Abstract
A key tenet of tissue engineering is the principle that the scaffold can perform the dual roles of biomechanical and biochemical support through presentation of the appropriate mediators to surrounding tissue. While growth factors have been incorporated into scaffolds to achieve sustained release, there are a limited number of studies investigating release of biologically active molecules from reactive two-component polymers, which have potential application as injectable delivery systems. In this study, we report the sustained release of platelet-derived growth factor (PDGF) from a reactive two-component polyurethane. The release of PDGF was bi-phasic, characterized by an initial burst followed by a period of sustained release for up to 21 days. Despite the potential for amine and hydroxyl groups in the protein to react with the isocyanate groups in the reactive polyurethane, the in vitro bioactivity of the released PDGF was largely preserved when added as a lyophilized powder. PUR/PDGF scaffolds implanted in rat skin excisional wounds accelerated wound healing relative to the blank PUR control, resulting in almost complete healing with reepithelization at day 14. The presence of PDGF attracted both fibroblasts and mononuclear cells, significantly accelerating degradation of the polymer and enhancing formation of new granulation tissue as early as day 3. The ability of reactive two-component PUR scaffolds to promote new tissue formation in vivo through local delivery of PDGF may present compelling opportunities for the development of novel injectable therapeutics.
1. Introduction
Wound healing is a tightly orchestrated sequence of events that is driven by intercellular communication via cytokines and growth factors. When healing goes awry, many of the consequences can be attributed to the alteration of these signaling mechanisms, and deficient wound healing can be corrected, in part, by administration of signal molecules to sites of tissue repair. Reorganization of damaged tissue requires restoration of appropriate architecture and biomechanical properties. Supplementing the tissue defect with biocompatible scaffolds that may be derived from natural or synthetic sources can augment this aspect. A key tenet of tissue engineering is the principle that the scaffold — like the native extracellular matrix — can perform the dual roles of biomechanical and biochemical support through presentation of the appropriate mediators to surrounding tissue.
PDGF is a co-factor with vascular endothelial growth factor (VEGF) for stable angiogenesis [1]. It is chemotactic and mitogenic for bone cells, mesenchymal stem cells, ligament, dermal fibroblasts, and fibroblast-like cells derived from the periodontal ligament and gingiva [2–4]. PDGF-BB is the most widely understood and utilized isoform for wound healing applications, and it has been reported to stimulate and enhance new tissue formation in both bone and soft tissue models [5–8]. Inasmuch as PDGF-BB is a universal ligand for the PDGF receptor, it has achieved moderate commercial success as a component of Regranex™, a carboxymethylcellulose (CMC) gel formulation of 100μg/ml rhPDGF-BB (for simplicity referred to as PDGF). Due to the bolus release of PDGF from the CMC gel, this topical agent, which is approved for treatment of diabetic foot ulcers, must be applied daily or more frequently at high concentrations. In contrast, preclinical studies have shown that a sustained release of PDGF enhances cellular infiltration and new tissue formation relative to a bolus release. In a mid-sagittal dorsal incision model in the rat, a nearly linear sustained release (e.g., up to 14 days) of PDGF from nanofibrous scaffolds [9] was reported to enhance new tissue formation at days 7 and 14 [8], while a bolus release (e.g., complete release after 4h) had a negligible effect [8, 10]. Likewise, gene delivery of PDGF has produced positive effects by sustained, low level release [11].
To address the clinical need for an improved delivery device for sustained release of PDGF, we are investigating the potential of polyurethane (PUR) scaffolds. Porous, biodegradable, and biocompatible PUR scaffolds have been reported to support cellular infiltration and new tissue formation in sub-cutaneous [12–15], cardiovascular [16, 17], and bone models [18, 19]. Furthermore, these materials have been shown to biodegrade at a controlled rate to nontoxic products in vitro [16, 17, 20, 21] and in vivo [14–16, 22] to non-cytotoxic decomposition products. In addition to its function as a matrix supporting the ingrowth of cells and new tissue, the PUR scaffold can also function as a drug delivery system [23, 24]. Biodegradable PUR scaffolds have been used for controlled release of growth factors such as bFGF [25] and PDGF-BB [12]. A distinct advantage of polyurethane scaffolds is the potential to inject the materials as a reactive liquid mixture which adheres to host tissue, conforms and expands to fill irregularly shaped wounds, and cures in situ to form an elastic porous scaffold [12, 26]. A polyisocyanate (or NCO-terminated prepolymer) mixed with a polyol (hydroxyl functionality) reacts to form a polymer network incorporating urethane linkages. Water reacts with the polyisocyanate to form a disubstituted urea and carbon dioxide, which functions as a blowing agent, thereby introducing pores into the material.
In a previous study, we have reported the release of PDGF from PUR scaffolds, wherein radiolabeled PDGF was incorporated as a dry powder into PUR scaffolds that were prepared from hexamethylene diisocyanate trimer (HDIt). The release profile was characterized by a burst followed by a period of sustained release, and the cumulative release of PDGF was found to be independent of the dosage [12]. However, the bioactivity of the released PDGF was not assessed. Proteins such as PDGF contain active hydrogens, including primary amine and hydroxyl groups, which can potentially react with the polyisocyanate, thereby denaturing the protein. Therefore, in this study, we used two strategies for delivering PDGF from PUR scaffolds to address the need to preserve the bioactivity of the released PDGF. In the first approach, PDGF was incorporated into PUR scaffolds as a powder in the presence of excipients (heparin and glucose). In the second approach, PDGF was bound to PLGA microspheres through heparin, the microspheres were coated with a gelatin layer to form granules, and the granules were then incorporated into PUR scaffold. The gelatin coating was designed to protect active hydrogens (e.g., primary amine and hydroxyl groups) in the growth factors from reacting with the polyisocyanate component of the PUR. We have measured the in vitro release kinetics of PDGF from two-component reactive PUR scaffolds, as well as the in vitro biological activity of released PDGF, using both strategies. Considering that there were no substantial differences in either the release profile or the biological activity of released PDGF between the two strategies, the simpler approach of adding PDGF as a powder was selected for in vivo experiments. PUR scaffolds with and without PDGF were implanted in excisional wounds in Sprague-Dawley rats. We reasoned that the initial burst release of PDGF would attract mesenchymal cells into the scaffold, while the sustained release of PDGF would promote tissue remodeling in the later stages of the healing process. PUR+PDGF scaffolds implanted in rats promoted substantial ingrowth of cells as early as day 3 post-implantation, followed by extensive new tissue formation and scaffold degradation at day 7 and almost complete healing at day 14. Interestingly, PDGF not only enhanced ingrowth of cells and new tissue, but also accelerated scaffold degradation relative to the negative control. Thus the ability of PUR scaffolds to deliver biologically active PDGF at sustained low levels for several weeks presents potentially compelling opportunities for these materials as a combined scaffold and delivery system for regenerative medicine.
