Abstract
Mitochondria are central players in the pathophysiology of ischemia-reperfusion. Activation of plasma membrane G-coupled receptors or the Na, K-ATPase trigger cytosolic signaling pathways that result in cardioprotection. Our working hypothesis is that the occupied receptors migrate to caveolae, where signaling enzymes are scaffolded into signalosomes that bud off the plasma membrane and migrate to mitochondria. The signalosome-mitochondria interaction then initiates intramitochondrial signaling by opening the mitochondrial ATP-sensitive K+ channel (mitoKATP). MitoKATP opening causes an increase in ROS production, which activates mitochondrial protein kinase C epsilon (PKCε), which inhibits the mitochondrial permeability transition (MPT), thus decreasing cell death. We review the experimental findings that bear on these hypotheses and other modes of protection involving mitochondria.
Keywords: mitochondrial KATP channel, protein kinase C, reactive oxygen species, permeability transition, signaling pathways
1. Mitochondria : the target for ischemia-reperfusion injury and cardioprotection
Mitochondria are effectors of both ischemia-reperfusion injury (IRI) and cardioprotection. As pointed out 30 years ago by Jennings and Ganote [1], the heart is strictly aerobic and consequently extremely vulnerable to a decrease in oxygen supply. Thus, ischemia causes profound and immediate mitochondrial derangements. These include cessation of ATP synthesis, inhibition of respiration, and a drop in ΔΨ. This is accompanied during ischemia by cellular changes, especially an increase in Ca2+ and phosphate, and, during reperfusion, by large increases in reactive oxygen species (ROS) originating from the respiratory chain [2, 3]. Together, these factors promote opening of the mitochondrial permeability transition (MPT), a high-conductance pore in the inner mitochondrial membrane, which is the main cause of necrotic cell death in IRI [4–8]. Consequently, as pointed out by Weiss, et al. [4], cardioprotection by preconditioning or postconditioning must ultimately involve the prevention of MPT.
In addition to their role as mediators of cell death, mitochondria have been shown to be major effectors of diverse self-defense mechanisms, including ischemic pre- and post-conditioning [7, 9–11]. These and other conditioning protocols have been shown to require opening of the mitochondrial ATP-sensitive K+ channel (mitoKATP) and inhibition of MPT opening. Since cardioprotection involves both mitoKATP opening and a decrease in MPT opening, it is reasonable to hypothesize that these two phenomena are part of the same signaling pathway. Indeed, this connection has been demonstrated [12], and will be discussed in this review.
This review describes our current understanding of the signaling mechanisms that originate at plasma membrane receptors, go to mitochondria, and terminate with MPT inhibition. For space reasons, we have not discussed mechanisms for the prevention of apoptosis. For different perspectives, readers are referred to excellent reviews by other authors [13–18].
2. Receptor-mediated signaling to open mitoKATP
2.1 Gi-protein coupled receptor (GPCR) pathways
Ischemic preconditioning (IPC) and ischemic postconditioning are receptor-mediated processes that are triggered by GPCR agonists released by the ischemic heart, primarily bradykinin, opioid peptides, and adenosine [19]. Other GPCR ligands, including acetylcholine, catecholemines, endothelin, and angiotensin II, are also cardioprotective [20–24], but they were found not to be physiological triggers of IPC [14]. A composite diagram of the GPCR signaling pathways is given in Fig. 1. GPCR signaling has been extensively studied by Downey and Cohen and their coworkers, and is the subject of an excellent review by these authors [14]. It should be emphasized that each Gi-coupled receptor ligand triggers its own unique signaling cascade. Opioids and acetylcholine instigate transactivation of the epidermal growth factor receptor (EGFR), leading to downstream activation of phosphtidylinostitol 3-kinase (PI3-K) and Akt. Bradykinin also induces activation of PI3-K and Akt, but without transactivation of EGFR. These two pathways then converge and ultimately lead to mitoKATP opening and production of ROS. The adenosine signaling pathway has not yet been fully characterized. MitoKATP opening is not involved during the trigger phase of adenosine preconditioning (i.e., when 5-HD administration brackets adenosine perfusion) [25], but mitoKATP opening is required during the mediator phase (i.e., when 5-HD administration precedes ischemia) [26–29].
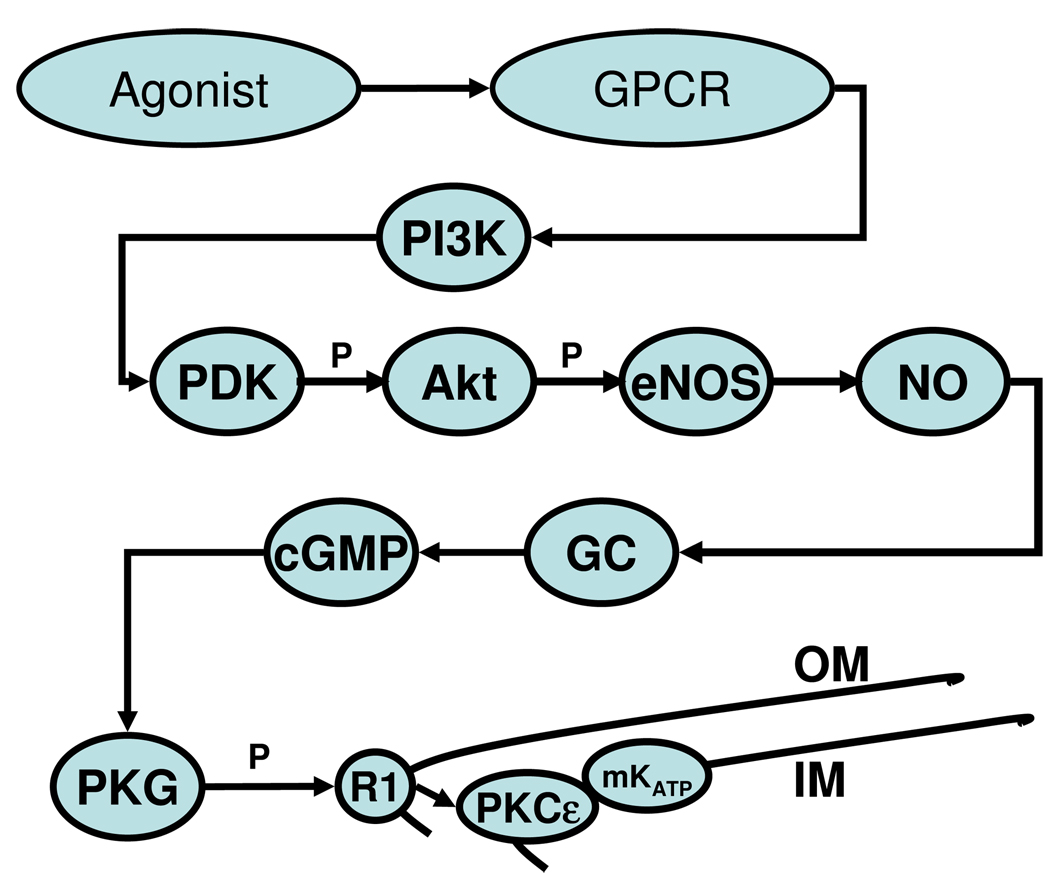
Figure 1. GPCR-mediated signaling to mitochondria.
Occupation of the GPCR leads to activation of PI3-kinase, phosphorylation of phosphatidylinositol bisphosphate, and activation of the phosphatidylinositol-dependent kinases (PDKs) [128]. PDKs then phosphorylate Akt, which initiates the remainder of the cytosolic signaling pathway: endothelial nitric oxide synthase (eNOS) is phosphorylated, leading to production of NO. NO stimulates guanylyl cyclase, and the cGMP produced activates protein kinase G (PKG) [129], which causes mitoKATP opening [62, 130–132].
Protection afforded by all of the trigger substances is blocked by PKC inhibitors, and PKC, probably PKCε, is thought to be a common target of cardioprotective signaling [14]. It has been difficult to localize the critical PKCε, because multiple PKCε isoforms participate in cardioprotection [30]. In ouabain signaling, PKCε acts proximally in conjunction with EGFR transactivation [31]. The adenosine A1 receptor is believed to directly stimulate PLC and PLD to activate PKC [21]. These PKCs are cytosolic. As discussed below, two PKCε isoforms regulate mitoKATP and MPT at the level of the inner mitochondrial membrane [32]. Thus, the physiological effect of PKCε activation depends entirely on its location and not on its biochemistry, which appears to be invariant.
