Abstract
TGFβ induces lens epithelial cells to undergo epithelial mesenchymal transition (EMT) and many changes with characteristics of fibrosis including posterior capsular opacification (PCO). Consequently much effort is directed at trying to block the damaging effects of TGFβ in the lens. To do this effectively it is important to know the key signaling pathways regulated by TGFβ that lead to EMT and PCO. Given that Wnt signaling is involved in TGFβ-induced EMT in other systems, this study set out to determine if Wnt signaling has a role in regulating this process in the lens. Using RT-PCR, in situ hybridization and immunolocalization this study clearly shows that Wnts 5a, 5b, 7b, 8a, 8b and their Frizzled receptors are upregulated in association with TGFβ-induced EMT and cataract development. Both rat in vitro and mouse in vivo cataract models show similar profiles for the Wnt and Frizzled mRNAs and proteins that were assessed. Currently it is not clear if the canonical β-catenin/TCF signaling pathway, or a non-canonical pathway, is activated in this context. Overall, the results from the current study indicate that Wnt signaling is involved in TGFβ-induced EMT and development of fibrotic plaques in the lens.
Keywords: Lens epithelium, Anterior subcapsular cataract, Wnt, TGFβ, Posterior capsular opacification
Introduction
Approximately 25 million people are bilaterally blind as a result of cataract, which is the most common cause of blindness in the world today (Chang et al., 2007). The most effective treatment currently for cataract is surgery, which involves removal of opaque lens material and insertion of a plastic intraocular lens into the capsular bag. Although initially very effective in restoring sight, a common postoperative complication of surgery is the development of a secondary cataract commonly known as posterior capsule opacification (PCO). This is caused by fibrotic growth of residual lens epithelial cells left behind in the capsular bag after surgery (Wormstone, 2002). Studies with rat and mouse models showed that TGFβ induces lens fibrosis and cataract. TGFβ induces rodent lens epithelial cells to undergo epithelial mesenchymal transition (EMT) and acquire many features and markers characteristic of fibrosis including PCO (Hales et al., 1994, 1995; Liu et al., 1994; Lovicu and McAvoy, 2005; Lovicu et al., 2002, 2004a, 2004b). Studies on human lens cells in vitro, as well as analysis of post-operative cataract material, have also implicated TGFβ as a key inducer of fibrotic cataracts including PCO (Wormstone, 2002; Saika, 2004).
As PCO progresses, further treatment such as Nd-YAG laser capsulotomy is often given to try to restore some visual acuity; however, it is not without its complications and adds significant costs to what is the most common surgery carried out in Western countries (Saika, 2004; Bilotte and Berdeaux, 2004). Therefore, a major focus in cataract research in recent years has been directed at identifying ways of blocking the cataractous effects of TGFβ and promoting the normal lens epithelial phenotype (Wormstone et al., 2002; Saika et al., 2001; Schulz et al., 1996; Stump et al., 2006). However, to do this effectively it is important to know the key signaling pathway(s) regulated by TGFβ that leads to EMT and all the PCO-associated changes. This is central to devising molecular strategies to slow or prevent this common complication of modern cataract surgery.
TGFβ is a key regulator of many processes in both normal and pathological development (Nawshad et al., 2005). On TGFβ receptor activation, receptor Smads (Smad2 or Smad3) associate with Smad4. This Smad2/3-Smad4 complex then enters the nucleus to regulate transcription of target genes. Amongst the major effects of TGFβ signaling is induction of EMT and associated fibrosis (Akhurst and Derynck, 2001). Studies have also shown that Smad2 and Smad3 have non-redundant functions and that Smad3 appears to be the key Smad regulator of TGFβ-induced EMT/fibrosis (Yang et al., 2003; Roberts et al., 2006).
