Abstract
Although tamoxifen (TAM) is used for the front-line treatment and prevention of estrogen receptor-positive (ER+) breast tumors, nearly 40% of estrogen-dependent breast tumors do not respond to TAM treatment. Moreover, the positive response is usually of short duration, and most tumors eventually develop TAM-resistance. Overexpression of HER2 gene is associated with TAM-resistance of breast tumor, and suppression of HER2 expression enhances the TAM activity. Soy isoflavone genistein has been shown to have anti-cancer activities and suppress expression of HER2 and ERα. The objective of this study was to test the hypothesis that genistein may sensitize the response of ER+ and HER2-overexpressing breast cancer cells to TAM treatment. The combination treatment of TAM and genistein inhibited the growth of ER+/HER2-overexpressing BT-474 human breast cancer cells in a synergistic manner in vitro. Determination of cellular markers indicated that this synergistic inhibitory effect might be contributed in part from combined effects on cell-cycle arrest at G1 phase and on induction of apoptosis. Further determination of the molecular markers showed that TAM and genistein combination synergistically induced BT-474 cell apoptosis in part by synergistic downregulation of the expression of survivin, one of the apoptotic effectors, and downregulation of EGFR, HER2, and ERα expression. Our research may provide a novel approach for the prevention and/or treatment of TAM insensitive/resistant human breast cancer, and warrants further in vivo studies to verify the efficacy of genistein and TAM combination on the growth of ER+/HER2-overexpressing breast tumors and to elucidate the in vivo mechanisms of synergistic actions.
Keywords: synergy, apoptosis, survivin
INTRODUCTION
More than 60% of human breast tumors are estrogen receptor (ER)-positive (ER+) and depend on estrogens for growth. The anti-estrogen tamoxifen (TAM) is currently the first-line medicine for treatment of ER+ breast cancer in both pre- and post-menopausal women [1]. TAM has also been demonstrated its efficacy on the prevention of ER+ breast tumors [2]. However, not all ER+ breast cancers respond to TAM. In fact, nearly 40% of them do not respond to TAM despite the presence of ER in malignant tissues [3]. Moreover, the positive response is usually of short duration, and most tumors eventually develop TAM-resistance in 2-5 yr [4]. Therefore, more effective modalities are needed to enhance the efficacy of TAM on prevention and/or treatment of the progression of ER+ and TAM-insensitive or TAM-resistant breast cancer.
While it is generally acknowledged that the resistance of ER+ breast cancer cells to TAM is influenced by multiple factors, overexpression of the members of the human epidermal growth factor receptor (HER) family, especially HER2, has been shown to play a major role [5]. HER2 gene is amplified in breast tumors. Experimental studies suggest that HER2-positive tumors may be less responsive to endocrine treatments [6]. Although some clinical studies showed inconsistent and/or conflicting results, totality of clinical evidence by meta-analysis supports that HER2-positve breast cancer is less responsive to TAM treatment [6]. Suppression of HER2 receptor by using HER2 receptor inhibitor trastuzumab (herceptin) has been shown to significantly inhibit breast tumor growth in HER-2 overexpressing human breast cancer [7-9].
Since both HER-2 and ER are critical in the breast carcinogenesis and are validated preventive and therapeutic targets, it is possible that targeting both receptors simultaneously may have a potentiating effect on the prevention of breast cancer development and progression. Indeed, the experimental studies showed that suppression of HER2 enhanced the antiproliferative activity of TAM [10,11].
Epidemiological investigations suggest that soy consumption is associated with a reduced risk of breast cancer [12]. Experimental studies indicate that soy isoflavone genistein is in part responsible for the cancer prevention activity of soy. Genistein has various types of biological functions that may be related to its breast cancer prevention activity. Genistein competes with estrogens in binding to ERs and regulates estrogen-regulated gene expression [13], acts as a protein tyrosine kinase inhibitor [14], inhibits DNA topoisomerase II activity [15], suppresses angiogenesis [16], induces breast cancer cell apoptosis [17-20], and downregulates HER2 [19,20] and ERα expression [21]. Therefore, supplementation of genistein to suppress HER2 expression may sensitize the efficacy of TAM on prevention and/or treatment of ER+ and HER2-overexpressing breast cancer.
