Abstract
Dexamethasone (DX) induces apoptosis resistance in most solid malignant tumors during co-treatment with chemotherapy agents, such as camptothecin (CAM). In this study, we investigated the mechanism by which DX reduces chemotherapy efficiency in C6-glioma. DX reduced CAM-increased DNA fragmentation and caspase-3 activation. The DX’s protection was negated by RU486, an antagonist of glucocorticoid receptor (GR). DX itself increased anti-apoptotic gene, Bcl-xL expression, and its transcription factor, signaling transducer and activator of transcription 5 (Stat5), DNA binding activity and phospho-Stat5 expression. DX blocked the CAM-decreased Bcl-xL and phospho-Stat5 expression, and Stat5 binding activity. RU486 negated DX’s actions. To determine whether Stat5 regulates Bcl-xL expression in CAM -induced cell death, C6-glioma was infected with an adenovirus containing a constitutively activated Stat5-GFP (Ad-Stat5ca). Overexpression of Stat5ca increased Bcl-xL and decreased CAM-induced cell death compared to control adenovirus infected cells; whereas Stat5 siRNA decreased DX-induced Bcl-xL and increased cell death. Phospho-Stat5 expression was observed in the nuclear extract by co-immunoprecipitation with an anti-GR antibody, indicating that Stat5 and GR were interactive and formed a complex in the nuclei. These results suggest that DX’s prevention from CAM -induced apoptosis and RU486’s antagonism of DX’s protection may be through Stat5/Bcl-xL signal pathway regulated by a GR.
Keywords: Apoptosis, Bcl-xL, Camptothecin, C6-glioma, Dexamethasone, Stat5
Introduction
Glucocorticoids (GCs) such as dexamethasone (DX) are essential in the treatment of inflammatory disorders, such as rheumatoid arthritis, asthma and dermatitis, autoimmune diseases, tissue edema. These properties have made GCs one of the most frequently prescribed drugs worldwide. Moreover, GCs are commonly used as co-medication in cancer therapy [1]. In the early 1960’s, GCs were introduced for remission of induction of childhood leukemia [1]. Subsequently, the ability of GCs to efficiently kill lymphoid cells has led to their inclusion in all chemotherapy protocols for lymphoid malignancies [2]. GCs are also widely used as co-medication in cancer therapy for solid malignant tumors for their effectiveness in treating the malignant tumor or treatment-related edema, inflammation, pain, electrolyte imbalance, and to stimulate appetite, to prevent nausea and emesis, or toxic reactions caused by cytotoxic treatment [1, 3]. Before, during, and after chemotherapy for solid malignant tumors, GCs are given at varying doses to reduce acute toxicity in cancer patients, thus offering protection against the long-term effects of genotoxic drugs [3].
The cell type specific pro- and anti-apoptotic effects of GCs and its potential clinical implications have been mostly unknown until recently. GCs strongly induce apoptosis in cells of the hematological lineage, but also in some nonhematologic cells such as osteoblasts [4, 5]. In contrast, GCs support survival in several nonhematologic tissues, such as mammary gland [6, 7], ovary [8, 9], liver [10], fibroblasts [11, 12], and glioma [13-16]. Depending on the circumstances, GCs even exhibit pro- or anti-apoptotic potential in the same cell type. These GC-induced survival effects may become clinically relevant when they interfere with the effect of chemotherapeutics [17, 18]. While mechanisms of the chemotherapy agent, camptothecin’s (CAM) pro-apoptotic signaling are well studied, mechanisms by which GC plays an anti-apoptotic role in epithelial origin tumor cells are less well understood.
The most common glucocorticoids prescribed for brain tumors is dexamethasone (DX) [13, 19, 20]. DX has a dramatic effect on symptoms in patients with brain tumors by decreasing the blood-brain barrier permeability and the regional cerebral blood volume [21-23]. DX decreased edema in brain tumor may be counteract the action of vascular endothelial growth factor (VEGF) [24]. DX pre-treatment has been recently reported to interfere with apoptotic death in brain tumor cells by the transcriptional activation of a Bcl-xL gene [13-15, 25-27]. Patients treated with the combination of 1,3-Bis (2-chloroethyl)-1-nitrosourea (BCNU) and a high-dose of methylprednisolone have less effect than those treated with BCNU alone[18] leading to the claim that the beneficial effects of steroid treatment in patients with brain tumors must be weighed against the possibility that it may reduce the efficacy of chemotherapeutic drugs which act by inducing apoptosis.
Apoptosis, or programmed cell death (PCD), is mainly characterized by activation of caspases, mitochondrial depolarization, cell volume loss, chromatic condensation, and nucleosomal DNA fragmentation [28]. The members of Bcl-2 family of genes are regulators of apoptosis. The anti-apoptotic members of these proteins, such as Bcl-2 and Bcl-xL, enhance cell survival, while the pro-apoptotic members, such as Bax and Bcl-xS, promote cell death [29]. Bcl-xL is a protein that shares several anti-apoptotic features with Bcl-2, but the Bcl-x promoter is distinct from the Bcl-2 promoter and is under the control of different transcriptional activators [13-15, 25, 26]. Bcl-xL is thought to increase resistance to chemotherapy by inhibiting apoptosis. In a number of neoplasms including glioma, the expression of Bcl-xL promotes cell survival and decreases sensitivity to chemotherapy [14, 15].
The signaling transducer and activator of transcription 5 (Stat5) proteins are transcriptional factors. When activated Stats are dimerized and translocated into the nucleus, they bind to specific DNA elements, and activate the transcription of responsive genes. Seven mammalian Stat proteins (Stat1, Stat2, Stat3, Stat4, Stat5A, Stat5B, and Stat6) have been isolated. Stat5, one of these Stat proteins, is activated by the IL-2 and 3 family, growth hormone, erythropoietin, and thrombopoietin [30-32]. Stat5 is also known to play a role in transcriptional regulation of Bcl-x gene [26, 33-38]. Studies on interaction between Stats and other transcriptional regulators show that Stat5 may interact with YY-1, Sp1, C/EEPβ and GR [26, 35]. The mechanism by which DX prevents chemotherapy agent-induced apoptosis has not been fully understood. In this study, we investigated how Stat5 regulated the chemotherapy agent, CAM-induced apoptosis, especially regulated Bcl-xL expression in C6-glioma cells.
Materials and Methods
All the chemicals were purchased from Sigma (St. Louis, MO) unless otherwise specified. Polyclonal rabbit anti-Phospho-Stat5 (Tyr694) antibody was from Cell Signaling, (Beverly, MA). Monoclonal mouse anti-Stat5 antibody was from BD Transduction Laboratories, (San Diego, CA). Mouse anti-actin serum and GR antibody were from Santa Cruz Biotechnology, (Santa Cruz, CA). Secondary anti-mouse and anti-rabbit antibody conjugated with alkaline phosphatase was from Promega, (Madision, WI). Rat C6-glioma cells (American Type Culture Collection, Rockland, MD) were grown in HAM’s F-12 medium (BioWhittaker, Walkersville, MD) supplemented with 15% horse serum (BioWhittaker, MD), 2.5% fetal bovine serum (BioWhittaker, MD), 0.15% sodium biocarbonate, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life technologies, Grand Island, NY).
Treatment of cells
Camptothecin (topoisomerase I inhibitor), Dexamethasone (DX) and RU486 (Mifepristone, RU) were dissolved in dimethyl sulfoxide (DMSO) (10 mM as a stock solution). The final concentration of each drug was 2 μM. The cells were pre-treated with DX and/or RU486 (30 min before DX) for 6 hours or indicated time before CAM treatment.