Studies have also highlighted the importance of Smad-independent TGFβ-activated pathways (Derynck and Zhang, 2003). With particular importance for EMT, TGFβ has been shown to activate mitogen-activated protein kinase (MAPK), phosphatidlyinositol 3-kinase (PI3K) and Rho GTPases (Peinado et al., 2003; Yi et al., 2005). In addition, and in the context of mediating the cellular processes of EMT, TGFβ has been shown to stimulate the β-catenin/TCF pathway (Nawshad et al., 2005; Medici et al., 2006; Warner et al., 2005). β-catenin/TCF signaling is involved in regulating EMT in development and cancer (Brabletz et al., 2005; Reya and Clevers, 2005). β-catenin is tightly regulated by a multiprotein complex containing glycogen synthase kinase-3β (GSK3β). This complex phosphorylates β-catenin and targets it for degradation. Signaling events that activate Disheveled (Dvl) phosphorylate GSK3β and inhibit this cytostolic degradation machinery and promote stabilization of β-catenin. In this unphosphorylated form β-catenin can translocate to the nucleus and associate with the DNA binding factors, TCF or LEF, and regulate expression of target genes that influence many different cellular processes. Through binding to Frizzled receptors, members of the Wnt growth factor family are well known activators of Dvl and subsequently this, so called, canonical signaling pathway (Reya and Clevers, 2005). In relation to mechanisms of cross-talk between Wnt and TGFβ pathways, Smad3 has been shown to bind Dvl (Warner et al., 2003) and TGFβ/Smad activated ILK phosphorylates (inactivates) GSK3β and can lead to stabilization of β-catenin (Willis and Borok, 2007).
Studies on the lens, using in vitro and in vivo models have shown that exposure to TGFβ and subsequent EMT and fibrotic changes, are associated with translocation of Smad3 into the nucleus. This has been shown in the development of anterior subcapsular cataracts (involves EMT and fibrosis similar to PCO) that arise in mouse lenses cultured with TGFβ as well as in a mouse model where a wound healing response is induced by injury (Saika et al., 2001, 2004). In addition, studies with human lens cells have shown that nuclear translocation of Smad3 is associated with the EMT and the PCO that follows cataract surgery (Saika et al., 2002, 2004). However, recent studies have also shown that when a Smad3 knockout mouse is crossed with a transgenic mouse that overexpresses TGFβ1 in lens cells, they still undergo a strong EMT and fibrotic response (Bahn et al., 2006). This result indicates that Smad-independent signaling pathways may also have major roles in mediating TGFβ-induced EMT and fibrosis in the lens.
As already noted for other systems, TGFβ-induced EMT has been shown to be associated with β-catenin/TCF signaling and this is characterized by nuclear translocation of unphosphorylated (stabilized) β-catenin (Medici et al., 2006, Jian et al., 2006). Given this background, it is important to determine if TGFβ-activation of β-catenin/TCF signaling has a role in regulating EMT/fibrosis in the lens. In a first step to address this question, this study set out to investigate the effects of TGFβ on Wnt expression and signaling in rat lens explants and transgenic mice.
Materials and Methods
All procedures involving animals were carried out in accordance with the National Health and Medical Research Council (Australia) guidelines and conformed to the Association for Research in Vision and Ophthalmology Incorporated Resolution on the use of animals in Ophthalmic Research. All procedures involving animal and human tissues were approved by the Animal and Human Ethics Committees of the University of Sydney, Australia.
Tissue Collection and Processing
For transgenic studies, eyes were collected from transgenic mice (OVE853) that overexpress an active form of TGFβ1 specifically in the lens (see Lovicu et al., 2002, 2004a, 2004b; Srinivasan et al., 1998). Tissues were collected from postnatal day (P3) to P21 transgenic and wild-type mice. All tissues collected were fixed in 10% neutral buffered formalin (NBF) overnight, rinsed in PBS and processed for histology. At least two litters each of transgenic and wild-type mice were collected and from these at least 3 pairs of eyes were sectioned for analysis.
For tissue culture studies, Wistar rats between P19 and P25 were sacrificed by asphixiation and their eyes removed. Whole lenses were dissected and cultured for 5 days in medium 199 supplemented with 1ng/ml TGFβ2 (R&D Systems, Inc. Minneapolis, MN) as described earlier (Hales et al., 1995). Lens epithelial explants (a monolayer of lens epithelial cells attached to their natural basement membrane, the lens capsule) were prepared as described previously (Lovicu and McAvoy, 2001) and cultured with or without TGFβ2 for 3 days. For explants, TGFβ2 was used at a final concentration of 200 pg/ml as earlier studies showed this dose to be potent at inducing prominent cataractous changes in explants by 3 days of culture (Gordon-Thomson et al., 1998). For both whole lens and explant experiments culture dishes, each containing 2 lenses or explants, were cultured with or without TGFβ2 (controls). At least 5 dishes were in each group and experiments were repeated at least 3 times.
