | 0.97–2.02 | 0.073 |
| MV adjusted‡ | 1.0 | 1.34 | 1.00–1.79 | 1.72 | 1.17–2.52 | 0.005 |
| MV adjusted§ | 1.0 | 1.41 | 1.00–2.01 | 1.62 | 1.07–2.45 | 0.014 |
Total N = 533.
* The fracture model included age, clinic, education, BMI, alcohol use, baseline total hip BMD, fractures after age 50, health status, fall in past year, smoking, estrogen use, and calcium and vitamin D use.
† Further adjusted for grip strength, reaction time lower limb, groove pegboard score, vibration and touch, visual acuity, walk speed, chair stands, and trail making B score.
‡ The falls model included age, clinic, education, BMI, alcohol use, health status, smoking, falls in past year, CNS active medication, estrogen use, and calcium and vitamin D use.
§ Further adjusted for grip strength, reaction time lower limb, groove pegboard score, vibration and touch, visual acuity, walk speed, chair stands, and trail making B score.
Falls
During the 4-yr follow-up period, the risk of falls was higher among women with higher blood lead (Table 3). In age- and clinic-adjusted models, women with the highest blood lead had a 40% increased risk of fall. The trend across groups was statistically significant in the multivariable-adjusted model where women in the highest blood lead category were at 72% increased risk of falling compared with women with the lowest lead levels (IRR = 1.72; 95% CI = 1.17–2.52; p trend = 0.005). Women in the medium blood lead category were at 34% increased risk of falls (IRR = 1.34; 95% CI = 1.00, 1.79; p = 0.050). Further adjustment for neurological and neuromuscular variables did not change the significance of our results (IRR = 1.62; 95% CI: 1.07, 2.45; p trend = 0.014). In multivariable-adjusted models, the risk of falls increased by 9% per SD increase in blood lead (IRR = 1.09; 95% CI = 1.00, 1.21; p = 0.075).
DISCUSSION
In this cohort of older women, circulating levels of blood lead ≥8 μg/dl were associated with lower total hip BMD, and an increased risk of falls and nonspine fractures. We found an association with fractures and falls at blood levels ≥8 μg/dl (median = 9 μg/dl), a level previously thought to be safe. The association with nonspine fractures and falls was not explained by poor cognitive or physical function, tests of vibration or touch sensitivity, balance, or visual acuity. Indeed, fractures and falls are multifactorial, and adjustment for other factors that could confound the association tended to strengthen the association with lead.
Although mean U.S. blood lead levels have decreased dramatically over the past 30 yr, as documented by repeated National Health and Nutrition Examination Surveys from 1988 to 2002, lead toxicity continues to be a public health problem for older individuals with higher lifetime environmental lead exposure.(48,49) More specifically, older women, who grew up when environmental exposures were higher because of leaded gasoline, paint, and unregulated industrial lead use, may have absorbed more lead from the environment and thus have a higher overall body burden of stored lead.(1)
We studied blood lead levels that reflect both recirculation from endogenous sources as well as more recent exposures. Studies report that skeletal contribution to blood lead levels ranges from 40% to 70% in adults.(50–54) In the Normative Aging Study of older men, blood lead was moderately correlated to bone lead levels for both trabecular and cortical bone (rho = 0.43, p < 0.01 for patella and rho = 0.34, p < 0.01 for tibia).(55) It has been proposed that lead released from the skeleton is preferentially partitioned into plasma and maybe more readily bio-available.(56) In middle-aged to elderly women (47–74 yr), an increase of 19 μg/g of lead in tibia was associated with an increase in blood lead level of 1.7 μg/dl, which corresponds to a 0.09-μg/dl increase in blood lead per 1 μg/g of bone mineral.(57)
In our study, baseline hip BMD levels but not calcaneal BMD were lower among women with higher blood lead levels. BMD was also significantly inversely associated with blood lead in the general population after adjusting for other factors associated with blood lead.(6,58,59) We found that greater lead levels were associated with faster rates of hip bone loss, but the results were not statistically significant. On the other hand, the association between blood lead and calcaneal bone loss was statistically significant whereby women in the high blood lead group experienced a greater rate of calcaneal bone loss than women in the low blood lead group. The calcaneus contains almost exclusively trabecular bone, and our findings are consistent with a higher rate of bone turnover in trabecular bone, leading to higher blood lead levels.(60)
Previous research, although limited, have not shown consistent relationships between lead and skeletal outcomes. Longitudinal analyses of postmenopausal women with occupational lead exposure showed that biomarkers for bone resorption were significantly higher in women who had higher blood and calcaneus bone lead.(61,62) Vertebral BMD was significantly inversely associated with blood lead even 10–18 yr after occupational exposure.(5,61–63) A more recent finding showed higher lead with higher bone lead but no relationship to blood lead.(18) Taken together, more research is clearly needed to further our understanding of the relationships of lead to skeletal outcomes.
