Abstract
Conditionally replicating adenoviruses (CRAds) have many advantages as agents for cancer virotherapy and have been safely employed in human clinical trials. However, replicating adenoviruses have been limited in their ability to eliminate tumors by oncolysis. Thus, the efficacy of these agents must be improved. To this end, CRAds have been engineered to express therapeutic transgenes that exert antitumor effects independent of direct viral oncolysis. These transgenes can be expressed under native gene control elements, in which case placement within the genome determines the expression profile, or they can be controlled by exogenous promoters. The therapeutic transgenes used to arm replicating adenoviruses can be broadly classified into three groups. There are those that mediate killing of the infected cell, those that modulate the tumor microenvironment, and those with immunomodulatory functions. Overall, the studies to date in animal models have shown that arming a CRAd with a rationally chosen therapeutic transgene can improve its antitumor efficacy over that of an unarmed CRAd. However, a number of obstacles must be overcome before the full potential of armed CRAds can be realized in the human clinical context. Hence, strategies are being developed to permit intravenous delivery to disseminated cancer cells, to overcome the immune response and to enable in vivo monitoring of the biodistribution and activity of armed CRAds.
Keywords: cancer therapy, oncolysis, replicating adenoviruses, virotherapy
Introduction
In recent years, oncolytic viruses have been increasingly studied as potential cancer therapeutics, a field known as virotherapy. Oncolytic viruses replicate selectively in cancer cells, thereby amplifying the initial inoculum, and destroy the cells by lysis. The viral progeny are then released, enabling them to infect neighboring cells, which results in multiple self-perpetuating rounds of infection, replication, lysis and spread throughout the tumor, all while sparing normal cells. Additionally, viral replication within a tumor may help mobilize the immune system by inducing the release of cytokines and by liberating tumor antigens.1 A number of clinical trials have already been conducted that are based on oncolytic viruses, including those that are naturally selective for tumor cells, such as reovirus and Newcastle disease virus, and those that have been made selective by genetic manipulation, such as adenovirus and herpes simplex virus.2
Conditionally replicating adenoviruses
Human adenovirus serotype 5 (Ad5), of species C, has a number of features that make it particularly attractive as a platform for oncolytic virus construction. It is not associated with any serious disease.3 Moreover, the Ad5 genome has been well characterized, allowing for relatively easy genetic modification. In this regard, modifications have been made to capsid proteins, especially the fiber, to achieve efficient and specific infection of tumor cells.4 Finally, it can be grown to high titers and is relatively stable in the bloodstream, two features that allow the adenovirus to be administered systemically, which raises the possibility of treating distant metastases.
Conditionally replicating adenoviruses (CRAds) can be engineered to selectively replicate within tumor cells by two broad strategies. In the first, deletions are made to the adenovirus genome that prevent replication in normal cells, but which allow replication in tumor cells with genetic defects that complement the deleted viral gene functions. The earliest and most widely used example of this is the dl1520 virus, otherwise known as Onyx-015.5 This virus lacks the E1B-55k gene and was intended to replicate selectively within p53-deficient tumor cells. However, later investigations into the mechanics of Onyx-015 replication have revealed that the late functions of E1B-55k, namely the nuclear export of viral mRNAs and shutoff of host protein translation, are the major determinants of its selectivity.6 Another example is the so-called Δ24 mutant, in which a 24-bp deletion in the CR2 region of E1A results in a protein that is unable to bind and inactivate the retinoblastoma tumor suppressor/cell cycle regulator protein.7 This modification precludes efficient viral replication in cells with an intact G1-S phase checkpoint.
The second broad strategy used to construct CRAds is to control the expression of genes involved in viral replication with a tumor- or tissue-selective promoter (TSP). Typically, this is done by placing the viral E1A gene, the first to be expressed in replication, under the control of an exogenous promoter that is active in a particular cancer. The earliest example of this was the use of the prostate-specific antigen (PSA) promoter in the prostate cancer-targeted virus CN706.8 Other promoters employed in this way include the L-plastin (for breast and ovarian cancer),9,10 tyrosinase (for melanoma),9 osteocalcin (for bone metastases of prostate cancer),11 and Cox-2 (for gastric cancer) promoters.12 The human telomerase reverse transcriptase (hTERT) promoter has also been used to control expression of E1A and restrict viral replication to a range of telomerase-positive cancer cells.13,14 Alternatively, a TSP can be used to direct the expression of other early viral genes critical for replication, such as E1B15 or E4,16 either alone or in addition to E1A.
Rationale for armed CRAds
CRAds have already been used clinically. The most widely used thus far has been Onyx-015,17 although others such as CN706, formerly known as CV706,18 and the related CG787019 have been used as well. These trials have involved a variety of tumors and routes of administration. Overall, the results of these trials have indicated that while oncolytic adenoviruses are safe, they are unable to significantly alter the course of the disease. In light of these findings, it is evident that the potency of CRAds must be improved. One means of augmenting antitumor efficacy utilizes the CRAd as a platform for the delivery of a therapeutic transgene, thereby creating a so-called armed replicating adenovirus, in which the input dose of transgene is amplified by replication of the virus.20
Placement of transgenes
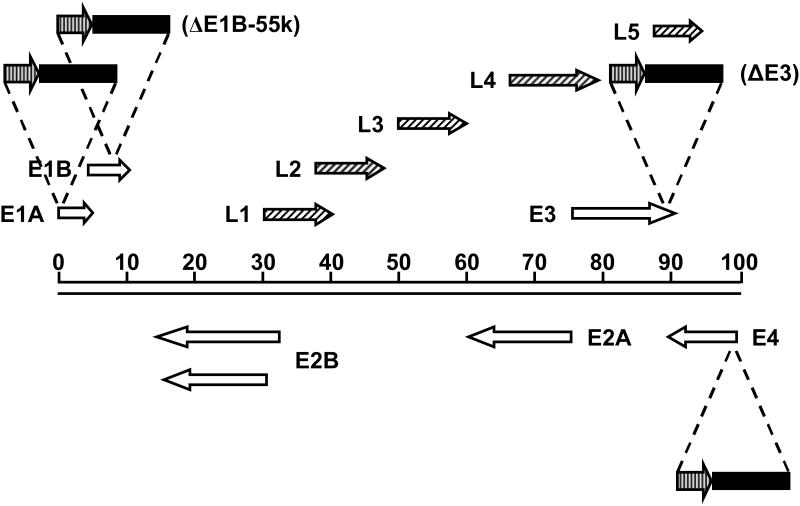
There are two key considerations that must be taken into account when inserting a therapeutic transgene into a CRAd. First, given that replication is a critical feature of a CRAd, it is important that an insert not disrupt this replication, either by activity of the transgene itself, or by the alterations made to the genome by the insert. Secondly, the packaging limitations of the virus must be considered. Ad5 has a genome of approximately 36kb, and can stably encapsidate genomes up to 38kb, or 105% of the wild-type genome.21 Thus, the size of any putative insert is limited. Because of this limitation, space within the adenoviral genome must be used economically. This can be achieved in part by utilizing the endogenous gene expression machinery (promoter, polyadenylation and splicing signals). Since adenoviral genes are expressed in well-characterized patterns (Figure 1), this strategy allows the amount and kinetics of transgene expression to be predicted. Thus, a transgene can be inserted into the adenoviral genome in a location that yields the ideal expression profile for that particular gene.
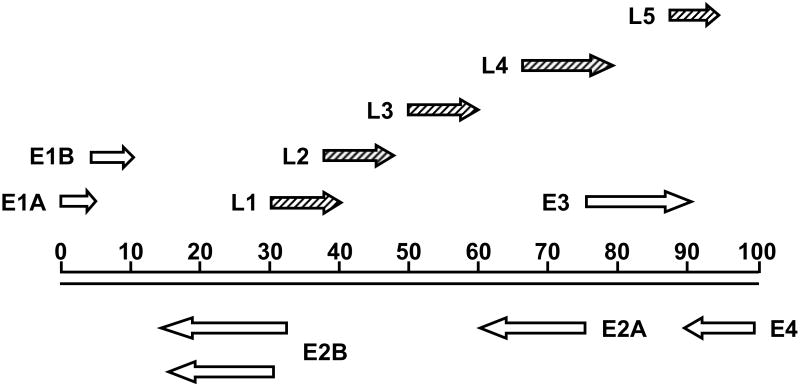
Figure 1.
Schematic diagram of the Ad5 genome. The Ad5 genome is approximately 36 kb long, divided into 100 map units. The direction of transcription is indicated by arrows. Open arrows represent early transcripts; diagonally striped arrows represent late transcripts.
Transgenes under control of endogenous viral gene control elements
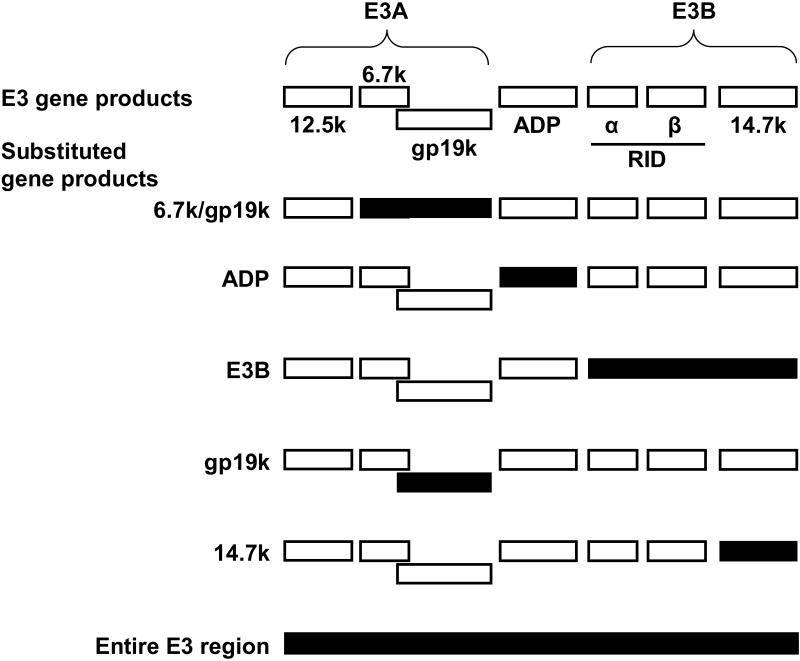
In general, armed CRAds employing native expression elements fall into two categories. In the first category, a viral gene unnecessary for replication in the target cells is replaced with a transgene. In this scenario, the native gene control elements flanking the deleted viral gene are left intact and therefore direct expression of the inserted transgene. Viruses in this category have been developed under the observation that the E3 transcription unit is not necessary for viral replication. Thus, early armed CRAds were based on E3-deleted platforms (Figure 2). One of the first involved the replacement of the entire E3 coding region with a transgene for interferon. In this case, the E3 promoter and termination site were retained, and therefore directed the expression of the transgene.22 In a similar approach, Doronin et al.23 deleted the entire E3 region and then re-inserted the gene for adenovirus death protein (ADP), an E3 gene product responsible for efficient cell lysis and viral release.24 This allowed ADP to be expressed solely by the major late promoter (MLP), leading to high levels of expression, late in the infection cycle.23 Later studies have exploited the complexity of the E3 transcription unit, which encodes multiple overlapping mRNAs dependent on variable splicing events that are expressed with different timing. This raises the possibility of arming a CRAd with multiple transgenes. Hawkins et al. constructed a number of armed CRAds in which transgenes were substituted for the 6.7k/gp19k, ADP, or E3B genes, respectively. It was found that in each case, the expression of the transgene mimicked that of the replaced viral gene with respect to timing and levels, and that effects on the surrounding viral genes were minimal.25-27 While this study involved the replacement of 6.7k/gp19k or E3B as a unit, it is also possible to replace the gp19k28 or (E3B) 14.7k29 genes individually. Furthermore, it has also been demonstrated that multiple transgenes can be inserted into the E3 transcription unit, while maintaining the expression of the remaining E3 genes.30,31 In order to identify additional sites for transgene insertion, Jin et al. utilized a transposon-based system to scan the adenovirus genome for insertion sites that did not compromise the viral life cycle. In this study, a reporter transgene was linked to a splice acceptor site such that expression depended on endogenous promoters. A variety of insertion sites were discovered, located primarily between E3 and the adjacent L5 gene and in and around the E4 gene. In every case, transcripts originated from the MLP. It is important to note, however, that in order to accommodate the transposon, the viral genome used was E3-deleted. Thus, additional insertion sites within E3 may be possible.32 Finally, transgenes have also been expressed from the L3 region of the genome.33 This strategy, described by Robinson et al., also relied on a splice acceptor sequence but retained all viral genes. In a comparison of E3 14.7k and L3 as insertion sites, both yielded high levels of transgene expression with expression from L3 being more strictly dependent on viral DNA replication.33
Figure 2.
