Abstract
This review is based on the honor of receiving the Discovery Award from the Society of Free Radical Biology and Medicine. The review is reflective and presents our thinking which led to experiments that yielded novel observations. Critical questioning of our understanding of oxygen free radicals in biomedical problems led us to use and develop more direct and extremely sensitive methods. This included nitrone free radical spin-trapping and HPLC-electrochemical detection. This technology led to the pioneering use of salicylate to trap hydroxyl free radicals and show increased flux in ischemia/reperfused brain regions and to also first sensitively detect 8-hydroxy-droxyguanosine (8-OHdG) in oxidative-damaged DNA and help assess its role in cancer development. We demonstrated that Methylene Blue (MB) photo-induced formation of 8-hydroxy-guanine in DNA and RNA and discovered that MB sensitively photo-inactivated RNA viruses including HIV and the West Nile Virus. Studies in experimental stroke led us to serendipitously discover that α-phenyl-tert-butylnitrone (PBN) was neuroprotective if given after the stroke. This led to extensive commercial development of NXY-059, a PBN derivative, for the treatment of stroke. More recently we discovered that PBN-nitrones have potent anti-cancer activity and are active in preventing hearing loss caused by acute acoustical trauma.
Introduction and Overview
Delving into the scientific unknown is not for the faint of heart, but it is how discoveries are made that lead to deeper understanding. The early pioneers of free radical biology, both thinkers and experimentalists such as Chance, Michaelis, Harman, Commoner and the team of Gerschman and Gilbert, made critical observations and bold postulates in the early 1950s that free radical processes were involved in biological processes [1]. The fundamental facts available were limited, but nonetheless it was clear to Gerschman and Gilbert that the toxic side effects of oxygen were similar to the damage caused by x-rays [2]. Harman early on postulated that free radicals had major consequences to fundamental biological processes, including aging [3] and cancer [4]. Decades later we are still trying to understand the full extent of his early prescient theories. Thus, this was the state of affairs in a fledgling research field that was destined to become a hot-bed of research activity that increased in momentum from the late 1960s following the discovery of superoxide dismutase by McCord and Fridovich {9433, 9451} up to the present time. It was during this early time (1969) that I began working on oxygen and biological processes in Britton Chance’s lab which led to pioneering observations on the fundamental processes of oxygen formation from water in green plant photosynthesis [7]. Shortly thereafter I spent another important time period (1971-1974) doing research in Barry Commoner’s laboratory, who in 1954 had made the first observation of free radicals in biological systems using electron paramagnetic resonance methods [8]. The experience in Commoner’s laboratory, where we showed that free radicals of ascorbate were observed using electron paramagnetic resonance of tissues which were concurrently undergoing x-irradiation [9], fueled my increasing interest in the field of free radical processes in biomedical problems. After setting up my own laboratory at the Oklahoma Medical Research Foundation in 1974, our research early on focused on the initiation phase of cancer, which led to several observations pertinent to free radicals in carcinogen metabolism [10-14]. The role of free radicals in cancer development and other age-related diseases became a major part of our research effort for the next 34 years, during which time advancements in the field of free radical biology and the surprising results of our experiments yielded ever-increasing interest in the role of reactive oxygen and nitric oxide species in biomedical problems. Serendipitous findings that arose in trying to understand oxidative damage in experimental brain ischemia-reperfusion caused us to have an increased focus on taking our basic science findings to practical applications in the commercial development of therapeutic treatment of stroke. This effort led to increased focus on various biomedical problems, including cancer development and protection from acute acoustical trauma inducted hearing loss, and to the development and utilization of different talents in order to achieve our goals. We recently reviewed some aspects of this effort {35437}.
In my career I have had the fortunate opportunity to conduct research on various, sometimes seemingly unrelated, areas of research. However in hindsight, oxygen has been a central theme throughout. Figure 1 presents a list in chronological order of some of the various areas where our research effort has contributed to the field and has helped open up a deeper degree of understanding. In this review only those areas of research will be discussed which I perceive to have been the most original and/or useful to the research field at large or to represent an important concept in research approach. Some areas will be discussed to help honor the excellent creative scientists I have had the good fortune to work with and to learn valuable lessons from in both creative approaches and the courage to ask difficult questions. The goal of this perspective review of our research effort is to provide a concept-based report that will be helpful, especially to younger scientists, in order to fuel confidence in the importance of creativity and the value of searching for a deeper understanding by questioning existing explanations. This approach leads to discoveries that open up creative opportunities both in basic science as well as translational science.
Figure 1.

List of research areas where our research contributed novel findings.
P680- Oxygen Formation in Photosynthesis
The importance of oxygen formation during photosynthesis is one of the most fundamental processes in all of biology. This area attracted my attention during graduate studies at Purdue University and it was my privilege to conduct post doctorate research in this area with Don Devault and Britton Chance at the University of Pennsylvania. Devault and Chance had discovered that light-driven electron transfer occurred via tunneling mechanisms at liquid helium temperatures in photosynthetic bacteria in 1966 [16]. They demonstrated that electrons were transferred at very low temperatures (from 120 K to at least 4 K) in a temperature-independent manner [16, 17] from cytochrome to the photosynthetic reaction center which had been rendered electron deficient by photon capture. The fact that electron flow at very low temperatures is temperature independent, means that the electrons “tunnel” through the activation energy barriers. This was a revolutionary discovery in biological systems and has been reviewed in a formal framework by Devault [17].
In the context of these fundamental discoveries in photosynthetic bacteria, I was led into the problem of studying light-activated electron flow at low temperatures in green plants by trying to understand abnormalities previously observed in the oxidation of cytochrome f in green plants by Chance’s laboratory. In order to study this properly we utilized the optical apparatus designed, constructed and assembled by Don Devault which involved a Q-switched 20 ns ruby laser system as the light activation pulse [7, 16]. The optical apparatus that Devault built required the development of electronic circuitry components which were not available commercially at that time. His creative approach and design is described in detail by Parson [18] and Seibert [19] upon the occasion of honoring his life.
The studies I conducted with Devault required a low-temperature dewar silver-coated in a particular pattern to accommodate the activation light pulses as well as the path for the analyzing light beam. Construction of this dewar from a blank, including proper pattern-silvering, was my duty to master as a rite that must be passed to be initiated into Don Devault’s “creation of all existing technology from basic principles” approach to fundamental basic science pursued in his lab. Another obstacle to be overcome was the availability of activation laser pulses of the proper wavelength. Since the ruby laser emitted a 694 nm light pulse, it was necessary to obtain a light pulse outside of the 650-700nm range, because pilot studies and previous work had shown that optical changes of at least one reaction center (P700) of the two green plant photosynthetic reaction centers absorbed in this range and it was thought, but not really known, that the second reaction center might absorb in this range also. Purchasing a laser system that emitted light outside of the 670-700 nm range was out of the question. Therefore a completely new approach was conceived and designed. This consisted of blasting the 20 ns ruby laser pulses into a quartz optical cell containing hydrogen gas under high pressure (60 atm). The optical cell emitted a 539 nm green laser pulse generated as a result of the ruby laser pulse causing Raman stimulation of the hydrogen molecules to yield the first anti-Stokes line at 539 nm [7]. Therefore after proper filtering out of the blow through ruby light pluses and selectively allowing the green laser light pluses to pass, we obtained activation light pulses outside of the 650-700 nm range. This then made it possible to measure the kinetics of absorbance changes in the 650-700nm range.