2. Materials & Methods
2.1. Materials
Polyvinyl alcohol (PVA), glycolide, and D,L-lactide were obtained from Polysciences (Warrington, PA). The tertiary amine catalyst TEGOAMIN33, which comprises a solution of 33 wt% triethylene diamine (TEDA) in dirpropylene glycol, was received from Goldschmidt (Hopewell, VA) as a gift. Polyethylene glycol (PEG, 600 Da) was purchased from Alfa Aesar (Ward Hill, MA). Dichloromethane (DCM), methanol, trifluoroacetic acid (TFA) and glucose were from Acros Organics (Morris Plains, NJ). Hexamethylene diisocyanate trimer (HDIt, Desmodur N3300A) was received as a gift from Bayer Material Science (Pittsburgh, PA). PDGF-BB was received as a gift from Amgen (Thousand Oaks, CA). Sodium iodide (Na125I) for radiolabeling was purchased from New England Nuclear (part of Perkin Elmer, Waltham, MA). Stannous octoate catalyst was received from Nusil technology (Overland Park, KS). 1-ethyl–3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) was from Advanced ChemTech (Louisville, Kentucky). Reagents for cell culture were all purchased from HyClone (Logan, UT). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Prior to use, glycerol and PEG 600 were dried at 10 mm Hg for 3 hours at 80 °C, and ε-caprolactone was dried over anhydrous magnesium sulfate, while all other materials were used as received.
2.2. Radiolabeling of PDGF-BB
PDGF-BB was labeled with radioactive iodine (125I) using IODO-BEADS Iodination Reagent (Pierce Biotechnology, Rockford, IL) in accordance with previously published techniques [12]. IODO-beads containing approximately 1 mCi Na125I were incubated in 1 ml reaction buffer for 5 minutes under room temperature, followed by addition of 50 μg PDGF to the reaction solution and incubation for another 25 minutes. The solution was then removed from the IODO-BEADS reaction tube and the Iodine-labeled PDGF (125I-PDGF) was separated in a Sephadex disposable PD-10 desalting column (Sigma-Aldrich). Eluted fractions were collected and Cobra II Autogamma counter (Packard Instrument Co, Meridien, CT) was adopted to determine the fractions containing the 125I-PDGF.
2.3. Amine-terminated poly(L-lactide-co-glycolide) (PLGA-NH2) synthesis
Hydroxyl-terminated poly(L-lactide-co-glycolide) (PLGA-OH, 50/50 lactide/glycolide, MW 20,000) copolymers were synthesized by ring-opening polymerization of D,L-lactide and glycolide using a dried polycaprolactone triol (MW 300) starter in the presence of stannous octoate catalyst as described previously [20, 22]. The polymer was then dissolved in DCM and reacted with N-t-Boc-glycine (4 mM) and DMAP (1.2 mM). To this mixture 4 mM of DCC was added, and the reaction mixture stirred in ice/water bath for 24 h. After filtering the dicyclohexylurea by-product, the filtrate was precipitated in methanol, filtered, and dried. The amine groups were de-protected by reacting the polymer in a solution of TFA in DCM for 3 h to remove the t-Boc groups, followed by precipitation in methanol, filtration, and drying. Structure of the the PLGA-NH2 was verified by NMR (Bruker 300 MHz).
2.4. Fabrication of PLGA-Heparin-PDGF microspheres
A microcapillary device was used to generate the PLGA-NH2 microspheres [27]. An oil phase comprising a solution of PLGA-NH2 in DCM (5% wt) was fed to the concentric tube (0.1 inch in diameter), while an aqueous carrier stream (2% w/v PVA) surrounded the emerging jet of the PLGA-NH2/DCM solution, which was sprayed into a solution of 2% PVA. After stirring ~3 hour to evaporate the DCM, the particles were filtered, washed with Deionized water, and lyophilized for 24 h. The particle size was measured to be 50 μm using an Olympus BX60 microscope.
Heparin (activity: 170 USPU/mg) was covalently bound to the surfaces of PLGA-NH2 microspheres using standard carbodiimide techniques [10, 28]. Briefly, heparin (0.33 g/g PLGA-NH2) was incubated with PLGA-NH2 microspheres in a pH 5.5 buffer solution (0.1M MES containing 0.5M NaCl). To activate the –COOH groups of the heparin, NHS (6 mmol) and EDC (6 mmol) were added to the solution, followed by reaction in an ice water bath for 24 h. The resulting PLGA-Hp microspheres were filtered, washed, and lyophilized. The amount of heparin conjugated to the microsphere surface was measured to be 0.15 wt% using the toluidine blue method [28]. PDGF-BB (labeled and/or non-labeled) was loaded onto PLGA-Hp microspheres by incubating in PBS for 1 h, followed by centrifugation to separate the particles. The loading efficiency was measured to be 80% by ELISA (R&D systems).
2.5. Gelatin coating of PLGA-Heparin-PDGF microspheres
PLGA-Heparin-PDGF microspheres (210 mg) were mixed with 70 μl gelatin solution (5%) and forced through a 300-μm sieve to yield gelatin coated granules. The granules were then dried at 4 °C for 2 days.
2.6. Polyurethane scaffold synthesis and characterization
Polyester triols (900 Da) were prepared from a glycerol starter and a backbone comprising 60wt% ε-caprolactone, 30wt% glycolide, and 10wt% D,L-lactide as published previously [20, 22]. The reaction was carried out by mixing these components in a 100-ml reaction flask with mechanical stirring under argon in the presence of stannous octoate (0.1 wt%) catalyst for 40 hours at 140 °C. The product was then dried under vacuum at 80 °C overnight. PUR scaffolds were synthesized by one-shot reactive liquid molding of hexamethylene diisocyanate trimer (HDIt; Desmodur N3300A) and a hardener comprising polyol (half polyester triol 900-Da and half PEG 600-Da), 1.5 parts per hundred parts polyol (pphp) water, 4.5 pphp TEGOAMIN33 tertiary amine catalyst, 1.5 pphp sulfated castor oil stabilizer, and 4.0 pphp calcium stearate pore opener. The isocyanate was added to the hardener, mixed for 30 seconds in a Hauschild SpeedMixer™ DAC 150 FVZ-K vortex mixer (FlackTek, Inc., Landrum, SC), and the resulting liquid mixture poured into a cylindrical mold where it cured as a free-rise foam after ~20 min [20, 22]. The targeted index (the ratio of NCO to OH equivalents times 100) was 115.
Scanning electron microscopy (Hitachi S-4200 SEM, Finchampstead, UK) was utilized to measure the pore size and determine the internal pore morphology of the polyurethane scaffolds. The core porosity was calculated from the measure density as reported previously [20]. The glass transition temperature (Tg) of the scaffolds was evaluated by dynamic mechanical analysis (DMA) in compression mode with a temperature sweep of −80 °C to 100 °C, at a frequency of 1 Hz, 20-μm amplitude, 0.3-% strain, and 0.2-N static force [12].