Schematic diagram of the Ad5 E3 region showing transgene insertion sites. Open boxes represent gene products encoded by the non-essential E3 transcription unit. Solid boxes represent therapeutic transgenes inserted into the E3 transcription unit in place of native genes. Transgenes are under control of endogenous E3 gene expression elements.
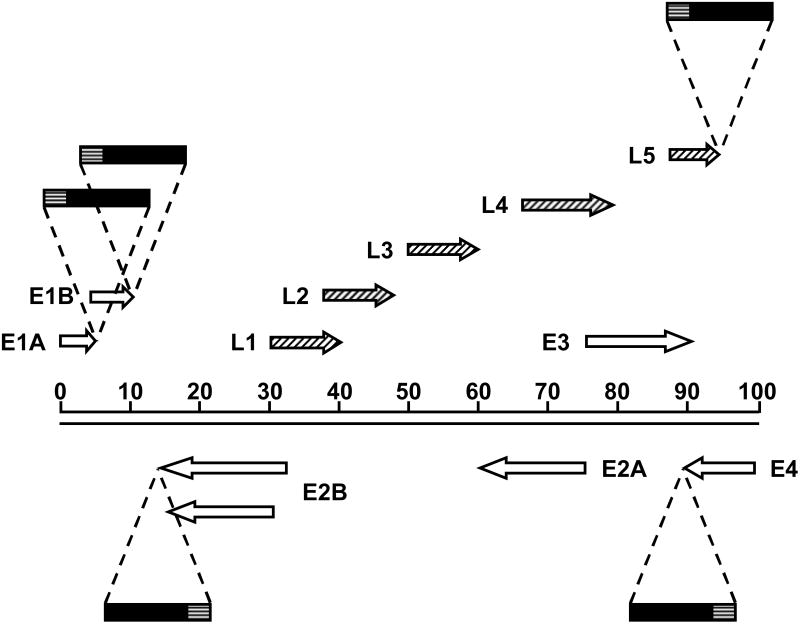
The second category of armed CRAds employing endogenous gene control elements are those in which a transgene is linked to a viral gene by an internal ribosome entry site (IRES), which allows both genes to be expressed as a single transcript (Figure 3). This allows the transgene to be expressed in a similar fashion to the gene to which it is linked, both in amount and timing. The most common example of this strategy is to link a therapeutic transgene to the viral fiber gene. This location has been used by several different groups to express p53,34 yeast cytosine deaminase (yCD)35 and nitroreductase,36 respectively. In each case, it was found that the inserted transgene was expressed late in the infection cycle, and to high levels. Late expression has a number of advantages. Expressing a transgene after viral DNA replication avoids the complication of using cytotoxic transgenes that can interfere with viral replication. Secondly, allowing viral replication to proceed before expression of a transgene amplifies the available transcript templates in a given cell. Finally, by limiting expression of the transgene to cells that support replication of the CRAd, the problem of transgene expression in normal cells is avoided. However, the fiber gene is not the only viral gene that can be used to support the expression of a transgene via an IRES. In order to explore the expression of transgenes at earlier time points, Rivera et al. constructed three different viruses in which the luciferase reporter gene was linked to E1A, E2 and fiber, respectively. In each case, expression of the transgene closely mimicked that of the gene to which it was linked, both in timing and amount. However, the E1A-IRES-luciferase virus had attenuated expression of E1A, E1B and fiber. Overall, the use of fiber-IRES was shown to yield the highest expression of luciferase.37 Similarly, Wirth et al. used an IRES to link expression of enhanced green fluorescent protein (EGFP) from the E1B promoter in a virus with E1A under the control of the hTERT promoter. The expression of EGFP from this virus was found to be more selective and longer sustained than that from the non-replicating control virus, in which GFP expression was driven by the CMV promoter.13
Figure 3.
Schematic diagram of the Ad5 genome showing insertion sites of transgenes separated by native viral genes by IRES sequences. The direction of transcription is indicated by arrows. Open arrows represent early transcripts; diagonally striped arrows represent late transcripts; solid boxes represent therapeutic transgenes; horizontally striped boxes represent IRES sequences. Transgenes are under control of endogenous viral gene expression elements.
Transgenes under control of exogenous promoters
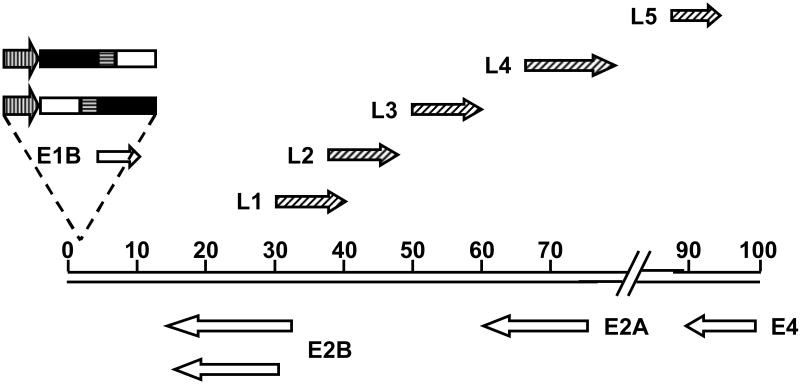
Armed CRAds have also been constructed in which the therapeutic transgene is under the control of a non-native promoter. In general, these armed CRAds are deleted for the E3 region, which is non-essential for viral replication. This deletion provides space within the genome, allowing the insertion of larger expression cassettes than could be accommodated by a wild-type genome. Armed CRAds with exogenously controlled expression cassettes can be divided into two classes. In the first, the exogenous promoter, either a TSP or constitutively active promoter, is used to drive the expression of a single transcript, with a therapeutic transgene linked by an IRES to the viral E1A gene (Figure 4). Thus, the transgene is expressed concurrently with E1A at the start of the viral life cycle. The order of the transgene, IRES and E1A within the cassette is flexible. For example, Ye et al. constructed a CRAd with an E1A-IRES-TRAIL cassette driven by the AFP promoter38 while Akbulut et al. constructed a CRAd with a CD-IRES-E1A cassette expressed from the L-plastin promoter.10 The constitutively active CMV39 and RSV40 promoters have also been used to control the expression of cassettes in which E1A is linked via an IRES to a transgene.
Figure 4.
Schematic diagram of E3-deleted Ad5 genome showing exogenous promoter driving the expression of a therapeutic transgene linked by an IRES to the viral E1A gene. The direction of transcription is indicated by arrows. Vertically striped arrows represent exogenous promoters; open boxes represent E1A gene; solid boxes represent therapeutic transgenes; horizontally striped boxes represent IRES sequences. Open arrows represent early transcripts; diagonally striped arrows represent late transcripts.
The second class of armed CRAds with expression cassettes containing exogenous promoters comprises those CRAds in which a TSP or constitutively active promoter drives the expression of the transgene alone (Figure 5). Two locations within the adenoviral genome have most often served as sites for transgene insertion. Many investigators have inserted transgenes in the place of the E1B-55k gene.41,42 This strategy is based on a desire to improve the efficacy of Onyx-015, and depends on the deletion of E1B-55k for selectivity of replication. Many of these viruses lack the E3 region of the genome,41 while others retain ADP and the rest of E3, in order to further improve potency.42 Other groups have inserted transgenes in the E3 region of the genome of viruses in which the E3 region has either been entirely43 or partially44,45 deleted. In the latter case, the deletion of E3B alone allows the retention of the ADP gene. In addition to E1B-55k and E3, other locations within the viral genome can also be used for transgene insertion, such as 5′ to E1A46 or the right end of the genome (3′ to E4).47,48 Moreover, Kretschmer et al. used the transposon-based system mentioned earlier to uncover yet other insertion sites for promoter-containing expression cassettes. At least seven sites were identified that allowed the recovery of viruses, including sites within the E1A promoter, between E1A and E1B, within the E1B gene, and within the junction between the fiber gene and the E4 gene.49 As before, the genomic backbone was E3-deleted, meaning that additional insertion sites within E3 may be possible. Further work will be necessary to fully characterize these viruses with respect to oncolytic potency and gene expression.
Figure 5.
Schematic diagram of the Ad5 genome showing insertion sites of transgenes driven by exogenous promoters. The direction of transcription is indicated by arrows. Open arrows represent early transcripts; diagonally striped arrows represent late transcripts; vertically striped arrows represent exogenous promoters; solid boxes represent therapeutic transgenes.
Therapeutic transgenes employed in armed CRAds
CRAds have been armed with a variety of transgenes, representing diverse strategies for the eradication of a tumor. In this review, the various transgenes will be organized into three broad categories according to the focus of their activity. The first group will be comprised of transgenes that directly enhance the killing of the cancer cell, including those that affect only the infected cell, those with bystander effects, and those that enhance viral spread. The second group will include transgenes that modulate the tumor microenvironment, and thus effect tumor killing indirectly. The final group will consist of transgenes that stimulate an immune response to the tumor. Thus, the focus of the following discussion will begin with the tumor cell before moving to the tumor microenvironment and then to the interaction between the tumor and the immune system.
Transgenes that enhance cell killing
Many of the transgenes selected for CRAds are intended to bring about the death of the infected cell, independent of direct viral oncolysis. A number of different mechanisms of action have been employed. Several investigators have utilized interfering nucleic acids directed against cell cycle regulators in order to stimulate cell death. One group has generated a series of related viruses in which antisense cDNAs directed against the genes encoding polo-like kinase 1 (plk1),50 checkpoint kinase 1 (chk1),51 and checkpoint kinase 2 (chk2)52 have been inserted in place of the E3 6.7/gp19k gene in a genome with a Δ24-like mutation in E1A. In each case, the intravenous administration of virus along with cisplatin led to a reduction in metastasis and an improvement in survival (versus unarmed controls) in orthotopic models of hepatocellular carcinoma. In a similar approach, Zhang et al. developed an armed CRAd by cloning an expression cassette consisting of the PolIII (H1) promoter and the DNA for a small interfering RNA (siRNA) against the oncogene K-ras into the E3B region of Onyx-411. This combination of siRNA treatment and viral oncolysis yielded an additive antitumor effect in vitro, while maintaining the viral replication and selectivity of the parent vector. This virus also proved more effective than the unarmed control at suppressing tumor growth in vivo, when injected into subcutaneous tumors.44
Others have sought to stimulate apoptosis of the infected cell. Cui et al. showed that CRAds armed with suppressor of cytokine signaling 3 (socs3) mediated more effective tumor growth suppression than a CRAd expressing a reporter gene from the same backbone, in a subcutaneous model of hepatocellular carcinoma.53 One group has generated a series of E1B-55k-deleted CRAds armed with different pro-apoptotic transgenes such as second mitochondria-derived activator of caspases (Smac),54 X-linked inhibitor of apoptosis protein (XIAP)-associated factor 1 (XAF1),55 suppressor of tumorigenicity-13 (ST13),56 an siRNA against the antiapoptotic factor apollon57 or the antioxidant enzyme manganese superoxide dismutase (MnSOD)58 which has been shown by some to act as an antiproliferative and apoptosis inducer in cancer cells. Each of these viruses demonstrated significant oncolytic activity both in vitro and upon intratumoral injection in vivo, while some have induced synergistic oncolytic effects when combined with the chemotherapeutic agents 5-fluorouracil (5-FU)57,59,60 and cisplatin.60
The above-mentioned transgenes enhance only the killing of the infected cell. This is a significant limitation to their utility because current CRAds are unable to spread throughout entire tumors. However, the reach of an oncolytic virus can be extended by arming with therapeutic genes that can act on uninfected cells. To date, the most widely used transgenes for the enhancement of cell killing are the “suicide genes” that encode prodrug-converting enzymes, which convert non-toxic prodrugs into toxic metabolites, thereby avoiding the systemic toxicities associated with conventional chemotherapy. An important feature of suicide gene therapy is the “bystander effect”, in which the toxic metabolites of the prodrug diffuse away from the expressing cell and kill neighboring cells. Whereas replication-defective vectors have previously been used to deliver suicide genes, a lack of transduction efficiency limited the potency of these vectors. It was thought that the use of replicating adenoviruses would offer improved tumor transduction and thus enhanced potency over the earlier vectors.