High bone turnover has been associated with increased risk of fracture.(64) The association between lead and fracture may be caused by a direct effect of lead on bone strength or may simply be a marker of high bone turnover. Small studies of bisphosphonates(50) and hormone therapy(5) showed that treatments that reduce bone turnover resulted in a lower skeletal lead release.(50) In our study, women who reported using estrogen had lower lead levels. Thus, antiresorptive treatments could reduce lead exposure in older adults.
A genetic polymorphism has been identified in the vitamin D receptor gene (VDR) that can influence the accumulation of lead in bone.(65) A negative correlation has also been observed between lead and vitamin D.(3) Low vitamin D has been linked to hip and nonspine fractures and falls.(66,67) In our study, women with the highest blood lead were less likely to take vitamin D supplements at baseline in comparison with women with lower blood lead, suggesting that circulating vitamin D levels may also be lower in this group. Moreover, we observed a significant inverse association in blood lead level and number of years women took vitamin D supplements (p < 0.019). We were, however, unable to adjust for circulating vitamin D levels.
Lead exposure could predispose to fractures because of increased risk of falls because it affects neuromuscular and sensory function and balance.(68,69) Blood lead levels as low as 8 μg/dl were associated with poorer performance on tests of visual perception,(70) psychomotor speed, and manual dexterity.(22,70) Neuromotor dysfunction in lead workers has been reported at average blood lead levels of 14.4 μg/dl.(71) Using computerized static posturography, the effect of lead on postural balance was examined in 49 male lead workers, with mean (range) lead levels of 18 (7–36) μg/100 g. Compared with healthy non–lead-exposed controls, lead-exposed workers had significantly greater postural sway.(72) Thus, our finding of an association between blood lead and falls is consistent with these biological mechanisms. Although our relatively crude measures of balance and sensory and motor function did not differ across lead groups, adjustment for these factors tended to strengthen these associations. Future research should consider more sensitive tests (e.g., of postural sway rather than balance [by a tandem stand]) to further evaluate the effects of lead.
There are a number of strengths to our study. We used a prospective study design and studied a well-characterized cohort of community-dwelling older women. We categorized blood lead levels based on previous published results.(22) Follow-up was nearly complete, and all fractures were validated by review of radiology reports and/or medical records. However, this study does have some limitations. Our participants were likely to be healthier than average community-dwelling older women because they were volunteers. We measured blood lead only once but our approach is similar to other prospective studies of sex hormones predicting fractures.(73) We had no information on occupational exposures but we adjusted for a number of important covariates, including cognitive, neuromuscular function, and sensory. BMD measurements could seem artificially higher in lead-exposed individuals because of co-contamination with cadmium or strontium.(74) BMD increased with increasing bone lead levels, especially when BMD was measured on old pencil beam scanners, similar to the scanners used in our study.(75,76) Thus, our ability to adjust for BMD and test whether the association with fracture is independent of BMD may be limited. Finally, we used blood lead as the measure of lead exposure variable, but blood lead reflects only 40–70% of the skeletal lead burden. Future studies should include measures of skeletal lead, a better measure of the cumulative dose of lead. Finally, our results may not be generalizable to older nonwhite women or to men.
In conclusion, high blood lead levels were associated with an increased risk of falls and fractures in older women extending the negative health consequences of lead to include osteoporotic fractures.
ACKNOWLEDGMENTS
JAC and NK thank Lily Lui for assistance with the statistical analysis of the data. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1.
Footnotes
Dr Cauley has received research support from Merck & Company, Eli Lilly & Company, Pfizer Pharmaceuticals, and Novartis Pharmaceuticals. She has also received consulting fees from Eli Lilly & Company and Novartis Pharmaceuticals. Dr Cummings receives research support from Amgen, Pfizer, Novartis, and Eli Lilly and Co. and consulting fees or honoraria from Eli Lilly and Co., Zelos, Merck and Co., Novartis, GlaxoSmithKline, Procter & Gamble, and Aventis.