Preparation of samples (isolated chloroplasts or portions of whole leaves) and the positioning of the sample cuvette for analysis was done in complete darkness to rigorously exclude stray photons. This is because the samples, after loading into the cuvette, were immersed in liquid nitrogen prior to positioning into the holder for analysis, and therefore every stray photon hitting the frozen sample was captured and hence the reaction centers were irreversibly oxidized. This also meant that after one activation laser pulse the sample was irreversibly altered and could not be used again. Therefore fresh samples were required for each data point collected, which became very labor intensive especially when defining the activation spectrum over a large wavelength range. Photomultiplier traces for each sample were captured on an oscilloscope and the traces photographed onto Polaroid film and then analyzed by hand.
The results we obtained during this research effort proved to be important in understanding green plant photosynthesis [7]. The research was important in establishing that P680 was the reaction center of the so-called second photo-act of photosynthesis, i.e., the reaction center where oxygen is formed from water. Also, our research established that electron flow from cytochrome b559 to P680 was kinetically temperature independent from 80 to 220 K. As with many novel results, our studies were repeated by several laboratories and independently challenged using other approaches. With time our results have been proven to be correct and are still considered important in this field.
Do Oxygen Radicals Exist? Novel Approaches and New Methods Required
Important Question
A defining time in a scientist’s career appears to arise when one begins to really look deep into and question the validity of the basic tenets of the chosen field of research. This occurred to me in the late 1970s and early 1980s after I had already received and completed several NIH grants. The questions that began to plague me can best be stated as: A) Do oxygen free radicals really exist and are they really important in biological and biomedical processes? B) If they are important, then brain should be the organ the most susceptible and therefore why not focus on it? These questions arose primarily because I became acutely aware of the lack of direct rigorous methods then in practice to identify and measure oxygen free radicals in biological systems. Wrestling with these questions plagued me until I began to take concrete creative approaches to address them. This turned out to be one of the most important turning points in my research career.
Spin Trapping
In order to address these fundamental questions it was important to utilize new approaches and new methods that took several years to develop and yield results pertinent to the original questions. The very short lifetime of most oxygen free radicals have always highlighted the severe limitations to the methodological approaches available to study them. The spin trapping method which we began to use early on in 1975 [20] was very useful to us in many biochemical research problems [21-24]. However, despite its usefulness in biochemical and chemical research problems, it had severe limitations to seriously address the real question of whether free radicals existed and were important in living systems. The most serious limitation of the spin-trapping approach is the fact that the spin-trapped free radical product (spin-adduct) is readily reduced by biological systems (Fig 2) and hence is rendered non-observerable by electron paramagnetic resonance (EPR) methods. Using EPR we demonstrated that the EPR spectrum of the trapped hydroxyl free radical adduct of 5,5-dimethyl-1-pyrroline-1-oxide (DMPO) was rapidly rendered EPR silent by synaptosomes [25]. We showed that the DMPO-OH adduct was rapidly chemically reduced by the mitochondria in the synaptosomes. This meant that it should then be possible to use oxidation methods to reverse this process and hence uncover the original nitroxyl spin-adducts for measurement by EPR. Many attempts to accomplish this using various approaches proved unsuccessful.
Figure 2.
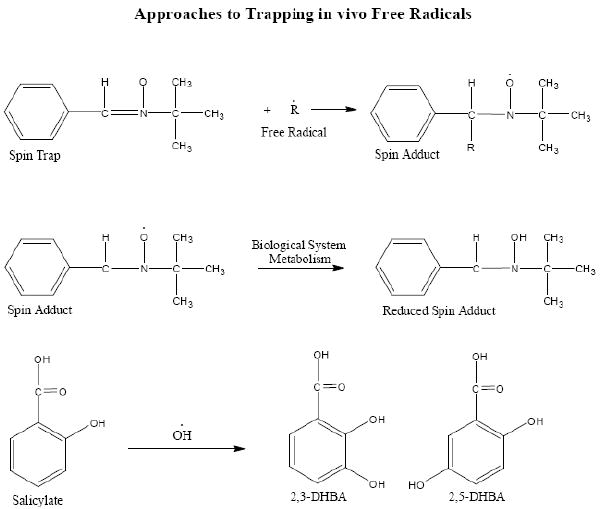
Equations illustrating the use of the spin-trap α-phenyl-tert-butylnitrone (PBN) to trap a free radical and the subsequent metabolic reduction of the spin-adduct to a species that is not observable by electron paramagnetic resonance. Salicylate trapping of hydroxyl free radicals yields the 2,3 and 2,5-dihydroxy benzoic acid compounds noted as 2,3 and 2,5 –DHBA.
Spin Trapping and HPLC-Electrochemical Detection
An approach we investigated was to utilize an HPLC-Electrochemical Detection (HPLC-ED) methodology approach whereby we could in principle detect not only the free radical spin adduct in the “EPR visible” nitroxide state but also the reduced “EPR silent” spin adduct. We tested this approach using a simple chemical system where UV light was used to produce hydroxyl free radicals from H2O2 that were trapped using DMPO [26]. HPLC-ED methodology demonstrated that not only could the DMPO spin-trapped hydroxyl free radical spin-adduct be observed as a separate HPLC peak, also visible by EPR, but that it was completely separated from the ascorbate-reduced DMPO-trapped hydroxyl radical species that was EPR-silent. Therefore, the HPLC-electrochemical detection methodology made it possible, in principle, to determine the spin-trapped free radicals formed in vivo even if they were reduced and became EPR-silent. As promising as this technology and approach was in a purely chemical system, in biological systems we chose to pursue other approaches simply because we could not find a solvent system that would cleanly remove the DMPO-free radical spin-adducts from the biological milieu.
HPLC-Electrochemical Detection and Salicylate Hydroxyl Radical Trapping
The very rapid reactivity of hydroxyl free radicals contributes greatly to their extremely low concentration in vivo. Because the HPLC-ED technology has inherent extreme sensitivity, this methodology was vital to help resolve the role of free radicals in biomedical problems. The chemical literature on hydroxyl free radical reactivity guided us to determine if phenol could be used as a trap. The fact that the specific reaction products of hydroxyl free radicals with phenol could be detected by electrochemical detection methods prompted us to use phenol as a first approach. This approach was successful [27]. However, we realized that phenol was extremely toxic to biological systems so we began to consider a chemical somewhat similar in reactivity to phenol yet well tolerated by biological systems. Fortunately, we utilized salicylate and found that the hydroxyl free radical reaction products 2,3- and 2,5-dihydroxy benzoate (2,3- and 2,5-DHBA) were readily extracted from biological milieu and eluted separate from salicylate by HPLC, and were readily and sensitively detected by electrochemical methods [27]. Our first attempt to utilize salicylate as a trap for hydroxyl free radicals in vivo was in the adriamycin-treated rat model [28]. Adriamycin was known to be very toxic to heart because of oxidative processes which limited its use as an effective cancer therapeutic agent. Rats were administered a bolus of 100 mg/kg salicylate during an adriamycin treatment regimen known to cause damage to the heart. Tissue extracts analyzed by HPLC-ED showed that heart, muscle, lung, brain and blood tissue had from 100-fold to 3-fold higher levels respectively of 2,5-DHBA when compared to normal non-adriamycin-treated rat tissue. This study clearly demonstrated that salicylate was useful as a hydroxyl free radical trap in vivo. As it turned out later, the salicylate trapping method was very important in our studies on stroke.