In this study, we evaluated the effects of genistein and TAM combinations on the growth inhibition of ER+/HER2-overexpressing BT-474 human breast cancer cells in vitro and on modulation of related cellular and molecular markers.
MATERIALS AND METHODS
Culture of BT-474 Human Breast Cancer Cell Line
ER+/HER2-overexpressing BT-474 human breast cancer cell line was purchased from the American Type Culture Collection (Bethesda, MD). Cells were routinely maintained as monolayer cultures in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, 2μmol of L-glutamine/mL, 100 U of penicillin/mL, and 100 μg of streptomycin/mL in a 95% air, 5% carbon dioxide, and water-saturated atmosphere.
Growth Inhibition Assays
Cells (1 × 106 cells/flask) were seeded into 25 cm2 cell culture flasks. After overnight settlement, the cells were treated with the indicated concentrations of TAM and/or genistein dissolved in 1:1 of dimethyl sulfoxide (DMSO) and ethanol (final DMSO and ethanol concentration ≤0.1% by volume) for different times (48, 72, and 96 h). Cells were trypsinized and counted with Z1 Coulter Particle Counter (Beckman Coulter Company, Miami, FL). All assays were completed in triplicate, and experiments were replicated at least once.
The nature of combined effect was determined by using the published methods [22,23], based on the principles described by Chou and Talalay [24]. In brief, the expected value of combination effect between treatment 1 and treatment 2 was calculated as [(observed treatment 1 value)/(control value)] × [(observed treatment 2 value)/(control value)] × (control value); and the combination index was calculated as the ratio of (expected value)/(observed value). A ratio of >1 indicated a synergistic effect, and a ratio of <1 indicated a less than additive or antagonistic effect [23].
Analysis of Cell-Cycle Progression
Cells were grown under conditions as described above, treated in duplicate with genistein, TAM and the combinations for different times (48 and 72 h), harvested by trypsinization and centrifugation, washed twice with PBS and fixed in 70% ice-cold ethanol at -20°C. Cells were stained with propidium iodide (Sigma, St. Louis, MO, 50 μg/mL) solution containing 50 μg/mL of RNase A and incubated at 37°C in dark for 30 min. The DNA content, as reflected by the fluorescence signal of propidium iodide, was measured by using FACScans (Becton-Dickinson, Immunocytometry Systems, Mountview, CA). The experiments were repeated at least once.
Cell Apoptotic Death Assays
DNA fragmentation was used to assess apoptotic cell death by DNA ladder analysis. After appropriate treatments for 72 h, the cells were harvested, washed with PBS solution at 4°C, and suspended in lysis buffer (10 mM of Tris-HCl, pH7.5, 10 mM of EDTA, and 0.2% Triton X-100). After incubation for 15 min at 4°C, samples were centrifuged at 13 000g for 10 min at 4°C. The supernatant containing the fragmented DNA was precipitated with 0.5 M of NaCl and 1 volume of isopropanol for at least 1 h at -70°C. Samples were centrifuged at 13 000g for 10 min at 4°C, and the pellet was washed once with 70% ethanol, and air-dried. The precipitates were dissolved in 10 μL of TE-RNase (0.1 mg/mL) and incubated at 37°C for 30 min. The samples were electrophoresed through a 1% agarose gel.
To quantitate DNA fragmentation, cells were treated with genistein and/or TAM, and collected daily from Day 1 to Day 5. Quantitation of DNA fragmentation was conducted by using the Cell Death Detection enzyme-linked immunosorbent assay (ELISA) Plus Kit (Boheringher Mannheim, Indianapolis, IN), following the procedures recommended by the manufacturer.
Real-Time Quantitative Polymerase Chain Reaction (PCR) Analysis
Real-time quantitative PCR primers were designed with Beacon Designer 2.0 software (Palo Alto, CA). Total RNA was isolated by using Qiagen RNeasy Mini Kit (Qiagen, Inc., Valencia, CA). First-strand cDNA synthesis used 1 mmol/L of oligo dT of 18-mer (Invitrogen, Carlsbad, CA), and 1.0 μg of total RNA per 30 μL reaction with Ready-To-Go You-Prime First-Strand Beads (Amersham Biosciences, Piscataway, NJ). For validation of the efficiency of the real-time PCR assay, fourfold dilution series was generated with the cDNA. The relative expression was quantitated by using the Ct method. The equivalent of 0.15 μL of the cDNA generated in its synthesis step was used per reaction. Sequences of the primers used in this study were as follows: ERα gene, forward 5′-AGGAGACTCGCTACTGTGC-3′ and reverse 5′-ACTGGTTGGTGGCTGGAC-3′; HER2 gene, forward 5′-ATCTTAGACGAAGCATACG-3′ and reverse 5′-TTGGCAATCTGCATACAC-3′; and GAPDH gene, forward 5′-GAGTCCACTGGCGTCTTC-3′ and reverse 5′-GGAGGCATTGCTGATGATC-3′.