DNA fragmentation
A Cell Death Detection enzyme-linked immunosorbent assay ELISA kit (Roche Applied Science, Germany) was used to quantify DNA fragmentation after induction of cell death. This assay determines the levels of histone-assoicated DNA fragments including mono- and oligonucleosomes in cell lysates [39-41], based on a sandwich-enzyme-immunoassay protocol using a monoclonal mouse-anti-DNA and histone antibody. Analysis was performed according to manufacturer’s instructions.
Caspase-3 activity
Caspase-3 specific activity was determined by using a colorimetric CaspACE™ Assay System (Promega) following manufacturer’s instruction. Briefly, cells were seeded onto culture plates and treated with DX and/or RU486 with or without CAM. Harvested cells were subjected to cell lysist buffer, incubated on ice for 20 minutes, and the supernatant fraction was collected for use as cell extract. The cell extract was incubated with 50 μM caspase-3 substrate, Ac-DEVE-p-NA for 4 h at 37°C. The amounts of released pNA were measured in a microplate reader at 405 nm. Protein concentrations in cell lysate were determined. Caspase-3 specific activities were expressed as pmol of pNA /μg protein/hour.
Cell death assay
LDH was measured as previously described after various drug treatments [42].
Western Blot
Cytoplasm and nuclear proteins were isolated from C6-glioma cells as described previously [43, 44]. Samples (30-50 μg of protein) were electrophoresed onto a 10-12% SDS-PAGE and transferred to polyvinylidenedifluoride (PVDF) membranes. The membranes were blocked in a TBST buffer containing 20 mM Tris-HCl, 5% nonfat milk, 150 mM NaCl, and 0.05% Tween 20, pH 7.5, for 1 hr at room temperature. Thereafter, the blot was incubated with a primary mouse anti-Bcl-xL (1:1,000), rabbit anti-phospho Stat5 (1:100), mouse anti-Stat5 (1:200), or mouse anti-actin antiserum (1:1,000), respectively overnight at 4 °C. The membrane was washed with TBST three times at 10 min intervals, incubated with the second antibody (anti-rabbit or anti-mouse IgG conjugated with alkaline phosphatase; 1:5,000 dilution) at room temperature for 1 hr, and then washed three times each at 10 min intervals with TBST. The color reaction was developed by the Blot AP System according to the technical manual provided by Promega.
Isolation of nuclear proteins
C6-glioma cells were collected after the treatment. Nuclear proteins were extracted following the high-salt method previously described [45]. Homogenization and extraction conditions have been described in detail elsewhere[43].
Electrophoretic mobility shift assay (EMSA)
Gel shift assays to assess Stat5 binding activity have been described in detail elsewhere [43, 46]. The following consensus oligonucleotides were used Stat5: 5’-TTTGGAGAAAGGCATTTCGGAGAAAAG-3’ (sense) and 3’-AAACCTCTTTCCGTAAAGCCTCTTTTC-5’ (antisense). The oligonucleotide probes were labeled with γ-32P ATP according to Promega technical bulletin number 106. The binding reaction was performed in a total volume of 20 μl containing the binding buffer (10 mM Tris-HCl, 20 mM NaCl, 1 mM DTT, 1 mM EDTA, 5% glycerol, at pH 7.6), 0.0175 pmol of labeled probe (>10,000 cpm), 20 μg of nuclear protein and 1 μg of poly dIdC. After incubation for 20 min at room temperature, the mixtures were subjected to electrophoresis in a nondenaturing 6% polyacrylamide gel at 180 V for 2 hrs under low ionic strength conditions. The gel was dried and subjected to autoradiography as described previously [43, 45].
Quantitative activated Stat5 by ELISA
Stats activities were determined by TransAM Stats family kits from Active Motif (Carlsbad, CA). All assays were performed following the manufactory instruction after the nuclear protein extraction [43].
Construction of full length of Stat 5a
Full length Stat5a cDNA [47] was obtained by RT-PCR using total RNA from rat as template. The following oligonucleotide primer pairs were used for PCR reactions: 5’-AGGTGAACAGCCATGGCGGGC-3’ and 5’-TCAGGACAAGGAGCTTCTGGC-3’ for Stat5a. PCR fragments of Stat5a were cloned into pGEM-T Easy vector (Promega Co). After DNA sequence verification, the fragments were subcloned (see below).
Construction of constitutively active Stat5 mutant (Stat5ca)
Constitutively active Stat5a was made by mutating histidine 298 to arginine and serine 710 to phenylalanine from the full length of Stat5a using site-directed mutagenesis. Histidine 298 is located upstream of the putative DNA binding domain and serine 710 is located in the transactivation domain. The double mutant (H298R and S710F) of Stat5a from full length Stat5a has been reported to be constitutively active [47]. Mutagenesis was performed using the QuickChange site-directed mutagenesis kit (200519) from Stratagene. The parental wild-type plasmids were removed by digestion with selective restriction enzymes. The oligonucleotides used for mutagenesis are as follows: 5’- CGCAGGGCTGAGCGCCTG TGCCAGCAG -3’ for H298R, and 5’- GTTTGTCAATGCTTTTGCAGATGCTGGAG -3’ for S710F (underlines represent mutated bases). DNA sequences were verified.
Construction of adenovirus vectors
The pMX-IRES-GFP vector [48] was digested with NotI-AfIII to obtain IRES-GFP, which was then subcloned into NotI-AfIII sites of the p-Shuttle vector (K1650-1, Clontech). The pGEM-T vector carrying Stat5ca was digested with NotI-NotI and subcloned into the p-Shuttle vector. After sequence verification, the p-Shuttle vectors (containing Stat5ca-IRES-GFP and IRES-GFP), were subcloned into the Adeno-X expression system (Clontech) using PI-SceI and I-Ceu I. The resultant Adeno-X DNAs containing Stat5 was transformed into E coli for amplification, and were purified and analyzed by PCR. Recombinant Adeno-X viral DNAs were confirmed, and amplified. The recombinant Adeno-X DNAs were linearized by digestion with PacI for transfection.
Production of adenovirus in mammalian cells
Ten μg of recombinant Adeno-X DNAs carrying Stat5ca-IRES-GFP and IRES-GFP were used to transfect HEK 293 cells at a density of 2 × 106 per 60 mm dish with calcium phosphate (K2051-1 Calphos Mammalian transfection kit, B& D Bioscience). Transfected cells were monitored with GFP fluorescence. The two adenoviruses were collected from the transfected 293 cells 10-14 days after transfection and were amplified for another 10-14 days for high-titer stocks. To concentrate adenovirus, we used a BD Adeno-X virus purification kit (K1654-1), and titers were determined by using Adeno-X rapid titer kits (K1653-1, Clontech). Infectious units (ifu/ml) were calculated for each well as follows: (infected cells/field) × (fields/well)/volume virus (ml) × (dilution factor). We obtained titers in the range of 108 - 1010 ifu/ml.
Infection of C6-glioma with adenovirus: 1×105 C6-glioma cells grown in plates or dishes for 24 hrs were infected with the virus for 48hrs at a final titer of 107 ifu/ml. The culture medium was changed, and cultures received various treatments.