DNA Oxidation – 8-OHdG Measurement by HPLC-ED
Direct measurement of unique oxidation products formed by the reaction of oxygen free radicals with in vivo molecular targets is another important approach to probe into their role in biological systems. Early on we began to explore the reaction of hydroxyl free radicals with DNA using a complex of DNA-ferrous iron-hydrogen peroxide [22]. Evidence of hydroxyl free radical attack on the sugar moieties of DNA was demonstrated. Utilizing various nucleotides in the presence of ferrous iron when reacted with hydrogen peroxide, we demonstrated that hydroxyl free radicals were formed and that the di- and tri-phosphate nucleotides complexed with Fe2+ and as such effectively slowed down ferrous iron oxidation to the ineffective ferric iron complex [23, 24]. The fact that di- and tri-nucleotides complexed with ferrous iron in a manner such that Fe2+ reacted with H2O2 to form hydroxyl free radicals suggested to us that these free radicals could also possibly react with the nucleotide bases to form unique oxidation products. Simultaneously we became aware that Kasai and Nishimura had just discovered a new oxidation adduct of guanine in DNA, namely 8-hydroxyl-2-deoxyguanosine (8-OHdG) and had demonstrated formation of it in DNA which had been treated with Fe and reducing agents to form oxygen free radicals [29]. We thought that it may be possible to detect 8-OHdG using HPLC-ED methods. Kasai and Nishimura graciously provided us with a small sample of 8-OGdG. Using this sample we were the first to show that 8-OHdG was very sensitively detected using HPLC-ED [30]. At the time, we first showed that 8-OHdG could be detected so sensitively no one knew if 8-OHdG was formed in vivo. However soon we, as well as others, were able to clearly demonstrate that low levels of 8-OHdG was present in the DNA of living systems and that it increased after subjecting the biological system to increased amount of oxidative stress [31]. We showed that exposure of isolated DNA to hydroxyl free radicals formed by exposure of H2O2 to UV light caused the formation of significant levels of 8-OHdG in the DNA [32].
HPLC methodology made it possible to detect sub-nanomole levels of 8-OHdG. We recognized early that the methods utilized in the isolation and treatment of DNA influenced the yield of 8-OHdG [33]. The HPLC-ED methodology was to become a very important tool to help elucidate the role of 8-OHdG in biomedical problems, especially its role in cancer initiation and development. Early on we studied conditions known to influence cancer and noted that there was a strong direct relationship of enhanced 8-OHdG levels in DNA and the development of cancer in experimental models of carcinogenesis where oxygen free radicals were strongly implicated [34-37]. The role of 8-OHdG in cancer development became a very important area of experimental research that is much too extensive to review here. Summarizing, it was soon demonstrated that the presence of 8-OHdG in DNA caused mutations [38]. With time, it was also demonstrated that three classes of DNA repair enzymes were present in biological systems: two specifically engaged in removing 8-OHdG from DNA and another which mediates the degradation of the 8-OH-guanine triphosphate nucleotides thus preventing this oxidized base from being inserted into newly forming DNA. The presence of these three enzyme systems is prima facia evidence that nature considers it important to assure that 8-OHdG is removed from DNA or is prevented from being formed de novo from the oxidized purine base nucleotide.
Methylene Blue Photo-inactivation of RNA Viruses
In June 1986, we began a series of experiments that rather rapidly lead to two original findings, namely: A) that methylene blue (MB) in the presence of light mediates the formation of 8-hydroxyguanine in DNA and RNA; and B) that MB photo-inactivates RNA viruses very effectively. Having in hand the HPLC-ED technology to sensitively measure 8-OHdG in DNA, it was straightforward to demonstrate that MB in the presence of light caused the formation of large levels of 8-OHdG in DNA [39, 40]. During this time (mid-1980s) the possibility of an AIDS pandemic was center stage. Spurred by this concern, we speculated that MB could photo-inactivate the HIV virus. We tested this notion using R17, a model RNA virus, and demonstrated that R17 was very sensitively photo-inactivated by MB. For example, we noted R17 was inactivated by over 8 logs in the presence of 2 μM MB illuminated for 5 min by white light. These observations are summarized in my patent entitled “Phototherapy Using Methylene Blue” [41]. With the expertise of Dr. Raymond Schinazi, we then demonstrated that this approach was also valid in the MB-mediated photo-inactivation of HIV in biological fluids. We also patented these discoveries [42]. More recently, we demonstrated that the West Nile Virus (WNV) is photo-inactivated by MB [43] and that MB photo-inactivation abolishes WNV infectivity in a mouse model [44].
Studies were carried out to understand the mechanisms involved in MB photo-mediation of: A) 8-OHdG formation in DNA; and B) the basis of RNA virus inactivation. In the case of MB photo-mediation of 8-OHdG formation in isolated DNA, we demonstrated that oxygen was necessary for this to occur [39]. We ruled out a role for superoxide, hydrogen peroxide and hydroxyl free radicals based on the fact that scavengers of these intermediates had no effect on the yield of 8-OHdG [39]. Based on the enhanced levels of 8-OHdG obtained in the presence of D2O versus H2O, we concluded that singlet oxygen was an important intermediate. This is consistent with the fact that it is well known that singlet oxygen is produced in the photolysis of MB in the presence of oxygen (Fig 3). Since MB is one of several members of the thiozin dyes, we carried out studies to determine the relative potencies of each of these dyes in the photo-mediation of 8-OHdG formation in isolated DNA. We found the relative potency to be as such: Methylene Blue > Azure B > Azure A > Toluidine Blue > Thionin [40].
Figure 3.
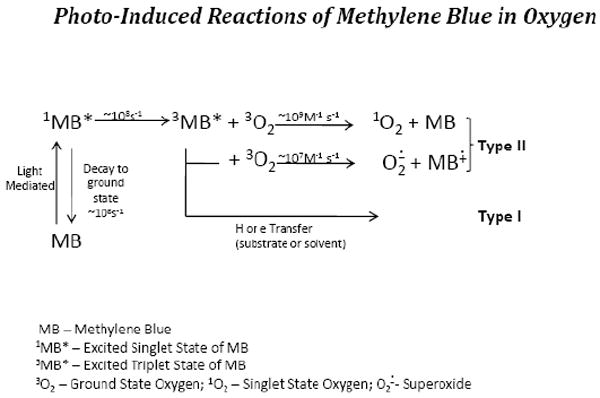
Equations noting the photo-induced formation of the Methylene blue (MB) excited singlet species which can either decay back to ground state MB or form the excited triplet state of MB which will react with ground state oxygen to form singlet oxygen.
In studies utilizing double-stranded super-coiled DNA plasmids, we demonstrated that MB plus light caused the formation of large levels of 8-OHdG as well as single strand nicks [45]. Single strand nicks occurred approximately 17-fold less frequently than did the formation of 8-OHdG and were reduced by magnesium ions. The role of hydroxyl free radicals was ruled out as being responsible for the strand nicks. It was thought that single strand breaks were dependent on the presence of 8-OHdG in the super-coiled DNA plasmids, yet the exact mechanistic basis was not established. Utilizing the single-stranded M13mp2 bacteriophage system the mutations induced by MB plus light was studied [46]. Large levels of 8-OHdG was generated by MB plus light in these plasmids, however the results showed that mutagenesis was highly specific and dependent on the SOS response in the bacteria into which the lesioned DNA plasmids were inserted. The results showed that the mutations were due to G→C transversions not due to the G→T transversions expected from the presence of 8-OHdG. It was concluded that the mutations were not due to the presence of 8-OHdG per se but were due to unidentified lesions generated by active oxygen species.