2.7. Incorporation of protein in the polyurethane scaffold
BSA-FITC (2.5 μg) was co-dissolved with varying amounts of glucose (0 mg, 0.5 mg, 2 mg, or 5 mg) and the resulting solution lyophilized. The lyophilized powder was added to the hardener component before mixing with the isocyanate to prepare the PUR scaffolds (each scaffold weighed 100 mg). For the foams including BSA-FITC in solution, the appropriate amount of BSA-FITC was dissolved in the water and the resulting solution added to the hardener. Prior to reaction with the isocyanate, 0.5 mg glucose powder was also added to the hardener. PDGF-BB (2.5 μg, labeled and/or non-labeled) was co-dissolved and lyophilized with excipients (heparin, glucose, or a combination of both) in order to stabilize the protein during lyophilization and scaffold synthesis. The heparin dosage was 50 μg per foam and the glucose dosage 2 mg per 100 mg scaffold. As described above, the lyophilized powder was added to the hardener component before mixing with the isocyanate to prepare the PUR scaffolds. For the foams containing gelatin-coated granules, 15wt% granules were mixed well with the hardener components before reacting with isocyanate.
2.8. In vitro release
An appropriate amount of sample (PLGA particles, gelatin-coated granules, and scaffolds) was immersed in 1 ml release medium (α-MEM containing 1% BSA) contained in polypropylene vials sealed by O-rings. The total protein amount in the release system was controlled at 2.5 μg by adding the appropriate mass of PUR foam to the medium. α-MEM and BSA were included to mimic the cellular growth environment and minimize adsorption of PDGF onto the scaffolds and vials.
For the PUR foam samples containing BSA-FITC, the medium was refreshed from each vial at the time points as indicated in the figures, and the released BSA-FITC concentration was measured by emission fluorescence at 530 nm after excitation at 485 nm using a BIO-TEK FL600 microplate fluorescence reader.
For the samples containing PDGF-BB, the medium was refreshed every 24 h. An extra centrifugation step was required before changing the medium of the particle and granule samples. The PDGF concentration was determined by either Human PDGF-BB Quantikine ELISA kit (R&D systems, Minneapolis, MN), or with a Cobra II Autogamma counter (Packard Instrument Co, Meridien, CT) for radio-labeled protein.
2.9. In vitro cell proliferation assay with released PDGF
MC3T3 mouse osteoblast precursor cells were seeded in 96-well plates at 1000 cells/well and incubated with α-MEM cell culture media containing 10% serum to promote cell attachment. After 24h, the media were replaced by media containing 0.5% serum and the PDGF releasates (or controls) were added at dosages of 3 and 10 ng/ml. Cell numbers were measured using a CyQUANT NF Cell Proliferation Assay kit from Invitrogen (Carlsbad, CA) at time points of 24 and 72h.
2.10. In vivo rat skin excisional wound test
The PUR/PDGF scaffolds were cut into 6mm × 2mm discs and implanted into 8mm full-thickness excisional wounds in the dorsal skin of adult male Sprague-Dawley rats. The loading of PDGF-BB in the scaffold was 0 μg (control), 1.8 μg (11.5 μg/ml scaffold), and 18 μg (11.5 μg/ml scaffold). Each was replicated 6 times. The rats were sacrificed 3, 7, and 14 days post-implantation. The harvested implants were fixed in formalin for 24 h, embedded in paraffin, and processed for histological evaluation with Mallory’s trichrome staining and hematoxylin & eosin (H&E) staining. Quantitative analysis of PUR degradation and granulation tissue was performed using Image Pro 6.2 (Media Cybernetics) by measuring the area percentage of polymer and new tissue within the scaffold based on trichrome images.
3. Results
3.1. In vitro release of BSA-FITC from PUR/BSA-FITC scaffolds
Previous studies have shown that dissolved proteins and peptides with active hydrogens (e.g., hydroxyl groups and amines) react with the polyisocyanate and are covalently bound to the polyurethane [29–31]. Reaction of hydroxyl and amine groups in the dissolved protein with isocyanate groups will result in the formation of urethane and urea bonds, which hydrolyze very slowly. Furthermore, reaction of the protein may result in denaturation and loss of biological activity. We therefore hypothesized that addition of the protein in solution would result in low cumulative release. To test this hypothesis, BSA-FITC was dissolved in water and added to the hardener component prior to mixing with HDIt. As shown in Figure 1, the cumulative release of BSA-FITC was <20% after 21 days, thereby suggesting that a substantial portion of the BSA reacts with the polyisocyanate and is covalently bound to the scaffold. To protect the protein from reacting with the polyisocyanate, BSA-FITC was lyophilized with a varying amount of glucose excipient and then added to the hardener as a powder. As shown in Figure 1, the total amount of protein released was significantly higher when added as a powder compared to addition in solution. The glucose dosage played an important role in the release profile; the presence of 0.5% and 2% glucose increased the total amount released after 21 days and increased the initial burst release. However, further increasing the glucose dosage to 5% decreased the total release.
Figure 1.
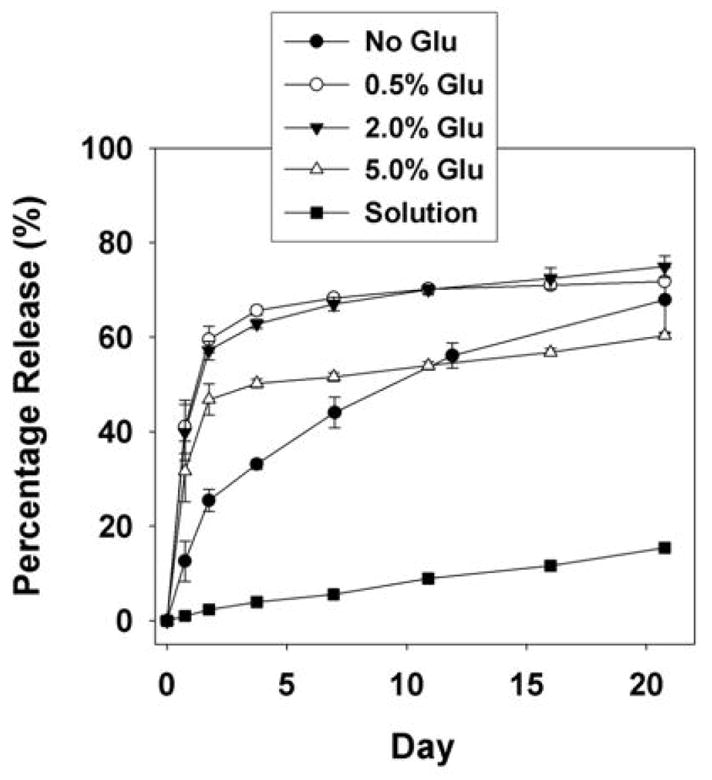
In vitro release profile of BSA-FITC from polyurethane scaffolds. BSA-FITC was incorporated in the scaffold as solution in the presence of 0.5% glucose, and as powder in presence of different weight percentages of glucose. Each data point represents the mean of three measurements ± SD.
3.2. In vitro release of PDGF-BB from PUR scaffolds
Preliminary PDGF release experiments were performed in the absence of glucose excipient in an attempt to minimize the burst release and maximize the sustained release. Even more so than the PUR/BSA-FITC scaffolds, addition of PDGF as a powder without the glucose excipient resulted in negligible protein release (data not shown), which may be due to conformational and other biochemical differences between BSA-FITC and PDGF. Based upon the data in Figure 1, 2 wt% glucose was added to the scaffolds to increase the cumulative release of PDGF. The release profile was monitored by two methods. In the first approach, PDGF was radioiodinated and a gamma counter was used to monitor the release kinetics. Release of PDGF was also measured by ELISA assay using the liquid releasates. Both methods yielded similar release profiles (Figure 2), characterized by a burst release on the first day. The ELISA assay detected a lower total release of 70%, compared with 85% for the 125I labeling method, which likely results from denaturation of some fraction of the released protein that cannot be detected by the antibody.