2.2 Non-GPCR pathways of protection - digitalis
Cardiac glycosides are classic inhibitors of the plasma membrane Na+/K+-ATPase, but this enzyme also has important non-canonical functions that are triggered by digitalis. Thus, ouabain interaction with the Na, K-ATPase activates src kinase, causing formation of a ”binary receptor” that phosphorylates and assembles other proteins into signaling modules that transmit signals to intracellular compartments [33, 34]. Ouabain signaling has been shown to depend on mitoKATP opening and mitochondrial ROS production [35]. Ouabain is cardioprotective in rat heart [31, 36, 37], and this cardioprotection is blocked by the mitoKATP blocker 5-hydroxydecanoate (5-HD), the ROS scavenger N-2-mercaptopropionylglycine (MPG), and the src kinase inhibitor PP2 [36]. It is interesting to note that, whereas inhibition of the pump and consequent increase in intracellular Na+ and Ca2+ is required for positive inotropy, ouabain cardioprotection occurs at doses (about 10 µM in rat) that do not produce significant enzyme inhibition [37] or increased contractility [31, 36, 37]. These distinctions further emphasize the dissociation of the pumping and signaling functions of Na,K-ATPase. Ouabain cardioprotection does not depend on guanylyl cyclase or PKG activities, showing that this signaling pathway differs from that triggered by GPCR agonists [36]. Ouabain-induced inotropy also requires mitoKATP opening and ROS production [36, 38].
The rat heart Na,K-ATPase exhibits a low sensitivity to cardiac glycosides; however, we have observed qualitatively similar phenomena in the ouabain-sensitive rabbit heart. Thus, cardioprotection occurs at lower ouabain doses than those required for inotropy, and both cardioprotection and inotropy require mitoKATP opening (S. Pierre, unpublished data).
3. From receptor to mitochondria by signalosomes
We propose that cardioprotective signals are transmitted to mitochondria by signalosomes, which are vesicular, multimolecular signaling complexes that are assembled in caveolae and deliver signals to the mitochondrial outer membrane (MOM) [39]. A diagram of the signalosome hypothesis is presented in Fig. 2.
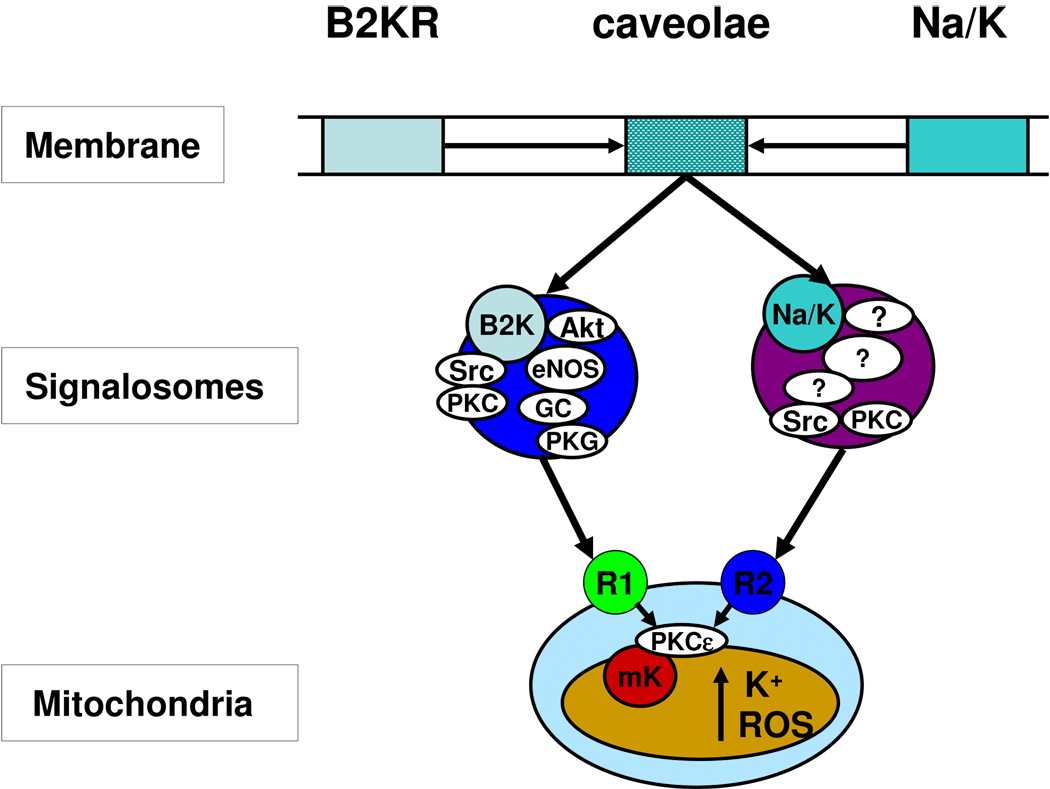
Figure 2. Signal transmission by signalosome.
It is proposed that interaction of bradykinin (and other GPCR agonists) or ouabain with their plasma membrane receptors induces formation of a vesicular caveolar signaling platform (signalosome) that phosphorylates receptor (R1 and R2) on the mitochondrial outer membrane (MOM). The terminal kinase of the bradykinin signalosome is PKG. The terminal kinases of the ouabain signalosome are PKCε and Src kinase. Following phosphorylation of the MOM receptor, the signal is transmitted across the intermembrane space to activate PKCε on the mitochondrial inner membrane. PKCε, in turn opens mitoKATP by a phosphorylation reaction [32].
3.1 Rationale
Signaling cascades such as the one portrayed in Fig. 1 must occur rapidly and precisely. We consider it unlikely that these spatio-temporal requirements can be met by random diffusional collisions. Cytosolic proteins are extensively hydrated, and the organization of this water causes a phase separation from bulk cytosolic water. Minimization of the phase boundary, in turn, causes proteins to coalesce within their common hydration phase [40]. If the proteins of the cardioprotective signaling pathway were randomly distributed in the cytosol, they too would coalesce within the hydration phase, with losses of directionality and specificity. Accordingly, we suggest that the signaling cascade is compartmentalized to promote metabolic channeling and that the entire reaction sequence moves through the cytosol as a unit. This hypothesis agrees with and extends the proposal by Ping and coworkers [41, 42] that intracellular signaling involves assembly and regulation of multiprotein complexes.
3.2 The signalosome hypothesis
Upon activation, GPCR migrate to caveolae, where caveolins organize and compartmentalize receptors and signaling molecules [43–49]. Caveolae are 50–100 nm membrane invaginations that are rich in cholesterol, sphingolipids, and caveolin proteins [50]. EGFR, the Src family of tyrosine kinases, G-protein α-subunits, PKC isoforms, and transporters such as the Na/K-ATPase have been found to associate with caveolins, which also regulate the activity of many of these proteins [50–56]. Caveolar assembly of the signaling platform is followed by budding off and internalization [57]. Receptor endocytosis has been observed for both GPCRs [45, 47, 58–60] and the α1-subunit of Na,K-ATPase [61]. We propose, therefore, that the receptor-specific signaling platform is assembled in caveolae, then separates and internalizes as a signalosome. The signalosome migrates via the cytoskeleton to mitochondria, where it binds to receptors on the MOM, designated in Fig. 2 as R1 (for GPCR-induced signalosomes) and R2 (for ouabain-induced signalosomes). The terminal kinase of the signalosome phosphorylates its specific receptor, which causes the signal to be transmitted across the MOM and intermembrane space to PKCε1 on the mitochondrial inner membrane. This is followed by the intramitochondrial signaling pathway described in Section 4 and Fig. 4.