In addition to studying DNA plasmids as noted above, we did indepth studies of the mechanisms involved in MB plus light photo-induced lesions in RNA bacteriophages and viruses [43, 47-49]. In a study where we used MB as well as Rose Bengal, a dye also known to effectively generate singlet oxygen in light, we examined the role of singlet oxygen, hydroxyl free radicals, reaction temperature, and 8-hydroxyguanosine (8-OHG) formation in phage RNA in the photo-inactivation of both R17 and Qβ bacteriophages [47]. It was shown that addition of agents to quench hydroxyl free radicals had little effect on the amount of phage inactivation by MB plus light. The role of phage inactivation by MB plus light as well as 8-OHG formation, assayed in the phage RNA by HPLC-ED methods [50], was temperature independent in the 13°-37° C range. We found that MB was much more effective than Rose Bengal in the photo-inactivation of phages, but that the rate of phage inactivation was significantly increased for both MB and Rose Bengal when the reactions were carried out in D2O as compared to H2O. All of these observations strongly implicate that singlet oxygen is involved.
We conducted focused studies which were devoted to ascertaining the nature of the lethal lesions in the Qβ bacteriophage photo-inactivation with MB [48, 49]. Lethal hits were assessed by loss of the ability of treated phage to mediate killing when reinfected into bacteria. Qβ is a very simple RNA virus which consists of a porous protein coat that envelopes the RNA genome. Utilizing MB plus light, we demonstrated that lethal hits to Qβ were dependent on the presence of oxygen and that the lethal hits could not be accounted for by the number of 8-OHG formed per viron. We discovered that MB plus light mediated the formation of RNA-protein cross links. The cross links presumably involved the coat protein. This discovery came about by leaving out the proteinase K digestion step prior to phenol extraction of the phage RNA. RNA recovery was significantly decreased however, it was soon noted that the missing RNA was at the phenol aqueous interface as RNA-protein cross links. Further studies using infectious RNA assays demonstrated that the lethal lesions in Qβ phage following MB plus light treatment could be accounted for and resided in the RNA component but that the protein component of the viron contributed to the inactivation [49]. We attempted to extend these findings in Qβ to MB plus light mediated photo-inactivation of HIV viruses. We had only limited success in this effort. However, our studies in this area did clearly indicate that MB plus light killing of HIV could not be accounted for by the photo-inactivation of the viral reverse transcriptase per se [43].
Therapeutic Potential of Nitrones
We began working with the nitrones as spin-trapping compounds starting in 1975. It was in the latter part of the 1980s that we discovered their potential as therapeutics in experimental stroke. About 10 years ago, we discovered that the PBN nitrones were effective anti-cancer agents, and then more recently, we found that they are effective agents to protect against acute acoustical trauma induced hearing loss. The use of nitrones as therapeutics has been recently reviewed where these areas of research are covered in depth {35437}. Our purpose in this review is to discuss the background research effort and rationale leading up to the first discoveries and then briefly summarize the past effort in this area and the current status of the research ongoing.
Oxidative Damage to Brain
To fully understand why we serendipitously discovered that nitrones were effective in protecting from experimental stroke, it is necessary to have an understanding of our background research effort in oxidative damage to brain which took root in the late 1970s and early 1980s. We were one of the first labs to focus on oxidative damage mechanisms in brain. Our first experiments, which utilized brain homogenate, clearly showed that brain was very easily oxidized and that the presence of trace amounts of Fe was very important in causing brain peroxidation [51-55]. Results of the early experiments combined with a perusal of literature allowed us to rationalize that brain would be extremely sensitive to oxidative damage principally because: A) brain consumes about 20% of the total oxygen demand of the whole body which is an inordinate amount based in its weight; B) brain is significantly enriched in highly unsaturated fatty acids (22:6 and 20:4) compared to other tissues and as such these fatty acids are potentially extremely sensitive to peroxidation: and C) brain has areas that are enriched in Fe as well as ascorbate and is low in the anti-oxidant enzyme catalase. Utilizing synaptosomes isolated from striatum tissue, we showed that synthesis of dopamine from tyrosine was lost in direct proportion to the amount of lipid peroxidation which occurred when these subcelluar organelles were induced to perioxidize by increasing amounts of the addition of ADP-chelated Fe and ascorbate [55]. We also demonstrated that with an increasing oxygen atmosphere, lipid peroxidation was increased in the homogenate of isolated brain regions that had the highest amount of total iron levels [51]. All of these observations reaffirmed our original predictions that brain is an organ that is poised to excessive oxidative damage. This means that any abnormality that caused an imbalance of the normal balance in oxidative damage control can set off a series of events that can lead to excessive oxidative damage.
Convergence of Important Scientific Developments
During this time interval (mid-1980s) there was a convergence of scientific developments that made the time ripe for us to begin to test our ideas on the susceptibility of brain to oxidative damage in an animal model. The important scientific developments were: A) our early demonstrations that brain tissue was extremely sensitive to oxidative damage; B) we had in hand the HPLC-ED technology that allowed us to sensitively detect increased flux of hydroxyl free radicals using salicylate as a trap; C) we began collaboration with Dr. John Carney who had experience with an excellent animal model (Mongolian gerbil) of experimental stroke and was at the University of Oklahoma Health Sciences Center therefore locally available and actively desirous of collaborating with us; and D) a novel theory was put forth by Dr. Joe McCord [56], based on primary observations [56, 57] in cat small intestine, that re-oxygenation of ischemic tissue caused a large increase in the flux of oxygen free radicals. I was quite skeptical of the theory put forth by McCord and the convergence of the four developments noted above made it possible to rigorously test these ideas in the reperfused stroke brain model. Utilizing the excellent Mongolian gerbil stroke model with salicylate as a trap in our first experiments, we proved very clearly that an increased flux of hydroxyl free radicals was produced in the ischemia/reperfused brain tissue only and not in those brain areas that had not undergone ischemia/reperfusion [58]. This was the first direct rigorous proof of this concept in an experimental animal model of stroke and was widely viewed with great interest in the scientific community.
Following these exciting results, our labs began collaborating with Dr. Earl Stadtman’s laboratory. Their lab had extensively studied oxidative damage to proteins and aging. Therefore protein oxidative damage measurements as well as glutamine synthase activity and neutral protease activity were parameters which were measured in young and old gerbil brains before and after experimental stroke. Additionally acute and chronic administration of the PBN was also utilized as another parameter. These collaborative efforts led rapidly to several published reports [59-63] that yielded several important novel findings. A summary of the novel observations we made are presented in Figure 4. We found that older gerbils were more susceptible to stroke than were younger gerbils and that the amount of oxidized protein in the brain of older gerbils was increased compared to younger gerbils. The older brains had lower activity of glutamine synthase as well as neutral protease, an enzyme system that catabolizes proteins, which was discovered in Stadtman’s laboratory and is now an extremely active area of research under the general area of proteasomes. Chronic administration of PBN (30mg/kg daily) for 14 days reversed many of the parameters of older gerbils back to the levels noted in younger gerbils, as noted in Figure 4. These are remarkable results that really excited us as well as the research field in general. Another striking and important observation that we made was the demonstration that administration of a bolus of PBN before giving the gerbils a stroke caused significant protection from stroke-induced brain damage. This observation led us to test if PBN given shortly (up to 1 hour) after a stroke would offer protection. We made the striking observation that PBN did protect if given up to 1 hour after a stroke [62, 64]. These results were soon reproduced and extended by the laboratory of Phillis [65, 66].