Figure 2.
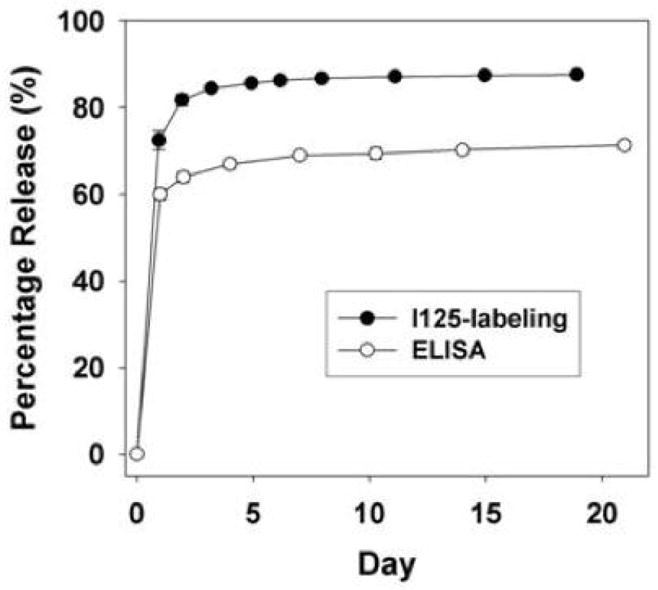
In vitro release profile of PDGF from polyurethane scaffolds including PDGF powder (PUR/PDGF) in the presence of 0.05% heparin and 2% glucose. Release kinetics were measured by (A) gamma counting and (B) ELISA.
To mitigate the burst release, another strategy was adopted to achieve controlled release of PDGF from polyurethane scaffolds. Heparin was bound to the surface of microparticles made from amine-terminated PLGA (average size 50 μm) using standard carbodiimide techniques. PDGF was then loaded onto the particle surface through heparin binding. As shown in Figure 3, the total release of PDGF from the particles alone at day 21 was determined to be 80% and 72% by 125I labeling and ELISA respectively. However, when the PLGA-Heparin-PDGF microparticles were incorporated in the polyurethane scaffold, less than 10% of the total protein was released over 21 days (data not shown), a finding which suggests that the surface-immobilized protein reacted with the polyisocyanate. To protect the protein on the surface of the PLGA microspheres from chemical modification, the microspheres were granulated by mixing the particles with a small amount of gelatin solution followed by forcing the mixture through a 48-mesh sieve to form 110-μm granules. The granules were then added to the hardener component before reacting with isocyanate to form PUR/G-PDGF polyurethane scaffolds. The PDGF release from gelatin-coated granules was similar to that of heparin-PLGA particles when detected by 125I labeling (Figure 3A), but release was lower when measured by ELISA (Figure 3B). Compared with the release profiles from PUR/PDGF scaffolds (Figure 2 and 3), the PUR/G-PDGF scaffolds exhibited lower burst and more sustained release when measured by radioiodination. However, when measured by ELISA, the total release was lower for the PUR/G-PDGF scaffolds, which again suggests that the isocyanate reaction may adversely affect the activity of the protein, despite the presence of a gelatin coating.
Figure 3.
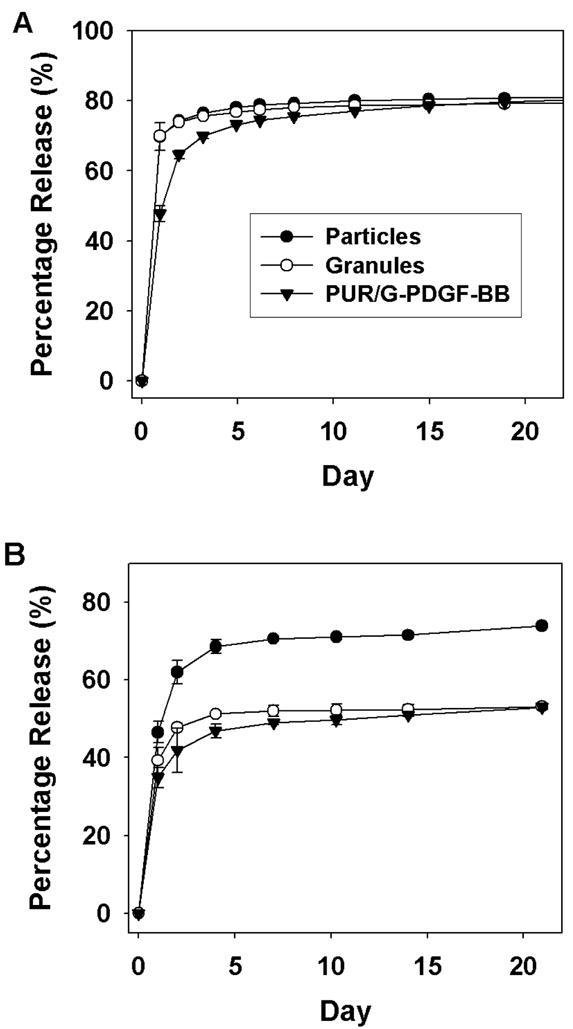
In vitro release profile of PDGF from PLGA particles, granules, and polyurethane scaffolds containing granules (PUR/G-PDGF). Release kinetics were measured by (A) gamma reading and (B) ELISA.
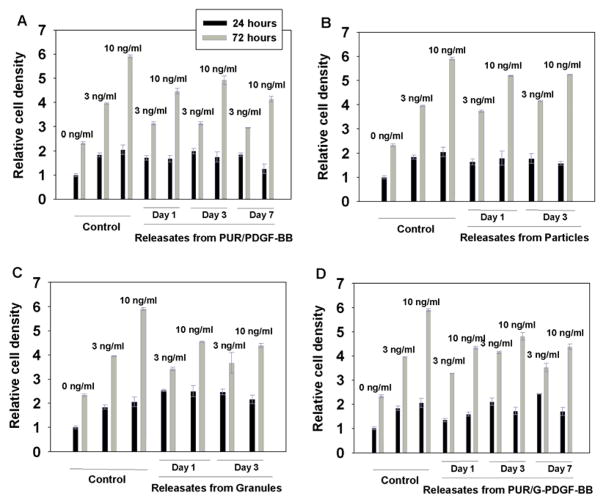
3.3. In vitro cell proliferation assay of released PDGF
To assess the in vitro bioactivity of PDGF released from the polyurethane scaffolds, releasates at days 1, 3, and 7 from PUR/PDGF and PUR/G-PDGF scaffolds, and at day 1 and 3 from particles and granules, were lyophilized and reconstituted to the same PDGF concentration, as determined by ELISA. The releasates were then tested for mitogenic activity using MC3T3 cells. After plating, the serum content was decreased from 10% to 0.5% to reduce background, and the reconstituted releasates were added at dosages of 3 and 10 ng/ml. Fresh PDGF acted as a positive control. Cell numbers were measured at time points of 24 and 72h by staining the DNA and measuring fluorescence on a plate reader. Figure 4 shows that the released PDGF from all the samples tested (PUR/PDGF, particles, granules, and PUR/G-PDGF) was biologically active, promoting proliferation of MC3T3 cells in a time-dependent manner. At the 72h time point, a statistically significant, dose-dependent effect was observed. There was no statistically significant difference in the samples at the 24h time point. While the addition of released PDGF yielded statistically significant higher cell numbers compared to the negative control, the cell numbers were less than the positive control. For the PUR/PDGF material, the data of the cell proliferation assay indicated that the released PDGF exhibited ~20% decreased activity.