RNA interference
Stat5 siRNA was used to knockdown Stat5 (Qiagen). Duplex siRNA sequences are as follows: sense (5’-GCAGUCGACGGAUACGUGAdTdT-3’) and antisense (5’-UCACGUAUCCGUCGACUGCdTdT-3’). A nonspecific duplex was used as a control siRNA which did not affect Stat5 mRNA levels relative to the untransfected controls. siRNAs in Opti-MEM medium was mixed with lipofectamine 2000 in Opti-MEM medium (Invitrogen) for 20 min, then added to glioma-C6 cultures (without antibiotics) for 48 hours (final concentration was 200 nM). After transfection, the medium was changed to DMEM/F12 and DX (2μM) was added for 6 hours followed by CAM (2 μM) for 24 hours. Cytitoxicity was measured by LDH and the cells were collected for determining Bcl-xL protein level.
Co-immunoprecipitation
Nuclear extract was pre-cleaned with Protein A sepharose and the protein was incubated with a rabbit anti-GR antibody (Santa Cluz, CA)at a concentration of 2 μg/ml at 4 °C overnight. Protein A sepharose was added to the antigen-antibody mixture and incubated with a gentle agitator for another 1-2h. The immunoprecipitates were washed with 500 μl of the lysis buffer containing 0.5 M NaCl for three times and with 500 μl of lysis buffer (without NaCl) once only, then resuspended and boiled in SDS loading buffer, separated on an 10% SDS-polyacrylamide gel, transferred to PVDF membrane, and further analyzed by Western blotting using rabbit anti-phospho-Stat5.
Results
Dexamethasone prevented the CAM-induced DNA fragmentation in a time-dependent manner
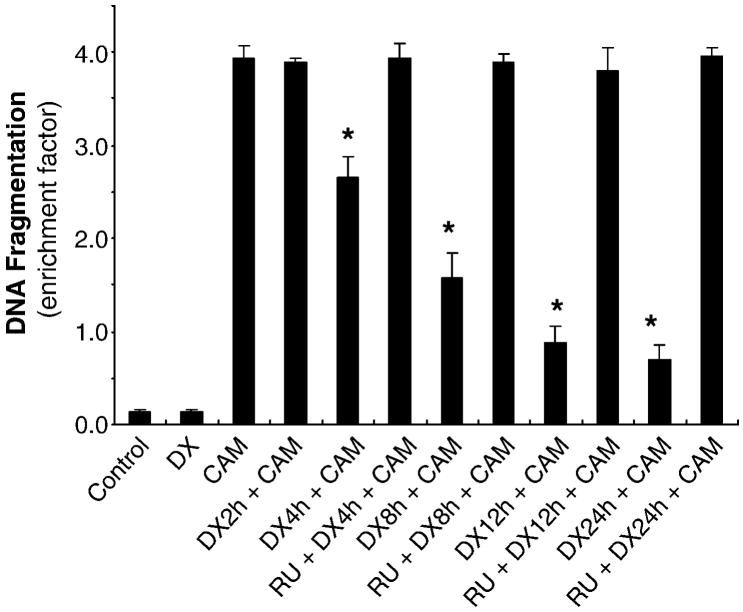
C6-glioma cells treated with CAM increased DNA fragmentation determined by a Cell Death Detection ELISA kit, which detects the levels of histone-associated DNA fragments, including mono- and oligo-nucleosomes in cell lysate. CAM (2 μM) showed a significant increase in DNA fragmentation (23 folds over the control, Fig 1). DX (2 μM) pre-treatment interfered with CAM-induced apoptotic death. The extent of inhibition of CAM-induced DNA fragmentation by DX was dependent on the time of DX pre-exposure (before CAM) starting at 4hrs and gradually increasing up to 24 hrs. Pre-treatment with DX for 2hrs did not have any protection (Fig 1). RU 486, a glucocorticoid receptor antagonist, completely negated the DX protection when added 30 min prior to DX (Fig 1).
Fig. 1.
DX-inhibiting CAM-increased DNA fragmentation depends on pre-exposure time. C6-glioma was pretreated with DX (2 μM) for 2, 4, 8, 12 and 24 h followed by CAM (2 μM) treatment for another 20 h. The cell lysate was collected for DNA fragmentation by ELISA kit. The value expressed as mean ± SD with duplicates in three experiments. P < 0.01 compared DX + CAM treatment to CAM treatment only.
Glucocorticoid receptor (GR) regulated the CAM-induced apoptosis
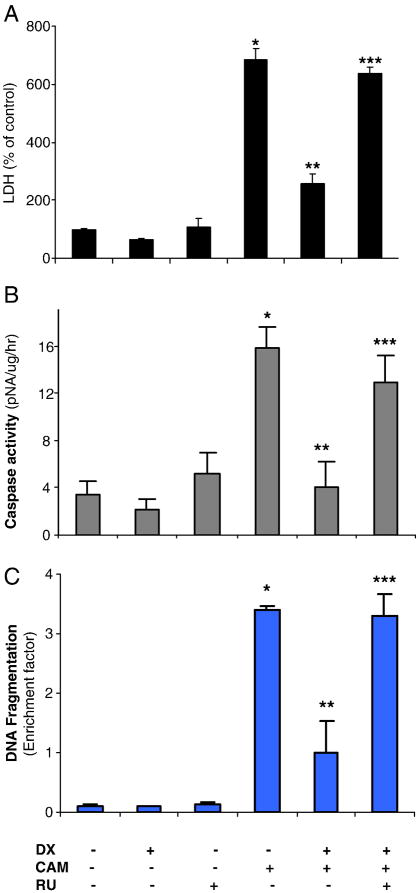
Chemotherapy agent, CAM, has been known to induce tumor cells apoptosis [10, 49-51]. To assess whether GR is involved in regulation of the CAM-induced cell death, we treated the cells with DX (6hrs) and/or RU 486 (30 min before DX) with or without CAM. We measured LDH level in the medium 24hrs after CAM treatment. CAM (2μM) significantly increased LDH release by 6 fold over the control. DX reduced LDH release and RU486 reversed DX’s effect significantly (Fig 2A). We also measured the caspase-3 activity by the colorimetric caspase-3 assay with CaspACE™ using Ac-DEVE-p-NA as a substrate. CAM alone increased caspase-3 activity up to 15.9 pmol pNA/μg/hour, compared to 3.5 pmol pNA/μg/hour in untreated control cells. DX completely blocked the CAM-induced caspase-3 activation. RU486 reversed the DX effect (Fig 2B). DX and RU 486 alone did not have any significant effect on caspase-3 activation. We also determined the levels of histone-associated DNA fragments. CAM showed significantly increased DNA fragmentation (26 folds over the control, Fig 2C). While DX (2 μM) reduced this effect significantly. RU486 reversed the protective effect of DX (Fig 2C). DX and RU486 alone did not show any significant toxicity.
Fig. 2.
GR regulates CAM-induced cell death determined by LDH, caspase-3 activity and DNA fragmentation. C6-glioma was pretreated with RU (2 μM) for 30 min followed by DX (2 μM) for 6 h and then followed by CAM (2 μM) treatment for another 20 h. The medium was collected for LDH assay (A). The LDH levels expressed as % of the control level (each value-basal (medium only)/control-basal) with triplicates in three experiments. The cells were collected for assay of caspase-3 activity determined by colorimetric kit (B). The caspase-3 activity expressed as pNA/ug protein/hr with mean ± SD in triplicates of three experiments. DNA fragmentation was determined by ELISA and was expressed as enrichment factor with triplicates in three experiments (C). The data were expressed as mean ± SD in triplicates of three experiments. P < 0.01, compared CAM to control; compared CAM to DX+CAM; compared RU + DX + CAM to DX + CAM.