PCR reactions were performed in the final volume of 25 μL containing 20 mM of Tris-HCl, pH 8.4, 50 mM of KCl, 200 μM each dNTP, 0.5 μM each primer, 3.5 mM of MgCl2, and 1 × SYBR Green (Bio-Rad, Hercules, CA). Three replicates were used. PCR was carried out with an initial 3 min denature at 95°C followed by 40 cycles of a combined annealing and extension step at 60°C for 30 s, and denaturation at 95°C for 10 s. Following the completion of the PCR amplification reaction, a melting curve analysis was performed by heating the sample to 95°C programmed for 0 s followed by cooling down to 50°C for 1 min and inclemently heating the samples to 95°C with each step of increasing 1°C and a duration of 10 s while the fluorescence was measured continuously. GAPDH was used as a reference house-keeping gene for internal control. All real-time PCR reactions were run on the iCycler iQ single color system (Bio-Rad). To check the amplification specificity, real-time PCR products were subjected to electrophoresis on a 1% agarose gel, stained with ethidium bromide, visualized, and photographed under ultraviolet illumination. The experiments were repeated at least once.
Western Blot Analysis
Cells were treated with genistein (25 μM), TAM (5 μM) and the genistein and TAM combination for 3 d, and the cell lysates were prepared in a lysis buffer containing 100 mM of KCl, 20 mM of HEPES (pH 7.9), 10 mM of EDTA, 10% glycerol, 1 mM of phenylmethylsulfonyl fluoride, 40 μg/mL of leupeptin, and 1 μg/mL of pepstatin A. Western blot analysis was performed according to the standard procedure with the chemiluminescence ECL Western blotting kit (Amersham Pharmacia Biotech, Piscataway, NJ), and the density of the protein band was quantitated with Quantity One software (Bio-Rad), as described previously [23]. Primary antibodies used in this study include ERα (monoclonal, 1:400), EGFR (polyclonal, 1:200), HER2 (polyclonal, 1:200), HER3 (polyclonal, 1:200), and survivin (monoclonal, 1:100) antibodies from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Statistical Analyses
Results were expressed as means ± standard deviation (SD), and were initially evaluated by analysis of variance followed by Fisher's protected least-significant difference [25] to determine statistical significance among treatment groups with the Statview 5.0 (Abacus Concepts, Inc., Berkeley, CA) program. A probability level of P < 0.05 was considered statistically significant.
RESULTS
Effects of TAM and Genistein Combinations on BT-474 Cell Growth
We first evaluated the effects of TAM and genistein, alone and in combinations, on the growth of BT-474 cells. Genistein (3.125, 6.25, 12.5, and 25 μM) treatment alone showed both time-dependent (data not shown) and dose-dependent (Figure 1, 96 h) effects on growth inhibition of BT-474 cells. TAM also inhibited BT-474 cell growth (Figure 1), but its inhibitory effect on the growth of BT-474 cell was much less than that of other TAM-sensitive cell line such as MCF-7 (data not shown). Treatment of BT-474 cells with the TAM and genistein combination resulted in a more significant growth inhibition than TAM or genistein treatment alone (Figure 1). Treatment of BT-474 cells with the 5 μM TAM and 25 μM genistein combination significantly reduced cell growth by 96%. The theoretic additive inhibitory values were calculated, as shown in Figure 1. The observed inhibitory effects of the combination treatments were greater than the theoretical additive effects, suggesting that the combination of genistein and TAM have a synergistic effect on the growth inhibition of BT-474 cells.
Figure 1.