Figure 4.
In vitro cell counts measured for MC3T3 cells incubated in PDGF releasates from (A) PUR/PDGF scaffolds, (B) microspheres incorporating PDGF, (C) granules, and (D) PUR/G-PDGF scaffolds. MC3T3 cells were treated with varying dosages of PDGF (0, 3, and 10 ng/ml) for 24 and 72 hours after attachment of the cells to the surface of the well plates. The control treatment comprised fresh PDGF. CyQUANT assay was carried out to measure cell number.
3.4. Porosity and pore size distribution of polyurethane scaffolds
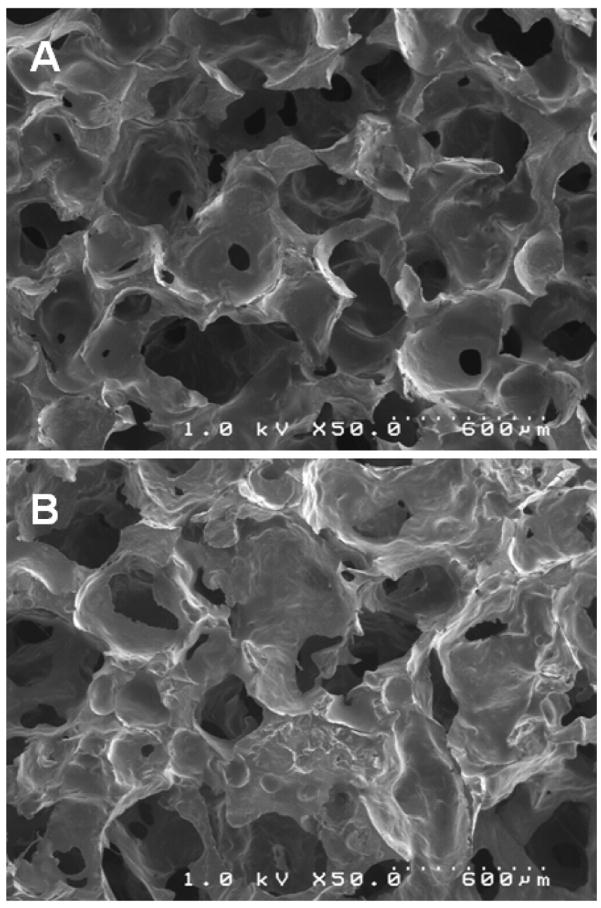
The polyurethane scaffold containing 2% glucose (Figure 5A) was porous with interconnected pores as evidenced by SEM imaging. The pore size was polydisperse and on the order of several hundred microns. The presence of 15 wt% granules in the PUR scaffold (Figure 5B) did not substantially change the internal pore morphology. PUR/G scaffolds had a slightly lower density, thus the core porosity was higher than that of the PUR. The porosities of PUR and PUR/G scaffolds were calculated to be 87.4% and 85.2% respectively (Table 1).
Fig. 5.
Scanning electronic microscopic images of polyurethane scaffold containing (A) 2% glucose, and (B) 15% granules.
Table 1.
PUR scaffold properties: density, porosity, and glass transition temperature (measured by DMA). The data for PUR containing 2% glucose (PUR) and 15 wt% granules (PUR-Granules) are listed.
| Density (kg·m−3) | Porosity (vol-%) | Tg (°C) | |
|---|---|---|---|
| PUR | 178.4 ± 8.9 | 85.2 ± 0.7 | 24.5 ± 0.6 |
| PUR-Granules | 152.2 ± 7.4 | 87.4 ± 0.6 | 34.0 ± 1.7 |
As shown in Figure 6, both materials exhibit glass transitions (Tg) less than 37°C, with Tg measured to be 24.5°C and 34.0°C for PUR and PUR/G respectively (Table 1). The scaffolds exemplify the characteristics associated with rubbery elastomers, including the glassy region below Tg, the glass transition region, and the rubbery plateau region above Tg.
Fig. 6.
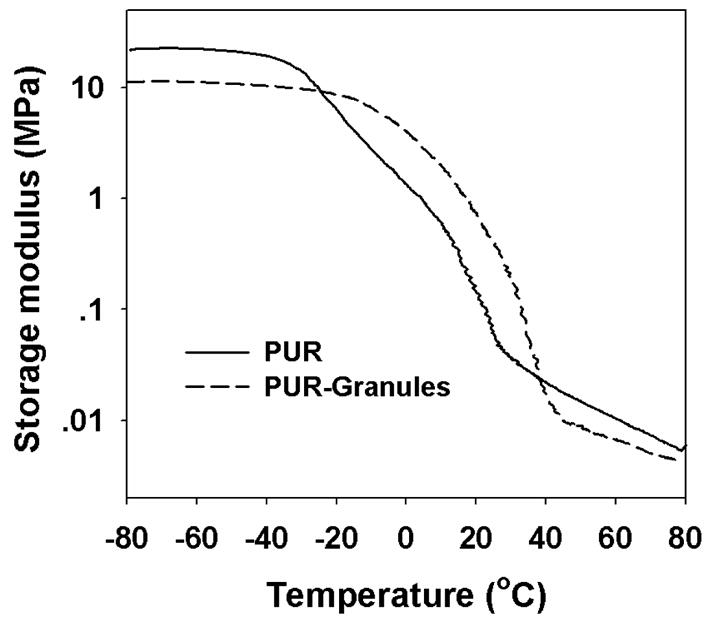
Temperature sweeps of (A) PUR, and (B) PUR/G scaffolds measured by DMA.
