Abstract
A growing body of research describes cancers other than breast and ovarian in families with BRCA1/2 mutations, but the prevalence and characteristics of pancreatic cancer in these families has not been well described. This study was designed to: 1) estimate the prevalence of pancreatic cancer in BRCA1/2 positive families; 2) ascertain age of onset and gender distribution of pancreatic cancer in this cohort; and 3) compare age and gender characteristics of pancreatic cancer in BRCA1/2 positive families with those of the general population. Within the UCSF Cancer Risk Program cohort, 24/219 (11.0%) BRCA1 and 17/156 (10.9%) BRCA2 families had at least 1 individual with pancreatic cancer. In the 24 BRCA1 families, median age of diagnosis was 59 (range 45-80) in males, and 68 (range 38-87) in females (male:female ratio = 2.00). In the 17 BRCA2 families, median age of diagnosis was 67 (range 39-78) in males and 59 (range 46-81) in females (male:female ratio = 1.11). The SEER database, which describes cancer characteristics in a representative sample of the US population, reports a median age of 70 in males and 74 in females (male:female ratio = 0.96) over the same time period. Additionally, mean ages of diagnosis of pancreatic cancer in BRCA1/2 families differ significantly from the SEER mean (p = 0.0014 for BRCA1 and p = 0.011 for BRCA2 by unpaired t-test). Our findings suggest that families with early onset pancreatic cancer and features of hereditary breast and ovarian cancer should be considered for BRCA1/2 testing.
Keywords: BRCA1, BRCA2, Genetic Testing, Hereditary Cancer, Pancreatic Cancer
INTRODUCTION
Although most pancreatic cancer is sporadic, 5-10% of cases show familial clustering, and in twin studies heritable factors account for up to 36% of pancreatic cancer risk [1]. Carriers of BRCA1/2 mutations, who are at very high risk for hereditary breast and ovarian cancer [2, 3], also face an increased risk of pancreatic cancer compared with the general population. For BRCA1 carriers, this relative risk (RR) is estimated to be 2-fold higher (RR = 2.26, 95% confidence intervals 1.26, 4.06) [4]. For BRCA2 carriers, this relative risk is approximately 3- to 4-fold higher (RR = 3.51, 95% confidence intervals 1.87, 6.58) [5]. Epidemiologic studies [4-6], as well as a study examining BRCA2 loss of heterozygosity in pancreatic cancer tissue [7], have established a reliable link between BRCA2 carriers and an increased pancreatic cancer risk. For BRCA1 carriers, the association with increased pancreatic cancer risk is not as well defined. A recent observational study, however, suggests that some BRCA1 families may have an increased predisposition to pancreatic cancer [8].
Prior studies of pancreatic cancer risk in BRCA1/2 carriers are limited by small numbers of pancreatic cancer outcomes, retrospective data collection, and incomplete verification of pancreatic cancer. We attempted to mitigate these limitations by systematically examining the prevalence of pancreatic cancer in a large cohort of families with BRCA1 and BRCA2 mutations. Because of increasing evidence suggesting that BRCA1/2 carriers are predisposed to a number of different cancers [5, 9, 10], our program has carefully assessed for, and verified pancreatic cancer histories in all probands and their relatives over the last 8 years. The purpose of this study is to estimate the prevalence of pancreatic cancer in BRCA1/2 positive families and to characterize the presentation of pancreatic cancer in these families.
MATERIALS AND METHODS
Cancer Risk Program and Study Population Ascertainment
The Cancer Risk Program (CRP) at the University of California, San Francisco (UCSF) is a clinical and research program that provides cancer risk assessment and recommendations, including genetic counseling and testing, to families at high risk of hereditary cancer. Population characteristics of the CRP have been described elsewhere [11]. Approximately two-thirds of CRP patients are referred by oncologists and surgeons, and about one-third of CRP patients are either self-referred or are referred by primary care providers and gynecologists.
All patients referred to the CRP are mailed a detailed questionnaire about their family history and potential risk factors for cancer, which they are instructed to bring to their first visit. At the initial “intake and education” visit, each patient receives education about hereditary cancers, a review of the baseline questionnaire, and a detailed 3-4 generation pedigree collected by a genetic counselor. A thorough assessment of cancer risk is provided at the second visit, and BRCA1/2 testing, when appropriate, is offered at that time. Test results are disclosed at the third visit, typically 1 month after the second visit. Research participants receive follow-up by phone, in person, and by mailed questionnaires. Prospective and retrospective cancer diagnoses are confirmed with pathologic reports or medical records, when available. Deaths are confirmed by death certificates and/or medical records.