Figure 4.

List of observations we made in the study of the Mongolian Gerbil model of stroke.
PBN-Nitrones as Therapeutics in Stroke
The discovery we made in 1988 that a bolus of PBN given up to one hour after starting the reperfusion of brain in experimental stroke was effective in curtailing brain damage was patented [64]. It was obvious to Dr. Carney and myself that the PBN-nitrones could become an effective treatment after a stroke and a such could also have commercial potential. Over a four-year period our ongoing research efforts added significant potential to the PBN-nitrone technology as detailed in Figure 4. Also during this time interval we had many futile visits with many small and major pharmaceutical companies in an attempt to license the technology to them. Because Dr. Huber Warner at the National Institutes of Aging had great interest in our technology, we were introduced to Paul Glenn, who had formed a private foundation (Paul F. Glenn Foundation for Aging Research) to help fund research in aging. He and his board of advisors saw in our research significant value in understanding brain aging, and in February, 1992, awarded a $50,000 grant (unsolicited) to us to conduct more research in this area. Through this small grant several PBN derivatives were synthesized and tested in the stroke model and other neurodegenerative models. This money was the catalytic leverage leading to the early synthesis and then the eventual development of a compound (2,4-disulfopheny-N-tert-butylnitrone) that was later to be called NXY-059 as the candidate drug for therapeutic treatment of stroke in humans. Also, as a result of our interface with the Glenn Foundation and advisors, in 1992 we formed Centaur Pharmaceuticals, Inc., as a company to explore the commercial potential of PBN-nitrones for neurodegenerative diseases. As a result of the extensive research and development conducted at Centaur and the partnership that was formed with AstraZeneca, the experimental drug NXY-059 was developed for the treatment of stroke. We have summarized this effort in a great detail in a recent review {35437} and therefore only the most important major aspects are presented in Figure 5 and only briefly discussed here.
Figure 5.

Time-line of the discovery and major research and development stages of PBN-nitrones for the treatment of stroke.
Several years of studies were devoted to exploring PBN derivatives in rodent preclinical experiments. The PBN derivative 2,4-disulfophenyl-N-tert-butylnitrone (NXY-059) was chosen based on its enhanced potency of neuroprotection in stroke and its excellent safety profile in experimental animals and human phase 1 and phase 2 clinical trials. A key study was the demonstration that NXY-059 provided significant neuroprotection if administered 4 hr after a stroke in a marmoset model. Based on all of the available information, world-wide phase 3 clinical efficacy trials sponsored by AstraZeneca began in 2003 with at first a smaller 1700 stroke patients (SAINT I) study. This study showed significant protection based on a modified Rankin scale if NXY-059 was administered within 6 hrs after the stroke. This study was followed up with a much larger 3200 stroke patients (SAINT II) trial that utilized many more clinical centers world-wide. Much to the surprise of many people the SAINT II trial failed to show significance. This caused AstraZeneca to cease development of NXY-059. In our recent review {35437} we discussed the several critiques that have been written on these studies.
PBN-Nitrones as Anti-cancer Agents
In 1997 we began a series of experiments that led to the demonstration that PBN had anti-cancer activity. We summarized this in a recent review {35437}. Most of my scientific career has been devoted to studying free radicals and cancer development. One of the most desirable goals in this area of research is to be able to work on a carcinogenesis model where cancer develops and the only known agent responsible is free radicals. In my mind the choline deficiency-induced hepatocellular carcinoma is the best model that fits this criteria. I had not had the opportunity to really work on this model until 1997. It was then that Dr. Dai Nakae from Dr. Yoichi Konishi’s laboratory in Nara, Japan, visited our laboratory. They had already demonstrated that certain antioxidants suppressed the early stages of cancer development in the choline deficiency model. I convinced them to try PBN in a pilot study. Therefore, the experiments started off with a short-term pilot study conducted in collaboration with Dr. Dai Nakae at the Nara Medical School in Nara, Japan, using the choline deficiency amino acid defined diet (CDAA). This diet when fed to male rats causes the induction of hepatocellular carcinoma in about one year. Cancer development in this model is closely coupled to enhanced levels of oxygen free radical processes. Administration of PBN at 3 different levels in the drinking water for 12 weeks clearly demonstrated that this nitrone had potent activity to inhibit the early stages of carcinogenesis [67]. The most remarkable findings were that administration of PBN: A) caused the preneoplastic lesions to be significantly smaller; B) decreased the oxidative damage to DNA as assessed by 8-OHdG content and; C) decreased lipid peroxidation in the target tissue. Follow up studies demonstrated that long term PBN administration significantly decreased the amount of frank hepatocellular carcinomas and as such acts as an anti-cancer agent [68-70]. In these studies, we also demonstrated that PBN when given in a long term study was not carcinogenic itself. This has been demonstrated in mouse models also.
Mechanistically we do not understand why PBN is an anti-cancer agent, however, we think important clues were obtained in these studies. We observed that PBN administration caused enhanced death of the cells within the preneoplastic nodules but had no effect, or even perhaps a palliative effect, on the normal surrounding cells [68, 71]. The differential influence of PBN on killing the cells that are preneoplastic, which can eventually develop into frank tumors, versus showing no, or even a protective, effect on the normal surrounding cells, is remarkable and important. The root cause of this is still not understood. We did show that inducible nitric oxide synthase (iNOS) may be involved and that PBN suppresses the induction of this enzyme in liver undergoing cancer development [72].
It is clear that PBN has wide-ranging anti-cancer effect in other experimental cancer models. Dr. Rheal Towner and I have shown that PBN has anti-cancer activity in an experimental model of glioblastoma [73, 74]. Derivatives of PBN are now being extensively investigated in this model with the long-term goal of developing one derivative for human studies. In preliminary studies we have also shown that PBN has anti-cancer activity in the APCmin model of colon cancer.
PBN-Nitrones and Hearing Loss Protection
Ample background research has demonstrated that free radical processes are involved in the damage to cochlea caused by exposure to intense noise itself or even more so in combination with environmental toxins. Based on this we helped and encouraged Dr. Lawrence Fechter to utilize PBN to determine if it had preventive effects. His research showed that PBN had some small protective effect in rat models using intense noise in combination with certain environmental toxins [75-77]. I was able to become more directly involved in this area by working with Dr. Richard Kopke and his colleagues at the Hough Ear Institute beginning in 2003. This fruitful association has yielded some exciting results with chinchillas where acute acoustical trauma (AAT) is induced using broadband noise. Our research in this area has been recently reviewed {35437}. We have discovered that hearing loss can be largely prevented by administering 4-hydroxy-PBN (4-OHPBN) in combination with N-Acetylcystine (NAC) 4 hours, or even longer, after AAT [78]. The combined 4-OHPBN plus NAC is much more effective than either alone, in fact, the protective effect is synergist such that much less of each drug is required. These are very important observations which we are actively pursuing as potential therapeutic approaches to protecting military personnel.