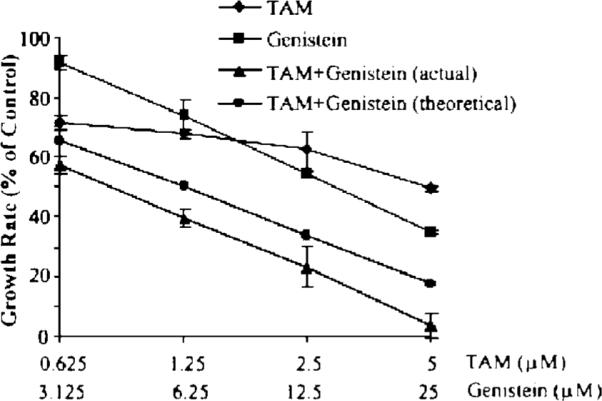
Effects of TAM and genistein on the growth of BT-474 cells in vitro. Cells were treated with the indicated concentrations of TAM and/or genistein dissolved in 1:1 of DMSO and ethanol for 3 d; the cells were counted with Z1 Coulter Particle Counter. Theoretic additive inhibitory values in % of control = % of control for TAM treatment multiplied by % of control for genistein treatment. Results are expressed as mean ± SD, n = 3.
Effects of TAM and Genistein Combinations on BT-474 Cell-Cycle Progression
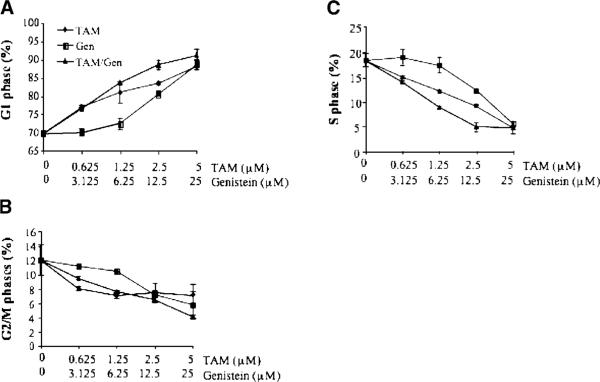
The effects of TAM and genistein treatments, alone and in combination, on cell-cycle progression of BT-474 cells were determined with flow cytometry. The dynamic changes in G1, S, and G2/M are shown in Figure 2A-C, respectively. Genistein and TAM treatments alone significantly increased cell proportions at G1 phase (except genistein treatments at 3.125 and 6.25 μM), and genistein and TAM combinations had even greater G1 arrest effect, suggesting a potentiating effect between genistein and TAM on G1 arrest. Subsequently, the treatments reduced cells at S (Figure 2B) and G2/M (Figure 2C) phases due to G1 arrest.
Figure 2.
Dose-dependent responses of BT-474 cell at G1 (A), S (B), and G2/M (C) phases to the treatments in vitro. Cells were treated and cycle distributions were measured by flow cytometry. Results are expressed as mean ± SD, n = 3.
Effects of TAM and Genistein Combinations on Apoptosis of BT-474 Cells
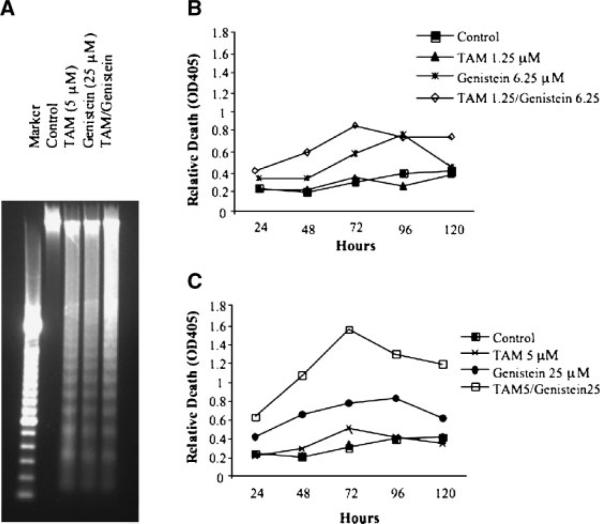
A characteristic DNA ladder was observed in BT-474 cells following treatment with TAM (5 μM), genistein (25 μM), or the TAM and genistein combination (Figure 3A) for 72 h, as compared with the control. ELISA was used to quantitate both dose- and time-dependent effects of TAM and genistein combinations on DNA fragmentation levels. With few exceptions, treatments resulted in a dose-dependent induction of DNA fragmentation (Figure 3B and C). The standard error bars (less than 10% of the means) were not shown in the figure for a clearer presentation. The treatments also showed a time-dependent induction of DNA fragmentation from Days 1-3 (Figure 3B and C). With few exceptions, both time- and dose-dependent inductions of DNA fragmentation were synergistically enhanced by the combination treatments (Figure 3B and C).