For this study, we reviewed the pedigrees of all research participants with a positive BRCA1/2 test between August 1998 and August 2006. This review identified BRCA1/2 positive families and individuals with pancreatic cancer within first-, second-, and third-degree relatives of known BRCA1/2 carriers. The median year of diagnosis of pancreatic cancer for these individuals was 1992 (range 1945–2004). The age of diagnosis of pancreatic cancer, and the gender, for these individuals was confirmed by a review of available medical records, including death certificates and pathology reports.
BRCA1/2 Mutation Carrier Designation
We classified the BRCA1/2 mutation carrier status of each individual with pancreatic cancer as definite (100% probability), very probable (91-99% probability), probable (50-90% probability), or <50% probability. Definite carriers included individuals who tested positive for a known BRCA1/2 mutation or those who were obligate carriers (directly in the line of descent between 2 known BRCA1/2 mutation carriers). Very probable carriers, probable carriers, and <50% carriers were classified according to their prior probability of carrying a BRCA1/2 mutation as determined by BRCAPRO software (http://astor.som.jhmi.edu/BayesMendel/brcapro.html) [12]. Some limitations of this software include its inability to assess relatives beyond second-degree and to apply clinical judgment to pedigree reading and analysis. Individuals with pancreatic cancer and a prior probability of carrying a BRCA1/2 mutation of <50% were excluded from further analyses.
Statistical Analysis
Pancreatic cancer prevalence, age of diagnosis, and gender characteristics were summarized using means, standard deviations, medians, ranges, and ratios. Unpaired t-tests with 2-tailed analyses were used to compare the mean ages of diagnosis of pancreatic cancer in BRCA1 and BRCA2 carriers to data from the SEER database [13, 14].
RESULTS
Prevalence and Mutation Frequencies
Of the 1312 families who received BRCA1/2 testing during the study period, 219 families had a deleterious mutation in BRCA1, and 156 families had a deleterious mutation in BRCA2. Of the 219 BRCA1 positive families, 24 (11%) had at least 1 relative with pancreatic cancer, including 6 families (2.7%) with more than 1 relative diagnosed with pancreatic cancer. Of the 156 BRCA2 positive families, 17 (11%) had at least 1 relative with pancreatic cancer, including 1 family (0.6%) with more than 1 relative diagnosed with pancreatic cancer (Figure 1). From these BRCA1/2 families, 4 individuals with pancreatic cancer were definite carriers, 12 were very probable carriers, and 33 were probable carriers. Nine individuals were excluded from age and gender analyses because their probability of carrying the family mutation was <50% (Table 1).
Figure 1.
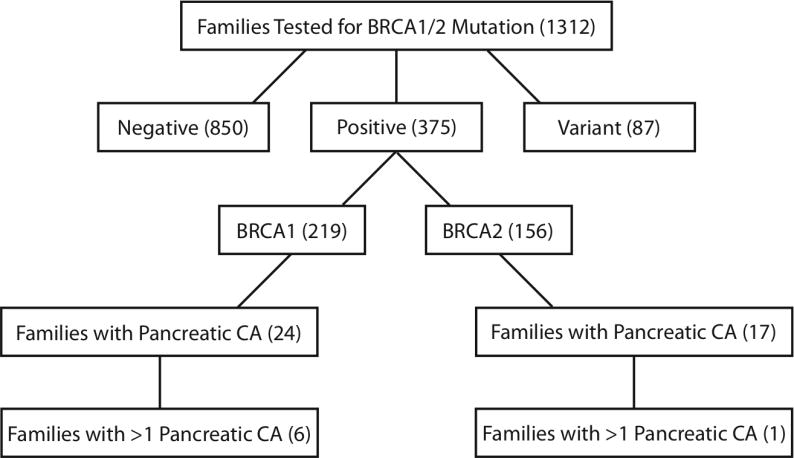
Flow diagram of the UCSF Cancer Risk Program Cohort. Numbers within parentheses indicate total number of families within the respective categories.
Table 1.