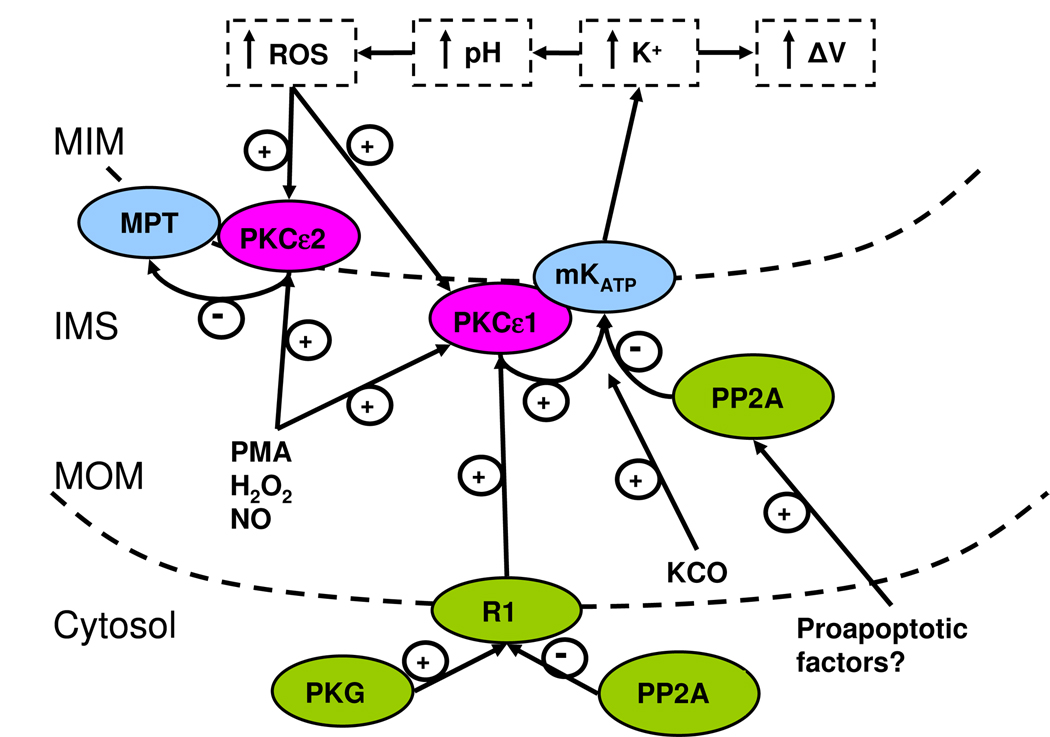
Figure 4. The intramitochondrial signaling pathways.
The pathways leading to mitoKATP opening, ROS production, and MPT inhibition are shown. Signals arising from Gi-coupled receptors are delivered to mitochondria via the terminal kinase PKG. Signals arising from ouabain action upon the Na-K ATPase are delivered to mitochondria via two terminal kinases, PKCε and Src. The terminal kinases are localized in the signalosomes. PKG phosphorylates an unknown MOM receptor, “R1”, whereas PKCε and Src act in conjunction to activate a distinct MOM receptor, “R2”. These MOM receptors transmit the signal by an unknown mechanism to PKCε1 located at the inner membrane. The activated PKCε1 phosphorylates and opens mitoKATP. PKCε1 activity is likely to be counteracted physiologically by Ser/Thr protein phosphatases (PPase) such as PP2A. MitoKATP opening via PKCε1 or by KATP channel openers such as diazoxide causes K+uptake, increased matrix pH, and increased H2O2 production from Complexl. H2O2 produced by mitoKATP activity now diffuses and activates both PKCε1 and PKCε2. PKCε2 inhibits MPT, thus reducing cell necrosis and infarct size. The figure is from Costa and Garlid [32].
3.3 Experimental evidence for signalosome-mediated mitoKATP opening
We developed protocols for purifying signalosomes from hearts subjected to various preconditioning or postconditioning protocols and then assayed their functional activity [39]. When the signalosome fractions were added to mitochondria from untreated hearts, they caused mitoKATP opening, as shown in Fig. 3. Based on the finding that functionally active signalosomes were obtained from hearts exposed to bradykinin, ouabain, ischemic preconditioning, and ischemic postconditioning (Fig. 3), we conclude that this is a general mechanism of signal transmission. Signalosome preparations also inhibited MPT when added to mitochondria from untreated hearts. The signalosomes were dissolved by the cholesterol binding agent methyl-β-cyclodextrin and were resistant to Triton X-100. These properties support their origin in caveolae. Electron microscopy reveals that the signalosomes are 100–140 nm in diameter and can be decorated with immunogold labeled caveolin 3 antibodies [39]. The signalosome induced by bradykinin stimulation contains eNOS, guanylyl cyclase, and cGMP-dependent protein kinase (PKG), and we were able to demonstrate the participation of each of these enzymes in the mitoKATP assay when proper substrates were supplied (Quinlan and Garlid, unpublished).
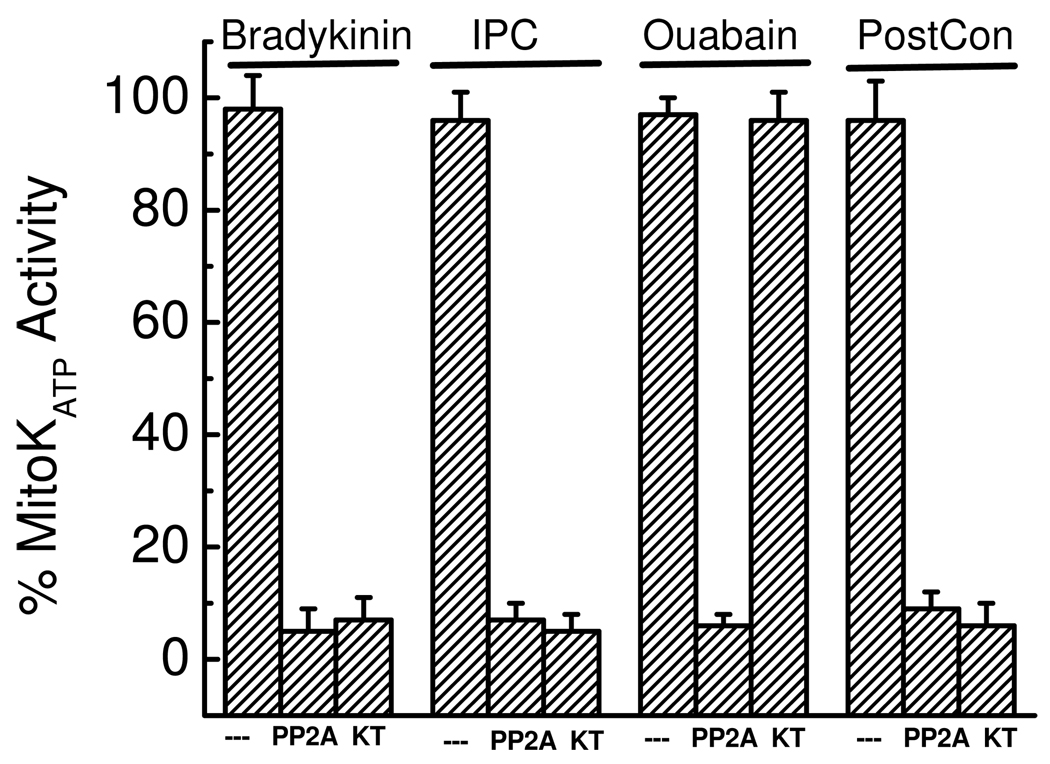
Figure 3. Signalosomes from treated hearts induce mitoKATP opening in mitochondria from untreated hearts.
Shown are changes in “MitoKATP activity (%)” when signalosome fractions from rat hearts treated with bradykinin, ouabain, ischemic preconditioning, or ischemic postconditioning were added to assay mitochondria from untreated rat hearts. Ischemic preconditioning consisted of two 5 min ischemia-reperfusion cycles, and ischemic postconditioning consisted of six 10 s cycles of ischemia-reperfusion. Also shown are the effects of PP2A (11 ng/mL) and KT5823 (“KT”, 0.5 µM) added to the assay. Note that PP2A cannot cross the MOM of intact mitochondria, so its effect is on the MOM. Data are averages ± SD of 3 to 4 independent experiments. The figure is from Quinlan, et al. [39].
3.4 Signalosomes phosphorylate MOM receptors
As shown in Fig. 3, addition of signalosomes from preconditioned hearts to mitochondria from non- preconditioned hearts results in activation of the mitoKATP. Activity of signalosomes induced by GPCR-mediated protection (bradykinin, IPC, and postconditioning) is inhibited by KT5823, and we conclude that GPCR signalosomes use PKG as the terminal kinase that interacts with mitochondria. In contrast, the activity of signalosomes induced by ouabain is not inhibited by KT5823, in agreement with the finding that KT5823 does not block protection by ouabain [36]. Activity of the ouabain signalosome is inhibited by preincubation with εV1–2 and PP2 (both are required), indicating that the terminal kinases are PKCε and Src kinase. We have found that recombinant PKG [32, 62] or recombinant PKCε plus Src (Quinlan and Garlid, unpublished) can also induce mitoKATP opening in isolated mitochondria.
That the signalosomes interact with the MOM was demonstrated by the finding that neither the signalosomes nor the recombinant terminal kinases induce mitoKATP opening in mitoplasts lacking the MOM [32, 39]. That signalosomes interact by phosphorylation was demonstrated by the finding that activity was blocked in the presense of the Ser/Thr phosphatase PP2A (Fig. 3). The ouabain signalosome was also blocked by tyrosine phosphatase, confirming the action of its Src kinase.