Figure 3.
Effects of TAM and genistein on apoptosis of BT-474 cells in vitro. Apoptosis was determined by DNA ladder (A) and DNA fragmentation (B, C) of BT-474 cells following the treatments with TAM and genistein alone or in combination. BT-474 cells were treated with 5 μM TAM, 25 μM genistein, and the TAM (5 μM) and genistein (25 μM) combination for 3 d and genomic DNA was isolated and subject to 1% agarose gel electrophoresis analysis (A). The SD of each data point (less than 10% of the mean) in B and C are not added in the figure for clear presentation.
Effects of TAM and Genistein Combinations on Apoptotic Gene Survivin and Its Protein Expression
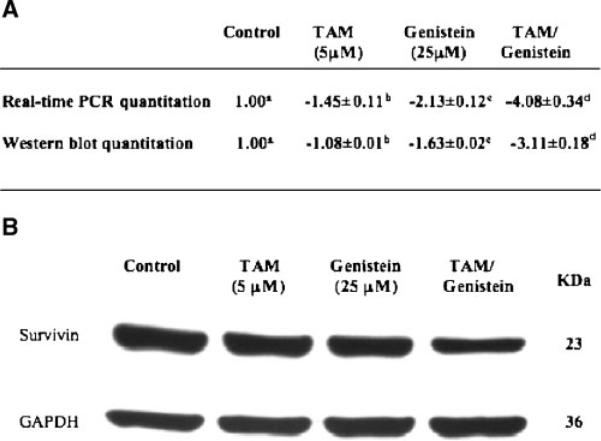
Since the synergistic inhibitory effect of the combined treatment was characterized by a synergistic induction of cell apoptosis, we explored some common apoptotic effects, and found that the inhibition apoptotic protein, also named survivin, was synergistically downregulated by the combined treatment in both its mRNA and protein levels (Figure 4). BT-474 cells were treated with 5 μM TAM, 25 μM genistein, and the combination for 3 d before samples were collected for analyses. Survivin mRNA and protein levels were quantitated with real-time PCR and Western blot analyses, respectively, and the results were expressed as signal log ratio (SLR, log2[treatment expression level/control expression level]). In either quantitation, the SLR value in the combination group was greater than the sum of SLR values in TAM and genistein groups (Figure 4A), suggesting that TAM and genistein combination synergistically downregulate survivin expression. These results suggest that downregulation of survivin expression and/or function may contribute, at least in part, to the synergistic inhibitory effect of genistein and TAM combination on induction of BT-474 cell apoptosis.
Figure 4.
Effects of TAM and genistein on survivin expression. Both mRNA and protein levels of surviving were measured, and the relative folds of change were the means of signal log ratios (SLR, log2[treatment expression level/control expression level]) ± standard deviation after being normalized to GAPDH (A). The experiments were duplicated and repeated at least once. Within each method in subpart A, the means without a common superscript letter are significantly different with P values at least <0.05. A representative of protein expression is shown in panel B.