Two of the earliest enzyme/prodrug systems to be used in replicating adenoviruses are the herpes simplex virus thymidine kinase/gancyclovir (HSV-TK/GCV) and the cytosine deaminase/5-fluorocytosine (CD/5FC) systems. Wildner et al. conducted a series of studies on CRAds in which the E1B-55k gene had been replaced with HSV-tk. These studies showed that the TK/GCV system can enhance the potency of a replicating adenovirus both in vitro39 and when delivered intratumorally in vivo, although the timing of GCV administration was of critical importance.39,61 This improved potency was also seen by another group using a CRAd with TK in place of E3 gp19k, injected into subcutaneous tumors.28 However, other investigations have shown that GCV administration does not improve the potency of TK-armed adenoviruses with wild-type E1B, in both subcutaneous and intraperitoneal tumor models.62,63 It has since been demonstrated in similar models that efficacy in this system is highly dependent on the E1B-55k gene, since TK/GCV can enhance the oncolytic potency of adenoviruses that lack E1B-55k but can limit the potency of the inherently more oncolytic CRAds that express E1B-55k.64,65 Additionally, the amount of GCV given may also be important. While this ability of GCV to inhibit replication of a TK-armed CRAd is a limitation with respect to oncolysis, it allows the system to be used as a safety mechanism to prevent uncontrolled dissemination.66
The bacterial CD gene converts the prodrug 5-FC into the cytotoxic agent 5-fluorouracil (5-FU). Because 5-FU can be incorporated into RNA as well as DNA, it is toxic to both non-dividing and dividing cells and is well suited for use against heterogeneous human tumors. When used to arm a CRAd, the CD/5-FC system has been shown to yield an additive cytotoxic effect in vitro10 and to improve survival upon intratumoral injection in subcutaneous models of colon10 and breast cancers.67 Zhan et al. developed a CRAd with CD in the place of E3B. In a subcutaneous model of prostate cancer, this virus was delivered intravenously to demonstrate an antitumor response that was enhanced by the use of 5FC.68 One CD-armed CRAd carried an additional transgene, heat shock protein 3 (hsp3), which was inserted in place of E3 for the purpose of stimulating an antitumor immune response; its utility was demonstrated by injection into subcutaneous tumors in a syngeneic, immunocompetent murine model of melanoma.69 In another study by the same group, the CD/5FC system improved the cytotoxicity of a CRAd when a relatively low multiplicity of infection (MOI) was used, but had no effect on cytotoxicity at a higher MOI, an effect that was seen both in vitro and following injection of subcutaneous tumors in vivo.70 As with TK/GCV, this probably reflects the inhibitory effect of CD/5FC on viral replication. Finally, at least two groups have used the yeast version of CD (yCD), which has greater catalytic activity than the bacterial version, to arm CRAds directed against colon cancer, 35 and in an orthotopic model of glioma.7
In an effort to further enhance potency while conserving genome space, a fusion gene incorporating both TK and CD has been created. One of the earliest armed replicating adenoviruses was developed by Freytag et al., who armed an E1B-55k-deleted virus with a CD/TK fusion gene. The initial study showed that the use of prodrugs enhanced the cytotoxicity of a replicating adenovirus but also inhibited viral replication. Furthermore, the suicide genes sensitized cancer cells to radiation.41 In a later set of in vivo studies with this virus, double suicide gene therapy was more effective than either gene alone, and the addition of radiation led to cures in some animals bearing either subcutaneous or intramuscular cervical carcinoma xenografts.71 These encouraging results led to the first clinical trials involving the delivery of a transgene by a replicating adenovirus. These phase I clinical trials for prostate cancer established the safety of an intratumorally-delivered armed, replicating adenovirus either alone72 or in combination with radiation therapy.73 There was evidence of efficacy in some patients, which may have translated into long term benefit.74 This virus was later improved by the use of a yCD gene fused to a mutant version of TK, which is more active than the wild-type version. The resulting virus was then further adapted to carry either human sodium iodide symporter (hNIS) for imaging purposes75 or ADP, which enhanced the potency and spread of the virus when injected into intramuscular tumors in vivo.76 As with the earlier version, radiation enhanced tumor response to the virus in vivo77 and safety was established in a clinical trial.78
In addition to the more widely used enzyme/prodrug systems mentioned previously, several other systems have been used in replicating adenoviruses. A CRAd armed with uracil phosphoribosyl transferase (UPRT), which converts 5-FU to more toxic metabolites, enhanced survival in a biliary cancer model in which both cells and virus were administered intraperitoneally. This effect, however, required optimal timing of prodrug administration, which varied among the cell lines used.79 UPRT has also been used in the form of a CD/UPRT fusion gene. This study, by Bernt et al., also included a replicating adenovirus armed with a secretory human β-glucuronidase, an enzyme that converts the water-soluble prodrug 9-aminocamptothecin to the membrane-permeable metabolite 9-aminocamptothecin glucuronide, a topoisomerase I inhibitor. There were indications that the prodrug therapy enhanced viral replication and spread in liver metastases, which was the case for both viruses following intravenous delivery.80 Other examples of prodrug-converting enzymes used to arm CRAds include the carboxypeptidase G2/ZD2767P and nitroreductase/CB1954 systems, each of which can kill both dividing and non-dividing cells. The former system, which does not inhibit viral replication, was shown to improve survival after a single intravenous administration in subcutaneous models of both hepatocellular81 and colon carcinomas.82 The latter system improved the cytotoxicity of a CRAd in vitro, but inhibited viral replication and thus far has offered only minimal improvements in survival in vivo upon intravenous36 or intratumoral delivery.83 Used in a different viral platform and in conjunction with a different prodrug (SN 28343), however, a CRAd expressing NTR exhibited potent antitumor activity when delivered intravenously to subcutaneous lung carcinoma xenografts.84 Finally, the carboxylesterase/CPT-11 system has been used in a CRAd by at least two groups to enhance cytotoxicity in vitro48 and improve survival in vivo following intravenous delivery to subcutaneous cervical carcinoma xenografts.85
Some transgenes used to augment cell killing do so by enhancing viral spread. In one instance, this has been accomplished using a native adenoviral gene, the adenovirus death protein (ADP). A critical step in the adenovirus infectious cycle is the lysis of the infected cell, which releases progeny virions that can then propagate the infection. In the wild-type adenovirus, this process is mediated chiefly by ADP. While ADP is not required for lysis of the infected cell, its presence makes the release and spread of viral progeny much more efficient.24 Doronin et al. sought to exploit this important property by reinserting ADP into the E3 region of two E3-deleted CRAds, designated KD1 (lacking all of E3) and KD3 (lacking all of E3 except 12.5k). As expected, KD1 and KD3 overexpressed ADP and exhibited enhanced release and spread versus controls with wild-type levels of ADP expression.23 Furthermore, KD1 and KD3, injected intratumorally, were more effective than a control with wild-type levels of ADP (dl01/07) at suppressing subcutaneous tumor growth in vivo in selected cases. The concept of using ADP to arm a CRAd was further explored in studies by this group employing derivatives of these viruses in which the E4 gene was placed under the control of TSPs such as the surfactant protein B16 and hTERT promoters,86 as well as a synthetic TCF promoter.87 Additionally, this family of viruses has been used in conjunction with radiation, resulting in greater tumor cell killing than either treatment modality alone, both in vitro and when injected into subcutaneous lung cancer tumors in vivo.88 Finally, another group found that placing ADP under the control of the CMV promoter in an E3-deleted genome yielded a more oncolytic virus than one with ADP under the control of the major late promoter.89
The tumor suppressor p53 has been used as a therapeutic gene to induce apoptosis, enhance viral release, and improve the oncolytic potency of a CRAd with a Δ24-modified genome in several different cancer cell lines of diverse origins, independent of the p53 status of the cell line.43 The enhanced potency of this p53-armed CRAd was also demonstrated in vivo by intratumoral injection into subcutaneous xenografts of primary glioma90 and neuroblastoma cells.91 The potency of both the armed and unarmed version of this CRAd can be enhanced by radiation therapy to the extent that both viruses were equally effective in a glioma model.92 While these studies relied on early expression of p53 from the simian virus 40 early promoter, another group showed that the late expression of p53 from an ADP-deleted CRAd also enhanced oncolysis, viral release and spread without impairing viral replication. Furthermore, a CRAd armed with p53 was shown to be more potent than the CRAd retaining ADP in several lung cancer cell lines, but less potent in normal fibroblasts.34 However, some cancer cells are resistant to the oncolysis-enhancing effect of p53.43 The biological basis of the resistance is degradation of p53 by factors such as murine double minute 2 (MDM2),93 human papillomavirus (HPV) E6 protein,94 or adenovirus E1B-55k.95 Hence, this resistance has been overcome by the development of p53 variants that are unaffected by these degradation pathways93-95 or by the use of small molecule inhibitors of MDM2.96 It should also be noted that p53 has the potential for other antitumor effects beyond those mentioned above. The expression of p53 alters the expression of factors that influence angiogenesis, exerting a bystander effect on untransduced cells by inhibiting the growth of new blood vessels needed to sustain a growing tumor.97-99 Therefore, while the antiangiogenic properties of p53 have not yet been directly studied in the context of a CRAd, these properties make p53 an attractive transgene for further study.
The HIV env gene has also been used to enhance the spread of a CRAd. HIV env encodes a precursor protein that undergoes proteolytic processing to yield two fusogenic membrane glycoproteins that can then induce the formation of syncytia among cells expressing CD4 and an appropriate coreceptor. An HIV env-expressing adenovirus was first developed by Dewar et al. by placing the gene under the control of the E3 promoter in an E3-deleted Ad5 genome.100 Later, Li et al. used CD4+ HeLa cells in a model system to explore the possibility of using this virus as an anticancer therapeutic for CD4+ cancers such as CD4+ T cell lymphomas and malignant histiocytosis. It was found that syncytium formation had no effect on adenoviral replication, and that the expression of HIV env improved the dispersion of both adenoviral gene products and progeny virions.101
Another protein used to enhance the spread of a CRAd is TNF-related apoptosis-inducing ligand (TRAIL). TRAIL is a transmembrane protein that is processed into a soluble molecule able to induce apoptosis in a variety of cancer cell types, while not causing any toxicity in vivo. Sova et al. demonstrated that a TRAIL-armed CRAd exhibits enhanced oncolysis and spread in a variety of tumor cell types in vitro. The enhanced spread was also shown in vivo, and led to the elimination of pre-established liver metastases of colon cancer by the intravenously administered CRAd.40 This armed CRAd also exhibited enhanced antitumor efficacy in a subcutaneous model of glioblastoma102 and has been used in conjunction with an adenoviral vector expressing the immune-stimulating factor FMS-like tyrosine kinase 3 ligand (Flt3L) in a syngeneic murine model of breast cancer. The addition of Flt3L to treatment with a TRAIL-expressing CRAd had an additive antitumor effect upon intratumoral injection, but did not completely eliminate established subcutaneous tumors.103 Another group has used TRAIL-armed CRAds in combination with chemotherapeutic agents such as cisplatin104 and 5-FU,105 in conjunction with non-replicating vectors expressing the NFkB inhibitor CYLD106 and the k5 domain of the antiangiogenic factor plasminogen,107 and in combination with CRAds expressing Smac,54 MnSOD,58 IL-24,108 and an siRNA against XIAP.109 In general, intratumoral injection of CRAds armed with TRAIL yield antitumor efficacy in vivo that can be synergistically enhanced by other factors. In some cases, pre-established subcutaneous tumors have been eliminated by these combination therapies.54,58,105-108
Modulation of the tumor microenvironment
The transgenes discussed above all augment killing of cancer cells. However, a tumor is a complex entity composed not only of cancerous cells, but also other cell types such as inflammatory cells, fibroblasts, and endothelial cells. This heterogeneity results from the complex processes of invasion and angiogenesis, during which malignant cells recruit normal cells from the nearby stroma to migrate toward and sustain the growing tumor. Thus, both tumors and metastases require the active participation of the stroma for the remodeling of the extracellular matrix and ingrowth of new blood vessels.110 In light of this, armed CRAds have been developed that carry transgenes that modulate this microenvironment, thereby acting on the cancerous cells indirectly. CRAds carrying transgenes directed against the tumor stroma can be organized into two categories: those that modulate the extracellular matrix, and those that inhibit angiogenesis.