DX increased Bcl-xL expression and Stat5 activation
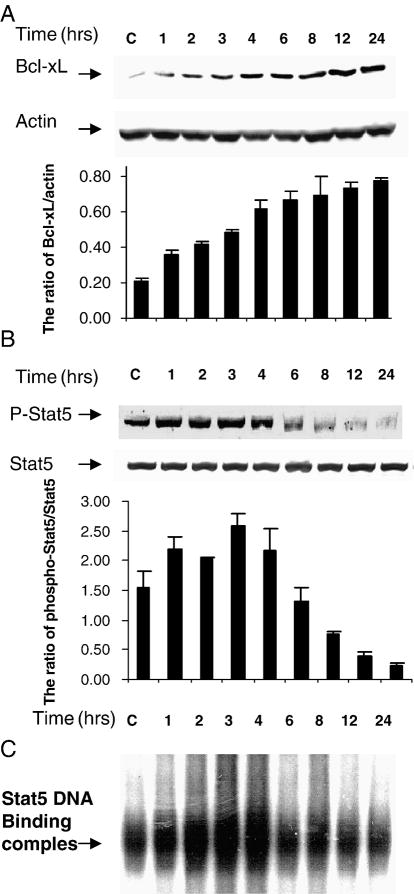
It has been reported that DX inhibits apoptosis through increasing Bcl-xL expression in C6- glioma cells [15, 52]. In order to compare the change of Bcl-xL, Phospho-Stat5 and Stat5 binding activity after DX treatment, we did time-courses for each. We found that DX alone increased Bcl-xL expression starting at 2hrs and gradually increasing up to 24hrs (Fig 3A). To further study whether Stat5 is involved in the regulation of DX- up-regulated Bcl-xL expression, we examined phosphorylated-Stat5 (phospho-Stat5) expression determined by Western Blot and Stat5 binding activity determined by an electrophoretic mobility shift assay (EMSA). Phospho-Stat5 expression in nucleus was increased at 1h and declined at 6hrs after DX treatment. Total Stat5 in cytoplasm was not changed 1-24h after DX treatment (Fig 3B). Densitometry assay showed that Bcl-xL levels were increased gradually and the ratio of phospho-Stat5/Stat5 increased at 1h and declined at 6hrs (Fig 3A and 3B). Stat5 DNA binding activity was also increased starting at 1h and declining at 6h after DX treatment. The change of Stat5 binding activity by DX was paralleled to that of the phospho-Stat5 level in the nuclear extract (Fig 3C). These results imply that the elevation of Bcl-xL level may be regulated by activation of its transcription factor, Stat5.
Fig. 3.
DX increases Bcl-xL, phospho-Stat5 expression and Stat5 DNA binding activity in a time-dependent manner. C6-glioma was treated with DX (2 μM) for 1, 2, 3, 4, 6, 8, 12 and 24 h. Cytoplasm proteins for Bcl-xL and Stat5 expression and nuclei protein for phospho-Stat5 expression were extracted for Western blot analysis blotted with anti-Bcl-xL (A), Stat5 and Phospho-Stat5 antibodies (B). Densitometry was performed with Quantity one software (Bio-Red) and expressed as ratio of Bcl-xL/actin and ratio of phospho-Stat5/Stat5 with mean ± SD from three blots (A and B). Stat5 DNA binding activity in nuclei protein was determined by EMSA (C).
GR was involved in the CAM altered Bcl-xL expression and Stat5 activation
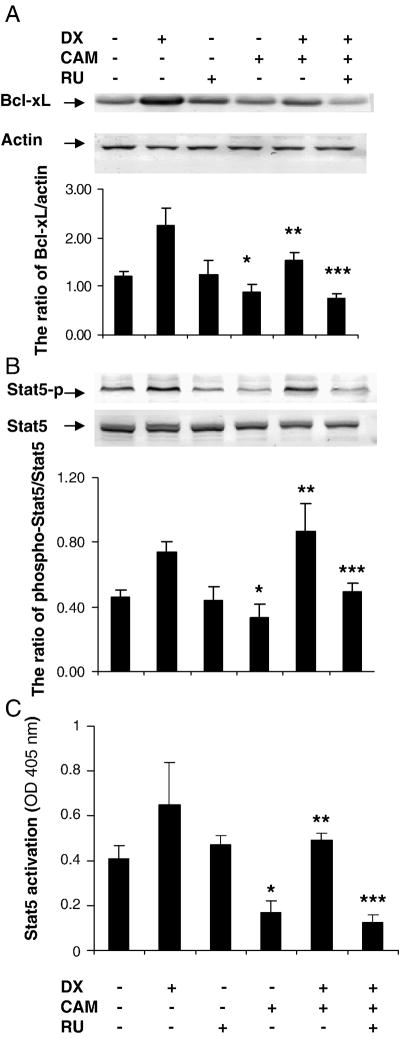
C6-glioma cells were treated with CAM (2 μM) for 24hrs with and without pretreatment of DX (6hrs) and/or RU486 (30 min before DX). Bcl-xL and phospho-Stat5 expression was decreased after CAM treatment. DX blocked the reduction of Bcl-xL expression and the ratio of phospho-Stat5/Stat5 induced by CAM. RU486 reversed DX’s effects (Fig 4A and B). We measured Stat5 activity after the same treatment as above using a sensitive and quantitative transcription factor ELISA kit (Active Motif). CAM reduced Stat5 activation significantly as compared to the control. DX blocked this effect and RU486 antagonized the DX’s action (Fig 4C). These results indicate that GR may be involved in regulation of Stat5/Bcl-xL pathway.
Fig. 4.
GR regulates Bcl-xL and phospho-Stat5 expression. C6-glioma was pretreated with RU (2 μM) for 30 min, DX (2 μM) for 6 h followed by CAM (2 μM) treatment for another 20–24 h (A). Cytoplasm protein was extracted for Western blotting for Bcl-xL (A) and Stat5 (B). Nuclear protein was extracted for Phospho-Stat5 expression (B) by Western blot. Actin served as control for equal loading protein. Densitometry was performed with Quantity one software (Bio-Red) and expressed by ratio of Bcl-xL/actin and phospho-Stat5/Stat5 with mean ± SD from three blots (A and B). Stat5 activity was determined by ELISA kit (Active Motif). The value of Stat5 activity (OD) was expressed as mean ± SD (each value minus basal value) with triplicates in two experiments (B). P < 0.05, compared CAM to control; P < 0.01 compared DX + CAM to CAM and compared RU + DX + CAM to DX + CAM.