We have not yet determined the molecular identity of R1. R2 is an endogenous MOM MAP kinase, as revealed by Western blot showing increased phosphorylation of p38 MAPK (Thr 180/Tyr 182) after the heart was treated with ouabain and functional studies showing that the MAP kinase inhibitor SB203580 blocked mitoKATP opening by the ouabain signalosome (Quinlan and Garlid, unpublished studies).
3.5 Signal transmission from MOM to PKCε1
Signalosome-dependent mitoKATP opening is also blocked by the PKCε inhibitors chererythrine and εV1–2 [62], confirming a role for PKCε (“PKCε1” in Fig. 2), which is discussed in the next section. Signaling from R1 or R2 to PKCε1 is not prevented by MPG, and therefore this step does not involve ROS. This is all we know at this stage about the nature of the link between the MOM receptors and PKCε1.
4. Intramitochondrial signaling
The diagram in Fig. 4 summarizes several years of studies on intramitochondrial signaling [12, 32, 39, 62–66]. The primary function of this pathway is to inhibit MPT opening, which is widely considered to be the cause of cell death after ischemiareperfusion [5, 6, 11].
4.1 Step one - opening mitoKATP by activation of PKCε1
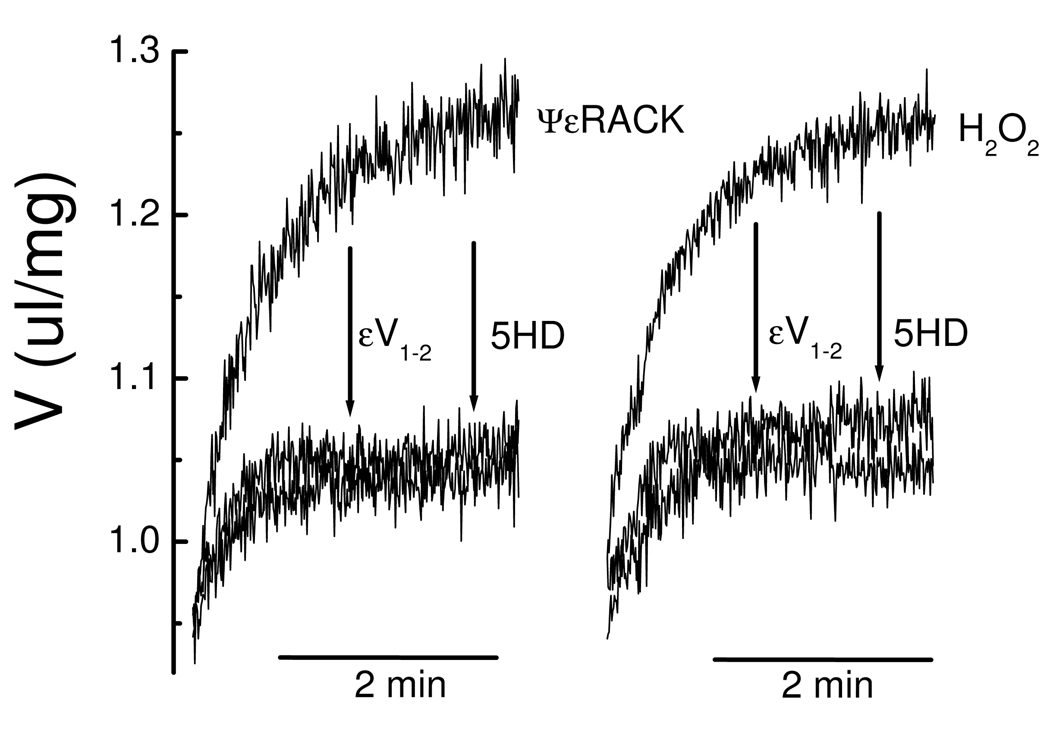
Ischemic preconditioning, ischemic postconditioning, and pharmacological preconditioning by plasma membrane receptor agonists cause mitoKATP opening by activating a PKCε that is constitutively expressed in mitochondria and associated with the mitochondrial inner membrane [64]. The PKCε-specific peptide agonist ΨεRACK and the PKCε agonists H2O2, NO, and phorbol 12-myristate 13-acetate (PMA) each open mitoKATP (see Fig. 5) [32]. That these agents were acting via PKCε (“PKCε1” in Fig. 4) was verified by showing that the PKCε-specific binding antagonist εV1–2 blocked all four modes of PKCε activation of mitoKATP but did not block mitoKATP opening by diazoxide [32, 64]. Neither the PKCε-specific peptide antagonist, δV1–1 nor a scrambled analog of εV1–2 had any effect on H2O2-dependent mitoKATP opening. Moreover, the PKC inhibitor Gö6983, which inhibits PKCα, PKCβ, PKCγ, and PKCζ, did not block PKCε-dependent mitoKATP opening, excluding a role for these isoforms [62].
Figure 5. PKCε–mediated mitoKATP opening.
Changes in mitochondrial matrix volume (V) are plotted versus time. Rat heart mitochondria (0.1 mg/ml) were suspended in standard assay medium [32]. H2O2 (2 µM) or ΨεRACK (0.5 µM) were added to medium in the presence of ATP (0.2 mM) approximately one second after the mitochondria. Other additions to the assay medium were 5-HD (0.3 mM) and εV1–2 (0.5 µM). Traces are representative of at least 5 independent experiments. The figure is from Costa and Garlid [32].
Jaburek, et al. [64] showed that ΨεRACK and εV1–2 open mitoKATP in liposomes reconstituted with partially purified mitoKATP. Thus, mitoKATP and PKCε copurify and remain associated during multiple purification steps carried out in the presence of Triton X-100. This, together with the finding that PKCε remains associated with mitochondria in mitoplasts [12], suggests that PKCε and mitoKATP are part of a functional complex. When given access in mitoplasts to the mitochondrial inner membrane (“MIM”), PP2A prevented mitoKATP-dependent swelling induced by PKCε agonists [32], and we conclude that PKCε-dependent mitoKATP opening requires phosphorylation, perhaps of mitoKATP itself.
PKCε requires anionic phospholipids for activity, a requirement met in this case by cardiolipin, which is abundant in mitochondria. PKCε is activated physiologically by diacylglycerol (or phorbol ester) or by a sulfydryl oxidizing agent, such as H2O2 [67] or NO [32]. PMA or H2O2 open up one of the two zinc fingers in PKCε [68, 69]. ΨεRACK, PMA, H2O2, or NO cause conformational changes that expose the substrate domain on PKCε and cause its binding to its RACK (receptor for activated C kinase [70]). ΨεRACK is a PKCε-specific peptide agonist that acts by regulating intramolecular PKCε binding, and εV1–2 is a PKCε-specific peptide antagonist that acts by preventing protein-protein interactions between PKCε and its binding protein [70–72]. Murriel and Mochly-Rosen [73] found that ΨεRACK protected cardiac cells from ischemic damage, whereas εV1–2 caused a loss of protection.
4.2 Step two - mitochondrial K+ uptake and its consequences
Once mitoKATP is opened, the increase in K+ uptake leads to several changes in the matrix. Electrophoretic K+ influx is balanced by electrogenic H+ efflux driven by the respiratory chain. Uncompensated, this would cause an increase of matrix pH of about 2 pH units. Partial compensation is provided by electroneutral uptake of substrate anions, such as phosphate. The compensation is partial because the concentration of phosphate in the cytosol is much lower than that of K+, and this imbalance leads to matrix alkalinization [65, 66, 74].
Matrix alkalinization now releases the K+/H+ antiporter from inhibition by matrix protons [75], causing K+ efflux to increase in response to increased K+ uptake until a new K+ steady state is achieved. The uncoupling caused by increased futile cycling of K+ induces is about 3% of the maximum activity of the electron transport chain. This low level of uncoupling reflects the low activity of mitoKATP, a property that is essential for mitochondrial survival. Thus, if we add sufficient valinomycin to double the mitoKATP-mediated K+ influx, the MOM ruptures with loss of cytochrome c[65].
Uptake of K+ salts and osmotically obligated water leads to increased matrix volume (“ΔV” in Fig. 4), which is the basis of the light scattering (LS) assay for mitoKATP activity [65]. LS is the only practical method to study this process in isolated mitochondria, because mitoKATP-dependent K+ flux is rapid (t1/2 ~ 30 s) and small in magnitude. This technique has been successfully employed by several laboratories to measure K+ flux in mitochondria [76–80].