Several armed CRAds developed thus far have carried transgenes that act upon extracellular matrix components. Lamfers et al. evaluated a Δ24 virus armed with tissue inhibitor of metalloproteinase 3 (TIMP3) in a murine model of glioma.111 TIMP3 inhibits the action of matrix metalloproteinases (MMPs), which are proteolytic enzymes that degrade extracellular matrix and basement membrane components. Because these processes release factors that promote tumor growth and angiogenesis, their inhibition by TIMP3 suppresses tumor invasiveness and angiogenesis. It was found that the TIMP3-armed virus exhibited enhanced oncolysis and suppression of cell proliferation in vitro. However, while intratumoral injection of the armed virus inhibited tumor growth and prolonged survival in vivo, TIMP3 expression did not improve efficacy over the control virus, in either subcutaneous or intracranial tumor models. This may have been due to the size of the tumors or redundancy in proteolytic cascades.111
A contrasting approach is the use of a transgene that promotes rather than inhibits the degradation of the extracellular matrix. Relaxin, a peptide hormone that downregulates expression of collagen and upregulates expression of MMPs, has been exploited by two groups with the goal of improving the spread of a CRAd through a tumor by reducing the presence of connective tissue barriers. Kim et al. armed a CRAd with relaxin by replacing the entire E3 region with a CMV-relaxin cassette,112 while Ganesh et al. replaced E3 14.7k with relaxin in a CRAd that also carried a gene for human GM-CSF and included a chimeric fiber pseudotyped with the Ad35 knob.31 Overall, arming the CRAds with relaxin enhanced viral spread in a variety of in vitro and in vivo tumor models, including intratumoral injection of subcutaneous tumors31 and intravenous delivery to orthotopic tumors,112 and improved survival of tumor-bearing animals in vivo. However, in the Ganesh study, which used a more potent ADP-expressing CRAd, the improvement in efficacy over an unarmed control virus was only seen in cells with poor transduction efficiency. Importantly, injection of subcutaneous tumors with a relaxin-armed CRAd yielded no increase in metastasis in one study,31 and reduced metastasis in the other study,112 although cells infected with this CRAd exhibited enhanced invasiveness in vitro.31
Other CRAds have been armed with transgenes that inhibit the process of angiogenesis. One group has constructed two similar armed CRAds by replacing the E1B-55k gene with a gene for murine endostatin, a potent antiangiogenic factor that inhibits endothelial cell proliferation. The endostatin-armed CRAds exhibited improved oncolysis versus unarmed controls and an enhanced ability to suppress tumor growth after intratumoral administration in subcutaneous in vivo models of hepatocellular carcinoma (using a viral platform with wild-type E1A)113 and gastric cancer (using a version with E1A under control of the human TERT promoter).114 Another group constructed a series of CRAds armed with short hairpin RNAs directed against vascular endothelial growth factor (VEGF)115 and/or interleukin 8116 or a zinc-finger protein targeted against the VEGF promoter.117 In all cases, the armed CRAds inhibited angiogenesis and demonstrated enhanced antitumor efficacy when injected intratumorally in subcutaneous models of glioma,115,117 hepatocellular carcinoma and lung carcinoma.116 Additionally, intrapleural administration of one of these CRAds inhibited lung metastases of breast cancer in a murine model.116 Finally, a CRAd armed with soluble Flt-1, an inhibitor of VEGF, exhibited enhanced potency over an unarmed control both in vitro and in vivo in a subcutaneous model of colon carcinoma.118
Immunomodulatory transgenes
The third class of transgenes used to arm CRAds comprises those that stimulate the immune system. In general, these factors are intended to recruit immune cells to the site of infection and induce their proliferation and activation. Many of these factors are also directly toxic to tumor cells or have antiangiogenic properties. Recruitment of the immune system has the potential to destroy not only the primary tumor, but also to mediate the clearance of metastatic disease and provide long term suppression of recurrence. In some cases, the efficacy of CRAds armed with immunomodulatory transgenes has been evaluated in immunocompetent models. However, many studies have relied on human xenograft tumors established in immunodeficient mice in which the full potential of these strategies could not be realized.
Two cytokines that recruit immune cells to the site of infection are monocyte chemotactic protein 3 (MCP-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF). MCP-3 was used by Bauzon et al. in a demonstration of multiple transgene expression from a CRAd (in a virus armed also with TNFα), although this study did not examine efficacy.30 GM-CSF has been used in numerous studies to improve the oncolytic potency of an intratumorally-injected CRAd, in some cases leading to the elimination of established subcutaneous tumors29,119,120 or, following intravesicular administration, elimination of orthotopic bladder cancer xenografts.120 In a study by Choi et al. using an immunocompetent murine model of melanoma, injection of a CRAd armed with murine versions of both GM-CSF and the T cell co-stimulatory molecule B7-1 into subcutaneous tumors led to disease-free survival in some animals, and also protected these animals from rechallenge.121
Other cytokines used to arm CRAds have multiple functions. As an example, TNFα increases MHC class I expression, is cytotoxic to tumor cells and can destroy tumor vasculature. Kurihara et al. showed that TNFα improved the ability of a CRAd to suppress tumor growth after injection into subcutaneous tumors in an immunodeficient murine model of breast cancer, an effect that lasted over 100 days.46 Interferons (IFNs), a class of cytokines, have also been used to arm CRAds. In the earliest study, an interferon consensus gene was inserted into the E3 region of a virus with wild-type E1 by Zhang et al. The IFN enhanced viral oncolysis both in vitro and after intratumoral injection in vivo, using subcutaneous human breast cancer or leukemia cell tumors in immunodeficient mice as well as Syrian hamsters with a syngeneic melanoma cell line.22 IFN-γ, which increases MHC class I expression, recruits immune cells and has antiangiogenic properties, was used in a study by Sarkar et al. In an immunodeficient murine model of pancreatic cancer, a CRAd armed with IFN-γ eliminated injected subcutaneous tumors as well as distant, non-injected xenografts in a majority of the treated animals. These distant tumors displayed evidence of viral replication. Secondly, while immune cell infiltration was not assayed, antitumor activity was demonstrated in vitro by splenic cells isolated from treated animals.122 Su et al. generated two CRAds armed with either human or murine IFN-γ. IFN-γ improved the potency of the CRAds in vivo. This tumor suppression was evidenced following intratumoral injection in both immunodeficient and immunocompetent subcutaneous models of hepatocellular carcinoma, with a greater effect seen in the immunocompetent model.123 Shashkova et al. armed an ADP-overexpressing CRAd with human IFN-α, which is similar to IFN-γ in function but also has apoptosis-inducing effects. This cytokine also improved potency of the CRAd, when injected into subcutaneous tumors, in both an immunodeficient murine model of hepatocellular carcinoma and an immunocompetent Syrian hamster kidney cancer model. It was also shown that IFN-α inhibited viral replication in normal human lung fibroblasts and decreased liver toxicity after intravenous administration in two animal models.124 Furthermore, when subcutaneous hepatocellular carcinoma xenografts were injected with this CRAd and a non-replicating vector expressing TRAIL, an improvement in survival and tumor growth suppression was exhibited.125
Several CRAds armed with interleukins have also been generated. IL-4, which has antiangiogenic as well as immune-activating properties, was used to arm a hypoxia-targeted CRAd by Post et al. In two immunodeficient murine models of glioma, the armed CRAd exhibited superior potency to the unarmed CRAd when injected into subcutaneous tumors. Infiltration of immune cells was also verified.126 As in other studies, the armed CRAd would be expected to be even more potent in a fully immunocompetent model. IL-12 not only stimulates the proliferation of immune cells, it also stimulates production of IFN-γ and TNFα. Lee et al. demonstrated that IL-12 improved the potency of a CRAd injected into subcutaneous tumors in an immunocompetent murine model of melanoma. Moreover, a CRAd armed with both IL-12 and B7-1 was shown to improve antitumor efficacy and survival even further. Both viruses stimulated an antitumor immune response.127 IL-24, also known as melanoma differentiation-associated gene 7 (MDA-7), stimulates the production of other cytokines such as IL-6, IFN-γ, TNFα, IL-1β, IL-12 and GM-CSF. In addition, it has potent antiangiogenic, apoptosis-inducing, and bystander effects. Sarkar et al. developed a CRAd armed with IL-24. Intratumoral administration of this armed CRAd eliminated primary subcutaneous tumors of breast128 or prostate129 cancer and either stimulated regression or eradication of distant tumors, in two immunodeficient murine models. This effect on distant tumors was shown to be due at least in part to viral replication. Furthermore, in the prostate xenograft model, the armed CRAd lysed cells overexpressing an apoptosis inhibitor, which were resistant to the activity of IL-24 alone.129 Other CRAds armed with IL-24 have demonstrated enhanced oncolytic potency versus control viruses upon intratumoral injection in subcutaneous models of leukemia130 and hepatocellular carcinoma, including some complete responses.131
Heat shock proteins are another class of immunomodulatory factors used to arm CRAds. In general, these proteins chaperone peptides to antigen-presenting cells and are employed to provoke an antitumor immune response. Additionally, heat shock proteins can attract and activate various immune cells. Heat shock protein 70 (hsp-70) improved the potency of a CRAd constructed by Huang et al., allowing the elimination of subcutaneous melanoma tumors injected intratumorally in an immunocompetent model. In separate experiments, the armed CRAd also protected treated mice from tumor rechallenge, and reduced the size of distant tumors. These effects were not reproduced in an immunodeficient model, indicating involvement of the immune system.132 Another group constructed a melanoma-targeted CRAd doubly armed with both CD and hsp-70. In an immunocompetent model of melanoma, the armed CRAd alone did not significantly suppress tumor growth following injection of subcutaneous tumors. However, mice treated with both the armed CRAd and 5-FC experienced tumor suppression superior to that yielded by a replicating virus expressing CD alone. Moreover, mice treated with both the armed CRAd and 5-FC were protected from tumor rechallenge while those treated with the armed CRAd alone were not, suggesting that robust oncolysis is required to stimulate an antitumor immune response.69 CRAds have also been armed with the heat shock protein gp96. In a study by Di Paolo et al., a gp96-armed CRAd suppressed tumor growth and stimulated an antitumor immune response in immunocompetent mice bearing subcutaneous tumors of infected TC-1 cells, an immortalized murine epithelial cell line expressing HPV16 E6 and E7. Two similar CRAds were generated, armed with either secretory or membrane-bound forms of gp96, with the secretory form being more efficacious. Treatment with cyclophosphamide, an inhibitor of regulatory T cells, enhanced this antitumor effect.133
Challenges and future directions
From the animal studies performed to date, it is evident that the rational design of arming strategies for CRAds can yield therapeutic benefit. In most of the studies reviewed here, armed CRAds have been shown to be more efficacious than unarmed CRAds with the same adenoviral backbone. Moreover, the antitumor efficacy of some armed CRAds can be further improved by the concurrent use of other treatment modalities such as radiation or chemotherapy. However, most in vivo studies of armed CRAds thus far have been conducted by direct intratumoral injection of subcutaneous xenografts established in immunodeficient mice. The use of these models reflects the difficulty in overcoming the obstacles associated with delivery in the human clinical context. Challenges remain with respect to systemic delivery, immune response to the armed CRAd, and the monitoring of injected CRAds. These are the same challenges facing the delivery of unarmed CRAds, and thus the same solutions will be applicable to both.