Mutation carrier designation for individuals in BRCA1 and BRCA2 families with pancreatic cancer.
| BRCA1 | BRCA2 | Total | |
|---|---|---|---|
| Definite Carriers (>99% Probability) | 3 | 1 | 4 |
| Very Probable Carriers (91-99% Probability) | 7 | 5 | 12 |
| Probable Carriers (50-90% Probability) | 20 | 13 | 33 |
| <50% Probability Carriers* | 6 | 3 | 9 |
| Total | 36 | 22 | 58 |
Excluded from age and gender analyses
The most common BRCA1 mutation in families with pancreatic cancer was the 187delAG mutation (n=10, 42% of BRCA1 families), and the second most common was the 5382insC mutation (n=3, 13% of BRCA1 families), both Ashkenazi Jewish founder mutations [15, 16]. The most common BRCA2 mutation in the study population was the 6174delT mutation (n=10, 59% of BRCA2 families), another Ashkenazi Jewish founder mutation. The proportion of BRCA1/2 families with pancreatic cancer that carry 1 of the 3 Ashkenazi Jewish founder mutations (56.1%) is considerably higher than the proportion of BRCA1/2 families without pancreatic cancer (31.3%).
Characteristics of Pancreatic Cancer in BRCA1 and BRCA2 Families
Within BRCA1 families, the mean age of diagnosis of pancreatic cancer was 62.9 (standard deviation 12.0) with a median age of 59 (range 45-80) in males and 68 (range 38-87) in females (male:female ratio = 2.00). Within BRCA2 families, the mean age of diagnosis of pancreatic cancer was 62.9 (standard deviation 11.7) with a median age of 67 (range 39-78) in males and 59 (range 46-81) in females (male:female ratio = 1.11). These data are summarized in Table 2 along with comparison data from the National Cancer Institute SEER database. Analysis of the data shows that the mean ages of diagnosis of pancreatic cancer in BRCA1 and BRCA2 families are significantly younger than the population mean (p=0.0014 for BRCA1 and p=0.011 for BRCA2).
Table 2.
Characteristics of pancreatic cancer in BRCA1 and BRCA2 families and comparison to the National Cancer Institute SEER data.
| Cancer Characteristics | BRCA1 | BRCA2 | SEER |
|---|---|---|---|
| Age at Diagnosis | |||
| All, Mean (Standard deviation) | 62.9 (12.0)* | 62.9 (11.7)** | 70.0 (12.1) |
| All, Median (Range) | 61 (38-87) | 66 (39-81) | 72 |
| Male, Median (Range) | 59 (45-80) | 67 (39-78) | 70 |
| Female, Median (Range) | 68 (38-87) | 59 (46-81) | 74 |
| Gender | |||
| Male | 20 | 10 | N/A |
| Female | 10 | 9 | N/A |
| Male to Female Ratio | 2.00 | 1.11 | 0.96 |
| Total | 30 | 19 | 31,318 |
Statistically significant difference compared to SEER (p=0.0014)
Statistically significant difference compared to SEER (p=0.011)
Pedigrees of BRCA1 and BRCA2 Families with Pancreatic Cancer
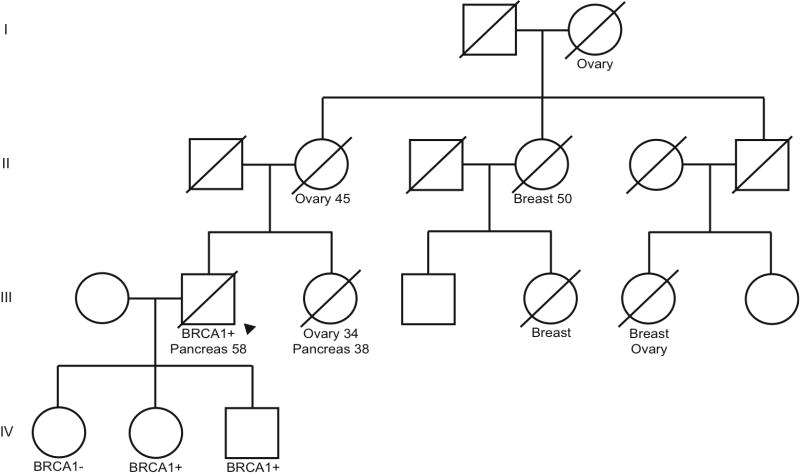
Within our study population, representative BRCA1 and BRCA2 family pedigrees are presented in Figures 2 and 3, respectively. In Figure 2, there are 2 family members with pancreatic cancer (III-2 and III-3) but only the male was tested and found to carry the BRCA1 (187delAG) family mutation, making him a definite mutation carrier. The probability that his sister (III-3) carried a BRCA1 mutation is >90% using BRCAPRO software. The face validity of this estimate is high, given the known family mutation and her confirmed young age at ovarian cancer diagnosis. This sister was classified as a very probable carrier. This pedigree exemplifies the young age of pancreatic cancer onset in BRCA1 positive men. It also illustrates the “competing mortality” of breast and ovarian cancer in many of the female relatives.
Figure 2.
Representative BRCA1 family pedigree. Arrow indicates proband. Ages of diagnosis are included for those that were confirmed.