A Reflective Perspective
I have been most fortunate to have the honor and pleasure to be a sojourner on the path of scientific adventure and discovery. This truly has been an age of spectacular achievements in biomedical sciences. The field of free radical biology and medicine has come from such a rudimentary knowledge base in the last 40 years to the present sophistication where scientific questions can be posed that could not have even been conceived earlier. Yet much is left to be discovered and translated into practical applications. Present day scientists, myself included, must continue to ask questions and develop new innovative technologies that will yield results that may undermine current models and create new models fully incorporating past observations. It is most amazing that science is almost never conducted with this lofty goal in mind. It is in the course of scientific inquiry when the experiments yield results that are not as predicted and methodological and protocol error have been eliminated that Nature may have presented a unique opportunity for what could lead to a deeper understanding and discoveries. It is true that prolonged attempts to understand why experiments produced puzzling results can lead to dead ends. I have been fortunate in that many of the serendipitous findings we have made came from follow-up experiments triggered by puzzling results. This is not to minimize the fact that many times the best planned experiments do yield the most amazing and fortuitous results which is a tribute to the scientific approach.
Acknowledgments
I would like to thank my parents, Aaron and Clarice Floyd, and my family, Marlene, Matthew and Patrick and the many teachers, mentors and colleagues who have helped me so much. I would especially like to thank the following: Carroll Dye, John Ragland, William Duncan, Al Ohlrogge, Britton Chance, Don Devault, Barry Commoner, Paul McCay, Lee Poyer, Peter Wong, Edward Schneider, Raymond Schinazi, John Carney, Huber Warner, Earl Stadtman, Paul Glenn, Mark Collins, Kirk Maples, Brian Frenzel, Charles Engels, Steinar Engelsen, Graham Crooke, Corey Goodman, Mike Kelly, Yashige Kotake, Dai Nakae, Kenneth Hensley, Rheal Towner, Richard Kopke and Donna Howell.
Monies to support the research was made possible by the support of grants from NIH and other federal agencies including the following NIH grants; GM12202, AG02599, CA18591, CA21542, CA40228, CA42854, ES04296, ES03067, NS23307, NS3577, CA082506, P20RR016478, CA82508 and NSF grant GM6556, contracts from the U S Air Force and DOD Office of Naval Research and grants from EPA R-814198 and CR-8127-10-01-0 and numerous NIH SBIR grants and commercial contracts from AstraZeneca to Centaur Pharmaceuticals as well as Oklahoma State seed grants OCAST H97-067, OARS AR052-041 and OCAST fMRI.002. I have been fortunate to have had the continuous support of the Oklahoma Medical Research Foundation and monies from the Merrick Foundation Chair for Aging Research for which I am very thankful.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hensley K, Floyd RA. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys. 2002;397:377–83. doi: 10.1006/abbi.2001.2630. [DOI] [PubMed] [Google Scholar]
- 2.Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–6. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Harman D. Mutation, Cancer, and Aging. Lancet. 1961;1:200–1. doi: 10.1016/s0140-6736(61)91371-x. [DOI] [PubMed] [Google Scholar]
- 5.McCord JM, Fridovich I. Superoxide dismutase: An enzymic function for erythrocuperin (Hemocuprein) J Biol Chem. 1969;244(22):6049–55. [PubMed] [Google Scholar]
- 6.McCord JM, Beauchamp CO, Goscin S, Misra HP, Fridovich I. Superoxide and superoxide dismutase. In: King TE, Mason HS, Morrison M, editors. Oxidases and related redox systems. Baltimore: University Park Press; 1971. pp. 51–76. [Google Scholar]
- 7.Floyd RA, Chance B, Devault D. Low temperature photo-induced reactions in green leaves and chloroplasts. Biochim Biophys Acta. 1971;226:103–12. doi: 10.1016/0005-2728(71)90182-4. [DOI] [PubMed] [Google Scholar]
- 8.Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174:689–91. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 9.Floyd RA, Brondson A, Commoner B. ESR signals during X-irradiation of tissue: Their characteristics and relationship to the cancerous state. Ann N Y Acad Sci. 1973;222:1077–86. doi: 10.1111/j.1749-6632.1973.tb15324.x. [DOI] [PubMed] [Google Scholar]
- 10.Floyd RA, Soong LM, Stuart MA, Reigh DL. Free radicals and carcinogenesis. Some properties of the nitroxyl free radicals produced by covalent binding of 2-nitrosofluorene to unsaturated lipids of membranes. Arch Biochem Biophys. 1978;185:450–7. doi: 10.1016/0003-9861(78)90188-1. [DOI] [PubMed] [Google Scholar]
- 11.Floyd RA. Free radicals in arylamine carcinogenesis. In: Pryor WA, editor. Free Radicals in Biology. NY: Academic Press; 1980. pp. 187–208. [Google Scholar]
- 12.Floyd RA. Free radicals in arylamine carcinogenesis. Natl Cancer Inst Monogr. 1981;58:123–31. [PubMed] [Google Scholar]
- 13.Floyd RA. Free radicals produced in a nitrosofluorene unsaturated lipid reaction. Experientia. 1977;33:197–8. doi: 10.1007/BF02124063. [DOI] [PubMed] [Google Scholar]
- 14.Floyd RA, Soong LM, Culver PL. Horseradish peroxidase/hydrogen peroxide catalyzed oxidation of the carcinogen N-hydroxy-N-acetyl-2-aminofluorene as effected by cyanide and ascorbate. Cancer Res. 1976;36:1510–9. [PubMed] [Google Scholar]
- 15.Floyd RA, Kopke RD, Choi CH, Foster SB, Doblas S, Towner RA. Nitrones as therapeutics. Free Radic Biol Med. 2008;45:1361–74. doi: 10.1016/j.freeradbiomed.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vault D, Chance B. Studies of photosynthesis using a pulsed laser. Biophysical Journal. 1966;6:825–47. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devault D. Quantum mechanical tunnelling in biological systems. Q Rev Biophys. 1980;13:387–564. doi: 10.1017/s003358350000175x. [DOI] [PubMed] [Google Scholar]
- 18.Parson WW. Don DeVault: A tribute on the occasion of his retirement. Photosynthesis Res. 1989;22:11–3. doi: 10.1007/BF00114762. [DOI] [PubMed] [Google Scholar]
- 19.Seibert M. Obituary. Photosynthesis Res. 1991;28:95–8. [Google Scholar]
- 20.Poyer JL, Floyd RA, McCay PB, Janzen EG, Davis ER. Spin trapping of the trichloromethyl radical produced during enzymic NADPH oxidation in the presence of carbon tetrachloride or carbon bromotrichloromethane. Biochim Biophys Acta. 1978;539:402–9. doi: 10.1016/0304-4165(78)90044-2. [DOI] [PubMed] [Google Scholar]