MitoKATP-dependent matrix alkalinization plays an essential role in intramitochondrial signaling. It causes Complex I to produce increased amounts of superoxide and its products, H2O2 and hydroxyl anion radical [66]. As seen below, the ROS produced by this mechanism play two important roles in cardioprotection, through their ability to activate PKCε.
We note that each of the consequences of mitoKATP opening are due specifically to the increased K+ influx. Thus, valinomycin (approximately 1 pmol/mg mitochondria) duplicates the effects of KCO on K+ uptake, respiration, matrix alkalinization, volume increase, and ROS production [65, 66].
4.3 Step three - activation of PKCε by endogenous ROS
4.3.1 ROS activation of PKCε2 and inhibition of MPT
The increased ROS activates a second mitochondrial PKCε, “PKCε2” in Fig. 4, which inhibits the mitochondrial permeability transition (“MPT”) in a phosphorylation-dependent reaction [12]. H2O2 and NO, but not superoxide, also activate PKCε2 and inhibit MPT [12, 32]. The dichotomy between protective and damaging ROS was strikingly demonstrated in an experiment in which 100 µM H2O2 plus 2 µM free Ca2+ were used to induce MPT opening in heart mitochondria. This ROS-induced MPT opening was inhibited when the mitochondria were first conditioned with 2 µM H2O2 [12]. Thus, cardioprotective mitoKATP opening leads to inhibition of MPT, and, therefore, to reduction of cell death after ischemia-reperfusion injury [5, 6, 11].
The evidence for two distinct mitochondrial PKCε is that the specific agonist ΨεRACK can activate PKCε1 and open mitoKATP, but it cannot activate the PKCε that regulates MPT [12]. This establishes a clear difference between the two PKCεs. Our tentative explanation for the observation is that PKCε2 faces the matrix side of the inner membrane. ΨεRACK is an anionic peptide that cannot enter the matrix, whereas εV1–2, which inhibits both PKCε1 and PKCε2, can readily enter the matrix.
4.3.2 ROS activation of PKCε1 and feedback mitoKATP opening
The mitoKATP-dependent increase in ROS plays an additional role in cardioprotection. Note in Fig. 4 that PKCε1 is bypassed when KCOs are administered to the heart; however, we have found that PKCε1 is soon activated by mitoKATP-dependent ROS, leading to a persistent phosphorylation-dependent open state of mitoKATP [32]. These data define a new, positive feedback loop for mitoKATP opening, whose existence, which has been suggested by a number of authors [81–83], means that mitoKATP may be either upstream or downstream of PKCε, depending on the triggering stimulus. We suggest that feedback phosphorylation of mitoKATP is the mechanism of memory, which is seen with all PC triggers [84, 85]. Thus, cardioprotective stimuli can be washed away from the system and the perfused heart remains protected from a major ischemic assault, thanks to phosphorylation of mitoKATP. We infer, but have not demonstrated, that mitoKATP opening is eventually reversed by an endogenous phosphatase (“PP2A” in Fig. 4) within the intermembrane space. For example, PP2A has been found in mitochondria where it is activated by proapoptotic factors [86].
4.4 Intramitochondrial signaling and the literature
The model in Fig. 4 helps to support and extend results of previous studies. Jiang et al [87] observed PKC and 5-HD regulation of the human cardiac mitoKATP in lipid bilayers. Garg and Hu [88] showed that PKCε modulates mitoKATP activity in cardiomyocytes and COS-7 cells. Penna et al [89] demonstrated that protection by postconditioning protection involves a redox mechanism and persistent activation of mitoKATP and PKC. Facundo et al. [80] showed that H2O2 induces mitoKATP activity in isolated mitochondria, but did not identify participation of PKCε. Zhang et al. [90] found that superoxide anion activated mitoKATP in planar bilayers, and we showed that this effect is mediated, not by superoxide, but by H2O2 acting indirectly via PKCε1 [32]. Sasaki, et al. [91] suggested that NO may open mitoKATP directly; however mitoKATP opening by NO is blocked by εV1–2 [32], showing that NO opens mitoKATP indirectly through PKCε1. Several authors have shown that exogenous and endogenous NO are cardioprotective and have attributed this effect to MPT inhibition [92–95]. Brookes, et al. [92] showed that NO inhibited MPT and cytochrome c release in isolated liver mitochondria. We showed that this effect occurs via activation of PKCε2 [32]. Korge, et al. [96] found that diazoxide prevented MPT opening and cytochrome c loss, and that both effects were mimicked by the PKC activator PMA and blocked by 5-HD. Kim, et al. [97] found that a cytosolic extract, together with cGMP and ATP, blocked MPT in isolated mitochondria, an effect that was blocked by PKG inhibition. Forbes, et al [98] and Pain, et al [85] found that N-acetylcysteine or MPG reversed the protective effect of diazoxide in perfused hearts. Our data suggests that blockade of protection occurred because mitoKATP-dependent ROS was scavenged and unavailable to activate PKCε2 and inhibit MPT. Lebuffe, e al [81] found that PMA-induced protection was blocked by 5-HD and that this block was reversed by coadministration of H2O2 and NO. This is also consistent with the model of Fig. 4 in that H2O2 and NO can bypass the blocked mitoKATP and act directly on PKCε2, thereby inhibiting MPT and protecting the heart.
5. Other mitochondrial mechanisms of cardioprotection
5.1 KATP channel openers (KCO)
KCOs have been shown to be cardioprotective in all species examined [99, 100]. The ability of KCOs to open mitoKATP in their therapeutic dose range was described in 1996 [101]. Diazoxide was 1000 times more potent in opening mitoKATP than in opening sarcKATP, making diazoxide a valuable tool to determine whether cardioprotection was mediated by the sarcolemmal or the mitochondrial KATP channel. It was found that diazoxide was as effective as cromakalim in protecting the heart. Moreover, diazoxide protection, unlike that mediated by cromakalim, was not accompanied by APD shortening, thus demonstrating that cardioprotection was not due to sarcKATP opening. These findings led to the hypothesis that mitoKATP is the receptor that mediates the cardioprotective effects of KCOs [101, 102]. KCOs act on the regulatory sulfonylurea receptors (SUR) of KATP channels. Pinacidil, cromakalim, and nicorandil are effective openers of cardiac KATP through their action on SUR2A, but ineffective on pancreatic beta cell KATP, which uses SUR1. Conversely, diazoxide is an effective opener of beta cell KATP, but ineffective on the cardiac channel [101, 103]. All KCOs we have examined open mitoKATP and protect the heart [65, 101, 102, 104–108].
5.2 glycogen synthase kinase -3β (GSK-3β)
The GSK-3β inhibitors lithium and SB 216763 are cardioprotective. IPC and diazoxide cause phosphorylation and inactivation of GSK-3β [83, 109], suggesting that GSK-3β may be downstream of mitoKATP. Inhibition of GSK-3β has no effect on MPT opening in isolated mitochondria [32], suggesting that the GSK isoform that interferes with cardioprotection resides outside of mitochondria. Importantly, cardioprotection by GSK-3β inhibition is blocked by 5-HD [110], indicating that the ultimate deleterious effect of GSK-3β activity may be to cause mitoKATP inhibition.
5.3 Amobarbital
Amobarbital, administered 1 min before 25 min global ischemia, is cardioprotective in rat, causing marked improvement of contractile function and reduction of infarct size [111]. Amobarbital is a short-acting barbiturate that is a classic, reversible inhibitor of Complex I at the rotenone site. Amobarbital treatment was associated with preservation of cytochrome c [111], which is otherwise released after ischemia-reperfusion, due in part to oxidative degradation of cardiolipin [112]. Cardioprotection by amobarbital is consistent with the authors’ overall hypothesis that ROS arising from Complex III during ischemia causes mitochondrial damage that contributes to myocardial injury during reperfusion [113].
5.4 Bromoenol lactone (BEL)
BEL, administered before global ischemia, is cardioprotective in rat [114] and rabbit [115], causing marked reduction of infarct size. BEL is a specific inhibitor of calcium-independent phospholipase A(2) (iPLA2), which is the major phospholipase A(2) in myocardium and is present in heart mitochondria [115]. Ischemia causes fatty acid accumulation in the heart, caused by phospholipases-mediated degradation of membrane phospholipids [112, 116, 117]. Protection by BEL was reversed, in both rat and rabbit hearts, by the simultaneous perfusion of 5-HD, implying participation of mitoKATP.