Systemic delivery
In cases in which armed CRAds can be used for localized tumors, direct intratumoral injection of the CRAd is the most efficient way to deliver a high number of viral particles. However, in the clinical setting, patients may have tumors that are inaccessible or are already metastatic at the time of detection. For these reasons, the systemic administration of armed CRAds remains an important goal. However, the systemic administration of adenoviruses is currently associated with a number of challenges. One problem is the sequestration of the virus by non-target organs. Upon intravenous delivery, the majority of the virus is taken up by the liver.4 Recent studies have demonstrated that adenoviral uptake by hepatocytes depends on binding of the hexon capsid protein to coagulation factor X (FX).134,135 Hence, it is now possible to design rational strategies to decrease liver uptake and thereby maintain CRAds in circulation for delivery to target tissues. It has been shown in mice that liver transduction can be reduced by treatment with the anti-coagulation drug warfarin or by genetic modification of the Ad5 hexon to ablate binding to FX. Furthermore, Ad serotypes have been identified that do not bind FX.135
Modification of the fiber capsid protein will also play an important role in developing targeted, injectable CRAds. Infection of a target cells depends upon primary binding of the fiber knob to the coxsackie and adenovirus receptor (CAR) on the cell surface. Many tumor cell types are CAR-deficient.136 Thus, the efficacy with which CRAds infect tumor cells can be increased by modifying the viruses to achieve efficient infection via CAR-independent pathways.137 Furthermore, the CAR-dependence of transduction contributes to sequestration of systemically delivered virus in non-target, CAR-expressing cells. Modification of adenoviral tropism can be accomplished by altering the fiber knob to redirect binding to alternate cellular receptors. Two general strategies have been developed to achieve this end. In the first, the virus is complexed with molecular bridges, either chemical conjugates or recombinant fusion proteins with specificity for both the knob and a cellular receptor. The second strategy relies on genetic modification of the fiber, either by pseudotyping (substitution of the Ad5 fiber in total or in part with that of another Ad serotype) or by the incorporation of ligands into the fiber knob. These strategies allow efficient transduction of CAR-deficient target cells.138
Physical barriers, such as the endothelium, must also be overcome for a systemically delivered CRAd to mediate efficient tumor cell transduction. The endothelial cell barrier may be circumvented through the use of carrier cells. Cell types that have a propensity to home to either primary or metastatic tumors, such as endothelial progenitors, mesenchymal stem cells, immune cells or cancer cells have all been used as carriers in animal studies.139 For example, mesenchymal stem cells can be infected with CRAds prior to inoculation into animals and can deliver CRAds to lung metastases of breast cancer.138 Carrier cells can also hide the CRAd from neutralization by circulating antibodies, an additional obstacle to systemic delivery. Neutralization by antibodies is a potentially significant problem, since most cancer patients will have pre-existing immunity to adenovirus. Additional strategies to shield the virus from neutralizing antibodies and decrease hepatotoxicity include coating the virus with polymers such as polyethylene glycol or HPMA,83 or relying on hexon chimerism with alternate Ad serotypes, especially rare or non-human serotypes to which patients will not previously have been exposed.138 In general, all the strategies mentioned above seek to prolong the half-life of a CRAd in the bloodstream, which is necessary to deliver the maximum number of particles to the target site. Furthermore, strategies to overcome one obstacle can provide benefit against another, as is the case with carrier cells.
Immune response
Aside from the role played by neutralizing antibodies, the immune system presents another set of challenges that must be overcome before armed CRAds can be established as anticancer therapeutics. The immune response to systemically administered CRAds occurs in two phases. During the innate phase, the majority of systemically administered CRAds are taken up and degraded by macrophages and Kupffer cells in the liver. These cells, along with dendritic cells, release a variety of pro-inflammatory cytokines within hours of transduction. This innate response is dose dependent, as it depends on capsid proteins and does not require adenoviral DNA replication. Therefore, the use of more potent armed CRAds will allow smaller viral doses to be administered and should reduce the acute inflammation. The transient depletion of Kupffer cells and macrophages by pharmacological means can also reduce the innate response. The second phase of the immune response becomes relevant 24 hours after administration as the cellular response is activated. This phase can potentially be reduced through the use of immunosuppressive drugs like cyclosporine. In an alternate approach, T cell activation can be blocked by antibodies specific to CD40 ligand or the co-stimulatory molecule B7.47,140 However, strategies that rely upon systemic immune suppression carry the inherent risk of leaving patients vulnerable to opportunistic infection. Ultimately, many of the issues related to the immune response to CRAds may be resolved through the increased understanding that will come with the use of more appropriate models. For reasons mentioned earlier, most studies of armed CRAds have been performed in immunodeficient mice, although more refined models currently exist. The use of immunocompetent mice69,103,121,123,127,132,133 as well as other models such as the cotton rat,141,142 Syrian hamster143 and pig models66 offer the potential to study the interaction between CRAds and an intact immune system. However, these species are not truly permissive for human Ad replication, and so limitations remain. One means of circumventing these limitations is by studying non-human adenoviruses in appropriate species. To this end, a conditionally replicating canine adenovirus for use against osteosarcoma in dogs has been described, allowing a CRAd to be studied in a syngeneic immunocompetent model of human disease.144 A better understanding of replicating adenoviruses in immunocompetent hosts will lead to improvements in vectors for clinical use.
Non-invasive monitoring of efficacy in living systems
Another area of interest within the field of CRAd research is that of monitoring their systemic distribution in vivo and spread throughout tumors, particularly if these agents are to be used clinically. Furthermore, there is also a desire to non-invasively monitor transgene expression and efficacy of armed CRAds in living systems. To this end, there are three general categories of reporters. The first consists of secreted reporters. These are soluble proteins that are not typically found in serum, allowing a non-invasive means of monitoring the persistence and efficacy of a CRAd. CRAds armed with human carcinoembryonic antigen,145 human chorionic gonadotropin β chain,146 and secreted placental alkaline phosphatase124 have all been generated. Because the serum levels of these proteins can be correlated to viral replication, they allow the persistence and efficacy of the armed CRAd to be determined non-invasively. However, secreted reporters do not reveal the distribution of armed CRAds in vivo. For these studies, CRAds armed with imaging molecules are needed. This second category of reporters includes factors that allow infected cells to be visualized. In some cases, these factors act upon injected substrates. For example, the luciferase enzyme, which activates a bioluminescent substrate, has been widely used as a tool to evaluate the transgene expression, tumor targeting and efficacy of replicating adenoviruses.37,147-150 Alternatively, the human sodium iodide symporter can be utilized to quantify gene expression from a CRAd following the administration of a radiological substrate.75,76,151,152 Other factors that allow visualization of infected cells rely on the production of a fluorescent protein by the cell and do not require the administration of a substrate. CRAds have been constructed that carry genes for green fluorescent protein (GFP),46,153,154 enhanced GFP (EGFP)155-157 and red fluorescent protein (RFP).124,158 Multifunctional reporters, such as thymidine kinase (TK) fused to GFP, have also been used to arm CRAds.159,160
The final category of reporters used to monitor replicating adenoviruses in vivo are those that have been incorporated into the viral capsid. Thus, gene transcription by the infected cell is not required for monitoring infection because the viral particle itself can be visualized. This has been accomplished by the fusion of imaging motifs to protein IX (pIX), a minor adenoviral capsid protein. Several different motifs have been used for this purpose, including the fluorescent proteins EGFP4,161,162 and RFP.163 Thymidine kinase has also been incorporated into the capsid of a replicating adenovirus by fusion with pIX either alone,164 or as part of a TK-luciferase fusion in a proof-of-principle study using a replication-defective vector.165 In both cases, the incorporated TK was shown to be functional with respect to its conversion of the prodrug gancyclovir as well as for the purpose of positron emission tomography imaging. As work continues in this field, other imaging modalities may also be discovered.
Conclusions
Adenoviruses have many advantages as replicating agents, including the ability to infect a wide range of cells, stability in the bloodstream, and an acceptable safety profile. The well-characterized Ad5 genome permits a number of genetic modifications, allowing infection and replication to be targeted to cancer cells. However, CRAds are rarely able to eliminate entire tumors by viral replication alone, illustrating the need for more potent agents. To this end, several locations within the Ad5 genome have been utilized to arm CRAds with therapeutic transgenes, which can be placed under exogenous or endogenous control. Overall, the studies to date in animal models have shown that arming a CRAd with a rationally chosen therapeutic transgene can improve its antitumor efficacy over that of an unarmed CRAd. It is now possible to envision future armed CRAds for clinical use that include many features to enhance their efficacy. Future CRAds could be armed with transgenes with different modes of action, increasing their potential to eliminate complex human tumors. Additional improvements in efficacy will result from advances made as the challenges of systemic delivery, immune response to the CRAd, and non-invasive monitoring are addressed.
Acknowledgments
Supported by NIH grants R01 CA108585, R01 CA083821, R01 CA111569 and T32 CA075930.