Figure 3.
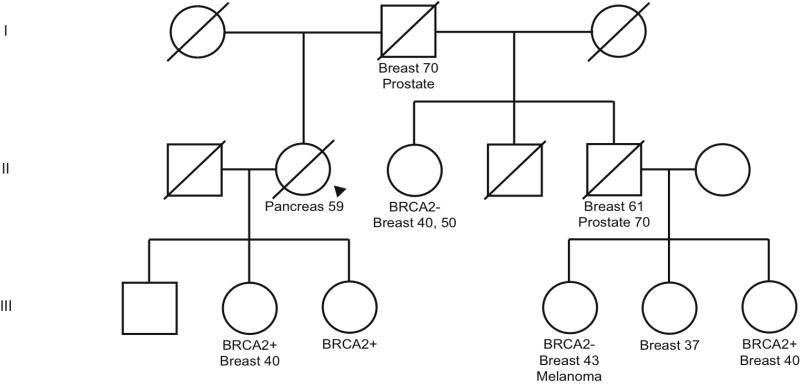
Representative BRCA2 family pedigree. Arrow indicates proband. Ages of diagnosis are included for those that were confirmed.
In Figure 3, there is 1 family member with pancreatic cancer (II-2) who is in the direct line of descent between 2 groups of family members with the same BRCA2 (6174delT) mutation, carried by her children (III-2 and III-3) and a half-niece (III-6). This places the proband (II-2) as an obligate carrier, thus classified as a definite carrier. This pedigree shows, again, a young age at pancreatic cancer diagnosis, and competing mortalities of breast and ovarian cancer in female relatives.
DISCUSSION
A number of studies have shown that the third most common cancer associated with BRCA1/2 mutations is pancreatic cancer [15]. This study is the largest to date examining pancreatic cancer as the primary outcome in families with BRCA1/2 mutations. In our retrospective cohort, approximately 11% of families with BRCA1/2 mutations had at least 1 relative with pancreatic cancer, consistent with previously published reports [8, 16].
The population characteristics of BRCA1/2 mutation carriers with pancreatic cancer differed from those of individuals with pancreatic cancer in the general population. Generally, individuals with pancreatic cancer from families with known BRCA1/2 mutations were younger, and in BRCA1 families, showed a 2:1 male:female ratio. This ratio in BRCA1 families differs from SEER data, which shows a 0.96:1 male:female ratio. One possible explanation for the predominance of pancreatic cancer in males in BRCA1 families is the competing mortality for breast and ovarian cancer in their female relatives [17, 18]. The risk of ovarian cancer in BRCA1 families is estimated to be as high as 66%, as compared with 27% in BRCA2 families. The aggressive nature of the disease may contribute to a significantly increased risk of mortality in BRCA1 positive women [19]. For this reason, we believe that recommendations for genetic testing to members of a family with a strong breast and ovarian cancer history should also include male relatives, as they represent an understudied group of at-risk individuals.
In most BRCA1/2 testing programs, less than 10% of the individuals tested are men, even though there is an equal gender distribution in the population of male and female BRCA1/2 carriers. Because an estimated 500,000 Americans carry BRCA1 mutations alone [20], the population-attributable risk of cancer in both male and female BRCA1/2 carriers is significant. Also, a growing number of studies show that BRCA1/2 families are affected by cancers other than breast and ovarian [5, 9, 10], and males are likely to be the group most at-risk for these cancers.
As BRCA1/2 testing becomes more widely utilized, particularly in people unaffected by cancer, it is important to inform carriers that they are at risk for early cancer detection. The importance of screening for early cancer detection is growing given the general aging of the population coupled with improvements in treating other chronic diseases such as heart disease and diabetes. These factors contributing to increased longevity make it more likely that BRCA1/2 mutation carriers will develop other cancers, such as pancreatic, in their advanced age. While data support early screening for breast and ovarian cancer in BRCA1/2 carriers [21], pancreatic cancer screening is not currently recommended outside of research protocols [22]. We agree that any screening program should be conducted in a research setting and that patients should be educated about not only the procedure-related risks of screening but also the risks associated with the work-up of false positive results.