5.5 Hydrogen sulfide
H2S, administered before global ischemia, is cardioprotective in rat [118–120]. H2S is synthesized in the heart and other tissues by cystathionine λ -lyase. Cytoplasmic [H2S] is determined by the balance between its constitutive production and its oxidation by mitochondria. When tissue oxygen levels fall, H2S oxidation decreases, and [H2S] increases, and Olson, et al. [121] consider H2S to be the oxygen sensor of cells. Protection by H2S was abolished by chelerythrine, implicating participation of PKC [119]. Infarct size reduction by H2S was also abolished by glibenclamide and 5-HD, implicating participation of mitoKATP in H2S protection [120]. H2S increased the open probability of sarcolemmal KATP in cardiomyocytes [122] and may have a similar effect on mitoKATP.
5.6 Mitochondrial aldehyde dehydrogenase (ALDH2)
An activator of ALDH2, administered before global ischemia, is cardioprotective in rat, causing marked reduction of infarct size [123]. This recent discovery was the result of a directed proteomic search. The authors speculate that protection by ALDH2 activation is due to its metabolism of cytotoxic aldehydes, such as 4-hydroxynonenol.
5.7 Ca2+-activated mitochondrial K+ channel (mitoKCa)
NS1619, an activator of the large conductance mitoKCa is cardioprotective in guinea pig, and protection is blocked by the inhibitor paxilline [124–126]. Sato, et al [127] found that there was no cross-talk between mitoKATP and mitoKCa — that is, paxilline blocked effects of NS1619 but not diazoxide, and 5-HD blocked effects of diazoxide but not NS1619. Cao et al. [125] observed similar absence of cross-talk in cardioprotection experiments. The latter findings suggest distinct channels with distinct pharmacology and suggest that these two channels constitute alternative mechanisms for raising matrix K+ and generating ROS.
6. Summary
Recent years have brought robust advances in our understanding of cardiac signal transduction during cardioprotection against ischemia-reperfusion and the pivotal role of mitochondria in these processes. This review exposes our current understanding of the mechanistic link between plasma membrane receptors and MPT, the ultimate mitochondrial target of cardioprotection. We suggest that interaction of the cardioprotective ligand with its receptor induces the formation of a signaling platform that is scaffolded by caveolins, that contains the activated enzymes of the pathway, and that is delivered to mitochondria as a signalosome. We believe that the signalosome mechanism [39] provides a means to resolve the mystery of receptor-specificity described by Downey, et al. [14]: “It is still a mystery how identical Gi proteins, when activated by binding of the different agonists to their individual receptors, can initiate such distinct signaling pathways.”
This review also points to several areas that require further investigation as to how signalosomes are directed to mitochondria, how cytoskeleton is involved, and what receptors are involved at the mitochondrial level. Even in light of these questions, the elucidation of the interactions among signaling components and mitochondria is a valuable tool for understanding the molecular controls in the decision between cell survival and cell death.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jennings RB, Ganote CE. Mitochondrial structure and function in acute myocardial ischemic injury. Circ Res. 1976;38:I80–I91. [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 5.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 6.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 8.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. Epub 2006 Feb 23. [DOI] [PubMed] [Google Scholar]
- 9.Argaud L, Loufouat J, Gateau-Roesch O, Gomez L, Robert D, Ovize M. Persistent Inhibition of Mitochondrial Permeability Transition by Preconditioning During the First Hours of Reperfusion. Shock. 2008;28:28. doi: 10.1097/SHK.0b013e31816a1c1c. [DOI] [PubMed] [Google Scholar]
- 10.Gateau-Roesch O, Argaud L, Ovize M. Mitochondrial permeability transition pore and postconditioning. Cardiovasc Res. 2006;70:264–273. doi: 10.1016/j.cardiores.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. Epub 2005 Jan 10. [DOI] [PubMed] [Google Scholar]
- 12.Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which mitoKATP opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- 13.Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Cepsilon (PKCepsilon): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007;35:1052–1054. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- 14.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 15.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 16.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70:212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Liem DA, Honda HM, Zhang J, Woo D, Ping P. Past and present course of cardioprotection against ischemia-reperfusion injury. J Appl Physiol. 2007;103:2129–2136. doi: 10.1152/japplphysiol.00383.2007. Epub 2007 Aug 2. [DOI] [PubMed] [Google Scholar]
- 18.Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail Rev. 2007;12:249–260. doi: 10.1007/s10741-007-9028-z. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MV, Downey JM. Myocardial preconditioning promises to be a novel approach to the treatment of ischemic heart disease. Annual Review Of Medicine. 1996;47:21–29. doi: 10.1146/annurev.med.47.1.21. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee A, Locke-Winter C, Rogers KB, Mitchell MB, Brew EC, Cairns CB, Bensard DD, Harken AH. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circulation Research. 1993;73:656–670. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Tsuchida A, Cohen MV, Downey JM. Pretreatment with angiotensin II activates protein kinase C and limits myocardial infarction in isolated rabbit hearts. J Mol Cell Cardiol. 1995;27:883–892. doi: 10.1016/0022-2828(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Gallagher KP, Downey JM, Cohen MV. Pretreatment with endothelin-1 mimics ischemic preconditioning against infarction in isolated rabbit heart. J Mol Cell Cardiol. 1996;28:579–588. doi: 10.1006/jmcc.1996.0054. [DOI] [PubMed] [Google Scholar]
- 23.Krieg T, Cui L, Qin Q, Cohen MV, Downey JM. Mitochondrial ROS generation following acetylcholine-induced EGF receptor transactivation requires metalloproteinase cleavage of proHB-EGF. J Mol Cell Cardiol. 2004;36:435–443. doi: 10.1016/j.yjmcc.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Krieg T, Qin Q, McIntosh EC, Cohen MV, Downey JM. ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol. 2002;283:H2322–H2330. doi: 10.1152/ajpheart.00474.2002. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. Circ Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- 26.Van Winkle DM, Chien GL, Wolff RA, Soifer BE, Kuzume K, Davis RF. Cardioprotection provided by adenosine receptor activation is abolished by blockade of the KATP channel. Am J Physiol. 1994;266:H829–H839. doi: 10.1152/ajpheart.1994.266.2.H829. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Sasaki N, O'Rourke B, Marban E. Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: A key step in ischemic preconditioning? Circulation. 2000;102:800–805. doi: 10.1161/01.cir.102.7.800. [DOI] [PubMed] [Google Scholar]
- 28.Reshef A, Sperling O, Zoref-Shani E. Opening of KATP channels is mandatory for acquisition of ischemic tolerance by adenosine. Neuroreport. 2000;11:463–465. doi: 10.1097/00001756-200002280-00007. [DOI] [PubMed] [Google Scholar]
- 29.Headrick JP, Gauthier NS, Morrison R, Matherne GP. Cardioprotection by KATP channels in wild-type hearts and hearts overexpressing A(1)-adenosine receptors. Am J Physiol. 2000;279:H1690–H1697. doi: 10.1152/ajpheart.2000.279.4.H1690. [DOI] [PubMed] [Google Scholar]
- 30.Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP Signaling in Pre- and Postconditioining: The Role of Mitochondria. Cardiovasc Res. 2008;77:344–352. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 31.Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas C, Garlid KD, Dos Santos P, Xie Z. Ouabain triggers preconditioning through activation of the Na,k-ATPase signaling cascade in rat hearts. Cardiovasc Res. 2007;73:488–496. doi: 10.1016/j.cardiores.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa ADT, Garlid KD. Intramitochondrial signaling: interactions among mitoKATPPKCε, ROS, and MPT. Am J Physiol. 2008;295:H874–H882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 34.Pierre SV, Xie Z. The Na,K-ATPase Receptor Complex: Its Organization and Membership. Cell Biochem Biophys. 2006;46:303–316. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- 35.Tian J, Liu J, Garlid KD, Shapiro JI, Xie Z. Involvement of Mitogen-activated Protein Kinases and Reactive Oxygen Species in the Inotropic Action of Ouabain on Cardiac Myocytes. A Potential Role for Mitochondrial KATP Channels. Mol Cell Biochem. 2003;242:181–187. [PubMed] [Google Scholar]