Overexpression or knockdown of Stat5 on Bcl-xL expression and cell cytotoxicity
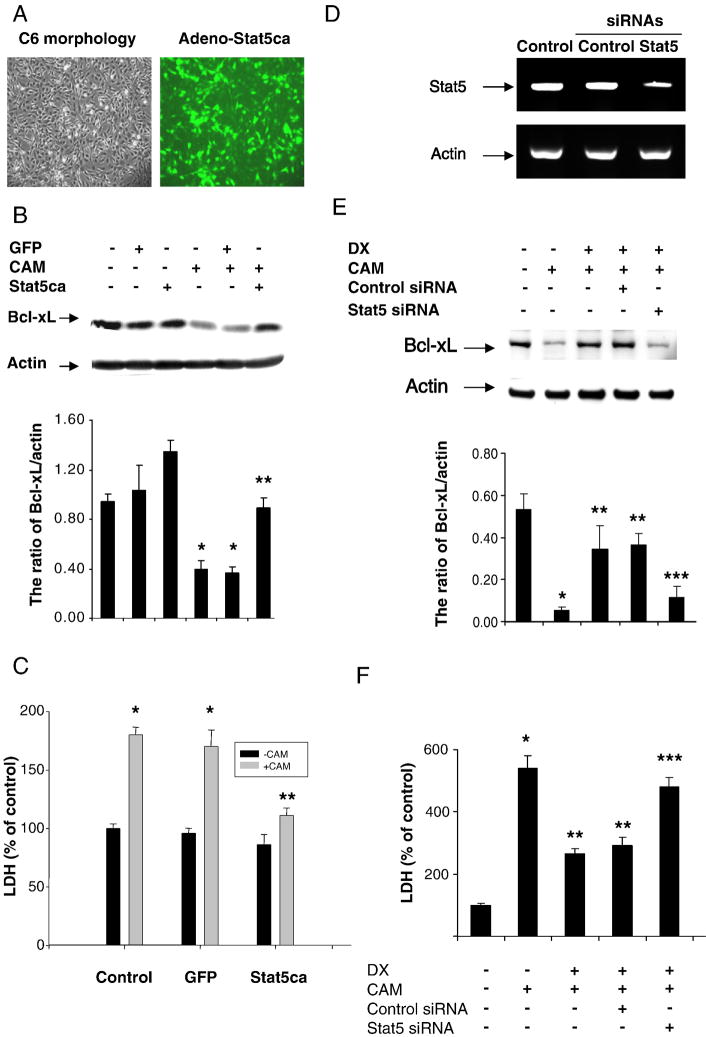
To determine whether Stat5 regulates Bcl-xL expression and whether Stat5 affects CAM-induced cell death, we constructed adenoviruses carrying constitutively activated Stat5 gene-GFP (Ad-Stat5ca) and Ad-GFP only as a control. C6-glioma cells were infected with the adenoviruses. The transduction efficiency was about 80-90% shown as in Fig 5A. Bcl-xL expression level was higher in the C6-glioma infected with Ad-Stat5ca than that in the C6 cells infected with the control virus only in the presence or absence of CAM treatment (Fig 5B). Accordingly, CAM-induced cell death was less in the C6 infected with Ad-Stat5ca than that in C6 infected with the control Ad-GFP only (Fig 5C). To further confirm Stat5 regulating Bcl-xL and cell death, we used siRNA to knockdown Stat5 expression. Stat5 siRNAs treatment resulted in an attenuation of DX-induced Bcl-xL (Fig. 5E) detected by Western blot and DX’s protective activity determined by LDH assay (Fig. 5F), concomitant with knockdown of Stat5 mRNA (Fig. 5D).
Fig. 5.
Effect of Constitutively activated Stat5 (Stat5ca) or Stat5 siRNA on CAM-induced cell death. C6-glioma was infected with adenovirus containing constitutively activated Stat5 (Stat5ca)-GFP and control GFP for 24 h and then were treated with CAM (2 μM) for another 24 h. The adenovirus infected cells demonstrated green fluorescence (A). Bcl-xL expression was detected by Western blot with or without CAM treatment. Densitometry was performed and the data were expressed by ratio of Bcl-xL/actin with mean ± SD from three blots (B). The cell death was measured by LDH assay with or without CAM treatment (C). Stat5 siRNA (200 nM) applied to C6-glioma for 48 h and the cells treated with DX for 6 h followed by CAM. mRNA was extracted and RT-PCR performed to detect Stat5 mRNA levels (D). The cytoplasmic proteins were extracted for determining Bcl-xL by Western blot (E). The data were expressed as ratio of Bcl-xL/actin from three blots. The cell death was measured by LDH assay. The LDH levels expressed as % of the control level in three experiments with triplicates. P < 0.01, compared CAM to control; compared infection of adeno-Stat5ca + CAM to control adeno-GFP + CAM or compared DX + CAM with or without Control siRNA with CAM; compared Stat5 siRNA + CAM + DX to Control siRNA + CAM + DX.
GR was associated with Stat5 in nuclei
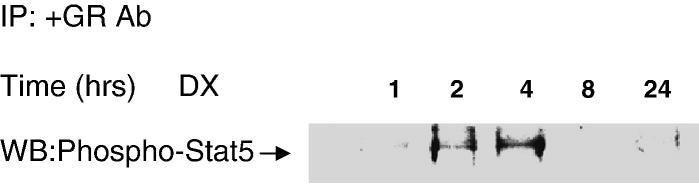
Glucocorticoids have been shown to act as a transcriptional co-activator for Stat5 and enhance Stat5-dependent transcription [53-55]. We examined whether a GR can be physically associated with Stat5 in C6-glioma cells after DX treatment. Nuclear extracts were prepared and co-immunoprecipitated with a GR specific antibody; immunoprecipitates were developed on Western Blots with a phospho-Stat5 specific antibody. Untreated cells showed minimal activity of phospho-Stat5 whereas the cells treated with DX for 4hrs increased phosphorylated Stat5 expression, and declined after 8hrs (Fig 6).
Fig. 6.
GR interacts with Stat5 in nucleus. 500 mg of nuclei protein of C6-glioma was collected at various times after DX 2 μM treatment and was pre-incubated with anti-GR antibody (2 μg/ml) overnight. The protein was developed by Western blot with phospho-Stat5 antibody.
Discussion
Glucocorticoid (GC) induces resistance to chemotherapy agent-induced apoptosis in epithelial origin tumor cells. GCs have been found to interfere with death receptor pathways, such as CD95-I, TRAIL, FADD and caspase-8 and caspase-3 activities [56]. Pretreatment with DX prevents chemotherapy-induced depolarization of mitochondrial membrane potential and caspase-9 activities [39, 57]. DX-mediated suppression of apoptosis in tumor cells has been reported through a cross-talk between GR and other transcription factors, such as AP-1, NF-κB, and NF-AT to activate anti-apoptotic genes or inhibit pro-apoptotic genes [58-60]. DX is also reported to reduce temozolomide (TMZ)-increased calpain activity, the ratio of Bax/Bcl-2 and apoptosis [16]. Calpain has been known to cleave Stats as functional dominant-negative proteins in platelets and mast cells [61]. This may be another mechanism for DX resistant to chemotherapy. It has not been reported that Stat5 regulated GCs-induced Bcl-xL expression via GR in glioma cells. The detailed mechanism for inhibition of apoptosis by GCs is not fully clear [62, 63].
Although it has been known that DX-induced resistance to chemotherapy agents is via enhancing the anti-apoptotic gene, Bcl-xL gene in glioma cells [15, 52, 64] and other solid malignant cells [65-67]. The mechanism by which DX increases the Bcl-xL level has not been fully understood. In this study we investigated whether GR is involved in transcriptional regulation of Bcl-xL expression in C6- glioma cells. Our results showed that DX resisted the CAM-induced apoptosis depending on the time of DX’s pretreatment. In shorter treatments, such as 2hrs prior to CAM, DX did not have any protection on CAM- induced apoptosis implying the resistance needs some process to be established. We found that DX treatment for 4-24 hours reduced CAM cytotoxicity. The pre-treated time is similar to others (8-24hrs) [14]. The difference may depend on the concentration of DX. RU 486 significantly reversed DX’s protection indicating that GR is involved in DX’s resistance.