References
- 1.Ries SJ, Brandts CH, Chung AS, Biederer CH, Hann BC, Lipner EM, et al. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl1520 (ONYX-015) Nat Med. 2000;6:1128–1133. doi: 10.1038/80466. [DOI] [PubMed] [Google Scholar]
- 2.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 3.Chu RL, Post DE, Khuri FR, Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin Cancer Res. 2004;10:5299–5312. doi: 10.1158/1078-0432.CCR-0349-03. [DOI] [PubMed] [Google Scholar]
- 4.Le LP, Rivera AA, Glasgow JN, Ternovoi VV, Wu H, Wang M, et al. Infectivity enhancement for adenoviral transduction of canine osteosarcoma cells. Gene Ther. 2006;13:389–399. doi: 10.1038/sj.gt.3302674. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells [see comments] Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 6.O'Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Conrad C, Miller CR, Ji Y, Gomez-Manzano C, Bharara S, McMurray JS, et al. Delta24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther. 2005;12:284–294. doi: 10.1038/sj.cgt.7700750. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Research. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 9.Zhang L, Akbulut H, Tang Y, Peng X, Pizzorno G, Sapi E, et al. Adenoviral vectors with E1A regulated by tumor-specific promoters are selectively cytolytic for breast cancer and melanoma. Mol Ther. 2002;6:386–393. doi: 10.1006/mthe.2002.0680. [DOI] [PubMed] [Google Scholar]
- 10.Akbulut H, Zhang L, Tang Y, Deisseroth A. Cytotoxic effect of replication-competent adenoviral vectors carrying L-plastin promoter regulated E1A and cytosine deaminase genes in cancers of the breast, ovary and colon. Cancer Gene Ther. 2003;10:388–395. doi: 10.1038/sj.cgt.7700579. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara S, Wada Y, Gardner TA, Egawa M, Park MS, Hsieh CL, et al. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61:6012–6019. [PubMed] [Google Scholar]
- 12.Ono HA, Davydova JG, Adachi Y, Takayama K, Barker SD, Reynolds PN, et al. Promoter-controlled infectivity-enhanced conditionally replicative adenoviral vectors for the treatment of gastric cancer. J Gastroenterol. 2005;40:31–42. doi: 10.1007/s00535-004-1490-y. [DOI] [PubMed] [Google Scholar]
- 13.Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, et al. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181–3188. [PubMed] [Google Scholar]
- 14.Su CQ, Sham J, Xue HB, Wang XH, Chua D, Cui ZF, et al. Potent antitumoral efficacy of a novel replicative adenovirus CNHK300 targeting telomerase-positive cancer cells. J Cancer Res Clin Oncol. 2004;130:591–603. doi: 10.1007/s00432-004-0577-4. [DOI] [PubMed] [Google Scholar]
- 15.Savontaus MJ, Sauter BV, Huang TG, Woo SL. Transcriptional targeting of conditionally replicating adenovirus to dividing endothelial cells. Gene Ther. 2002;9:972–979. doi: 10.1038/sj.gt.3301747. [DOI] [PubMed] [Google Scholar]
- 16.Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V, et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol. 2001;75:3314–3324. doi: 10.1128/JVI.75.7.3314-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 18.DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- 19.Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105:1169–1172. doi: 10.1172/JCI9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bett AJ, Prevec L, Graham FL. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993;67:5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JF, Hu C, Geng Y, Selm J, Klein SB, Orazi A, et al. Treatment of a human breast cancer xenograft with an adenovirus vector containing an interferon gene results in rapid regression due to viral oncolysis and gene therapy. Proc Natl Acad Sci U S A. 1996;93:4513–4518. doi: 10.1073/pnas.93.9.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WS. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins LK, Hermiston T. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the E3B region. Gene Ther. 2001;8:1142–1148. doi: 10.1038/sj.gt.3301509. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins LK, Hermiston TW. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the ADP region. Gene Ther. 2001;8:1132–1141. doi: 10.1038/sj.gt.3301508. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins LK, Johnson L, Bauzon M, Nye JA, Castro D, Kitzes GA, et al. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the 6.7 K/gp19 K region. Gene Ther. 2001;8:1123–1131. doi: 10.1038/sj.gt.3301507. [DOI] [PubMed] [Google Scholar]
- 28.Nanda D, Vogels R, Havenga M, Avezaat CJ, Bout A, Smitt PS. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001;61:8743–8750. [PubMed] [Google Scholar]
- 29.Zhu M, Bristol JA, Xie Y, Mina M, Ji H, Forry-Schaudies S, et al. Linked tumor-selective virus replication and transgene expression from E3-containing oncolytic adenoviruses. J Virol. 2005;79:5455–5465. doi: 10.1128/JVI.79.9.5455-5465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauzon M, Castro D, Karr M, Hawkins LK, Hermiston TW. Multigene expression from a replicating adenovirus using native viral promoters. Mol Ther. 2003;7:526–534. doi: 10.1016/s1525-0016(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 31.Ganesh S, Gonzalez Edick M, Idamakanti N, Abramova M, Vanroey M, Robinson M, et al. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer Res. 2007;67:4399–4407. doi: 10.1158/0008-5472.CAN-06-4260. [DOI] [PubMed] [Google Scholar]
- 32.Jin F, Kretschmer PJ, Hermiston TW. Identification of novel insertion sites in the Ad5 genome that utilize the Ad splicing machinery for therapeutic gene expression. Mol Ther. 2005;12:1052–1063. doi: 10.1016/j.ymthe.2005.07.696. [DOI] [PubMed] [Google Scholar]
- 33.Robinson M, Ge Y, Ko D, Yendluri S, Laflamme G, Hawkins L, et al. Comparison of the E3 and L3 regions for arming oncolytic adenoviruses to achieve a high level of tumor-specific transgene expression. Cancer Gene Ther. 2008;15:9–17. doi: 10.1038/sj.cgt.7701093. [DOI] [PubMed] [Google Scholar]
- 34.Sauthoff H, Pipiya T, Heitner S, Chen S, Norman RG, Rom WN, et al. Late expression of p53 from a replicating adenovirus improves tumor cell killing and is more tumor cell specific than expression of the adenoviral death protein. Hum Gene Ther. 2002;13:1859–1871. doi: 10.1089/104303402760372954. [DOI] [PubMed] [Google Scholar]
- 35.Fuerer C, Iggo R. 5-Fluorocytosine increases the toxicity of Wnt-targeting replicating adenoviruses that express cytosine deaminase as a late gene. Gene Ther. 2004;11:142–151. doi: 10.1038/sj.gt.3302148. [DOI] [PubMed] [Google Scholar]
- 36.Lukashev AN, Fuerer C, Chen MJ, Searle P, Iggo R. Late expression of nitroreductase in an oncolytic adenovirus sensitizes colon cancer cells to the prodrug CB1954. Hum Gene Ther. 2005;16:1473–1483. doi: 10.1089/hum.2005.16.1473. [DOI] [PubMed] [Google Scholar]
- 37.Rivera AA, Wang M, Suzuki K, Uil TG, Krasnykh V, Curiel DT, et al. Mode of transgene expression after fusion to early or late viral genes of a conditionally replicating adenovirus via an optimized internal ribosome entry site in vitro and in vivo. Virology. 2004;320:121–134. doi: 10.1016/j.virol.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Ye X, Lu Q, Zhao Y, Ren Z, Ren XW, Qiu QH, et al. Conditionally replicative adenovirus vector carrying TRAIL gene for enhanced oncolysis of human hepatocellular carcinoma. Int J Mol Med. 2005;16:1179–1184. [PubMed] [Google Scholar]
- 39.Wildner O, Morris JC, Vahanian NN, Ford H, Jr, Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999;6:57–62. doi: 10.1038/sj.gt.3300810. [DOI] [PubMed] [Google Scholar]
- 40.Sova P, Ren XW, Ni S, Bernt KM, Mi J, Kiviat N, et al. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol Ther. 2004;9:496–509. doi: 10.1016/j.ymthe.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH, Sun LY, et al. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res. 2003;13:481–489. doi: 10.1038/sj.cr.7290191. [DOI] [PubMed] [Google Scholar]
- 43.van Beusechem VW, van den Doel PB, Grill J, Pinedo HM, Gerritsen WR. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165–6171. [PubMed] [Google Scholar]
- 44.Zhang YA, Nemunaitis J, Samuel SK, Chen P, Shen Y, Tong AW. Antitumor activity of an oncolytic adenovirus-delivered oncogene small interfering RNA. Cancer Res. 2006;66:9736–9743. doi: 10.1158/0008-5472.CAN-06-1617. [DOI] [PubMed] [Google Scholar]
- 45.Wang ZG, Zhao W, Ramachandra M, Seth P. An oncolytic adenovirus expressing soluble transforming growth factor-beta type II receptor for targeting breast cancer: in vitro evaluation. Mol Cancer Ther. 2006;5:367–373. doi: 10.1158/1535-7163.MCT-05-0125. [DOI] [PubMed] [Google Scholar]
- 46.Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763–771. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carette JE, Overmeer RM, Schagen FH, Alemany R, Barski OA, Gerritsen WR, et al. Conditionally replicating adenoviruses expressing short hairpin RNAs silence the expression of a target gene in cancer cells. Cancer Res. 2004;64:2663–2667. doi: 10.1158/0008-5472.can-03-3530. [DOI] [PubMed] [Google Scholar]
- 48.Oosterhoff D, Pinedo HM, Witlox MA, Carette JE, Gerritsen WR, van Beusechem VW. Gene-directed enzyme prodrug therapy with carboxylesterase enhances the anticancer efficacy of the conditionally replicating adenovirus AdDelta24. Gene Ther. 2005;12:1011–1018. doi: 10.1038/sj.gt.3302492. [DOI] [PubMed] [Google Scholar]
- 49.Kretschmer PJ, Jin F, Chartier C, Hermiston TW. Development of a transposon-based approach for identifying novel transgene insertion sites within the replicating adenovirus. Mol Ther. 2005;12:118–127. doi: 10.1016/j.ymthe.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J, Gao Q, Chen G, Huang X, Lu Y, Li K, et al. Novel oncolytic adenovirus selectively targets tumor-associated polo-like kinase 1 and tumor cell viability. Clin Cancer Res. 2005;11:8431–8440. doi: 10.1158/1078-0432.CCR-05-1085. [DOI] [PubMed] [Google Scholar]
- 51.Gao Q, Zhou J, Huang X, Chen G, Ye F, Lu Y, et al. Selective targeting of checkpoint kinase 1 in tumor cells with a novel potent oncolytic adenovirus. Mol Ther. 2006;13:928–937. doi: 10.1016/j.ymthe.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Chen G, Zhou J, Gao Q, Huang X, Li K, Zhuang L, et al. Oncolytic adenovirus-mediated transfer of the antisense chk2 selectively inhibits tumor growth in vitro and in vivo. Cancer Gene Ther. 2006;13:930–939. doi: 10.1038/sj.cgt.7700967. [DOI] [PubMed] [Google Scholar]
- 53.Cui Q, Jiang W, Wang Y, Lv C, Luo J, Zhang W, et al. Transfer of suppressor of cytokine signaling 3 by an oncolytic adenovirus induces potential antitumor activities in hepatocellular carcinoma. Hepatology. 2008;47:105–112. doi: 10.1002/hep.21951. [DOI] [PubMed] [Google Scholar]
- 54.Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, et al. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39:1371–1381. doi: 10.1002/hep.20203. [DOI] [PubMed] [Google Scholar]
- 55.Qi R, Gu J, Zhang Z, Yang K, Li B, Fan J, et al. Potent antitumor efficacy of XAF1 delivered by conditionally replicative adenovirus vector via caspase-independent apoptosis. Cancer Gene Ther. 2007;14:82–90. doi: 10.1038/sj.cgt.7700992. [DOI] [PubMed] [Google Scholar]
- 56.Yang M, Cao X, Yu MC, Gu JF, Shen ZH, Ding M, et al. Potent antitumor efficacy of ST13 for colorectal cancer mediated by oncolytic adenovirus via mitochondrial apoptotic cell death. Hum Gene Ther. 2008;19:343–353. doi: 10.1089/hum.2007.0137. [DOI] [PubMed] [Google Scholar]
- 57.Chu L, Gu J, Sun L, Qian Q, Qian C, Liu X. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene Ther. 2008;15:484–494. doi: 10.1038/gt.2008.6. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res. 2006;66:4291–4298. doi: 10.1158/0008-5472.CAN-05-1834. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Qin X, Zhao L, Wang Y, Liu X, Yao L. Combination of ZD55-MnSOD therapy with 5-FU enhances antitumor efficacy in colorectal cancer. J Cancer Res Clin Oncol. 2007 doi: 10.1007/s00432-007-0273-2. [DOI] [PubMed] [Google Scholar]