REFERENCES
- 1.Vig EK, Hu H. Lead toxicity in older adults. J Am Geriatr Soc. 2000;48:1501–1506. [PubMed] [Google Scholar]
- 2.Pounds JG. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: A review. Neurotoxicology. 1984;5:295–331. [PubMed] [Google Scholar]
- 3.Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. Environ Health Perspect. 1991;91:17–32. doi: 10.1289/ehp.919117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silbergeld EK, Schwartz J, Mahaffey K. Lead and osteoporosis: Mobilization of lead from bone in postmenopausal women. Environ Res. 1988;47:79–94. doi: 10.1016/s0013-9351(88)80023-9. [DOI] [PubMed] [Google Scholar]
- 5.Potula V, Kaye W. The impact of menopause and lifestyle factors on blood and bone lead levels among female former smelter workers: The Bunker Hill Study. Am J Ind Med. 2006;49:143–152. doi: 10.1002/ajim.20262. [DOI] [PubMed] [Google Scholar]
- 6.Nash D, Magder LS, Sherwin R, Rubin RJ, Silbergeld EK. Bone density-related predictors of blood lead level among peri- and postmenopausal women in the United States: The Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2004;160:901–911. doi: 10.1093/aje/kwh296. [DOI] [PubMed] [Google Scholar]
- 7.Berlin K, Gerhardsson L, Borjesson J, Lindh E, Lundstrom N, Schutz A, Skerfving S, Edling C. Lead intoxication caused by skeletal disease. Scand J Work Environ Health. 1995;21:296–300. doi: 10.5271/sjweh.42. [DOI] [PubMed] [Google Scholar]
- 8.Bjora R, Falch JA, Staaland H, Nordsletten L, Gjengedal E. Osteoporosis in the Norwegian moose. Bone. 2001;29:70–73. doi: 10.1016/s8756-3282(01)00469-0. [DOI] [PubMed] [Google Scholar]
- 9.Gruber HE, Gonick HC, Khalil-Manesh F, Sanchez TV, Motsinger S, Meyer M, Sharp CF. Osteopenia induced by long-term, low- and high-level exposure of the adult rat to lead. Miner Electrolyte Metab. 1997;23:65–73. [PubMed] [Google Scholar]
- 10.Gonzalez-Riola J, Hernandez ER, Escribano A, Revilla M, Ca S, Villa LF, Rico H. Effect of lead on bone and cartilage in sexually mature rats: A morphometric and histomorphometry study. Environ Res. 1997;74:91–93. doi: 10.1006/enrs.1997.3760. [DOI] [PubMed] [Google Scholar]
- 11.Escribano A, Revilla M, Hernandez ER, Seco C, Gonzalez-Riola J, Villa LF, Rico H. Effect of lead on bone development and bone mass: A morphometric, densitometric, and histomorphometric study in growing rats. Calcif Tissue Int. 1997;60:200–203. doi: 10.1007/s002239900214. [DOI] [PubMed] [Google Scholar]
- 12.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 13.Shukla R, Bornschein RL, Dietrich KN, Buncher CR, Berger OG, Hammond PB, Succop PA. Fetal and infant lead exposure: Effects on growth in stature. Pediatrics. 1989;84:604–612. [PubMed] [Google Scholar]
- 14.Schwartz J, Angle C, Pitcher H. Relationship between childhood blood lead levels and stature. Pediatrics. 1986;77:281–288. [PubMed] [Google Scholar]
- 15.Ignasiak Z, Slawinska T, Rozek K, Little BB, Malina RM. Lead and growth status of schoolchildren living in the copper basin of south-western Poland: Differential effects on bone growth. Ann Hum Biol. 2006;33:401–414. doi: 10.1080/03014460600730752. [DOI] [PubMed] [Google Scholar]
- 16.Esserman L, Campbell M, Shoemaker M, Lobo M, Marx C, Benz C. Breast cancer inhibition by statins. J Clin Oncol. 2004;22(14S):97S. [Google Scholar]
- 17.Potula V, Kleinbaum D, Kaye W. Lead exposure and spine bone mineral density. J Occup Environ Med. 2006;48:556–564. doi: 10.1097/01.jom.0000222556.89044.90. [DOI] [PubMed] [Google Scholar]
- 18.Theppeang K, Glass TA, Bandeen-Roche K, Tood AC, Rohde CA, Links JM, Schwartz BS. Associations of bone mineral density and lead levels in blood, tibia, and patella in urban dwelling work. Environ Health Perspect. 2008;116:784–790. doi: 10.1289/ehp.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisskopf MG, Proctor SP, Wright RO, Schwartz J, Spiro A, III, Sparrow D, Nie H, Hu H. Cumulative lead exposure and cognitive performance among elderly men. Epidemiology. 2007;18:59–66. doi: 10.1097/01.ede.0000248237.35363.29. [DOI] [PubMed] [Google Scholar]