Bcl-xL, a member of the Bcl2 family, is an anti-apoptotic gene on mitochondria. Several transcription factors are present in the Bcl-xL promoter region, such as NF-kB, Stat3, Stat5, and AP-1. Glucocorticoids (GCs) have been shown to enhance NF-kB activity in MCF7 breast cancer cells [62]. In contrast, GCs also inhibit paclitaxel-induced apoptosis by inhibition of NF-kB activation[68, 69]. The role of GC-regulated NF-kB may be dependent on the cell types. Other transcription factors, Stat family, including Stat3 and Stat5, promote uncontrolled growth and survival through the interference of gene expression, including cyclin D1, c-Myc, Bcl-xL, Mcl-1, and survivin genes, and thereby contribute to oncogenesis [70]. DX increased Bcl-xL expression, is in a time-dependent manner. The change in ratio of phospho-Stat5/Stat5 and Stat5 binding activity were parallel with a peak time of 3-4hrs after DX treatment. Total Stat5 level did not change suggesting that the transcriptional up-regulation of Bcl-xL may be via activation of Stat5 phosphorylation and translocation from cytoplasm to nucleus. Dominant-negative Stat5 or antisense Stat5 inhibits growth and induces apoptosis in T47D-derived tumors in nude mice [71] and selected human leukaemic cell lines [72]. Knockdown Stat5 by siRNA decreased Bcl-xL expression and blocked DX’s attenuation of CAM-induced cell death. Our results are in agreement with others. Growth factor (GH) and prelactin (PRL) protect beta-cells against cytotoxic cytokines via STAT5-dependent mechanisms possibly at the level of Bcl-xL [73]. The survival of thrombopoietin-dependent leukaemia cell line UT-7/TPO and normal megakaryocytic progenitors is via the induction of Bcl-xL. Thrombopoietin induced the binding of Stat5 and subunits of nuclear factor kappa B, p50, and c-Rel to the Bcl-x gene promoter [74].
A relevant role for Stat5 and Bcl-xL has been known as apoptosis-regulatory proteins in the pathogenesis of lung cancer [38]. Stat5 is also a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells [48]. To our knowledge, DX increased Bcl-xL via the activation of Stat5 has not been reported. Whether GR is involved in regulating Stat5 activation and Bcl-xL expression has not been explored. Our results in this study showed that the CAM reduced Bcl-xL and phospho-Stat5 expression, and Stat5 binding activity were reversed by pre-treatment of DX. RU 486 blocked DX’s effects indicating that GR participates in Stat5 activation.
To further confirm that Stat5 mediates Bcl-xL, we applied a constitutively activated Stat5 mutant to C6-glioma cells with or without CAM treatment. A constitutively activated Stat5 mutant (Stat5ca) blocked the CAM-reduced Bcl-xL level in the infected C6-glioma indicating that Stat5ca regulated Bcl-xL expression directly. Stat5ca reduced CAM-induced cytotoxicity in the infected C6-glioma indicating that activation of the Stat5/Bcl-xL pathway reduced C6-glioma death. In contrast, knockdown of Stat5 by Stat5 siRNA blocked DX-induced Bcl-xL expression and DX’s protection. Therefore, DX resistance to CAM-induced apoptosis could be via Stat5 activation leading to Bcl-xL expression suggesting that DX resistance to the CAM-induced apoptosis may be through activation of the Stat5/Bcl-xL signaling pathway.
Using a protein co-immunoprecipitation method we showed that GR and phospho-Stat5 were associated and interacted in C6-glioma nuclear extracts indicating that GR may cross talk with Stat5 or co-activate Stat5 enhancing Bcl-xL expression. Immunoprecipitation with GR antibody showed that phospho-Stat5 expression was altered during the time period of DX treatment. This change was in agreement with phospho-Stat5 expression in nuclear extract by Western blot (Fig 3) indicating that GR and Stat5 were interacted in the cell nuclei.
In summary, DX treatment resistance to chemotherapy was through activation of the Stat5/Bcl-xL signaling pathway mediated by a GR. Therefore, it is necessary to re-evaluating the use of DX in conjunction with chemotherapy agents in clinical cancer therapy.
Acknowledgments
We thank Dr Jae Ho Cho for technical assistance and Mrs Johanna Caraway for revising the manuscript. This work is supported by grants from Monsanto (DYY) and NIH RO1 40625 (JX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rutz HP, Herr I. Interference of glucocorticoids with apoptosis signaling and host-tumor interactions. Cancer Biol Ther. 2004;3:715–718. doi: 10.4161/cbt.3.8.966. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11:S45–S55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 3.Rutz HP. Effects of corticosteroid use on treatment of solid tumours. Lancet. 2002;360:1969–1970. doi: 10.1016/S0140-6736(02)11922-2. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord. 2001;2:65–73. doi: 10.1023/a:1010007108155. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg MN, Dharmarajan AM, Waddell BJ. Glucocorticoids and progesterone prevent apoptosis in the lactating rat mammary gland. Endocrinology. 2002;143:222–227. doi: 10.1210/endo.143.1.8584. [DOI] [PubMed] [Google Scholar]
- 7.Feng Z, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J Cell Biol. 1995;131:1095–1103. doi: 10.1083/jcb.131.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasson R, Amsterdam A. Pleiotropic anti-apoptotic activity of glucocorticoids in ovarian follicular cells. Biochem Pharmacol. 2003;66:1393–1401. doi: 10.1016/s0006-2952(03)00489-1. [DOI] [PubMed] [Google Scholar]
- 9.Sasson R, Shinder V, Dantes A, Land A, Amsterdam A. Activation of multiple signal transduction pathways by glucocorticoids: protection of ovarian follicular cells against apoptosis. Biochem Biophys Res Commun. 2003;311:1047–1056. doi: 10.1016/j.bbrc.2003.10.097. [DOI] [PubMed] [Google Scholar]
- 10.Ikegami T, Matsuzaki Y, Al Rashid M, Ceryak S, Zhang Y, Bouscarel B. Enhancement of DNA topoisomerase I inhibitor-induced apoptosis by ursodeoxycholic acid. Mol Cancer Ther. 2006;5:68–79. doi: 10.1158/1535-7163.MCT-05-0107. [DOI] [PubMed] [Google Scholar]
- 11.Hammer S, Sauer B, Spika I, Schraut C, Kleuser B, Schafer-Korting M. Glucocorticoids mediate differential anti-apoptotic effects in human fibroblasts and keratinocytes via sphingosine-1-phosphate formation. J Cell Biochem. 2004;91:840–851. doi: 10.1002/jcb.10766. [DOI] [PubMed] [Google Scholar]
- 12.Viegas LR, Vicent GP, Baranao JL, Beato M, Pecci A. Steroid hormones induce bcl-X gene expression through direct activation of distal promoter P4. J Biol Chem. 2004;279:9831–9839. doi: 10.1074/jbc.M312402200. [DOI] [PubMed] [Google Scholar]
- 13.Das A, Banik NL, Patel SJ, Ray SK. Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and preventing proteolytic activities. Mol Cancer. 2004;3:1–10. doi: 10.1186/1476-4598-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorman AM, Hirt UA, Orrenius S, Ceccatelli S. Dexamethasone pre-treatment interferes with apoptotic death in glioma cells. Neuroscience. 2000;96:417–425. doi: 10.1016/s0306-4522(99)00565-5. [DOI] [PubMed] [Google Scholar]