- 60.Pan QW, Zhong SY, Liu BS, Liu J, Cai R, Wang YG, et al. Enhanced sensitivity of hepatocellular carcinoma cells to chemotherapy with a Smac-armed oncolytic adenovirus. Acta Pharmacol Sin. 2007;28:1996–2004. doi: 10.1111/j.1745-7254.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 61.Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]
- 62.Morris JC, Wildner O. Therapy of head and neck squamous cell carcinoma with an oncolytic adenovirus expressing HSV-tk. Mol Ther. 2000;1:56–62. doi: 10.1006/mthe.1999.0014. [DOI] [PubMed] [Google Scholar]
- 63.Lambright ES, Amin K, Wiewrodt R, Force SD, Lanuti M, Propert KJ, et al. Inclusion of the herpes simplex thymidine kinase gene in a replicating adenovirus does not augment antitumor efficacy. Gene Ther. 2001;8:946–953. doi: 10.1038/sj.gt.3301489. [DOI] [PubMed] [Google Scholar]
- 64.Wildner O, Morris JC. Therapy of peritoneal carcinomatosis from colon cancer with oncolytic adenoviruses. J Gene Med. 2000;2:353–360. doi: 10.1002/1521-2254(200009/10)2:5<353::AID-JGM130>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 65.Wildner O, Morris JC. The role of the E1B 55 kDa gene product in oncolytic adenoviral vectors expressing herpes simplex virus-tk: assessment of antitumor efficacy and toxicity. Cancer Res. 2000;60:4167–4174. [PubMed] [Google Scholar]
- 66.Wildner O, Hoffmann D, Jogler C, Uberla K. Comparison of HSV-1 thymidine kinase-dependent and -independent inhibition of replication-competent adenoviral vectors by a panel of drugs. Cancer Gene Ther. 2003;10:791–802. doi: 10.1038/sj.cgt.7700638. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Ye T, Maynard J, Akbulut H, Deisseroth A. Engineering conditionally replication-competent adenoviral vectors carrying the cytosine deaminase gene increases the infectivity and therapeutic effect for breast cancer gene therapy. Cancer Gene Ther. 2006;13:346–356. doi: 10.1038/sj.cgt.7700906. [DOI] [PubMed] [Google Scholar]
- 68.Zhan J, Gao Y, Wang W, Shen A, Aspelund A, Young M, et al. Tumor-specific intravenous gene delivery using oncolytic adenoviruses. Cancer Gene Ther. 2005;12:19–25. doi: 10.1038/sj.cgt.7700730. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Ye T, Sun D, Maynard J, Deisseroth A. Tumor-specific therapeutic effect induced by an oncolytic adenoviral vector containing heat shock protein 70 and prodrug activation genes. Gene Ther. 2006;13:1235–1243. doi: 10.1038/sj.gt.3302776. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, Deisseroth A. Oncolytic adenoviral vector carrying the cytosine deaminase gene for melanoma gene therapy. Cancer Gene Ther. 2006;13:845–855. doi: 10.1038/sj.cgt.7700962. [DOI] [PubMed] [Google Scholar]
- 71.Rogulski KR, Wing MS, Paielli DL, Gilbert JD, Kim JH, Freytag SO. Double suicide gene therapy augments the antitumor activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther. 2000;11:67–76. doi: 10.1089/10430340050016166. [DOI] [PubMed] [Google Scholar]
- 72.Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976. [PubMed] [Google Scholar]
- 73.Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- 74.Freytag SO, Stricker H, Peabody J, Pegg J, Paielli D, Movsas B, et al. Five-year Follow-up of Trial of Replication-competent Adenovirus-mediated Suicide Gene Therapy for Treatment of Prostate Cancer. Mol Ther. 2007;15:636–642. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- 75.Barton KN, Tyson D, Stricker H, Lew YS, Heisey G, Koul S, et al. GENIS: gene expression of sodium iodide symporter for noninvasive imaging of gene therapy vectors and quantification of gene expression in vivo. Mol Ther. 2003;8:508–518. doi: 10.1016/s1525-0016(03)00153-9. [DOI] [PubMed] [Google Scholar]
- 76.Barton KN, Paielli D, Zhang Y, Koul S, Brown SL, Lu M, et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol Ther. 2006;13:347–356. doi: 10.1016/j.ymthe.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Freytag SO, Barton KN, Brown SL, Narra V, Zhang Y, Tyson D, et al. Replication-competent Adenovirus-mediated Suicide Gene Therapy with Radiation in a Preclinical Model of Pancreatic Cancer. Mol Ther. 2007 doi: 10.1038/sj.mt.6300212. [DOI] [PubMed] [Google Scholar]
- 78.Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 79.Seo E, Abei M, Wakayama M, Fukuda K, Ugai H, Murata T, et al. Effective gene therapy of biliary tract cancers by a conditionally replicative adenovirus expressing uracil phosphoribosyltransferase: significance of timing of 5-fluorouracil administration. Cancer Res. 2005;65:546–552. [PubMed] [Google Scholar]
- 80.Bernt KM, Steinwaerder DS, Ni S, Li ZY, Roffler SR, Lieber A. Enzyme-activated Prodrug Therapy Enhances Tumor-specific Replication of Adenovirus Vectors. Cancer Res. 2002;62:6089–6098. [PubMed] [Google Scholar]
- 81.Schepelmann S, Hallenbeck P, Ogilvie LM, Hedley D, Friedlos F, Martin J, et al. Systemic gene-directed enzyme prodrug therapy of hepatocellular carcinoma using a targeted adenovirus armed with carboxypeptidase G2. Cancer Res. 2005;65:5003–5008. doi: 10.1158/0008-5472.CAN-05-0393. [DOI] [PubMed] [Google Scholar]
- 82.Schepelmann S, Ogilvie LM, Hedley D, Friedlos F, Martin J, Scanlon I, et al. Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res. 2007;67:4949–4955. doi: 10.1158/0008-5472.CAN-07-0297. [DOI] [PubMed] [Google Scholar]
- 83.Chen MJ, Green NK, Reynolds GM, Flavell JR, Mautner V, Kerr DJ, et al. Enhanced efficacy of Escherichia coli nitroreductase/CB1954 prodrug activation gene therapy using an E1B-55K-deleted oncolytic adenovirus vector. Gene Ther. 2004;11:1126–1136. doi: 10.1038/sj.gt.3302271. [DOI] [PubMed] [Google Scholar]
- 84.Singleton DC, Li D, Bai SY, Syddall SP, Smaill JB, Shen Y, et al. The nitroreductase prodrug SN 28343 enhances the potency of systemically administered armed oncolytic adenovirus ONYX-411(NTR) Cancer Gene Ther. 2007;14:953–967. doi: 10.1038/sj.cgt.7701088. [DOI] [PubMed] [Google Scholar]
- 85.Stubdal H, Perin N, Lemmon M, Holman P, Bauzon M, Potter PM, et al. A prodrug strategy using ONYX-015-based replicating adenoviruses to deliver rabbit carboxylesterase to tumor cells for conversion of CPT-11 to SN-38. Cancer Res. 2003;63:6900–6908. [PubMed] [Google Scholar]
- 86.Kuppuswamy M, Spencer JF, Doronin K, Tollefson AE, Wold WS, Toth K. Oncolytic adenovirus that overproduces ADP and replicates selectively in tumors due to hTERT promoter-regulated E4 gene expression. Gene Ther. 2005;12:1608–1617. doi: 10.1038/sj.gt.3302581. [DOI] [PubMed] [Google Scholar]
- 87.Toth K, Djeha H, Ying B, Tollefson AE, Kuppuswamy M, Doronin K, et al. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated wnt signaling. Cancer Res. 2004;64:3638–3644. doi: 10.1158/0008-5472.CAN-03-3882. [DOI] [PubMed] [Google Scholar]
- 88.Toth K, Tarakanova V, Doronin K, Ward P, Kuppuswamy M, Locke JE, et al. Radiation increases the activity of oncolytic adenovirus cancer gene therapy vectors that overexpress the ADP (E3-11.6K) protein. Cancer Gene Ther. 2003;10:193–200. doi: 10.1038/sj.cgt.7700555. [DOI] [PubMed] [Google Scholar]
- 89.Yun CO, Kim E, Koo T, Kim H, Lee YS, Kim JH. ADP-overexpressing adenovirus elicits enhanced cytopathic effect by induction of apoptosis. Cancer Gene Ther. 2005;12:61–71. doi: 10.1038/sj.cgt.7700769. [DOI] [PubMed] [Google Scholar]
- 90.Geoerger B, Vassal G, Opolon P, Dirven CM, Morizet J, Laudani L, et al. Oncolytic activity of p53-expressing conditionally replicative adenovirus AdDelta24-p53 against human malignant glioma. Cancer Res. 2004;64:5753–5759. doi: 10.1158/0008-5472.CAN-04-0499. [DOI] [PubMed] [Google Scholar]
- 91.Geoerger B, van Beusechem VW, Opolon P, Morizet J, Laudani L, Lecluse Y, et al. Expression of p53, or targeting towards EGFR, enhances the oncolytic potency of conditionally replicative adenovirus against neuroblastoma. J Gene Med. 2005;7:584–594. doi: 10.1002/jgm.703. [DOI] [PubMed] [Google Scholar]
- 92.Idema S, Lamfers ML, van Beusechem VW, Noske DP, Heukelom S, Moeniralm S, et al. AdDelta24 and the p53-expressing variant AdDelta24-p53 achieve potent anti-tumor activity in glioma when combined with radiotherapy. J Gene Med. 2007;9:1046–1056. doi: 10.1002/jgm.1113. [DOI] [PubMed] [Google Scholar]
- 93.van Beusechem VW, van den Doel PB, Gerritsen WR. Conditionally replicative adenovirus expressing degradation-resistant p53 for enhanced oncolysis of human cancer cells overexpressing murine double minute 2. Mol Cancer Ther. 2005;4:1013–1018. doi: 10.1158/1535-7163.MCT-05-0010. [DOI] [PubMed] [Google Scholar]
- 94.Heideman DA, Steenbergen RD, van der Torre J, Scheffner M, Alemany R, Gerritsen WR, et al. Oncolytic adenovirus expressing a p53 variant resistant to degradation by HPV E6 protein exhibits potent and selective replication in cervical cancer. Mol Ther. 2005;12:1083–1090. doi: 10.1016/j.ymthe.2005.06.443. [DOI] [PubMed] [Google Scholar]
- 95.Sauthoff H, Pipiya T, Chen S, Heitner S, Cheng J, Huang YQ, et al. Modification of the p53 transgene of a replication-competent adenovirus prevents mdm2- and E1b-55kD-mediated degradation of p53. Cancer Gene Ther. 2006;13:686–695. doi: 10.1038/sj.cgt.7700936. [DOI] [PubMed] [Google Scholar]
- 96.Graat HC, Carette JE, Schagen FH, Vassilev LT, Gerritsen WR, Kaspers GJ, et al. Enhanced tumor cell kill by combined treatment with a small-molecule antagonist of mouse double minute 2 and adenoviruses encoding p53. Mol Cancer Ther. 2007;6:1552–1561. doi: 10.1158/1535-7163.MCT-06-0631. [DOI] [PubMed] [Google Scholar]
- 97.Bouvet M, Ellis LM, Nishizaki M, Fujiwara T, Liu W, Bucana CD, et al. Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 1998;58:2288–2292. [PubMed] [Google Scholar]
- 98.Nishizaki M, Fujiwara T, Tanida T, Hizuta A, Nishimori H, Tokino T, et al. Recombinant adenovirus expressing wild-type p53 is antiangiogenic: a proposed mechanism for bystander effect. Clin Cancer Res. 1999;5:1015–1023. [PubMed] [Google Scholar]
- 99.Dameron KM, Volpert OV, Tainsky MA, Bouck N. The p53 tumor suppressor gene inhibits angiogenesis by stimulating the production of thrombospondin. Cold Spring Harb Symp Quant Biol. 1994;59:483–489. doi: 10.1101/sqb.1994.059.01.053. [DOI] [PubMed] [Google Scholar]
- 100.Dewar RL, Natarajan V, Vasudevachari MB, Salzman NP. Synthesis and processing of human immunodeficiency virus type 1 envelope proteins encoded by a recombinant human adenovirus. J Virol. 1989;63:129–136. doi: 10.1128/jvi.63.1.129-136.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li H, Haviv YS, Derdeyn CA, Lam J, Coolidge C, Hunter E, et al. Human immunodeficiency virus type 1-mediated syncytium formation is compatible with adenovirus replication and facilitates efficient dispersion of viral gene products and de novo-synthesized virus particles. Hum Gene Ther. 2001;12:2155–2165. doi: 10.1089/10430340152710504. [DOI] [PubMed] [Google Scholar]
- 102.Wohlfahrt ME, Beard BC, Lieber A, Kiem HP. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models. Cancer Res. 2007;67:8783–8790. doi: 10.1158/0008-5472.CAN-07-0357. [DOI] [PubMed] [Google Scholar]
- 103.Bernt KM, Ni S, Tieu AT, Lieber A. Assessment of a combined, adenovirus-mediated oncolytic and immunostimulatory tumor therapy. Cancer Res. 2005;65:4343–4352. doi: 10.1158/0008-5472.CAN-04-3527. [DOI] [PubMed] [Google Scholar]
- 104.Pan Q, Liu B, Liu J, Cai R, Wang Y, Qian C. Synergistic induction of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying TRAIL. Mol Cell Biochem. 2007 doi: 10.1007/s11010-007-9514-6. [DOI] [PubMed] [Google Scholar]
- 105.Qiu S, Ruan H, Pei Z, Hu B, Lan P, Wang J, et al. Combination of Targeting Gene-ViroTherapy with 5-FU enhances antitumor efficacy in malignant colorectal carcinoma. J Interferon Cytokine Res. 2004;24:219–230. doi: 10.1089/107999004323034097. [DOI] [PubMed] [Google Scholar]