- 20.Dogu O, Louis ED, Tamer L, Unal O, Yilmaz A, Kaleagasi H. Elevated blood lead concentrations in essential tremor: A case-control study in Mersin, Turkey. Environ Health Perspect. 2007;115:1564–1568. doi: 10.1289/ehp.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis ED, Jurewicz EC, Applegate L, Factor-Litvak P, Parides M, Andrews L, Slavkovich V, Graziano JH, Carroll S, Todd A. Association between essential tremor and blood lead concentration. Environ Health Perspect. 2003;111:1707–1711. doi: 10.1289/ehp.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muldoon SB, Cauley JA, Kuller LH, Morrow L, Needleman HL, Scott J, Hooper FJ. Effects of blood lead levels on cognitive function of older women. Neuroepidemiology. 1996;15:62–72. doi: 10.1159/000109891. [DOI] [PubMed] [Google Scholar]
- 23.Payton M, Riggs KM, Spiro A, III, Weiss ST, Hu H. Relations of bone and blood lead to cognitive function: The VA Normative Aging Study. Neurotoxicol Teratol. 1998;20:19–27. doi: 10.1016/s0892-0362(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 24.Chia SE, Chia HP, Ong CN, Jeyaratnam J. Cumulative blood lead levels and nerve conduction parameters. Occup Med (Lond) 1996;46:59–64. doi: 10.1093/occmed/46.1.59. [DOI] [PubMed] [Google Scholar]
- 25.Maizlish NA, Parra G, Feo O. Neurobehavioural evaluation of Venezuelan workers exposed to inorganic lead. Occup Environ Med. 1995;52:408–414. doi: 10.1136/oem.52.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz BS, Lee BK, Lee GS, Stewart WF, Lee SS, Hwang KY, Ahn KD, Kim YB, Bolla KI, Simon D, Parsons PJ, Todd AC. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with neurobehavioral test scores in South Korean lead workers. Am J Epidemiol. 2001;153:453–464. doi: 10.1093/aje/153.5.453. [DOI] [PubMed] [Google Scholar]
- 27.Lucchini R, Albini E, Cortesi I, Placidi D, Bergamaschi E, Traversa F, Alessio L. Assessment of neurobehavioral performance as a function of current and cumulative occupational lead exposure. Neurotoxicology. 2000;21:805–811. [PubMed] [Google Scholar]
- 28.Abbate C, Buceti R, Munao F, Giorgianni C, Ferreri G. Neurotoxicity induced by lead levels: An electrophysiological study. Int Arch Occup Environ Health. 1995;66:389–392. doi: 10.1007/BF00383145. [DOI] [PubMed] [Google Scholar]
- 29.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 30.Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44:M112–M117. doi: 10.1093/geronj/44.4.m112. [DOI] [PubMed] [Google Scholar]
- 31.Muldoon SB, Cauley JA, Kuller LH, Scott J, Rohay J. Lifestyle and sociodemographic factors as determinants of blood lead levels in elderly women. Am J Epidemiol. 1994;139:599–608. doi: 10.1093/oxfordjournals.aje.a117049. [DOI] [PubMed] [Google Scholar]
- 32.Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR. Hip and calcaneal bone loss increase with advancing age: Longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–1787. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 33.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res. 1992;7:625–632. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- 34.Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, Tao JL, Cummings SR. Factors associated with appendicular bone mass in older women. The Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1993;118:657–665. doi: 10.7326/0003-4819-118-9-199305010-00001. [DOI] [PubMed] [Google Scholar]
- 35.Orwoll ES, Bauer DC, Vogt TM, Fox KM. Axial bone mass in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1996;124:187–196. doi: 10.7326/0003-4819-124-2-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 36.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 37.Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC, Bowman PJ, Ensrud KE. Long-term prediction of incident hip fracture risk in elderly white women: Study of osteoporotic fractures. J Am Geriatr Soc. 2004;52:1479–1486. doi: 10.1111/j.1532-5415.2004.52410.x. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Magaziner J, Bassett SS, Hebel JR. Predicting performance on the Mini-Mental State Examination. Use of age- and education-specific equations. J Am Geriatr Soc. 1987;35:996–1000. doi: 10.1111/j.1532-5415.1987.tb04002.x. [DOI] [PubMed] [Google Scholar]