3.5. In vivo bioactivity of PUR/PDGF implants in rat skin excisional wounds
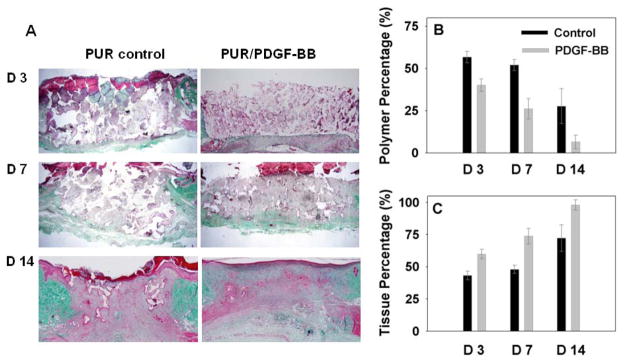
After PDGF release and bioactivity were measured in vitro, the PUR/PDGF scaffolds were implanted into excisional wounds on the backs of adult male Sprague-Dawley rats to test biological activity in a wound healing model. The PUR/PDGF delivery system was selected for in vivo studies based on several considerations. First, the differences in the shape of the release profiles for the PUR/PDGF and PUR/G-PDGF systems were relatively minor. Second, the in vitro bioactivity of the growth factor released from the two delivery systems was comparable; however, the cumulative release of PDGF from the PUR/PDGF system was almost 20% higher than that from the PUR/G-PDGF system, thereby requiring a lower dosage of growth factor. Third, the PDGF powder system was considerably simpler than the granule system and required fewer processing steps. Therefore, the PUR/PDGF system was selected for the in vivo studies. The loading of PDGF in the scaffolds was 0 μg (control), 1.8 μg, and 18 μg respectively. Preliminary experiments showed no statistically significant difference in new tissue ingrowth at the 18 μg dose compared to the 1.8 μg loading, so further experiments were performed at the lower dose only. After 3, 7, and 14 days post-implantation, the rats were sacrificed, and the harvested implants were processed for histology. As shown in Figure 7A (trichrome stain), PUR/PDGF scaffolds promoted significantly more new granulation tissue formation and polymer resorption compared to the blank PUR control at all time points. As the healing progressed, new extracellular matrix with dense collagen fibers filled the defect. At day 7, a remarkable level of new tissue infiltration and scaffold degradation was observed for the PDGF samples. At day 14, the polymer had almost completely resorbed, being replaced by new tissue accompanied by deposition of collagen. In addition, a complete epithelial layer had formed on the upper surface of the wound/implant site. The amounts of both remaining polymer and new tissue formation within the scaffold at different time points were analyzed quantitatively based on the area percentage measured from the histological images (Figures 7B and C). Statistical analysis between the control and PDGF groups (ANOVA with bonferoni correction, p≤0.05) showed that the amount of polymer within PDGF samples was significantly (p≤0.05) lower than the control scaffold, and that the new tissue formation within the PUR/PDGF scaffolds was significantly higher than the control at all time points.
Fig. 7.
Trichrome staining and quantitative evaluation of in vivo implants. In the representative images (A), the white, red, and green sections represent the presence of polymer, tissue, and collagen deposition respectively. The area percentage of polymer and new granulation tissue within the implants at each time point are shown in (B) and (C).
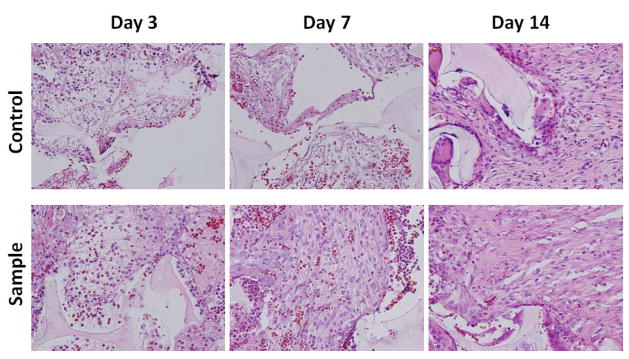
H&E staining (Figure 8) indicated the presence of both inflammatory cells (macrophages and neutrophils) and fibroblasts within the scaffold, and there was no substantial difference between the control and PUR/PDGF scaffolds in the relative proportion of these infiltrating cell populations. There was a typical neutrophil infiltration into the polymer scaffold at day 3 and 7. At day 14, the PDGF sample showed few definitive inflammatory cells while giant cells were observed around the remnant PUR scaffold in the control.
Fig. 8.
H&E staining of in vivo implants at 40X magnification. Representative tissue sections were photographed from 3, 7, and 14 day excisional wounds containing PUR scaffold alone (control) or scaffold containing 1.8μg PDGF-BB (sample). The scaffold is visible as a pale, acellular structure. At day 3, the interstices of the scaffold already contain a largely fibrinous matrix and an inflammatory infiltrate with both mononuclear cells and neutrophils. At day 7, the sample exhibits a notably higher proportion of spindle-shaped fibroblasts. At 14 days, both specimens show evidence of interstitial fibrous connective tissue, but it is more apparent and abundant, relative to the cellular component, in the treated group. The scaffold material is associated with giant cells that appear to be attempting to engulf the residual scaffold.
4. Discussion
The stabilization of therapeutic proteins in delivery systems is a challenging problem, due in part to the need to preserve the three-dimensional conformation of the protein. While proteins have been incorporated into polymer scaffolds to achieve sustained release, there are a limited number of studies investigating release of biologically active molecules from reactive two-component polymers. Ascorbic acid dissolved in glycerol was utilized as a chain extender to form a polyurethane network through reaction with a lysine diisocyanate (LDI) prepolymer. Ascorbic acid released from the polyurethane exhibited linear release kinetics [32]. However, the applicability of this approach with proteins is limited, due to the potential that the chemical reaction will covalently bind the protein to the polymer network as evidenced by the low cumulative release of BSA dissolved in water (Figure 1). However, adding the protein as a powder or coating the protein-loaded microspheres with gelatin protected the protein from reacting with the isocyanate, thus preserving its bioactivity. Addition of a glucose excipient significantly altered the release profile as observed for BSA-FITC (Figure 1), and an optimal glucose dosage exists that maximizes the cumulative release of BSA. Glucose and heparin were used as excipients when including PDGF powder into polyurethane scaffold. Glucose, which is in the form of solid particles that diffuse through the scaffold, has been proposed to act as a porogen which increases the diffusivity of the protein [33], as evidenced by the less than 10% cumulative release of PDGF in the absence of glucose (data not shown). Heparin is known to bind to several growth factors via electrostatic forces, thus enhancing the bioactivity of the protein [10, 28]. PEG 600 (50%), which is a liquid at ambient temperature, was included in the polyol component prior to polymerization with HDIt. The terminal hydroxyl groups on the PEG molecule react with the NCO groups to form urethane linkages, thereby covalently binding the PEG to the polymer network. Thus it is not likely that PEG acted as an excipient since the urethane bonds hydrolyze over a much longer time scale than that of protein release.
In the present study, we reasoned that sustained release of PDGF could be achieved by binding the protein to heparin-conjugated PLGA microspheres followed by gelatin granulation to protect the protein from reacting with the isocyanate during the formation of the scaffold. Binding of BMP-2 to heparin immobilized on the surface of PLGA scaffolds has yielded a nearly linear release profile for up to 14 days [10], and similar results have been reported for bFGF bound to heparin-conjugated PLGA nanospheres [28]. bFGF bound to heparin-conjugated porous PLGA microspheres showed a small burst release on the first day followed by sustained release for about 15 days [34]. Although we obtained a reduced burst and higher sustained release for PUR/G-PDGF compared with PUR/PDGF, the burst release was still higher than that reported previously for BMP-2 [10] and bFGF [34]. Furthermore, the ELISA assay (Figure 3B) showed decreased antibody binding activity of the released PDGF compared with the radiolabeling assay (Figure 3A), suggesting a slight loss in the protein activity during granulation.