- 36.Pasdois PP, Quinlan CL, Rissa A, Tariosse L, Vinassa B, Pierre SV, Dos Santos P, Garlid KD. Ouabain protects rat hearts against ischemia-reperfusion injury via a pathway involving src kinase, mitoKATP, and ROS. Am J Physiol. 2007;292:H1470–H1478. doi: 10.1152/ajpheart.00877.2006. [DOI] [PubMed] [Google Scholar]
- 37.D'Urso G, Frascarelli S, Zucchi R, Biver T, Montali U. Cardioprotection by Ouabain and Digoxin in Perfused Rat Hearts. J Cardiovasc Pharmacol. 2008;10:10. doi: 10.1097/FJC.0b013e3181884448. [DOI] [PubMed] [Google Scholar]
- 38.Garlid KD, Puddu PE, Pasdois P, ADT Costa, Beauvoit B, Criniti A, Tariosse L, Diolez P, Dos Santos P. Inhibition of cardiac contractility by 5-hydroxydecanoate and tetraphenylphosphonium ion: a possible role of mitoKATP in the response to inotropic stress. Am J Physiol. 2006;291:H152–H160. doi: 10.1152/ajpheart.01233.2005. [DOI] [PubMed] [Google Scholar]
- 39.Quinlan CL, ADT Costa, Costa CL, Pierre SV, Dos Santos P, Garlid KD. Conditioning the Heart Induces Formation of Signalosomes that Interact with Mitochondria to Open MitoKATP. Amer J Phys. 2008;295:H953–961. doi: 10.1152/ajpheart.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garlid KD. The state of water in biological systems. Int Rev Cytol. 2000;192:281–302. doi: 10.1016/s0074-7696(08)60530-6. [DOI] [PubMed] [Google Scholar]
- 41.Ping P, Zhang J, Pierce WM, Jr, Bolli R. Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 42.Vondriska TM, Pass JM, Ping P. Scaffold proteins and assembly of multiprotein signaling complexes. J Mol Cell Cardiol. 2004;37:391–397. doi: 10.1016/j.yjmcc.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 44.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 45.Prabhakar P, Thatte HS, Goetz RM, Cho MR, Golan DE, Michel T. Receptor-regulated translocation of endothelial nitric-oxide synthase. J Biol Chem. 1998;273:27383–27388. doi: 10.1074/jbc.273.42.27383. [DOI] [PubMed] [Google Scholar]
- 46.Escriche M, Burgueno J, Ciruela F, Canela EI, Mallol J, Enrich C, Lluis C, Franco R. Ligand-induced caveolae-mediated internalization of A1 adenosine receptors: morphological evidence of endosomal sorting and receptor recycling. Exp Cell Res. 2003;285:72–90. doi: 10.1016/s0014-4827(02)00090-3. [DOI] [PubMed] [Google Scholar]
- 47.Maniatis NA, Brovkovych V, Allen SE, John TA, Shajahan AN, Tiruppathi C, Vogel SM, Skidgel RA, Malik AB, Minshall RD. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ Res. 2006;99:870–877. doi: 10.1161/01.RES.0000245187.08026.47. [DOI] [PubMed] [Google Scholar]
- 48.Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta opioid receptors. Am J Physiol. 2006;291:H344–H350. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 49.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. Faseb J. 2007. In press. [DOI] [PubMed]
- 50.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 53.Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 54.Trincavelli ML, Tuscano D, Marroni M, Klotz KN, Lucacchini A, Martini C. Involvement of mitogen protein kinase cascade in agonist-mediated human A(3) adenosine receptor regulation. Biochim Biophys Acta. 2002;1591:55–62. doi: 10.1016/s0167-4889(02)00248-3. [DOI] [PubMed] [Google Scholar]
- 55.Franco R, Ciruela F, Casado V, Cortes A, Canela EI, Mallol J, Agnati LF, Ferre S, Fuxe K, Lluis C. Partners for adenosine A1 receptors. J Mol Neurosci. 2005;26:221–232. doi: 10.1385/JMN:26:2-3:221. [DOI] [PubMed] [Google Scholar]
- 56.Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res. 2004;94:1133–1141. doi: 10.1161/01.RES.0000126048.32383.6B. [DOI] [PubMed] [Google Scholar]
- 57.Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 58.Haasemann M, Cartaud J, Muller-Esterl W, Dunia I. Agonist-induced redistribution of bradykinin B2 receptor in caveolae. J Cell Sci. 1998;111:917–928. doi: 10.1242/jcs.111.7.917. [DOI] [PubMed] [Google Scholar]
- 59.Pizard A, Blaukat A, Muller-Esterl W, Alhenc-Gelas F, Rajerison RM. Bradykinin-induced internalization of the human B2 receptor requires phosphorylation of three serine and two threonine residues at its carboxyl tail. J Biol Chem. 1999;274:12738–12747. doi: 10.1074/jbc.274.18.12738. [DOI] [PubMed] [Google Scholar]
- 60.Trincavelli ML, Tuscano D, Cecchetti P, Falleni A, Benzi L, Klotz KN, Gremigni V, Cattabeni F, Lucacchini A, Martini C. Agonist-induced internalization and recycling of the human A(3) adenosine receptors: role in receptor desensitization and resensitization. J Neurochem. 2000;75:1493–1501. doi: 10.1046/j.1471-4159.2000.0751493.x. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 62.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein Kinase G Transmits the Cardioprotective Signal From Cytosol to Mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 63.Garlid KD. Cation transport in mitochondria--the potassium cycle. Biochim Biophys Acta. 1996;1275:123–126. doi: 10.1016/0005-2728(96)00061-8. [DOI] [PubMed] [Google Scholar]
- 64.Jaburek M, ADT Costa, Burton JR, Costa CL, Garlid KD. Mitochondrial PKCepsilon and mitoKATP co-purify and co-reconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 65.Costa ADT, Quinlan C, Andrukhiv A, West IC, Garlid KD. The direct physiological effects of mitoKATP opening on heart mitochondria. Am J Physiol. 2006;290:H406–H415. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 66.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from Complex I of the electron transport chain. Am J Physiol. 2006;291:H2067–H2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 67.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kazanietz MG, Bustelo XR, Barbacid M, Kolch W, Mischak H, Wong G, Pettit GR, Bruns JD, Blumberg PM. Zinc finger domains and phorbol ester pharmacophore. Analysis of binding to mutated form of protein kinase C zeta and the vav and c-raf proto-oncogene products. J Biol Chem. 1994;269:11590–11594. [PubMed] [Google Scholar]
- 69.Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277:44327–44331. doi: 10.1074/jbc.M205634200. [DOI] [PubMed] [Google Scholar]
- 70.Ron D, Mochly-Rosen D. An autoregulatory region in protein kinase C: the pseudoanchoring site. Proc Natl Acad Sci U S A. 1995;92:492–496. doi: 10.1073/pnas.92.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- 72.Souroujon MC, Mochly-Rosen D. Peptide modulators of protein-protein interactions in intracellular signaling. Nat Biotechnol. 1998;16:919–924. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 73.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 74.Garlid KD, Paucek P. Mitochondrial potassium transport: the K+ cycle. Biochim Biophys Acta. 2003;1606:23–41. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 75.Beavis AD, Garlid KD. Evidence for the allosteric regulation of the mitochondrial K+/H+ antiporter by matrix protons. J Biol Chem. 1990;265:2538–2545. [PubMed] [Google Scholar]
- 76.Pastore D, Stoppelli MC, Di Fonzo N, Passarella S. The existence of the K(+) channel in plant mitochondria. J Biol Chem. 1999;274:26683–26690. doi: 10.1074/jbc.274.38.26683. [DOI] [PubMed] [Google Scholar]
- 77.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Opening of mitochondrial KATP channels enhances cardioprotection through the modulation of mitochondrial matrix volume, calcium accumulation, and respiration. Am J Physiol. 2004;287:H1967–H1976. doi: 10.1152/ajpheart.00338.2004. Epub 2004 Jul 8. [DOI] [PubMed] [Google Scholar]
- 78.Riess ML, Costa AD, Carlson R, Jr, Garlid KD, Heinen A, Stowe DF. Differential increase of mitochondrial matrix volume by sevoflurane in isolated cardiac mitochondria. Anesth Analg. 2008;106:1049–1055. doi: 10.1213/ane.0b013e318167875e. [DOI] [PubMed] [Google Scholar]
- 79.Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: Implications for ischemic preconditioning. Biochim Biophys Acta. 2008. [DOI] [PMC free article] [PubMed]
- 80.Facundo HT, de Paula JG, Kowaltowski AJ. Mitochondrial ATP-sensitive K(+) channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med. 2007;42:1039–1048. doi: 10.1016/j.freeradbiomed.2007.01.001. Epub 2007 Jan 8. [DOI] [PubMed] [Google Scholar]
- 81.Lebuffe G, Schumacker PT, Shao ZH, Anderson T, Iwase H, Vanden Hoek TL. ROS and NO trigger early preconditioning: relationship to mitochondrial KATPchannel. Am J Physiol. 2003;284:H299–H308. doi: 10.1152/ajpheart.00706.2002. [DOI] [PubMed] [Google Scholar]