RT-PCR
RT-PCR was carried out on P19-25 rat lenses cultured with TGFβ2 for 5 days. Total RNA was extracted from dissected lens capsule (with adherent epithelial cells) using Tri Reagent (Sigma, Sydney Australia). First-strand cDNA synthesis was carried out using 2 μg of RNA with a reverse transcription system (Promega, Sydney, Australia) according to the manufacturer’s instructions. RT-PCR for Wnts 5a, 5b, 7a, 7b, 8a and 8b was carried out as described previously (Stump et al., 2003).
In Situ Hybridisation
The expression patterns of mRNA transcripts for Wnts 5a, 5b, 7a, 7b, 8a, 8b and Frizzled 2 in rat lenses and in lenses of transgenic and wildtype mice, were examined by in situ hybridisation using digoxygenin-labeled riboprobes (Stump et al., 2003). Sense and anti-sense probes were prepared as described previously (Stump et al., 2003). The in situ hybridisation procedures were conducted as previously described for digoxigenin-labeled riboprobes (de Iongh et al., 2001).
Immunofluorescence
For both transgenic and cultured lenses, 6μm mid-sagittal sections were used for immunolocalization of Wnts 5a, 7b and Frizzleds (antibodies from Santa Cruz Biotechnology, CA; for Frizzled, the H-300/sc-9196 antibody was used as it recognizes Frizzled proteins 1–10). A monoclonal antibody (Dako, Glostrup, Denmark) was used to localize α-smooth muscle actin. Activated (unphosphorylated) β-catenin was localized in lens epithelial explants with an antibody to the unphosphorylated form (Clone 8E7, Upstate, Lake Placid, NY). For immunofluorescent labeling, explants were fixed in methanol. Following standard blocking and washing procedures explants or whole lenses were incubated overnight at 4°C with the primary antibody then incubated with an appropriate secondary antibody (Alexa 488 or Alexa 594; Invitrogen, Mount Waverly, Vic, Australia) for 60 minutes at room temperature (diluted 1:1000 with PBS/BSA). In each case antibodies were used at the concentrations recommended by the provider. Explants were counterstained with propidium iodide (100 ng/ml; Invitrogen) for 2–10 minutes to visualize cell nuclei. Fluorescent labeling was visualized using a laser-scanning confocal microscope, Axioskop 2 - LSM 5 Pascal (Carl Zeiss, Jena, Germany) and accompanying software.
Results
Wnt expression studies
mRNA expression
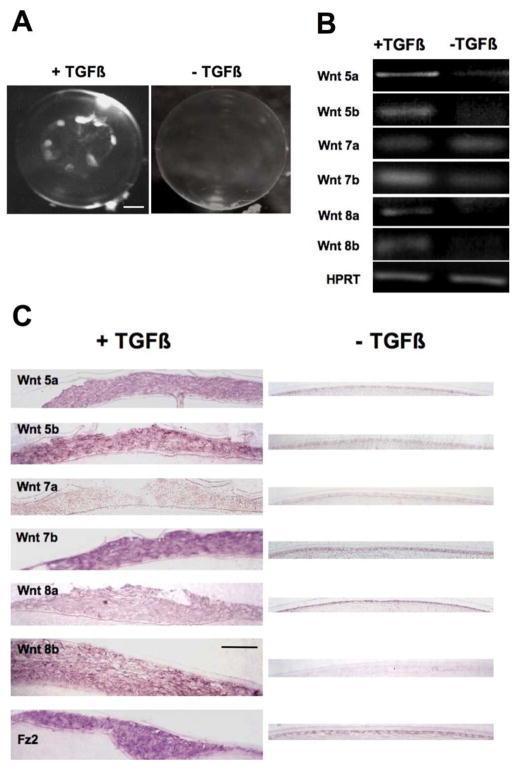
When whole rat lenses are cultured with TGFβ2 prominent opaque subcapsular plaques develop by 5 days (Figure 1A). These have been shown in earlier studies to have the same morphological and molecular features of anterior subcapsular cataract in humans (Hales et al., 1995). As shown by RT-PCR and in situ hybridization, Wnts 5a, 5b, 7b, 8a, 8b and the Frizzled 2 receptor are more strongly expressed in these TGFβ-induced subcapsular plaques than in untreated lenses (Figure 1B, C). In fact, out of the six Wnts assessed, only Wnt 7a is not upregulated in the subcapsular plaques compared with controls. The in situ hybridization analysis also shows that the strong expression of Wnts and Frizzled 2 extends throughout the subcapsular plaques (Figure 1C).