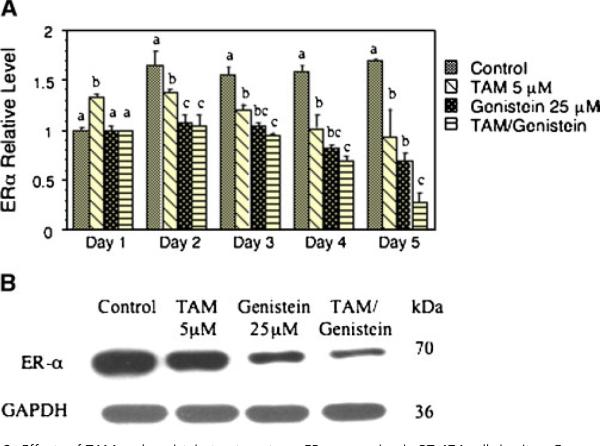
Effects of TAM and Genistein Combinations on Expression of the HER Family and ER-α
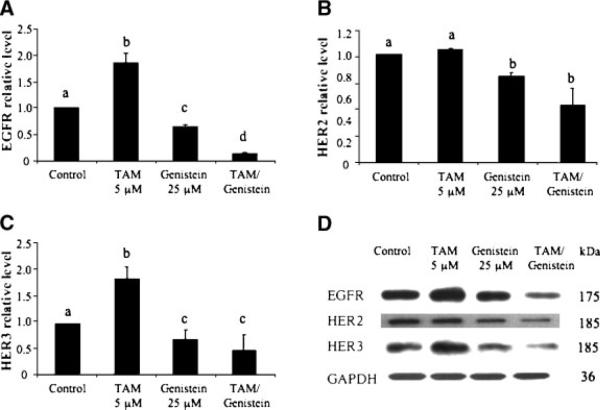
We also studied putative genes and/or protein products, such as the HER family and ERα, that might be associated with TAM-insensitivity/resistance of breast cancer. We first determined the effects of genistein and TAM on protein expression of the HER family. TAM at 5 μM significantly stimulated the expression of epidermal growth factor receptor (EGFR, also known as HER1) (Figure 5A) and HER3 (Figure 5C) proteins, whereas its effect on HER2 protein was not significant (Figure 5B). Genistein treatment at 25 μM, on the other hand, significantly downregulated proteins levels of EGFR (Figure 5A), HER2 (Figure 5B), and HER3 (Figure 5C). In particular, the combination of genistein and TAM synergistically inhibited the expression of these proteins (Figure 5A-C). The real time quantitation showed the similar effects of TAM and genistein on the mRNA levels of EGFR, HER2, and HER3 to that of protein levels, and TAM and genistein combination further downregulated mRNA expression of these genes in a synergistic manner (data not shown). These results suggest that the TAM and genistein combination may synergistically inhibit the growth of ER+/HER2-overexpressing human breast cancer cells in part by synergistic modulation of EGFR, HER2, and HER3.
Figure 5.
Effects of TAM and genistein treatments on the protein expression of EGFR (A), HER2 (B), and HER3 (C) in BT-474 cells in vitro. BT-474 cells were treated with TAM and genistein alone, or in combination. The proteins were detected by Western blot analysis and quantitated by image analysis. Results are expressed as mean ± SD. Each experiment was duplicated and repeated at least twice. Within each panel, the means without a common superscript letter are significantly different with P values at least <0.05. A representative of protein expression for EGFR, HER2, and HER3 with indicated treatments for 3 d is shown in panel D.
With few exceptions, TAM (5 μM) or genistein (25 μM) alone downregulated expression of ERα mRNA (Figure 6A), and the TAM and genistein combination further enhanced this downregulation (Figure 6A). The expression of ERα protein showed the similar results as that of the ERα mRNA (Figure 6B, Day 3). Further analysis indicated that the TAM and genistein combination had an additive effect on ER-α expression.
Figure 6.
Effects of TAM and genistein treatments on ERα expression in BT-474 cells in vitro. Gene expression was quantitated by real-time PCR. Results are expressed as mean ± SD (A). Each experiment was duplicated and repeated at least twice. Within each time point, the means without a common superscript letter are significantly different with P values at least <0.05. A representative of protein expression for ERα with indicated treatments for 3 d is shown in panel B. [Color figure can be viewed in the online issue, which is available at www.interscience. wiley.com.]
DISCUSSION
In the prevention and therapy of ER+ human breast cancer, the efficacy of the antiestrogen TAM has been well established. However, inherited or acquired hormone resistance reduces TAM activity, and treatment-associated side effects limit use of high doses TAM. Therefore, more effective modality is needed to sensitize TAM on ER+/TAM-insensitive breast tumors. Combination treatment is one of the developmental trends for increasing the efficacy and/or minimizing the side effects. In this study, we found that the genistein and TAM combination had a synergistic inhibitory effect on the growth of ER+/HER2-overexpressing BT-474 human breast cancer cells in vitro in part via the synergistic effect on induction of apoptosis, and that the molecular mechanisms of this synergistic action might be in part via modulation on the expression of the apoptotic suppressor survivin and the HER family members (EGFR, HER2, and HER3).