- 40.Teng EL, Chui HC. The modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 41.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 42.Bleecker ML, Ford DP, Vaughan CG, Lindgren KN, Tiburzi MJ, Walsh KS. Effect of lead exposure and ergonomic stressors on peripheral nerve function. Environ Health Perspect. 2005;113:1730–1734. doi: 10.1289/ehp.8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sands ML, Schwartz AV, Brown BW, Nevitt MC, Seeley DG, Kelsey JL. Relationship of neurological function and age in older women. The study of osteoporotic fractures. Neuroepidemiology. 1998;17:318–329. doi: 10.1159/000026186. [DOI] [PubMed] [Google Scholar]
- 44.Bleecker ML, Ford DP, Lindgren KN, Scheetz K, Tiburzi MJ. Association of chronic and current measures of lead exposure with different components of brainstem auditory evoked potentials. Neurotoxicology. 2003;24:625–631. doi: 10.1016/s0161-813x(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 45.Bleecker ML, Lindgren KN, Tiburzi MJ, Ford DP. Curvilinear relationship between blood lead level and reaction time. Differential association with blood lead fractions derived from exogenous and endogenous sources. J Occup Environ Med. 1997;39:426–431. doi: 10.1097/00043764-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Steele G, Kattouf V. Blood lead levels and vision. Optometry. 2000;71:217–220. [PubMed] [Google Scholar]
- 47.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991-1994. Environ Health Perspect. 1998;106:745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: The National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–2161. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- 50.Gulson B, Mizon K, Smith H, Eisman J, Palmer J, Korsch M, Donnelly J, Waite K. Skeletal lead release during bone resorption: Effect of bisphosphonate treatment in a pilot study. Environ Health Perspect. 2002;110:1017–1023. doi: 10.1289/ehp.021101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulson BL, Gillings BR. Lead exchange in teeth and bone—a pilot study using stable lead isotopes. Environ Health Perspect. 1997;105:820–824. doi: 10.1289/ehp.97105820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulson BL, Mahaffey KR, Jameson CW, Mizon KJ, Korsch MJ, Cameron MA, Eisman JA. Mobilization of lead from the skeleton during the postnatal period is larger than during pregnancy. J Lab Clin Med. 1998;131:324–329. doi: 10.1016/s0022-2143(98)90182-2. [DOI] [PubMed] [Google Scholar]
- 53.Gulson BL, Mahaffey KR, Mizon KJ, Korsch MJ, Cameron MA, Vimpani G. Contribution of tissue lead to blood lead in adult female subjects based on stable lead isotope methods. J Lab Clin Med. 1995;125:703–712. [PubMed] [Google Scholar]
- 54.Gulson BL, Pounds JG, Mushak P, Thomas BJ, Gray B, Korsch MJ. Estimation of cumulative lead releases (lead flux) from the maternal skeleton during pregnancy and lactation. J Lab Clin Med. 1999;134:631–640. doi: 10.1016/s0022-2143(99)90104-x. [DOI] [PubMed] [Google Scholar]
- 55.Tsaih SW, Korrick S, Schwartz J, Lee ML, Amarasiriwardena C, Aro A, Sparrow D, Hu H. Influence of bone resorption on the mobilization of lead from bone among middle-aged and elderly men: The Normative Aging Study. Environ Health Perspect. 2001;109:995–999. doi: 10.1289/ehp.01109995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cake KM, Bowins RJ, Vaillancourt C, Gordon CL, McNutt RH, Laporte R, Webber CE, Chettle DR. Partition of circulating lead between serum and red cells is different for internal and external sources of lead. Am J Ind Med. 1996;29:440–445. doi: 10.1002/(SICI)1097-0274(199605)29:5<440::AID-AJIM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 57.U.S. Environmental Protection Agency. Air Quality Criteria for Lead (Final) 2006. Available at http://oaspub.epa.gov/eims/eimscomm.getfile?p_download_id=459555. Accessed June 25, 2008.