Figure 1.
Expression of Wnt and Frizzled mRNAs in lenses cultured with TGFβ. (A) By 5 days culture with TGFβ2 rat lenses develop anterior subcapsular opacities, whereas in the absence of TGFβ, lenses remain transparent. (B) RT-PCR shows that capsule preparations (containing the lens epithelium and associated plaques) from lenses cultured with TGFβ2 express mRNA for Wnts 5a, 5b, 7b, 8a and 8b much more strongly than lenses cultured without TGFβ2. HPRT is used as a loading control. Of all the Wnts studied, Wnt 7a is the only isoform that is not upregulated by TGFβ2. (C) Histological sections that include the anterior pole of the lens show detail of plaques similar to those evident in (A). Representative images from an in situ hybridization analysis show that all the Wnts studied (with the exception of Wnt 7a) are all more strongly expressed in the subcapsular plaques of lenses cultured with TGFβ2 than in the epithelium of lenses cultured without TGFβ2. Expression of Frizzled 2 is also upregulated in the presence of TGFβ. Abbreviations: Fz2, Frizzled 2. Scale bar: A, 370 μm; C, 45 μm.
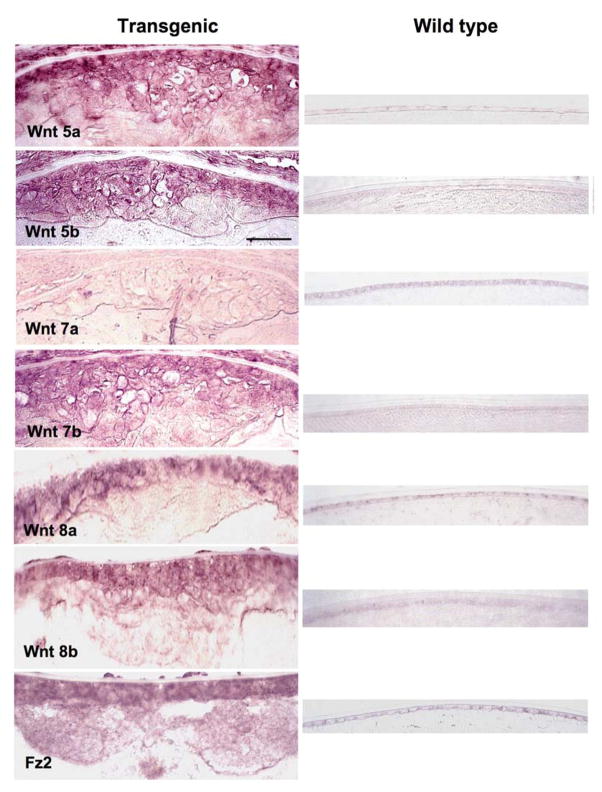
Similar plaques also develop in mice that overexpress active TGFβ1 specifically in the lens (Srnivasan et al., 1998). These transgenic mice reliably develop a single large anterior subcapsular plaque during postnatal development. Our earlier studies showed that subcapsular plaques in these mice are not only similar in morphology to those induced in rat lenses by TGFβ in vitro but also demonstrate a similar range of molecular markers for cataract including the expression of α-smooth muscle actin, desmin, collagen types I and III, fibronectin and tenascin (Lovicu et al., 2002; 2004a; 2004b). An in situ hybridisation analysis of the subcapsular plaques in these transgenic mice shows a similar profile of Wnt expression as in lenses cultured with TGFβ. Figure 2 shows stronger expression of Wnts 5a, 5b, 7b, 8a and 8b as well as Frizzled 2 in the anterior subcapsular plaques of transgenic lenses compared with the anterior lens epithelium of wild types. As with the cultured lenses, only Wnt 7a is unaffected by exposure to TGFβ (Figure 2).
Figure 2.
Wnt and Frizzled expression in transgenic lenses assessed by in situ hybridization. Representative images that include the anterior pole of the lenses of transgenic and wild-type mice show that Wnts 5a, 5b, 7b, 8a and 8b as well as Frizzled 2 are strongly expressed throughout the anterior subcapsular plaques of transgenic mice that overexpress TGFβ1 in the lens. All Wnts and Frizzled 2 are only weakly expressed in the epithelium of wild type lenses. In contrast to the other Wnts and Frizzled 2, Wnt 7a is only weakly, if at all, expressed in the anterior subcapsular plaque and is clearly not upregulated in comparison with the wild type. Abbreviations: Fz2, Frizzled 2. Scale bar: 50 μm.
