Abstract
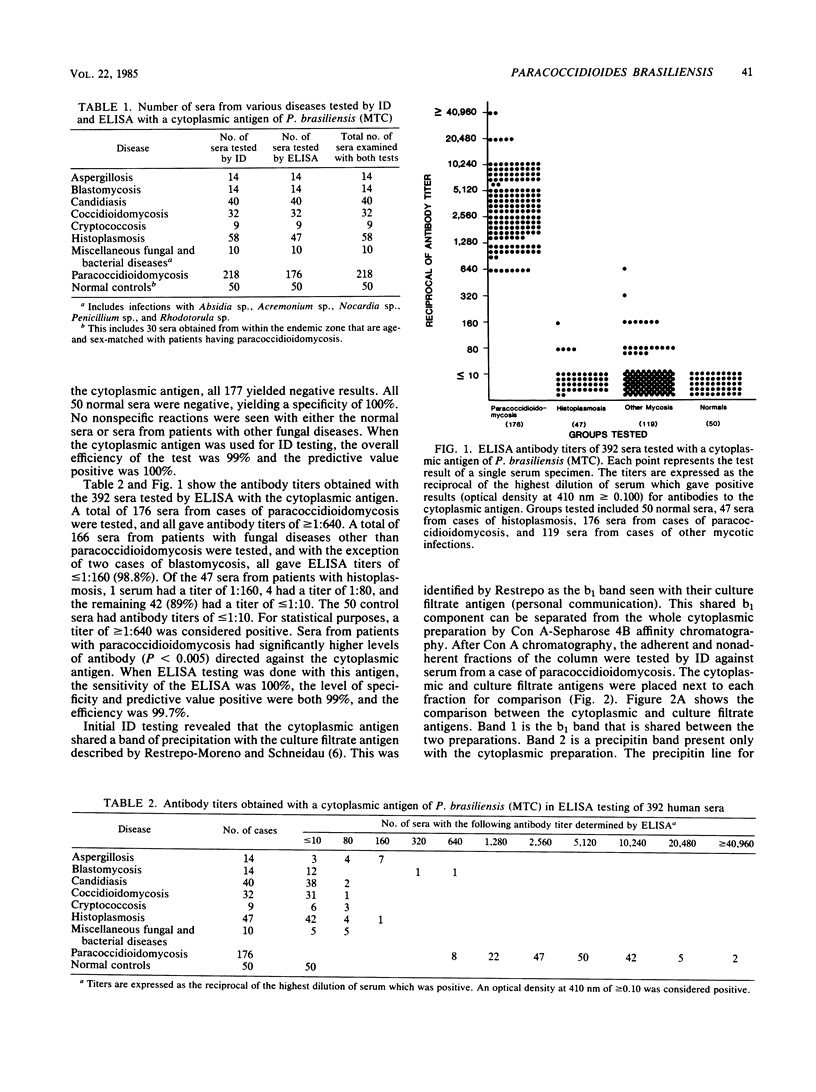
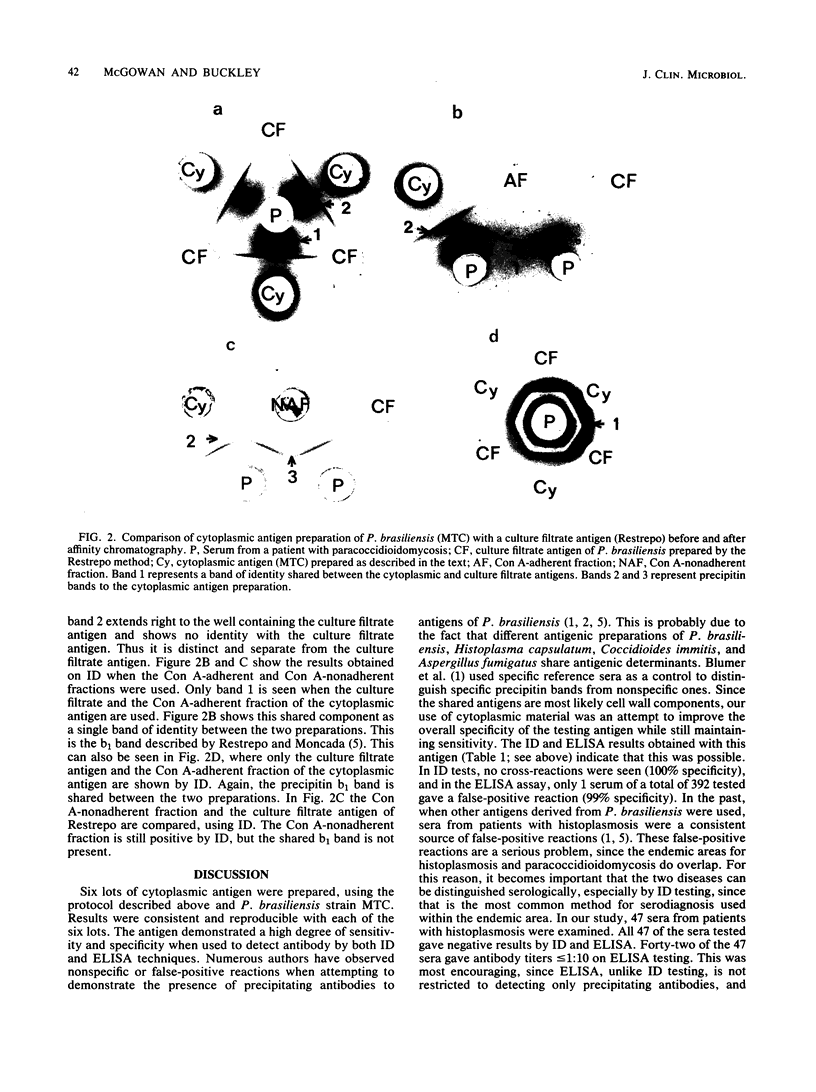
A cytoplasmic antigen of Paracoccidioides brasiliensis (strain MTC) prepared from the yeast phase grown in the chemically defined medium of McVeigh and Morton is described. This antigen can be easily prepared, does not vary from lot to lot, and can be lyophilized without loss of activity or potency. In the immunodiffusion test, the cytoplasmic antigen demonstrated a sensitivity of 97% and a specificity of 100% when tested against 218 sera from 139 cases of paracoccidioidomycosis. When 177 sera from patients with fungal diseases other than paracoccidioidomycosis were tested by immunodiffusion, there were no false-positive reactions. In an enzyme-linked immunosorbent assay, the antigen was equally effective in identifying cases, giving a sensitivity of 100% and a specificity of 99% when a 1:640 titer was considered the threshold for clinical significance. Antigenic components in the cytoplasmic extract of P. brasiliensis were examined after fractionation by concanavalin A-Sepharose 4B column chromatography. The fraction of the cytoplasmic antigen that binds to the concanavalin A column is material identical to the specific b1 (antigen 1) precipitin band described by A. Restrepo and L. H. Moncada (Appl. Microbiol. 