- 15.Ni Chonghaile T, Concannon CG, Szegezdi E, Gorman AM, Samali A. Dexamethasone inhibits apoptosis in C6 glioma cells through increased expression of Bcl-XL. Apoptosis. 2006;11:1247–1255. doi: 10.1007/s10495-006-7233-1. [DOI] [PubMed] [Google Scholar]
- 16.Sur P, Sribnick EA, Patel SJ, Ray SK, Banik NL. Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia. 2005;50:160–167. doi: 10.1002/glia.20168. [DOI] [PubMed] [Google Scholar]
- 17.Ochiai H, Pernell CT, Archer GE, Chewning TA, McLendon RE, Friedman HS, Sampson JH. Treatment of neoplastic meningitis with intrathecal 9-nitro-camptothecin. Neurol Med Chir (Tokyo) 2006;46:485–489. doi: 10.2176/nmc.46.485. discussion 489-490. [DOI] [PubMed] [Google Scholar]
- 18.Piette C, Munaut C, Foidart JM, Deprez M. Treating gliomas with glucocorticoids: from bedside to bench. Acta Neuropathol. 2006;112:651–664. doi: 10.1007/s00401-006-0100-x. [DOI] [PubMed] [Google Scholar]
- 19.Ito S, Rachinger W, Stepp H, Reulen HJ, Stummer W. Oedema formation in experimental photo-irradiation therapy of brain tumours using 5-ALA. Acta Neurochir (Wien) 2005;147:57–65. doi: 10.1007/s00701-004-0422-1. discussion 65. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro WR, Posner JB. Corticosteroid hormones. Effects in an experimental brain tumor. Arch Neurol. 1974;30:217–221. doi: 10.1001/archneur.1974.00490330025004. [DOI] [PubMed] [Google Scholar]
- 21.Hossmann KA, Hurter T, Oschlies U. The effect of dexamethasone on serum protein extravasation and edema development in experimental brain tumors of cat. Acta Neuropathol (Berl) 1983;60:223–231. doi: 10.1007/BF00691870. [DOI] [PubMed] [Google Scholar]
- 22.Jarden JO, Dhawan V, Moeller JR, Strother SC, Rottenberg DA. The time course of steroid action on blood-to-brain and blood-to-tumor transport of 82Rb: a positron emission tomographic study. Ann Neurol. 1989;25:239–245. doi: 10.1002/ana.410250306. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro WR, Hiesiger EM, Cooney GA, Basler GA, Lipschutz LE, Posner JB. Temporal effects of dexamethasone on blood-to-brain and blood-to-tumor transport of 14C-alpha-aminoisobutyric acid in rat C6 glioma. J Neurooncol. 1990;8:197–240. doi: 10.1007/BF00177352. [DOI] [PubMed] [Google Scholar]
- 24.Machein MR, Kullmer J, Ronicke V, Machein U, Krieg M, Damert A, Breier G, Risau W, Plate KH. Differential downregulation of vascular endothelial growth factor by dexamethasone in normoxic and hypoxic rat glioma cells. Neuropathol Appl Neurobiol. 1999;25:104–112. doi: 10.1046/j.1365-2990.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 25.Cuevas P, Diaz-Gonzalez D, Sanchez I, Lozano RM, Gimenez-Gallego G, Dujovny M. Dobesilate inhibits the activation of signal transducer and activator of transcription 3, and the expression of cyclin D1 and bcl-XL in glioma cells. Neurol Res. 2006;28:127–130. doi: 10.1179/016164106X97982. [DOI] [PubMed] [Google Scholar]
- 26.de Groot RP, Raaijmakers JA, Lammers JW, Koenderman L. STAT5-Dependent CyclinD1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol Cell Biol Res Commun. 2000;3:299–305. doi: 10.1006/mcbr.2000.0231. [DOI] [PubMed] [Google Scholar]
- 27.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 28.Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage (Review) Oncol Rep. 2005;14:595–599. [PubMed] [Google Scholar]
- 29.Kim R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem Biophys Res Commun. 2005;333:336–343. doi: 10.1016/j.bbrc.2005.04.161. [DOI] [PubMed] [Google Scholar]
- 30.Bovolenta C, Pilotti E, Mauri M, Turci M, Ciancianaini P, Fisicaro P, Bertazzoni U, Poli G, Casoli C. Human T-cell leukemia virus type 2 induces survival and proliferation of CD34(+) TF-1 cells through activation of STAT1 and STAT5 by secretion of interferon-gamma and granulocyte macrophage-colony-stimulating factor. Blood. 2002;99:224–231. doi: 10.1182/blood.v99.1.224. [DOI] [PubMed] [Google Scholar]
- 31.Packham G, White EL, Eischen CM, Yang H, Parganas E, Ihle JN, Grillot DA, Zambetti GP, Nunez G, Cleveland JL. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Kirken RA, Furian L, Janczewska S, Qu X, Hancock WW, Wang M, Tejpal N, Kerman R, Kahan BD, et al. Allograft rejection requires STAT5a/b-regulated antiapoptotic activity in T cells but not B cells. J Immunol. 2006b;176:128–137. doi: 10.4049/jimmunol.176.1.128. [DOI] [PubMed] [Google Scholar]
- 33.Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- 34.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 35.Dumon S, Santos SC, Debierre-Grockiego F, Gouilleux-Gruart V, Cocault L, Boucheron C, Mollat P, Gisselbrecht S, Gouilleux F. IL-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene. 1999;18:4191–4199. doi: 10.1038/sj.onc.1202796. [DOI] [PubMed] [Google Scholar]
- 36.Garcon L, Rivat C, James C, Lacout C, Camara-Clayette V, Ugo V, Lecluse Y, Bennaceur-Griscelli A, Vainchenker W. Constitutive activation of STAT5 and Bcl-xL overexpression can induce endogenous erythroid colony formation in human primary cells. Blood. 2006;108:1551–1554. doi: 10.1182/blood-2005-10-009514. [DOI] [PubMed] [Google Scholar]
- 37.Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol. 2000;12:543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Ceja SG, Reyes-Maldonado E, Vazquez-Manriquez ME, Lopez-Luna JJ, Belmont A, Gutierrez-Castellanos S. Differential expression of STAT5 and Bcl-xL, and high expression of Neu and STAT3 in non-small-cell lung carcinoma. Lung Cancer. 2006;54:163–168. doi: 10.1016/j.lungcan.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Meyer S, Eden T, Kalirai H. Dexamethasone protects against Cisplatin-induced activation of the mitochondrial apoptotic pathway in human osteosarcoma cells. Cancer Biol Ther. 2006;5:915–920. doi: 10.4161/cbt.5.8.2881. [DOI] [PubMed] [Google Scholar]
- 40.Duke RC, Cohen JJ, Chervenak R. Differences in target cell DNA fragmentation induced by mouse cytotoxic T lymphocytes and natural killer cells. J Immunol. 1986;137:1442–1447. [PubMed] [Google Scholar]
- 41.Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 42.Xu J, Chen S, Ku G, Ahmed SH, Xu J, Chen H, Hsu CY. Amyloid beta peptide-induced cerebral endothelial cell death involves mitochondrial dysfunction and caspase activation. J Cereb Blood Flow Metab. 2001;21:702–710. doi: 10.1097/00004647-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 43.An G, Lin TN, Liu JS, Xue JJ, He YY, Hsu CY. Expression of c-fos and c-jun family genes after focal cerebral ischemia. Ann Neurol. 1993;33:457–464. doi: 10.1002/ana.410330508. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, He L, Ahmed SH, Chen SW, Goldberg MP, Beckman JS, Hsu CY. Oxygen-glucose deprivation induces inducible nitric oxide synthase and nitrotyrosine expression in cerebral endothelial cells. Stroke. 2000;31:1744–1751. doi: 10.1161/01.str.31.7.1744. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- 46.Salminen A, Liu PK, Hsu CY. Alteration of transcription factor binding activities in the ischemic rat brain. Biochem Biophys Res Commun. 1995;212:939–944. doi: 10.1006/bbrc.1995.2060. [DOI] [PubMed] [Google Scholar]