The observational nature of this study only allows conclusions based on associations and thus, this study (and others of similar design), can not claim a causative link between BRCA1 and BRCA2 mutations and pancreatic cancer. Also, as 56% of the study population is of Ashkenazi Jewish ancestry it is unclear to what extent the conclusions of this study are applicable to the general population. Finally, most patient referrals for BRCA testing came from gynecology and breast clinics so there may be a referral bias toward breast and ovarian cancers. However, these limitations only highlight the fact that the 11% prevalence in this study is most likely an underestimate of the true prevalence of pancreatic cancers in BRCA1 and BRCA2 families. This study also excluded pancreatic cancer patients with sequence variants of unknown significance which may later be found to be pathologic [23]. This may further underestimate the prevalence found in this study. Also, there may be other unknown factors that place BRCA1/2 carriers of Ashkenazi Jewish ancestry at an even more elevated risk based on the findings of this study. This relationship has been proposed in a paper studying germline BRCA2 mutations in Ashkenazi Jewish patients with pancreatic cancer [24] and may warrant further investigation.
In conclusion, we found that pancreatic cancer differentially affects males in BRCA1/2 families and that males represent an underrepresented at-risk population outside of the usual population referred for BRCA1/2 testing. We recommend that males in families with a strong history of breast, ovarian, and pancreatic cancer be considered for BRCA1/2 testing along with their female relatives.
Acknowledgments
The original publication is published in Familial Cancer available at www.springerlink.com.
This publication was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
ABBREVIATIONS
- SEER
Surveillance Epidemiology and End Results
References
- 1.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 2.King MC, Marks JH, Mandell JB New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou AC, Pharoah PD, Narod S, et al. Breast and ovarian cancer risks to carriers of the BRCA1 5382insC and 185delAG and BRCA2 6174delT mutations: a combined analysis of 22 population based studies. Journal of Medical Genetics. 2005;42(7):602–3. doi: 10.1136/jmg.2004.024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson D, Easton DF Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 5.The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310–6. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 6.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, et al. Netherlands Collaborative Group on Hereditary Breast Cancer (HEBON) Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. Journal of Medical Genetics. 2005;42(9):711–9. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56(23):5360–4. [PubMed] [Google Scholar]
- 8.Lynch HT, Deters CA, Snyder CL, et al. BRCA1 and pancreatic cancer: pedigree findings and their causal relationships. Cancer Genet Cytogenet. 2005;158(2):119–25. doi: 10.1016/j.cancergencyto.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22(4):735–42. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Offit K, Levran O, Mullaney B, et al. Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst. 2003;95(20):1548–51. doi: 10.1093/jnci/djg072. [DOI] [PubMed] [Google Scholar]
- 11.Lee R, Beattie M, Crawford B, et al. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genetic Testing. 2005;9(4):306–12. doi: 10.1089/gte.2005.9.306. [DOI] [PubMed] [Google Scholar]
- 12.Berry DA, Iversen ES, Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701–12. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- 13.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute; Bethesda, MD: 2007. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site. [Google Scholar]
- 14.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15(4):704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 15.Greer JB, Whitcomb DC. Role of BRCA1 and BRCA2 mutations in pancreatic cancer. Gut. 2007;56(5):601–5. doi: 10.1136/gut.2006.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nature Medicine. 1996;2(11):1179–83. doi: 10.1038/nm1196-1179. [DOI] [PubMed] [Google Scholar]
- 17.Evans DG, Howell A. Are BRCA1- and BRCA2-related breast cancers associated with increased mortality? Breast Cancer Res. 2004;6(1):E7. doi: 10.1186/bcr748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Research. 2004;6(1):R8–R17. doi: 10.1186/bcr658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robles-Díaz L, Goldfrank DJ, Kauff ND, et al. Hereditary ovarian cancer in Ashkenazi Jews. Fam Cancer. 2004;3(34):259–64. doi: 10.1007/s10689-004-9552-0. [DOI] [PubMed] [Google Scholar]
- 20.Whittemore AS, Gong G, John EM, et al. Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic Whites. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2078–83. [PubMed] [Google Scholar]
- 21.Bermejo-Pérez MJ, Márquez-Calderón S, Llanos-Méndez A. Effectiveness of preventive interventions in BRCA1/2 gene mutation carriers: a systematic review. International Journal of Cancer. 2007;121(2):225–31. doi: 10.1002/ijc.22817. [DOI] [PubMed] [Google Scholar]
- 22.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4(6):766–81. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Chenevix-Trench G, Healey S, Lakhani S, et al. Genetic and histopathologic evaluation of BRCA1 and BRCA2 DNA sequence variants of unknown clinical significance. Cancer Res. 2006;66(4):2019–27. doi: 10.1158/0008-5472.CAN-05-3546. [DOI] [PubMed] [Google Scholar]
- 24.Ozcelik H, Schmocker B, Di Nicola N, et al. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nature Genetics. 1997;16(1):17–18. doi: 10.1038/ng0597-17. [DOI] [PubMed] [Google Scholar]