28:138-144, 1974).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumer S. O., Jalbert M., Kaufman L. Rapid and reliable method for production of a specific Paracoccidioides brasiliensis immunodiffusion test antigen. J Clin Microbiol. 1984 Mar;19(3):404–407. doi: 10.1128/jcm.19.3.404-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh I., Morton K. Nutritional studies of Histoplasma capsulatum. Mycopathol Mycol Appl. 1965 Apr 14;25(3):294–308. doi: 10.1007/BF02049917. [DOI] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Moncada L. H. Characterization of the precipitin bands detected in the immunodiffusion test for paracoccidioidomycosis. Appl Microbiol. 1974 Jul;28(1):138–144. doi: 10.1128/am.28.1.138-144.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarzabal L. A., Andrieu S., Bout D., Naquira F. Isolation of a specific antigen with alkaline phosphatase activity from soluble extracts of Paracoccidioides brasiliensis. Sabouraudia. 1976 Nov;14(3):275–280. doi: 10.1080/00362177685190411. [DOI] [PubMed] [Google Scholar]
- de Camargo Z. P., Guesdon J. L., Drouhet E., Improvisi L. Titration of antibodies to Paracoccidioides brasiliensis by erythro-immunoassay (EIA). Sabouraudia. 1984;22(1):73–77. [PubMed] [Google Scholar]