- 82.Kevin LG, Camara AKS, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol. 2003;284:H566–574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 83.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Downey JM, Cohen MV. Signal transduction in ischemic preconditioning. Adv Exp Med Biol. 1997;430:39–55. doi: 10.1007/978-1-4615-5959-7_4. [DOI] [PubMed] [Google Scholar]
- 85.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, MV Cohen, Downey JM. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 86.Klumpp S, Krieglstein J. Serine/threonine protein phosphatases in apoptosis. Curr Opin Pharmacol. 2002;2:458–462. doi: 10.1016/s1471-4892(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 87.Jiang MT, Ljubkovic M, Nakae Y, Shi Y, Kwok WM, Stowe DF, Bosnjak ZJ. Characterization of human cardiac mitochondrial ATP-sensitive potassium channel and its regulation by phorbol ester in vitro. Am J Physiol. 2006;290:H1770–H1776. doi: 10.1152/ajpheart.01084.2005. [DOI] [PubMed] [Google Scholar]
- 88.Garg V, Hu K. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels in mitochondrial inner membrane. Am J Physiol. 2007;293:H322–H332. doi: 10.1152/ajpheart.01035.2006. [DOI] [PubMed] [Google Scholar]
- 89.Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, Losano G, Pagliaro P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006;101:180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- 90.Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res. 2001. In press. [DOI] [PubMed]
- 91.Sasaki N, Sato T, Ohler A, O'Rourke B, Marban E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- 92.Brookes PS, Salinas EP, Darley-Usmar K, Eiserich JP, Freeman BA, Darley-Usmar VM, Anderson PG. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. J Biol Chem. 2000;275:20474–20479. doi: 10.1074/jbc.M001077200. [DOI] [PubMed] [Google Scholar]
- 93.Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology. 2004;39:1533–1543. doi: 10.1002/hep.20197. [DOI] [PubMed] [Google Scholar]
- 94.Wang G, Liem DA, Vondriska TM, Honda HM, Korge P, Pantaleon DM, Qiao X, Wang Y, Weiss JN, Ping P. Nitric oxide donors protect murine myocardium against infarction via modulation of mitochondrial permeability transition. Am J Physiol. 2005;288:H1290–H1295. doi: 10.1152/ajpheart.00796.2004. [DOI] [PubMed] [Google Scholar]
- 95.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 96.Korge P, Honda HM, Weiss JN. Protection of cardiac mitochondria by diazoxide and protein kinase C: implications for ischemic preconditioning. Proc Natl Acad Sci U S A. 2002;99:3312–3317. doi: 10.1073/pnas.052713199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide: a signaling molecule against mitochondrial permeability transition- and pH-dependent cell death after reperfusion. Free Radic Biol Med. 2004;37:1943–1950. doi: 10.1016/j.freeradbiomed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 98.Forbes RA, Steenbergen C, Murphy E. Diazoxide-Induced Cardioprotection Requires Signaling Through a Redox-Sensitive Mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 99.Grover GJ, McCullough JR, Henry DE, Conder ML, Sleph PG. Anti-ischemic effects of the potassium channel activators pinacidil and cromakalim and the reversal of these effects with the potassium channel blocker glyburide. J Pharmacol Exp Ther. 1989;251:98–104. [PubMed] [Google Scholar]
- 100.Grover GJ, Garlid KD. ATP-Sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- 101.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 102.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 103.Moreau C, Jacquet H, Prost AL, D'Hahan N, Vivaudou M. The molecular basis of the specificity of action of KATP channel openers. Embo J. 2000;19:6644–6651. doi: 10.1093/emboj/19.24.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem. 1998;273:13578–13582. [PubMed] [Google Scholar]
- 105.Puddu PE, Garlid KD, Monti F, Iwashiro K, Picard S, Dawodu AA, Criniti A, Ruvolo G, Campa PP. Bimakalim: A promising KATP channel activating agent. Cardiovasc Drug Rev. 2000;18:25–46. [Google Scholar]
- 106.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 107.Grover GJ, D'Alonzo AJ, Garlid KD, Bajgar R, Lodge NJ, Sleph PG, Darbenzio RB, Hess TA, Smith MA, Paucek P, Atwal KS. Pharmacologic characterization of BMS-191095, a mitochondrial KATP opener with no peripheral vasodilator or cardiac action potential shortening activity. J Pharmacol Exp Ther. 2001;297:1184–1192. [PubMed] [Google Scholar]
- 108.Oldenburg O, Yang XM, Krieg T, Garlid KD, Cohen MV, Grover GJ, Downey JM. P1075 opens mitochondrial KATP channels and generates reactive oxygen species resulting in cardioprotection of rabbit hearts. J Mol Cell Cardiol. 2003;35:1035–1042. doi: 10.1016/s0022-2828(03)00151-2. [DOI] [PubMed] [Google Scholar]
- 109.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 110.Gross ER, Hsu AK, Gross GJ. GSK3beta inhibition and K(ATP) channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol. 2007;102:341–349. doi: 10.1007/s00395-007-0651-6. Epub 2007 Apr 23. [DOI] [PubMed] [Google Scholar]
- 111.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. Epub 2006 Sep 21. [DOI] [PubMed] [Google Scholar]
- 112.Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H2770–H2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- 113.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. Epub 2007 Dec 12. [DOI] [PubMed] [Google Scholar]
- 114.Sargent CA, Wilde MW, Dzwonczyk S, Tuttle JG, Murray HN, Atwal K, Grover GJ. Glyburide-reversible cardioprotective effects of calcium-independent phospholipase A2 inhibition in ischemic rat hearts. Cardiovascular Research. 1996;31:270–277. [PubMed] [Google Scholar]
- 115.Williams SD, Gottlieb RA. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem J. 2002;362:23–32. doi: 10.1042/0264-6021:3620023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van der Vusse GJ, Roemen TH, Prinzen FW, Coumans WA, Reneman RS. Uptake and tissue content of fatty acids in dog myocardium under normoxic and ischemic conditions. Circ Res. 1982;50:538–546. doi: 10.1161/01.res.50.4.538. [DOI] [PubMed] [Google Scholar]
- 117.Van der Vusse GJ, Reneman RS, van Bilsen M. Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: possible causes and consequences. Prostaglandins Leukot Essent Fatty Acids. 1997;57:85–93. doi: 10.1016/s0952-3278(97)90497-x. [DOI] [PubMed] [Google Scholar]
- 118.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 119.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. Epub 2005 Oct 4. [DOI] [PubMed] [Google Scholar]
- 120.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury--Evidence for a role of K ATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. Epub 2005 Nov 21. [DOI] [PubMed] [Google Scholar]
- 121.Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. 2006;209:4011–4023. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol. 2007;85:1248–1253. doi: 10.1139/Y07-120. [DOI] [PubMed] [Google Scholar]
- 123.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 125.Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther. 2005;312:644–650. doi: 10.1124/jpet.104.074476. [DOI] [PubMed] [Google Scholar]
- 126.Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol. 2004;287:H2070–H2077. doi: 10.1152/ajpheart.00431.2004. [DOI] [PubMed] [Google Scholar]
- 127.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 128.Yang CM, Lin MI, Hsieh HL, Sun CC, Ma YH, Hsiao LD. Bradykinin-induced p42/p44 MAPK phosphorylation and cell proliferation via Src, EGF receptors, and PI3-K/Akt in vascular smooth muscle cells. J Cell Physiol. 2005;203:538–546. doi: 10.1002/jcp.20250. [DOI] [PubMed] [Google Scholar]
- 129.Oldenburg O, Cohen MV, Downey JM. Mitochondrial KATP channels in preconditioning. J Mol Cell Cardiol. 2003;35:569–575. doi: 10.1016/s0022-2828(03)00115-9. [DOI] [PubMed] [Google Scholar]
- 130.Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol. 2004;286:H468–H476. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 131.Oldenburg O, Critz SD, Cohen MV, Downey JM. Acetylcholine-induced production of reactive oxygen species in adult rabbit ventricular myocytes is dependent on phosphatidylinositol 3- and Src-kinase activation and mitochondrial KATP channel opening. J Mol Cell Cardiol. 2003;35:653–660. doi: 10.1016/s0022-2828(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 132.Krieg T, Philipp S, Cui L, Dostmann WR, Downey JM, Cohen MV. Peptide blockers of PKG inhibit ROS generation by acetylcholine and bradykinin in cardiomyocytes but fail to block protection in the whole heart. Am J Physiol Heart Circ Physiol. 2005;288:H1976–H1981. doi: 10.1152/ajpheart.00883.2004. [DOI] [PubMed] [Google Scholar]