- 106.Chu L, Gu J, He Z, Xiao T, Liu X. Adenoviral vector expressing CYLD augments antitumor activity of TRAIL by suppression of NF-kappaB survival signaling in hepatocellular carcinoma. Cancer Biol Ther. 2006;5:615–622. doi: 10.4161/cbt.5.6.2662. [DOI] [PubMed] [Google Scholar]
- 107.Liu XY, Qiu SB, Zou WG, Pei ZF, Gu JF, Luo CX, et al. Effective gene-virotherapy for complete eradication of tumor mediated by the combination of hTRAIL (TNFSF10) and plasminogen k5. Mol Ther. 2005;11:531–541. doi: 10.1016/j.ymthe.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Zhao L, Dong A, Gu J, Liu Z, Zhang Y, Zhang W, et al. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]
- 109.Pan Q, Liu B, Liu J, Cai R, Liu X, Qian C. Synergistic antitumor activity of XIAP-shRNA and TRAIL expressed by oncolytic adenoviruses in experimental HCC. Acta Oncol. 2008;47:135–144. doi: 10.1080/02841860701403053. [DOI] [PubMed] [Google Scholar]
- 110.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 111.Lamfers ML, Gianni D, Tung CH, Idema S, Schagen FH, Carette JE, et al. Tissue inhibitor of metalloproteinase-3 expression from an oncolytic adenovirus inhibits matrix metalloproteinase activity in vivo without affecting antitumor efficacy in malignant glioma. Cancer Res. 2005;65:9398–9405. doi: 10.1158/0008-5472.CAN-04-4264. [DOI] [PubMed] [Google Scholar]
- 112.Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 113.Li GC, Yang JM, Nie MM, Su CG, Sun LC, Qian YZ, et al. Potent antitumoral effects of a novel gene-viral therapeutic system CNHK300-mEndostatin in hepatocellular carcinoma. Chin Med J (Engl) 2005;118:179–185. [PubMed] [Google Scholar]
- 114.Zhang Q, Nie M, Sham J, Su C, Xue H, Chua D, et al. Effective gene-viral therapy for telomerase-positive cancers by selective replicative-competent adenovirus combining with endostatin gene. Cancer Res. 2004;64:5390–5397. doi: 10.1158/0008-5472.CAN-04-1229. [DOI] [PubMed] [Google Scholar]
- 115.Yoo JY, Kim JH, Kwon YG, Kim EC, Kim NK, Choi HJ, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15:295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 116.Yoo JY, Kim JH, Kim J, Huang JH, Zhang SN, Kang YA, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15:635–651. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 117.Kang YA, Shin HC, Yoo JY, Kim JH, Kim JS, Yun CO. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol Ther. 2008;16:1033–1040. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Z, Zou W, Wang J, Gu J, Dang Y, Li B, et al. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol Ther. 2005;11:553–562. doi: 10.1016/j.ymthe.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 119.Bristol JA, Zhu M, Ji H, Mina M, Xie Y, Clarke L, et al. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Mol Ther. 2003;7:755–764. doi: 10.1016/s1525-0016(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 120.Ramesh N, Ge Y, Ennist DL, Zhu M, Mina M, Ganesh S, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12:305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 121.Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13:1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- 122.Sarkar D, Su ZZ, Vozhilla N, Park ES, Randolph A, Valerie K, et al. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- 123.Su C, Peng L, Sham J, Wang X, Zhang Q, Chua D, et al. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-gamma gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 124.Shashkova EV, Spencer JF, Wold WS, Doronin K. Targeting Interferon-alpha Increases Antitumor Efficacy and Reduces Hepatotoxicity of E1A-mutated Spread-enhanced Oncolytic Adenovirus. Mol Ther. 2007;15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 125.Shashkova EV, Kuppuswamy MN, Wold WS, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Post DE, Sandberg EM, Kyle MM, Devi NS, Brat DJ, Xu Z, et al. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007;67:6872–6881. doi: 10.1158/0008-5472.CAN-06-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee YS, Kim JH, Choi KJ, Choi IK, Kim H, Cho S, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin Cancer Res. 2006;12:5859–5868. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- 128.Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, et al. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- 130.Qian W, Liu J, Tong Y, Yan S, Yang C, Yang M, et al. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–369. doi: 10.1038/sj.leu.2405034. [DOI] [PubMed] [Google Scholar]
- 131.Luo J, Xia Q, Zhang R, Lv C, Zhang W, Wang Y, et al. Treatment of cancer with a novel dual-targeted conditionally replicative adenovirus armed with mda-7/IL-24 gene. Clin Cancer Res. 2008;14:2450–2457. doi: 10.1158/1078-0432.CCR-07-4596. [DOI] [PubMed] [Google Scholar]
- 132.Huang XF, Ren W, Rollins L, Pittman P, Shah M, Shen L, et al. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res. 2003;63:7321–7329. [PubMed] [Google Scholar]
- 133.Di Paolo NC, Tuve S, Ni S, Hellstrom KE, Hellstrom I, Lieber A. Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 135.Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001;61:813–817. [PubMed] [Google Scholar]
- 137.Krasnykh V, Dmitriev I, Navarro JG, Belousova N, Kashentseva E, Xiang J, et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Res. 2000;60:6784–6787. [PubMed] [Google Scholar]
- 138.Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 139.Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: Molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toth K, Spencer JF, Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL, et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther. 2005;16:139–146. doi: 10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 142.Steel JC, Morrison BJ, Mannan P, Abu-Asab MS, Wildner O, Miles BK, et al. Immunocompetent syngeneic cotton rat tumor models for the assessment of replication-competent oncolytic adenovirus. Virology. 2007 doi: 10.1016/j.virol.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 144.Hemminki A, Wang M, Hakkarainen T, Desmond RA, Wahlfors J, Curiel DT. Production of an EGFR targeting molecule from a conditionally replicating adenovirus impairs its oncolytic potential. Cancer Gene Ther. 2003;10:583–588. doi: 10.1038/sj.cgt.7700606. [DOI] [PubMed] [Google Scholar]
- 145.Kanerva A, Zinn KR, Peng KW, Ranki T, Kangasniemi L, Chaudhuri TR, et al. Noninvasive dual modality in vivo monitoring of the persistence and potency of a tumor targeted conditionally replicating adenovirus. Gene Ther. 2005;12:87–94. doi: 10.1038/sj.gt.3302387. [DOI] [PubMed] [Google Scholar]
- 146.Rajecki M, Kanerva A, Stenman UH, Tenhunen M, Kangasniemi L, Sarkioja M, et al. Treatment of prostate cancer with Ad5/3Delta24hCG allows non-invasive detection of the magnitude and persistence of virus replication in vivo. Mol Cancer Ther. 2007;6:742–751. doi: 10.1158/1535-7163.MCT-06-0403. [DOI] [PubMed] [Google Scholar]
- 147.Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14:779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leyton J, Lockley M, Aerts JL, Baird SK, Aboagye EO, Lemoine NR, et al. Quantifying the activity of adenoviral E1A CR2 deletion mutants using renilla luciferase bioluminescence and 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography imaging. Cancer Res. 2006;66:9178–9185. doi: 10.1158/0008-5472.CAN-06-1539. [DOI] [PubMed] [Google Scholar]
- 149.Mocanu JD, Yip KW, Alajez NM, Shi W, Li JH, Lunt SJ, et al. Imaging the modulation of adenoviral kinetics and biodistribution for cancer gene therapy. Mol Ther. 2007;15:921–929. doi: 10.1038/mt.sj.6300119. [DOI] [PubMed] [Google Scholar]
- 150.Guse K, Dias JD, Bauerschmitz GJ, Hakkarainen T, Aavik E, Ranki T, et al. Luciferase imaging for evaluation of oncolytic adenovirus replication in vivo. Gene Ther. 2007;14:902–911. doi: 10.1038/sj.gt.3302949. [DOI] [PubMed] [Google Scholar]
- 151.Barton KN, Xia X, Yan H, Stricker H, Heisey G, Yin FF, et al. A quantitative method for measuring gene expression magnitude and volume delivered by gene therapy vectors. Mol Ther. 2004;9:625–631. doi: 10.1016/j.ymthe.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 152.Merron A, Peerlinck I, Martin-Duque P, Burnet J, Quintanilla M, Mather S, et al. SPECT/CT imaging of oncolytic adenovirus propagation in tumours in vivo using the Na/I symporter as a reporter gene. Gene Ther. 2007;14:1731–1738. doi: 10.1038/sj.gt.3303043. [DOI] [PubMed] [Google Scholar]
- 153.Davis JJ, Wang L, Dong F, Zhang L, Guo W, Teraishi F, et al. Oncolysis and suppression of tumor growth by a GFP-expressing oncolytic adenovirus controlled by an hTERT and CMV hybrid promoter. Cancer Gene Ther. 2006;13:720–723. doi: 10.1038/sj.cgt.7700944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fujiwara T, Kagawa S, Kishimoto H, Endo Y, Hioki M, Ikeda Y, et al. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int J Cancer. 2006;119:432–440. doi: 10.1002/ijc.21846. [DOI] [PubMed] [Google Scholar]
- 155.Ono HA, Le LP, Davydova JG, Gavrikova T, Yamamoto M. Noninvasive visualization of adenovirus replication with a fluorescent reporter in the E3 region. Cancer Res. 2005;65:10154–10158. doi: 10.1158/0008-5472.CAN-05-1871. [DOI] [PubMed] [Google Scholar]
- 156.Li X, Zhang YP, Kim HS, Bae KH, Stantz KM, Lee SJ, et al. Gene therapy for prostate cancer by controlling adenovirus E1a and E4 gene expression with PSES enhancer. Cancer Res. 2005;65:1941–1951. doi: 10.1158/0008-5472.CAN-04-3666. [DOI] [PubMed] [Google Scholar]
- 157.Ito H, Aoki H, Kuhnel F, Kondo Y, Kubicka S, Wirth T, et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst. 2006;98:625–636. doi: 10.1093/jnci/djj161. [DOI] [PubMed] [Google Scholar]
- 158.Huang Q, Zhang X, Wang H, Yan B, Kirkpatrick J, Dewhrist MW, et al. A novel conditionally replicative adenovirus vector targeting telomerase-positive tumor cells. Clin Cancer Res. 2004;10:1439–1445. doi: 10.1158/1078-0432.ccr-03-0122. [DOI] [PubMed] [Google Scholar]
- 159.Hakkarainen T, Hemminki A, Curiel DT, Wahlfors J. A conditionally replicative adenovirus that codes for a TK-GFP fusion protein (Ad5Delta24TK-GFP) for evaluation of the potency of oncolytic virotherapy combined with molecular chemotherapy. Int J Mol Med. 2006;18:751–759. [PMC free article] [PubMed] [Google Scholar]
- 160.Raki M, Hakkarainen T, Bauerschmitz GJ, Sarkioja M, Desmond RA, Kanerva A et al. Utility of TK/GCV in the context of highly effective oncolysis mediated by a serotype 3 receptor targeted oncolytic adenovirus. Gene Ther. 2007 doi: 10.1038/sj.gt.3302992. [DOI] [PubMed] [Google Scholar]
- 161.Le LP, Everts M, Dmitriev IP, Davydova JG, Yamamoto M, Curiel DT. Fluorescently labeled adenovirus with pIX-EGFP for vector detection. Mol Imaging. 2004;3:105–116. doi: 10.1162/15353500200404100. [DOI] [PubMed] [Google Scholar]
- 162.Le LP, Li J, Ternovoi VV, Siegal GP, Curiel DT. Fluorescently tagged canine adenovirus via modification with protein IX-enhanced green fluorescent protein. J Gen Virol. 2005;86:3201–3208. doi: 10.1099/vir.0.80968-0. [DOI] [PubMed] [Google Scholar]
- 163.Le LP, Le HN, Dmitriev IP, Davydova JG, Gavrikova T, Yamamoto S, et al. Dynamic monitoring of oncolytic adenovirus in vivo by genetic capsid labeling. J Natl Cancer Inst. 2006;98:203–214. doi: 10.1093/jnci/djj022. [DOI] [PubMed] [Google Scholar]
- 164.Li J, Le L, Sibley DA, Mathis JM, Curiel DT. Genetic incorporation of HSV-1 thymidine kinase into the adenovirus protein IX for functional display on the virion. Virology. 2005;338:247–258. doi: 10.1016/j.virol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 165.Matthews QL, Sibley DA, Wu H, Li J, Stoff-Khalili MA, Waehler R, et al. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol Imaging. 2006;5:510–519. [PMC free article] [PubMed] [Google Scholar]