The hallmark of the genistein and TAM combination treatment was synergistic induction of apoptosis (Figure 3). We explored some apoptotic effectors that were responsible for this phenomenon, and found that survivin was synergistically downregulated (Figure 4). Survivin, also known as inhibitor of apoptosis protein-1, is a member of a large family of genes that promote cell survival after apoptotic stimuli. Survivin is overexpressed in multiple types of tumors [26-28], and is shown to facilitate resistance of some lung cancer cell lines to gemcitabine [29]. Loss of survivin function results in apoptosis [30]. TAM is shown to inhibit the growth of colon cancer cell lines associated with down-regulation of survivin expression [31]. Genistein is also shown to inhibit survivin expression in B cell malignant cells [32] and prostate cancer cells [33]. However, the interactive effect of TAM and genistein on survivin expression has not been reported. Our results suggest that the synergistic downregulation of survivin expression/activity may be one of the molecular mechanisms by which the TAM and genistein combination synergistically induces apoptosis of ER+/HER2-overexpressing breast cancer cells. To the best of our knowledge, this is the first time to report the synergistic interaction between TAM and genistein on apoptosis via downregulation of survivin expression.
Overexpression and/or activation of the HER family, especially EGFR (HER1) and HER2/neu (erbB-2), have been causally associated with TAM resistance in human breast cancer cells. Forced overexpression of HER2 in breast cancer cells resulted in mitogen-activated protein kinase (MAPK) hyperactivity and TAM resistance [10]. Inhibition of expression and/or tyrosine kinase activity of HER2 and EGFR restored the growth inhibition effect of TAM [10,34]. In this study, we reported that genistein and TAM combination synergistically inhibited the expression of EGFR and HER2. We further found that genistein inhibited phosphorylation activation of EGFR and HER2 in vitro, but TAM did not, and TAM and genistein combinations did not further inhibit the HER family activation (data not shown). These findings suggest that the tyrosine kinase activity inhibition may be essential for the synergistic inhibitory effect of the TAM and genistein combination on ER+/HER2-oeverexpressing breast cancer cells.
Although inhibition of phosphorylation activation of the HER family was not shown to be responsible for synergistic inhibition of BT-474 cells by the TAM and genistein combination, it is possible that the TAM and genistein combination inhibits BT-474 cell growth in part by modulating interactions between the HER family and other pathways, such as the ER, insulin-like growth factor-I (IGF-I) and Akt pathways. HER2 and ER are believed to be important cell survival/death factors in human breast cancer cells. However, how HER2 and ERα interact to confer resistance to hormone treatment is not well understood. Previous studies showed that HER2 directly interacted with ER at cell membrane, and dissociation of HER2 from cell membrane ER resulted in induction of apoptosis by TAM [35]. We found in this study that genistein and TAM combinations down-regulated expression of HER2 and ERα in a synergistic and an additive manner, respectively (Figures 5 and 6). It is thus possible that the TAM and genistein combination may synergistically inhibit BT-474 cell growth by modulating the interactions between HER and ERα signaling pathways. We also studied the effects of genistein and TAM combinations on Akt and IGF-I signaling pathways, and the results suggest that these two pathways may not be responsible for the synergistic effect of genistein and TAM combination (data not shown).
In conclusion, we found that genistein and TAM combination had a synergistic inhibitory effect on the growth of ER+/HER2-overexpressing BT-474 human breast cancer cells in vitro in part via induction of apoptosis and cell-cycle arrest at G1 phase. Further determination of the molecular markers showed that TAM and genistein combination synergistically induced BT-474 cell apoptosis in part by synergistic downregulation of the expression of survivin, one of the apoptotic effectors, and downregulation of EGFR, HER2, and ERα expression. Our research may provide a novel approach for the prevention and/or treatment of TAM insensitive/resistant human breast cancer, and warrants further in vivo studies to verify the efficacy of genistein and TAM combination on the growth of ER+/HER2-overexpressing breast tumors and to elucidate the in vivo mechanisms of synergistic actions.
ACKNOWLEDGMENTS
This study was supported in part by Susan Komen's Breast Cancer Research Foundation BCTR0402749, and the United States Public Health Service RO3 CA112644 and RO1 AT00863.