In vitro cell proliferation analysis suggested that the released PDGF from PUR/PDGF and PUR/G-PDGF scaffolds had ~20% lower activity than fresh PDGF. Considering that the release profiles for both delivery strategies showed no substantial differences, the PUR/PDGF scaffold was selected for the in vivo study on the basis of simpler fabrication. We also reasoned that since PDGF functions in the early stages of cell proliferation [35], release of the growth factor in the first few days of healing would recruit cells to the scaffold and accelerate healing.
In previous studies, we have shown that two-component PUR scaffolds synthesized from aliphatic and lysine-derived polyisocyanates are biocompatible and degrade to non-cytotoxic decomposition products when implanted sub-cutaneously [12]. When implanted in excisional wounds, the scaffolds supported the ingrowth of cells and new tissue. The presence of PDGF within the implants shown in this report improved excisional wound healing significantly due to accelerated polymer degradation and enhanced new granulation tissue formation. There was little difference between the active and control scaffolds in terms of the inflammatory response at days 3 and 7, but there were significantly more inflammatory cells in the control than the PDGF sample at day 14. The presence of inflammatory cells may release reactive oxygen species in the early stages as well as enzymes such as cholesterol esterase, esterase, and carboxyl esterase [36]. Both these effects have been reported to degrade poly(ester urethane)s [37–39]. Both inflammatory cells and fibroblasts have been reported to play a role in the acceleration of wound healing by PDGF [40]. Considering that PDGF has been reported to attract neutrophils, macrophages, and fibroblasts [41], it is possible that the inflammatory cells attracted to the PUR/PDGF scaffolds accelerated degradation of the polymer through the reactive oxygen and enzymatic mechanisms described above. The enhanced formation of granulation tissue in the PUR/PDGF scaffolds relative to the control likely results from the recruitment of fibroblast cells which resulted in endogenous growth factor production, provisional extracellular matrix synthesis, fibroblast proliferation, and collagen synthesis [40, 42, 43].
Nanofibrous scaffolds incorporating PLGA microspheres encapsulated with PDGF have been shown to achieve nearly linear release kinetics of PDGF with a small burst release in vitro [9]. These materials have also been reported to promote chemokine expression and tissue neogenesis in mid-sagittal incision model of rat dorsa [8]. No significant tissue infiltration was observed at day 3, but significant tissue neogenesis was observed at day 7 and almost complete tissue penetration occurred at day 14 [8]. In related experiments, a surface absorption method for releasing PDGF was also investigated, which did not promote tissue neogenesis as well as the microsphere approach [8] due to the bolus release of growth factor (i.e., within several hours) [10]. These results underscore the importance of sustained release beyond the first few hours to accelerate wound healing. In the nanofiber experiments, two different PDGF dosages (11.9 μg/ml and 119 μg/ml scaffold) and release strategies (fast and slow) were investigated. Formation of new tissue was highest for the fast delivery system at the 119 μg/ml dosage. In contrast, for the PUR scaffolds, we observed no effect of PDGF dosage on new tissue formation at dosages of 11.5 μg/ml and 115 μg/ml scaffold. Furthermore, for the nanofiber scaffolds at the low PDGF dosage (11.9 μg/ml scaffold), new tissue formation was significantly higher for the slow release system (characterized by a burst release < 5% on day 1) compared to the fast release system (a burst release of 30% on day 1). Surprisingly, for the PUR/PDGF delivery system where the burst release on day 1 is 60%, we have observed significant new tissue formation promoted by PDGF as early as day 3, compared to day 7 for the nanofiber scaffolds. While the models used in the nanofiber and PUR studies differ, our data suggest that a burst release in the first few days followed by a lower sustained release may accelerate healing. It is important to note that the accelerated in vivo degradation of PUR/PDGF scaffolds is anticipated to substantially modify the release profile as compared to in vitro release assays. Stated another way, the observed accelerated degradation of the PUR scaffolds in the presence of PDGF in vivo may have a feedback effect, where the release of PDGF stimulates polymer degradation, which further increases the release of PDGF from the scaffold.
5. Conclusion
When released from a reactive two-component polyurethane scaffold in the first several days of the healing process, PDGF promotes cell proliferation, enhanced granulation tissue formation, and acceleration of scaffold degradation. The bioactivity of the released growth factor is substantially preserved in the presence of the chemical reaction between the isocyanate and hydroxyl groups. The ability of PUR scaffolds to promote new tissue formation in excisional wounds in rat skin, achieved through local delivery of PDGF, suggests the potential utility of biodegradable polyurethanes both as a supportive scaffold and as a protein delivery system for tissue restoration.
Acknowledgments
This work was funded by the National Institute on Aging (AG-06528), the Department of Veterans Affairs, the Orthopaedic Trauma Research Program (DOD-W81XWH-07-1-0211) and Vanderbilt University School of Engineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999 Oct;79(4):1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 2.Centrella M, McCarthy TL, Kusmik WF, Canalis E. Isoform-specific regulation of platelet-derived growth factor activity and binding in osteoblast-enriched cultures from fetal rat bone. J Clin Invest. 1992 Apr;89(4):1076–84. doi: 10.1172/JCI115687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piche JE, Graves DT. Study of the growth factor requirements of human bone-derived cells: a comparison with human fibroblasts. Bone. 1989;10(2):131–8. doi: 10.1016/8756-3282(89)90011-2. [DOI] [PubMed] [Google Scholar]
- 4.Gruber R, Varga F, Fischer MB, Watzek G. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002 Oct;13(5):529–35. doi: 10.1034/j.1600-0501.2002.130513.x. [DOI] [PubMed] [Google Scholar]
- 5.McGill JJ, Strates BS, McGuire MH. Stimulation of Osteogenesis by Platelet Derived Growth-Factor and A Transforming Growth-Factor. Clinical Research. 1991;39(3):A788-A. [Google Scholar]