Protein localization
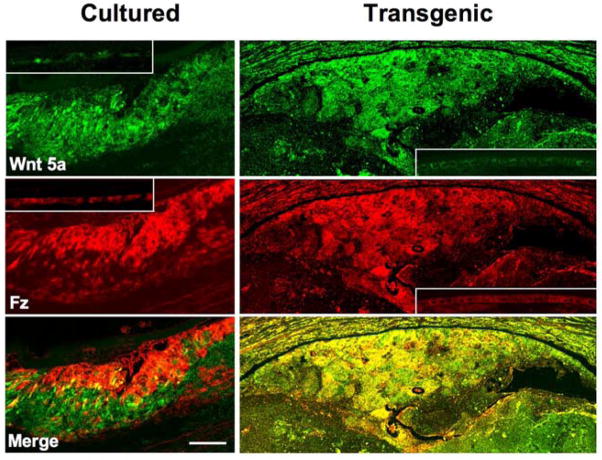
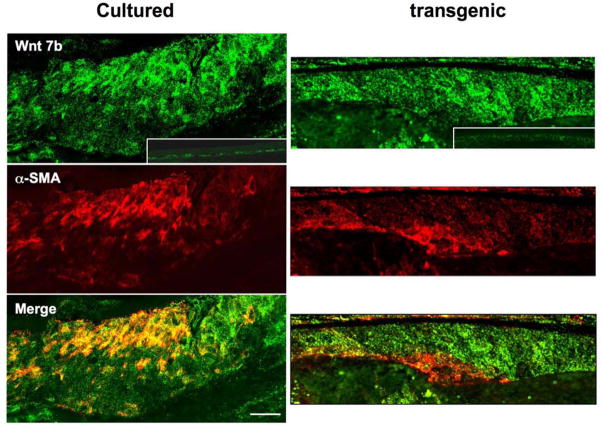
To determine if protein expression follows a similar pattern to mRNA, Wnt and Frizzled localization studies were conducted using available antibodies. Antibodies against Wnt 5a and Frizzleds (1–10) show strong immuno-reactivity in the TGFβ-induced subcapsular plaques in both cultured lenses and lenses of transgenic mice (Figure 3). Double labeling studies show that Wnt 5a and Frizzled receptors colocalize in the plaques; however, in the transgenics immuno-reactivity for ligand and receptor tends to extend throughout the plaques and this results in their colocalization being more pronounced than in the cultured lenses (Figure 3). It is not clear if these differences in Wnt 5a and Frizzled localization are a result of species differences and/or differences in presentation or potency of TGFβ in the different cataract models. Antibodies against Wnt 7b also show strong immuno-reactivity in TGFβ-induced subcapsular plaques in both cultured lenses and lenses of transgenic mice (Figure 4). As with Wnt 5a, immuno-reactivity for Wnt 7b tends to be more extensive throughout the transgenic plaques compared with plaques in cultured lenses. Alpha-smooth muscle actin, a key marker for EMT, is prominent in patches within the plaques and colocalizes with Wnt 7b in some groups of cells (Figure 4)
Figure 3.
Wnt 5a and Frizzled localization in cultured and transgenic lenses. Representative images that include the anterior pole of lenses cultured with TGFβ2 and lenses of transgenic mice that overexpress TGFβ1 show strong immuno-reactivity for Wnt 5a and Frizzleds in anterior subcapsular plaques. Comparison with the lens epithelium of controls (insets; no exposure to TGFβ) shows immuno-reactivity for Wnt 5a and Frizzleds is enhanced by exposure to TGFβ in both cultured and in transgenic lenses. Merging of the images shows co-localization of Wnt 5a and Frizzleds in both TGFβ cataract models; however, co-localization appears more uniform throughout the plaque in the transgenic model. Fz, Frizzled. Scale bar: A–F, 60 μm.
Figure 4.