Abbreviations
- DMSO
dimethyl sulfoxide
- EGFR
epidermal growth factor receptor
- HER
human epidermal growth factor receptor
- PCR
polymerase chain reaction
- SLR
signal log ratio
- SD
standard deviation
- TAM
tamoxifen
REFERENCES
- 1.Fisher B, Dignam J, Bryant J, et al. Five vs. more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: Twenty-eight years later. J Clin Oncol. 1995;13:513–529. doi: 10.1200/JCO.1995.13.2.513. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB. Endocrine therapy for advanced breast cancer: A review. Breast Cancer Res Treat. 1992;21:15–26. doi: 10.1007/BF01811960. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of HER2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 6.De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 8.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa H, Lenferink AE, Simpson JF, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 11.Argiris A, Wang CX, Whalen SG, DiGiovanna MP. Synergistic interactions between tamoxifen and trastuzumab (Herceptin) Clin Cancer Res. 2004;10:1409–1420. doi: 10.1158/1078-0432.ccr-1060-02. [DOI] [PubMed] [Google Scholar]
- 12.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 13.Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993;45:399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 14.Shao Z-M, Wu J, Shen Z-Z, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–4857. [PubMed] [Google Scholar]
- 15.Markovits J, Linassier C, Fosse P, et al. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 16.Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125:790S–797S. doi: 10.1093/jn/125.suppl_3.790S. [DOI] [PubMed] [Google Scholar]
- 17.Pagliacci MC, Smacchia M, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Growth-inhibitory effects of the natural phytoestrogen genistein in MCF-7 human breast cancer cells. Eur J Cancer. 1994;30A:1675–1682. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Bhuiyan M, Sarkar FH. Induction of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by genistein. Int J Oncol. 1999;15:525–533. doi: 10.3892/ijo.15.3.525. [DOI] [PubMed] [Google Scholar]
- 20.Katdare M, Osborne M, Telang NT. Soy isoflavone genistein modulates cell cycle progression and induces apoptosis in HER-2/neu oncogene expressing human breast epithelial cells. Int J Oncol. 2002;21:809–815. [PubMed] [Google Scholar]
- 21.Chen WF, Huang MH, Tzang CH, Yang M, Wong MS. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim Biophys Acta. 2003;1638:187–196. doi: 10.1016/s0925-4439(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: Inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190–2196. [PubMed] [Google Scholar]
- 23.Zhou J-R, Yu L, Mai Z, Blackburn GL. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int J Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enz Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Steel RGD, Torrie JH. Principles and procedures of statistics: A biometrical approach. McGraw-Hill book Company, Inc.; New York: 1980. [Google Scholar]
- 26.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki H, Toyoda M, Shinohara H, et al. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026–2032. doi: 10.1002/1097-0142(20010601)91:11<2026::aid-cncr1228>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Bao R, Connolly DC, Murphy M, et al. Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst. 2002;94:522–528. doi: 10.1093/jnci/94.7.522. [DOI] [PubMed] [Google Scholar]
- 29.Bandala E, Espinosa M, Maldonado V, Melendez-Zajgla J. Inhibitor of apoptosis-1 (IAP-1) expression and apoptosis in non-small-cell lung cancer cells exposed to gemcitabine. Biochem Pharmacol. 2001;62:13–19. doi: 10.1016/s0006-2952(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem. 1998;273:11177–11182. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama Y, Sakamoto H, Satoh K, Yamamoto T. Tamoxifen and gonadal steroids inhibit colon cancer growth in association with inhibition of thymidylate synthase, survivin and telomerase expression through estrogen receptor beta mediated system. Cancer Lett. 2000;161:63–71. doi: 10.1016/s0304-3835(00)00600-5. [DOI] [PubMed] [Google Scholar]
- 32.Mansour A, McCarthy B, Schwander SK, et al. Genistein induces G2 arrest in malignant B cells by decreasing IL-10 secretion. Cell Cycle. 2004;3:1597–1605. doi: 10.4161/cc.3.12.1293. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Koike H, Matsui H, et al. Genistein, a soy isoflavone, induces glutathione peroxidase in the human prostate cancer cell lines LNCaP and PC-3. Int J Cancer. 2002;99:846–852. doi: 10.1002/ijc.10428. [DOI] [PubMed] [Google Scholar]
- 34.Lichtner RB. Estrogen/EGF receptor interactions in breast cancer: Rationale for new therapeutic combination strategies. Biomed Pharmacother. 2003;57:447–451. doi: 10.1016/j.biopha.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 2002;97:306–312. doi: 10.1002/ijc.1614. [DOI] [PubMed] [Google Scholar]