- 6.Grotendorst GR, Martin GR, Pencev D, Sodek J, Harvey AK. Stimulation of Granulation-Tissue Formation by Platelet-Derived Growth-Factor in Normal and Diabetic Rats. Journal of Clinical Investigation. 1985;76(6):2323–9. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of Platelet-Derived Growth-Factor on Tibial Osteotomies in Rabbits. Bone. 1994;15(2):203–8. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 8.Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE, Ma PX, et al. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoSONE. 2008;3(3):e1729. doi: 10.1371/journal.pone.0001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei GB, Jin QM, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. Journal of Controlled Release. 2006;112(1):103–10. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon O, Song SJ, Kang SW, Putnam AJ, Kim BS. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28(17):2763–71. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z, Sugai JV, Jin Q, Chandler LA, Giannobile WV. Platelet-derived growth factor-B gene delivery sustains gingival fibroblast signal transduction. J Periodontal Res. 2008 Aug;43(4):440–9. doi: 10.1111/j.1600-0765.2008.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafeman AE, Li B, Yoshii T, Zienkiewicz K, Davidson JM, Guelcher SA. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. PharmRes. 2008;25(10):2387–99. doi: 10.1007/s11095-008-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett S, Connolly K, Lee DR, Jiang Y, Buck D, Hollinger JO, et al. Initial biocompatibility studies of a novel degradable polymeric bone substitute that hardens in situ. Bone. 1996 Jul;19(1):S101–S7. doi: 10.1016/s8756-3282(96)00130-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JY, Beckman EJ, Hu J, Yang GG, Agarwal S, Hollinger JO. Synthesis, biodegradability, and biocompatibility of lysine diisocyanate-glucose polymers. Tissue Engineering. 2002;8(5):771–85. doi: 10.1089/10763270260424132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JY, Beckman EJ, Piesco NP, Agarwal S. A new peptide-based urethane polymer: synthesis, biodegradation, and potential to support cell growth in vitro. Biomaterials. 2000;21(12):1247–58. doi: 10.1016/s0142-9612(00)00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan JJ, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of efastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. Journal of Biomedical Materials Research. 2002;61(3):493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 17.Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials. Biomaterials. 2005 Dec;26(35):7457–70. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 18.Gorna K, Gogolewski S. Biodegradable polyurethanes for implants. II. In vitro degradation and calcification of materials from poly(epsilon-caprolactone)-poly(ethylene oxide) diols and various chain extenders. Journal of Biomedical Materials Research. 2002;60(4):592–606. doi: 10.1002/jbm.10100. [DOI] [PubMed] [Google Scholar]
- 19.Gorna K, Gogolewski S. Preparation, degradation, and calcification of biodegradable polyurethane foams for bone graft substitutes. Journal of Biomedical Materials Research Part A. 2003;67A(3):813–27. doi: 10.1002/jbm.a.10148. [DOI] [PubMed] [Google Scholar]
- 20.Guelcher S, Srinivasan A, Hafeman A, Gallagher K, Doctor J, Khetan S, et al. Synthesis, In vitro degradation, and mechanical properties of two-component poly(ester urethane)urea scaffolds: Effects of water and polyol composition. Tissue Engineering. 2007;13(9):2321–33. doi: 10.1089/ten.2006.0395. [DOI] [PubMed] [Google Scholar]
- 21.Guelcher SA. Biodegradable polyurethanes: Synthesis and applications in regenerative medicine. Tissue Engineering Part B-Reviews. 2008;14(1):3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 22.Guelcher SA, Patel V, Gallagher KM, Connolly S, Didier JE, Doctor JS, et al. Synthesis and in vitro biocompatibility of injectable polyurethane foam scaffolds. Tissue Engineering. 2006;12(5):1247–59. doi: 10.1089/ten.2006.12.1247. [DOI] [PubMed] [Google Scholar]
- 23.Simmons A, Padsalgikar AD, Ferris LM, Poole-Warren LA. Biostability and biological performance of a PDMS-based polyurethane for controlled drug release. Biomaterials. 2008;29(20):2987–95. doi: 10.1016/j.biomaterials.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: A review. Journal of Pharmaceutical Sciences. 2008;97(8):2892–923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 25.Guan J, Stankus JJ, Wagner WR. Biodegradable elastomeric scaffolds with basic fibroblast growth factor release. Journal of Controlled Release. 2007;120(1–2):70–8. doi: 10.1016/j.jconrel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafeman AE, Zienkiewicz KJ, Davidson JM, Guelcher SA. Injectability of Biodegradable, Porous Polyurethane Scaffolds for Tissue Regeneration. TERMIS-NA 2008 Annual Conference; Abstract 2008. [Google Scholar]
- 27.Berkland C, Kipper MJ, Narasimhan B, Kim KK, Pack DW. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. Journal of Controlled Release. 2004;94(1):129–41. doi: 10.1016/j.jconrel.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Jeon O, Kang SW, Lim HW, Chung JH, Kim BS. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(L-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006;27(8):1598–607. doi: 10.1016/j.biomaterials.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 29.Lejeune KE, Mesiano AJ, Bower SB, Grimsley JK, Wild JR, Russell AJ. Dramatically stabilized phosphotriesterase-polymers for nerve agent degradation. Biotechnol Bioeng. 1997 Apr 20;54(2):105–14. doi: 10.1002/(SICI)1097-0290(19970420)54:2<105::AID-BIT2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Guan J, Wagner WR. Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules. 2005 Sep–Oct;6(5):2833–42. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taite LJ, Yang P, Jun HW, West JL. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J Biomed Mater Res B Appl Biomater. 2008 Jan;84(1):108–16. doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JY, Doll BA, Beckman EJ, Hollinger JO. Three-dimensional biocompatible ascorbic acid-containing scaffold for bone tissue engineering. Tissue Eng. 2003;9(6):1143–57. doi: 10.1089/10763270360728053. [DOI] [PubMed] [Google Scholar]
- 33.Park K. Controlled Drug Delivery: Challenges and Strategies. Springer; Netherlands: 1997. [Google Scholar]
- 34.Chung HJ, Kim HK, Yoon JJ, Park TG. Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery. Pharmaceutical Research. 2006;23(8):1835–41. doi: 10.1007/s11095-006-9039-9. [DOI] [PubMed] [Google Scholar]
- 35.Tatsuyama K, Maezawa Y, Baba H, Imamura Y, Fukuda M. Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. European Journal of Histochemistry. 2000;44(3):269–78. [PubMed] [Google Scholar]
- 36.Salthouse TN. Cellular enzyme activity at the polymer-tissue interface: a review. JBiomedMaterRes. 1976;10(2):197–229. doi: 10.1002/jbm.820100204. [DOI] [PubMed] [Google Scholar]
- 37.Santerre JP, Labow RS, Adams GA. Enzyme Biomaterial Interactions - Effect of Biosystems on Degradation of Polyurethanes. Journal of Biomedical Materials Research. 1993;27(1):97–109. doi: 10.1002/jbm.820270113. [DOI] [PubMed] [Google Scholar]
- 38.Labow RS, Duguay DG, Santerre JP. The Enzymatic-Hydrolysis of A Synthetic Biomembrane - A New Substrate for Cholesterol and Carboxyl Esterases. Journal of Biomaterials Science-Polymer Edition. 1994;6(2):169–79. doi: 10.1163/156856294x00293. [DOI] [PubMed] [Google Scholar]
- 39.Labow RS, Erfle DJ, Santerre JP. Neutrophil-Mediated Degradation of Segmented Polyurethanes. Biomaterials. 1995;16(1):51–9. doi: 10.1016/0142-9612(95)91096-h. [DOI] [PubMed] [Google Scholar]
- 40.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of Platelet-Derived Growth-Factor in Wound-Healing. Journal of Cellular Biochemistry. 1991;45(4):319–26. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 41.Chen G, Quinn LS. Partial Characterization of Skeletal Myoblast Mitogens in Mouse Crushed Muscle Extract. Journal of Cellular Physiology. 1992;153(3):563–74. doi: 10.1002/jcp.1041530318. [DOI] [PubMed] [Google Scholar]
- 42.Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. JCell Biol. 1989;109(1):429–40. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierce GF, Mustoe TA, Senior RM, Reed J, Griffin GL, Thomason A, et al. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. JExpMed. 1988;167(3):974–87. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]