- 21.Floyd RA, Soong LM. Spin trapping in biological systems. Oxidation of the spin trap 5,5-dimethyl-1-pyrroline-1-oxide by a hydroperoxide-hematin-system. Biochem Biophys Res Commun. 1977;74:79–84. doi: 10.1016/0006-291x(77)91377-8. [DOI] [PubMed] [Google Scholar]
- 22.Floyd RA. DNA-ferrous iron catalyzed hydroxyl free radical formation from hydrogen peroxide. Biochem Biophys Res Commun. 1981;99:1209–15. doi: 10.1016/0006-291x(81)90748-8. [DOI] [PubMed] [Google Scholar]
- 23.Floyd RA. Direct demonstration that ferrous ion complexes of di- and triphosphate nucleotides catalyze hydroxyl free radical formation from hydrogen peroxide. Arch Biochem Biophys. 1983;225:263–70. doi: 10.1016/0003-9861(83)90029-2. [DOI] [PubMed] [Google Scholar]
- 24.Floyd RA, Lewis CA. Hydroxyl free radical formation from hydrogen peroxide by ferrous iron-nucleotide complexes. Biochem. 1983;22:2645–9. doi: 10.1021/bi00280a008. [DOI] [PubMed] [Google Scholar]
- 25.Floyd RA. Hydroxyl free-radical spin-adduct in rat brain synaptosomes. Observations on the reduction of the nitroxide. Biochim Biophys Acta. 1983;756:204–16. doi: 10.1016/0304-4165(83)90093-4. [DOI] [PubMed] [Google Scholar]
- 26.Floyd RA, Lewis CA, Wong PK. High-pressure liquid chromatography-electrochemical detection of oxygen free radicals. Methods Enzymol. 1984;105:231–7. doi: 10.1016/s0076-6879(84)05030-8. [DOI] [PubMed] [Google Scholar]
- 27.Floyd RA, Watson JJ, Wong PK. Sensitive assay of hydroxyl free radical formation utilizing high pressure liquid chromatography with electrochemical detection of phenol and salicylate hydroxylation products. J Biochem Biophys Methods. 1984;10:221–35. doi: 10.1016/0165-022x(84)90042-3. [DOI] [PubMed] [Google Scholar]
- 28.Floyd RA, Henderson R, Watson JJ, Wong PK. Use of salicylate with high pressure liquid chromatography and electrochemical detection (LCED) as a sensitive measure of hydroyxl free radicals in adriamycin treated rats. J Free Radic Biol Med. 1986;2:13–8. doi: 10.1016/0748-5514(86)90118-2. [DOI] [PubMed] [Google Scholar]
- 29.Kasai H, Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984;12:2137–45. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: Sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1:163–72. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- 31.Floyd RA, Watson JJ, Harris J, West M, Wong PK. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986;137:841–6. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- 32.Floyd RA, West MS, Eneff KL, Hogsett WE, Tingey DT. Hydroxyl free radical mediated formation of 8-hydroxyguanine in isolated DNA. Arch Biochem Biophys. 1988;262:266–72. doi: 10.1016/0003-9861(88)90188-9. [DOI] [PubMed] [Google Scholar]
- 33.Floyd RA, West MS, Eneff KL, Schneider JE, Wong PK, Tingey DT, et al. Conditions influencing yield and analysis of 8-hydroxy-2’-deoxyguanosine in oxidatively damaged DNA. Anal Biochem. 1990;188:155–8. doi: 10.1016/0003-2697(90)90544-j. [DOI] [PubMed] [Google Scholar]
- 34.Floyd RA, West MS, Hogsett WE, Tingey DT. Increased 8-hydroxyguanine content of chloroplast DNA from ozone-treated plants. Plant Physiol. 1989;91:644–7. doi: 10.1104/pp.91.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy D, Floyd RA, Liehr JG. Elevated-8-hydroxydeoxyguanosine levels in DNA of diethylstilbestrol-treated syrian hamsters: Covalent DNA damage by free radicals generated by redox cycling of diethylstilbestrol. Cancer Res. 1991;51:3882–5. [PubMed] [Google Scholar]
- 36.Aiyar J, Berkovits HJ, Floyd RA, Wetterhahn KE. Reaction of chromium(VI) with glutathione or with hydrogen peroxide: Identification of reactive intermediates and their role in chromium(VI)-induced DNA damage. Environ Health Perspect. 1991;92:53–62. doi: 10.1289/ehp.919253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floyd RA. The Role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11:1447–50. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- 38.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G T and A C Substitutions. J Biol Chem. 1992;267:166–72. [PubMed] [Google Scholar]
- 39.Floyd RA, West MS, Eneff KL, Schneider JE. Methylene blue plus light mediates 8-hydroxyguanine formation in DNA. Arch Biochem Biophys. 1989;273:106–11. doi: 10.1016/0003-9861(89)90167-7. [DOI] [PubMed] [Google Scholar]
- 40.Floyd RA, West MS, Eneff KL, Schneider JE. Mediation of 8-Hydroxy-guanine formation in DNA by thiazin dyes plus light. Free Radic Biol Med. 1990;8:327–30. doi: 10.1016/0891-5849(90)90097-3. [DOI] [PubMed] [Google Scholar]
- 41.Floyd RA. Phototherapy using methylene blue. U.S.Patent 264,088[4,950,665] 1990 Aug 21;:1–8.
- 42.Floyd RA, Schinazi R. Thiazine dyes used to inactivate HIV in biological fluids. 251624[U.S. Patent 5,571,666] 1996 Nov 5;:1–10.
- 43.Floyd RA, Schneider JE, Jr, Dittmer DP. Methylene blue photoinactivation of RNA viruses. Antiviral Res. 2004;61:141–51. doi: 10.1016/j.antiviral.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Papin JF, Floyd RA, Dittmer DP. Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo. Antiviral Res. 2005;68:84–7. doi: 10.1016/j.antiviral.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Schneider JE, Price S, Maidt L, Gutteridge JMC, Floyd RA. Methylene blue plus light mediates 8-hydroxy-2’-deoxyguanosine formation in DNA preferentially over strand breakage. Nucleic Acids Res. 1990;18:631–5. doi: 10.1093/nar/18.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McBride TJ, Schneider JE, Floyd RA, Loeb LA. Mutations induced by methylene blue plus light in single-stranded M13mp2. Proc Natl Acad Sci USA. 1992;89:6866–70. doi: 10.1073/pnas.89.15.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider JE, Phillips JR, Pye Q, Maidt L, Price S, Floyd RA. Methylene blue and rose bengal photoinactivation of RNA bacteriophages: Comparative studies of 8-oxoguanine formation in isolated RNA. Arch Biochem Biophys. 1993;301:91–7. doi: 10.1006/abbi.1993.1119. [DOI] [PubMed] [Google Scholar]
- 48.Schneider JE, Jr, Tabatabaie T, Maidt L, Smith RH, Nguyen X, Pye Q, et al. Potential mechanisms of photodynamic inactivation of virus by methylene blue. Photochem Photobiol. 1998;67:350–7. [PubMed] [Google Scholar]
- 49.Schneider JE, Jr, Pye Q, Floyd RA. Qβ Bacteriophage photo-inactivated by methylene blue plus light involves inactivation of its genomic RNA. Photochem Photobiol. 1999;70:902–9. [PubMed] [Google Scholar]
- 50.Wong PK, Floyd RA. Photochemical synthesis of 8-hydroxy guanine nucleosides. Methods Enzymol. 1994;234:59–65. doi: 10.1016/0076-6879(94)34077-3. [DOI] [PubMed] [Google Scholar]