- 47.Onishi M, Nosaka T, Misawa K, Mui AL, Gorman D, McMahon M, Miyajima A, Kitamura T. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui AL, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. Embo J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma V, Lansdell TA, Peddibhotla S, Tepe JJ. Sensitization of tumor cells toward chemotherapy: enhancing the efficacy of camptothecin with imidazolines. Chem Biol. 2004;11:1689–1699. doi: 10.1016/j.chembiol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Wadkins RM, Bearss D, Manikumar G, Wani MC, Wall ME, Von Hoff DD. Topoisomerase I-DNA complex stability induced by camptothecins and its role in drug activity. Curr Med Chem Anticancer Agents. 2004;4:327–334. doi: 10.2174/1568011043352894. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida A, Takemura H, Inoue H, Miyashita T, Ueda T. Inhibition of glutathione synthesis overcomes Bcl-2-mediated topoisomerase inhibitor resistance and induces nonapoptotic cell death via mitochondrial-independent pathway. Cancer Res. 2006;66:5772–5780. doi: 10.1158/0008-5472.CAN-05-3916. [DOI] [PubMed] [Google Scholar]
- 52.Rieger J, Durka S, Streffer J, Dichgans J, Weller M. Gemcitabine cytotoxicity of human malignant glioma cells: modulation by antioxidants, BCL-2 and dexamethasone. Eur J Pharmacol. 1999;365:301–308. doi: 10.1016/s0014-2999(98)00883-8. [DOI] [PubMed] [Google Scholar]
- 53.Schorr K, Furth PA. Induction of bcl-xL expression in mammary epithelial cells is glucocorticoid-dependent but not signal transducer and activator of transcription 5-dependent. Cancer Res. 2000;60:5950–5953. [PubMed] [Google Scholar]
- 54.Stocklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- 55.Wyszomierski SL, Rosen JM. Cooperative effects of STAT5 (signal transducer and activator of transcription 5) and C/EBPbeta (CCAAT/enhancer-binding protein-beta) on beta-casein gene transcription are mediated by the glucocorticoid receptor. Mol Endocrinol. 2001;15:228–240. doi: 10.1210/mend.15.2.0597. [DOI] [PubMed] [Google Scholar]
- 56.Oh HY, Namkoong S, Lee SJ, Por E, Kim CK, Billiar TR, Han JA, Ha KS, Chung HT, Kwon YG, Lee H, Kim YM. Dexamethasone protects primary cultured hepatocytes from death receptor-mediated apoptosis by upregulation of cFLIP. Cell Death Differ. 2006;13:512–523. doi: 10.1038/sj.cdd.4401771. [DOI] [PubMed] [Google Scholar]
- 57.Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, Burger R, Munshi N, Ohtake Y, Saxena S, Anderson KC. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- 58.Baumann S, Dostert A, Novac N, Bauer A, Schmid W, Fas SC, Krueger A, Heinzel T, Kirchhoff S, Schutz G, Krammer PH. Glucocorticoids inhibit activation-induced cell death (AICD) via direct DNA-dependent repression of the CD95 ligand gene by a glucocorticoid receptor dimer. Blood. 2005;106:617–625. doi: 10.1182/blood-2004-11-4390. [DOI] [PubMed] [Google Scholar]
- 59.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 60.Novac N, Baus D, Dostert A, Heinzel T. Competition between glucocorticoid receptor and NFkappaB for control of the human FasL promoter. Faseb J. 2006;20:1074–1081. doi: 10.1096/fj.05-5457com. [DOI] [PubMed] [Google Scholar]
- 61.Hendry L, John S. Regulation of STAT signalling by proteolytic processing. Eur J Biochem. 2004;271:4613–20. doi: 10.1111/j.1432-1033.2004.04424.x. [DOI] [PubMed] [Google Scholar]
- 62.Machuca C, Mendoza-Milla C, Cordova E, Mejia S, Covarrubias L, Ventura J, Zentella A. Dexamethasone protection from TNF-alpha-induced cell death in MCF-7 cells requires NF-kappaB and is independent from AKT. BMC Cell Biol. 2006;7:1–12. doi: 10.1186/1471-2121-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mendoza-Milla C, Machuca Rodriguez C, Cordova Alarcon E, Estrada Bernal A, Toledo-Cuevas EM, Martinez Martinez E, Zentella Dehesa A. NF-kappaB activation but not PI3K/Akt is required for dexamethasone dependent protection against TNF-alpha cytotoxicity in L929 cells. FEBS Lett. 2005;579:3947–3952. doi: 10.1016/j.febslet.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 64.Zhang C, Wenger T, Mattern J, Ilea S, Frey C, Gutwein P, Altevogt P, Bodenmuller W, Gassler N, Schnabel PA, Dienemann H, Marme A, Hohenfellner M, Haferkamp A, Pfitzenmaier J, Grone HJ, Kolb A, Buchler P, Buchler M, Friess H, Rittgen W, Edler L, Debatin KM, Krammer PH, Rutz HP, Herr I. Clinical and mechanistic aspects of glucocorticoid-induced chemotherapy resistance in the majority of solid tumors. Cancer Biol Ther. 2007;6:278–287. doi: 10.4161/cbt.6.2.3652. [DOI] [PubMed] [Google Scholar]
- 65.Gascoyne DM, Kypta RM, Vivanco MM. Glucocorticoids inhibit apoptosis during fibrosarcoma development by transcriptionally activating Bcl-xL. J Biol Chem. 2003;278:18022–18029. doi: 10.1074/jbc.M301812200. [DOI] [PubMed] [Google Scholar]
- 66.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol. 2005;16:2615–2625. doi: 10.1681/ASN.2005020142. [DOI] [PubMed] [Google Scholar]
- 67.Wanke I, Schwarz M, Buchmann A. Insulin and dexamethasone inhibit TGF-beta-induced apoptosis of hepatoma cells upstream of the caspase activation cascade. Toxicology. 2004;204:141–154. doi: 10.1016/j.tox.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 68.Fan W, Sui M, Huang Y. Glucocorticoids selectively inhibit paclitaxel-induced apoptosis: mechanisms and its clinical impact. Curr Med Chem. 2004;11:403–411. doi: 10.2174/0929867043455990. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y, Johnson KR, Norris JS, Fan W. Nuclear factor-kappaB/IkappaB signaling pathway may contribute to the mediation of paclitaxel-induced apoptosis in solid tumor cells. Cancer Res. 2000;60:4426–4432. [PubMed] [Google Scholar]
- 70.Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- 71.Yamashita H, Nishio M, Fujii Y, Iwase H. Dominant-negative Stat5 inhibits growth and induces apoptosis in T47D-derived tumors in nude mice. Cancer Sci. 2004;95:662–665. doi: 10.1111/j.1349-7006.2004.tb03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baskiewicz-Masiuk M, Masiuk M, Machalinski B. The influence of STAT5 antisense oligonucleotides on the proliferation and apoptosis of selected human leukaemic cell lines. Cell Prolif. 2003;36:265–278. doi: 10.1046/j.1365-2184.2003.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jensen J, Galsgaard ED, Karlsen AE, Lee YC, Nielsen JH. STAT5 activation by human GH protects insulin-producing cells against interleukin-1beta, interferon-gamma and tumour necrosis factor-alpha-induced apoptosis independent of nitric oxide production. J Endocrinol. 2005;187:25–36. doi: 10.1677/joe.1.06086. [DOI] [PubMed] [Google Scholar]
- 74.Kirito K, Watanabe T, Sawada K, Endo H, Ozawa K, Komatsu N. Thrombopoietin regulates Bcl-xL gene expression through Stat5 and phosphatidylinositol 3-kinase activation pathways. J Biol Chem. 2002;277:8329–8337. doi: 10.1074/jbc.M109824200. [DOI] [PubMed] [Google Scholar]