Wnt 7b and α-smooth muscle actin localization in cultured and transgenic lenses. Representative images that include the anterior pole of lenses cultured with TGFβ2 and lenses of transgenic mice that overexpress TGFβ1 show strong immuno-reactivity for Wnt 7b and α-smooth muscle actin in anterior subcapsular plaques. Comparison with the lens epithelium of controls (insets; no exposure to TGFβ) shows immuno-reactivity for Wnt 7b is enhanced by exposure to TGFβ in both cultured and in transgenic lenses. Alpha-smooth muscle actin is prominent in patches within the plaques and merged images show colocalization with Wnt 7b only in some regions. α-SMA, α-smooth muscle actin. Scale bar: A–F, 60 μm.
Wnt/β-catenin signaling
Localization of unphosphorylated β-catenin
Wnt/β-catenin signaling is initiated when a Wnt ligand complexes with a Frizzled receptor and a LDL-related protein (Lrp) co-receptor (Logan and Nusse, 2004; Mikels and Nusse, 2006a). Formation of this complex leads to inhibition of glycogen synthase kinase-3β (GSK3β). Because active GSK3β phosphorylates β-catenin and targets it for destruction this ligand/receptor interaction leads to an increase in stabilised (unphosphorylated at residues Ser37 and Thr41) β-catenin. In this form, β-catenin can then associate with the DNA binding, T-cell factor (TCF), and in the nucleus regulate expression of target genes. Nuclear translocation of unphosphorylated β-catenin provides a convenient marker for activation of β-catenin/TCF signaling.
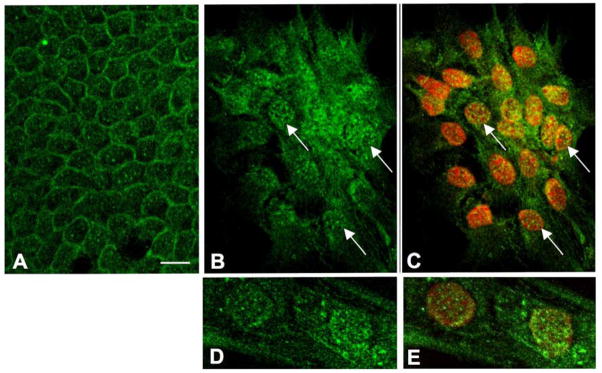
An antibody specific for unphosphorylated β-catenin showed that in control explants, β-catenin is predominantly localized to the cell margins (Figure 5A). In explants cultured with TGFβ for 3 days, the cobblestone packing is lost as cells become more spindle-shaped. This is a morphological feature of EMT in general and in particular for lens epithelial explant cultures is an important marker for cataractous change that becomes prominent after 3 days culture with TGFβ2 (Liu et al., 2004; Hales et al., 2004; Gordon-Thomson et al., 1998). In these spindle-shaped cells reactivity for β-catenin is evident throughout the cytoplasm and is particularly prominent in cell nuclei (Figure 5B, C). This is consistent with β-catenin switching from a predominantly adhesion function when it is associated with E-cadherin at the cell margins, to a signaling function when it translocates to the nucleus (Nelson and Nusse, 2004). Nuclear localization of unphosphorylated β-catenin is also evident after 1 day exposure to TGFβ2 (Figure 5D, E).
Figure 5.
Assay for Wnt/β-catenin signaling. (A) In the absence of TGFβ (controls), unphosphorylated (stabilized) β-catenin is predominantly associated with cell margins and is not prominent in nuclei; this is the case for controls at all times of culture examined. (B, C) Cells in lens epithelial explants exposed to 200 pg/ml TGFβ2 for 3 days have lost their polarity and cobblestone packing and exhibit irregular, spindle-shapes. Reactivity for unphosphorylated β-catenin is prominent in nuclei (arrows, nuclei are counterstained with propidium iodide in C). (D, E) Nuclear localization of unphosphorylated β-catenin is also evident after 1 day exposure to TGFβ2 (nuclei are counterstained with propidium iodide in E). Scale bar: A–C, 40 μm; D, E, 20 μm.