- 51.Floyd RA, Zaleska M, Harmon HJ. Possible involvement of iron and oxygen free radicals in aspects of aging in brain. In: Armstrong D, Sohal RS, Cutler RG, Slater TF, editors. Free Radicals in Molecular biology, Aging and Disease. NY: Raven Press; 1984. pp. 143–61. [Google Scholar]
- 52.Floyd RA, Zaleska MM. Involvement of activated oxygen species in membrane peroxidation: Possible mechanisms and biochemical consequences. In: Bors W, Saran M, Tait D, editors. Oxygen Radicals in Chemistry and Biology. Berlin: Walter de Gruyter & Co.; 1984. pp. 285–97. [Google Scholar]
- 53.Zaleska MM, Floyd RA. Regional lipid peroxidation in rat brain in vitro: possible role of endogenous iron. Neurochem Res. 1985;10:397–410. doi: 10.1007/BF00964608. [DOI] [PubMed] [Google Scholar]
- 54.Floyd RA. Oxygen radical mediated damage to brain tissue. In: Simic MG, Ward JF, Taylor KA, Von Sonntag C, editors. Oxygen Radicals in Biology and Medicine. 1988. pp. 1015–23. [DOI] [PubMed] [Google Scholar]
- 55.Zaleska MM, Nagy K, Floyd RA. Iron-induced lipid peroxidation and inhibition of dopamine synthesis in striatum synaptosomes. Neurochemistry. 1989;14:597–605. doi: 10.1007/BF00964867. [DOI] [PubMed] [Google Scholar]
- 56.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. Free Radicals and Ischemia. 1985;312(3):159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 57.Granger DN, McCord JM, Parks DA, Hollwarth ME. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986;90:80–4. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- 58.Cao W, Carney JM, Duchon A, Floyd RA, Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett. 1988;88:233–8. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- 59.Floyd RA, Carney JM. Protection against oxidative damage to CNS by α-phenyl-tert-butyl nitrone and other spin-trapping agents: A novel series of nonlipid free radical scavengers. In: Marangos PJ, Lal H, editors. Emerging Strategies in Neuroprotection. Boston, Basel, Berlin: Birkhauser; 1992. pp. 252–72. [Google Scholar]
- 60.Floyd RA. Oxidative damage to behavior during aging. Science. 1991;254:1597. doi: 10.1126/science.1684251. [DOI] [PubMed] [Google Scholar]
- 61.Stadtman ER, Starke-Reed PE, Oliver CN, Carney JM, Floyd RA. Protein modification in aging. In: Emerit I, Chance B, editors. Free Radicals and Aging. Basel/Switzerland: Birkhauser Verlag; 1992. pp. 64–72. [DOI] [PubMed] [Google Scholar]
- 62.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–97. [PubMed] [Google Scholar]
- 63.Carney JM, Starke-Reed PE, Oliver CN, Landrum RW, Chen MS, Wu JF, et al. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spacial memory by chronic administration of the spin-trapping compound N-tert-butyl-α-phenylnitrone. Proc Natl Acad Sci USA. 1991;88:3633–6. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carney JM, Floyd RA. Phenyl butyl nitrone compositions and methods for treatment of oxidative tissue damage. U.S. Patent # 5,025,032. 1991 Jun 18;:1–14.
- 65.Clough-Helfman C, Phillis JW. The free radical trapping agent N-tert-butyl-α-phenylnitrone (PBN) attenuates cerebral ischaemic injury in gerbils. Free Radic Res Commun. 1991;15:177–86. doi: 10.3109/10715769109049138. [DOI] [PubMed] [Google Scholar]
- 66.Cao X, Phillis JW. α-phenyl-tert-butyl-nitrone reduces cortical infarct and edema in rats subjected to focal ischemia. Brain Res. 1994;644:267–72. doi: 10.1016/0006-8993(94)91689-6. [DOI] [PubMed] [Google Scholar]
- 67.Nakae D, Kotake Y, Kishida H, Hensley KL, Denda A, Kobayashi Y, et al. Inhibition by phenyl N-tert-butyl nitrone on early phase carcinogenesis in the livers of rats fed a choline-deficient, L-amino acid-defined diet. Cancer Res. 1998;58:4548–51. [PubMed] [Google Scholar]
- 68.Floyd RA, Kotake Y, Hensley K, Nakae D, Konishi Y. Reactive oxygen species in choline deficiency induced carcinogenesis and nitrone inhibition. Mol Cell Biochem. 2002;234/235:195–203. [PubMed] [Google Scholar]
- 69.Nakae D, Uematsu F, Kishida H, Kusuoka O, Katsuda S, Yoshida M, et al. Inhibition of the development of hepatocellular carcinomas by phenyl N-tert-butyl nitrone in rats fed with a choline-deficient, L-amino acid-defined diet. Cancer Lett. 2004;206:1–13. doi: 10.1016/j.canlet.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Floyd RA, Kotake Y, Hensley K, Nakae D. Nitrone inhibition of cancer development. 819570[U.S. Patent 6,569,902] 2003 May 27;:1–17.
- 71.Nakae D, Kishida H, Enami T, Konishi Y, Hensley KL, Floyd RA, et al. Effects of phenyl N-tert-butyl nitrone and its derivatives on the early phase of hepatocarcinogenesis in rats fed a choline-deficient, l-amino acid-defined diet. Cancer Sci. 2003;94:26–31. doi: 10.1111/j.1349-7006.2003.tb01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Floyd RA, Kotake Y, Towner RA, Guo W-X, Nakae D, Konishi Y. Nitric Oxide and Cancer Development. J Toxicol Pathol. 2007;20:77–92. [Google Scholar]
- 73.Asanuma T, Doblas S, Tesiram YA, Saunders D, Cranford R, Yasui H, et al. Visualization of the protective ability of a free radical trapping compound against rat C6 and F98 gliomas with diffusion tensor fiber tractography. J Magn Reson Imaging. 2008;28:574–87. doi: 10.1002/jmri.21474. [DOI] [PubMed] [Google Scholar]
- 74.Doblas S, Saunders D, Tesiram Y, Kshirsager P, Pye Q, Oblander J, et al. Phenyl-tert-butyl-nitrone induces tumor regression and decreases angiogenesis in a C6 rat glioma model. Free Radical Biology & Medicine. 2007 doi: 10.1016/j.freeradbiomed.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Fechter LD, Liu Y, Pearce TA. Cochlear protection from carbon monoxide exposure by free radical blockers in the guinea pig. Toxicol Appl Pharmacol. 1997;142:47–55. doi: 10.1006/taap.1996.8027. [DOI] [PubMed] [Google Scholar]
- 76.Rao D, Fechter LD. Protective effects of phenyl-N-tert-butylnitrone on the potentiation of noice-induced hearing loss by carbon monoxide. Toxicol Appl Pharmacol. 2000;167:125–31. doi: 10.1006/taap.2000.8995. [DOI] [PubMed] [Google Scholar]
- 77.Fechter LD, Gearhart C, Shirwany NA. Acrylonitrile potentiates noise-induced hearing loss in rat. JARO. 2004;5:90–8. doi: 10.1007/s10162-003-4028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi CH, Chen K, Vasquez-Weldon A, Jackson RL, Floyd RA, Kopke RD. Effectiveness of 4-hydroxy phenyl N-tert-butylnitrone (4-OHPBN) alone and in combination with other antioxidant drugs in the treatment of acute acoustic trauma in chinchilla. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.02.005. [DOI] [PubMed] [Google Scholar]