- 58.Nash D, Magder L, Lustberg M, Sherwin RW, Rubin RJ, Kaufmann RB, Silbergeld EK. Blood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. JAMA. 2003;289:1523–1532. doi: 10.1001/jama.289.12.1523. [DOI] [PubMed] [Google Scholar]
- 59.Osterloh JD, Kelly TJ. Study of the effect of lactational bone loss on blood lead concentrations in humans. Environ Health Perspect. 1999;107:187–194. doi: 10.1289/ehp.99107187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garrido Latorre F, Hernandez-Avila M, Tamayo Orozco J, Albores Medina CA, Aro A, Palazuelos E, Hu H. Relationship of blood and bone lead to menopause and bone mineral density among middle-age women in Mexico City. Environ Health Perspect. 2003;111:631–636. doi: 10.1289/ehp.111-1241456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potula V, Henderson A, Kaye W. Calcitropic hormones, bone turnover, and lead exposure among female smelter workers. Arch Environ Occup Health. 2005;60:195–204. doi: 10.3200/AEOH.60.4.195-204. [DOI] [PubMed] [Google Scholar]
- 62.Popovic M, McNeill FE, Chettle DR, Webber CE, Lee CV, Kaye WE. Impact of occupational exposure on lead levels in women. Environ Health Perspect. 2005;113:478–484. doi: 10.1289/ehp.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potula V, Kaye W. Report from the CDC. Is lead exposure a risk factor for bone loss. J Womens Health (Larchmt) 2005;14:461–464. doi: 10.1089/jwh.2005.14.461. [DOI] [PubMed] [Google Scholar]
- 64.Looker AC, Bauer DC, Chesnut CH, III, Gundberg CM, Hochberg MC, Klee G, Kleerekoper M, Watts NB, Bell NH. Clinical use of biochemical markers of bone remodeling: Current status and future directions. Osteoporos Int. 2000;11:467–480. doi: 10.1007/s001980070088. [DOI] [PubMed] [Google Scholar]
- 65.Onalaja AO, Claudio L. Genetic susceptibility to lead poisoning. Environ Health Perspect. 2000;108(Suppl 1):23–28. doi: 10.1289/ehp.00108s123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB. Effect of Vitamin D on falls: A meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 67.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 68.Greenspan SL, Beck TJ, Resnick NM, Bhattacharya R, Parker RA. Effect of hormone replacement, alendronate, or combination therapy on hip structural geometry: A 3-year, double-blind, placebo-controlled clinical trial. J Bone Miner Res. 2005;20:1525–1532. doi: 10.1359/JBMR.050508. [DOI] [PubMed] [Google Scholar]
- 69.Iwata T, Yano E, Karita K, Dakeishi M, Murata K. Critical dose of lead affecting postural balance in workers. Am J Ind Med. 2005;48:319–325. doi: 10.1002/ajim.20220. [DOI] [PubMed] [Google Scholar]
- 70.Bleecker ML, Lindgren KN, Ford DP. Differential contribution of current and cumulative indices of lead dose to neuropsychological performance by age. Neurology. 1997;48:639–645. doi: 10.1212/wnl.48.3.639. [DOI] [PubMed] [Google Scholar]
- 71.Chia SE, Chia HP, Ong CN, Jeyaratnam J. Cumulative concentrations of blood lead and postural stability. Occup Environ Med. 1996;53:264–268. doi: 10.1136/oem.53.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yokoyama K, Araki S, Murata K, Morita Y, Katsuno N, Tanigawa T, Mori N, Yokota J, Ito A, Sakata E. Subclinical vestibulo-cerebellar, anterior cerebellar lobe and spinocerebellar effects in lead workers in relation to concurrent and past exposure. Neurotoxicology. 1997;18:371–380. [PubMed] [Google Scholar]
- 73.Whooley MA, Grady D, Cummings SR. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;337:1389–1390. [PubMed] [Google Scholar]
- 74.Nielsen SP, Slosman D, Sorensen OH, Basse-Cathalinat B, De Cassin P, Roux CR, Meunier PJ. Influence of strontium on bone mineral density and bone mineral content measurements by dual X-ray absorptiometry. J Clin Densitom. 1999;2:371–379. doi: 10.1016/s1094-6950(06)60402-2. [DOI] [PubMed] [Google Scholar]
- 75.Campbell JR, Rosier RN, Novotny L, Puzas JE. The association between environmental lead exposure and bone density in children. Environ Health Perspect. 2004;112:1200–1203. doi: 10.1289/ehp.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puzas JE, Campbell J, O'Keefe R, Schwartz E, Rosier R. Lead in the skeleton interferes with bone mineral measurements. J Bone Miner Metab. 2002;17:S314. [Google Scholar]