Discussion
The results of this study clearly show that several Wnts and Frizzled receptors were upregulated in association with TGFβ-induced cataract development. Both in vitro and in vivo cataract models showed similar profiles for the mRNAs and proteins that were assessed. How this upregulation of Wnts and Frizzleds is associated with TGFβ-induced EMT in the lens and the other features of subcapsular cataract is not clear at present. Other studies have shown that the Wnt pathway, through activation of β-catenin/TCF signaling, is involved in EMT in development and cancer (Brabletz et al., 2005; Reya and Clevers, 2005). TGFβ has also been shown to regulate Wnt expression in other systems and transcriptional cooperation between TGFβ and Wnt/β-catenin pathways have been shown to contribute to tumor progression (Roarty and Serra, 2007; Labbe et al., 2007). In addition to showing that TGFβ promotes expression of Wnts 5a, 5b, 7b, 8a, 8b and Frizzleds in lens cells, the current study shows that TGFβ promotes translocation of β-catenin from the cell margins to the nucleus. This is consistent with TGFβ promoting the canonical Wnt signaling function of β-catenin at the expense of its role in E-cadherin-mediated adhesion at cell margins (Nelson and Nusse, 2004).
It should also be borne in mind that Wnt and Frizzled upregulation may also be related to promotion of non-canonical Wnt signaling. Recently, because of the growing recognition of its critical role in many key developmental processes that involve intricate remodelling of the cell cytoskeleton, the Wnt/Planar Cell Polarity (PCP) pathway has received much attention (Karner et al., 2006; Jones and Chen, 2007). Signaling through the Wnt/PCP pathway involves Dvl (although through a different domain than for canonical β-catenin signaling), and activation of the small GTPases and the JNK signaling cascade (Logan and Nusse, 2004; Karner et al., 2006; Jones and Chen, 2007). Since reorganization of the cytoskeleton is a key feature of EMT the involvement of Wnt/PCP pathway would not be unexpected. In future studies it will be important to address this possibility and identify the Wnt signaling pathway(s) involved in mediating TGFβ-induced processes.
Whilst all the same Wnts were upregulated in the different cataract models, it was notable that Wnt 7a was the exception as it showed no enhanced expression in response to TGFβ. This observation may indicate that Wnt 7a is not involved in the same (or any) Wnt signaling pathway as are the other Wnts during the development of the fibrotic plaques. As Wnt 7a is often referred to as a canonical signaling Wnt (for example, see Cerpa et al., 2007), the lack of enhancement of Wnt 7a could be taken as a indication that canonical Wnt/β-catenin signaling is not the primary pathway in the development of EMT and fibrosis in the lens. However, such an interpretation needs to be treated cautiously as the designation of particular Wnts exclusively to canonical or non-canonical signaling pathways has recently been shown in many cases not to hold true. For example, recent studies have shown that Wnt 5a, which is commonly classed as a non-canonical signaling Wnt, has the potential to signal through the canonical β-catenin pathway, given the presence of appropriate co-receptors and cellular context (Mikels and Nusse, 2006a; 2006b).
Overall, the results from the current study indicate that Wnt signaling may be involved in TGFβ-induced EMT and development of subcapsular plaques. Further studies in this area are clearly warranted as identification of the key TGFβ-triggered pathways involved in the induction of EMT and associated fibrotic changes in lens cells is fundamental to identifying the best target(s) for pharmaceutical intervention in strategies to prevent PCO.
Acknowledgments
This work was possible because of support from the Sydney Foundation for Medical Research; NHMRC (Australia), NIH (USA, R01 EY03177) and Ophthalmic Research Institute of Australia. This research was undertaken as part of the Vision Cooperative Research Centre, New South Wales, Sydney, Australia, supported by the Australian Federal Government through the Cooperative Research Centres Programme. CC also acknowledges support from The Wenkart Foundation during his postgraduate studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhurst RJ, Derynck R. TGF-beta signalling in cancer - a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- Banh A, Deschamps PA, Gauldie J, Overbeek PA, Sivak JG, West-Mays JA. Lens-specific expression of TGF-beta induces anterior subcapsular cataract formation in the absence of Smad3. Invest Ophthalmol Vis Sci. 2006;47:3450–60. doi: 10.1167/iovs.05-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billotte C, Berdeaux G. Adverse clinical consequences of neodymium:YAG laser treatment of posterior capsule opacification. J Cataract Refract Surg. 2004;30:2064–71. doi: 10.1016/j.jcrs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alafaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. WNT-7alpha modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2007;283:5918–27. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- Chang MA, Congdon NG, Baker SK, Bloem MW, Savage H, Sommer A. The surgical management of cataract: barriers, best practices and outcomes. Int Ophthalmol. 2007 Aug 22; doi: 10.1007/s10792-007-9121-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Gordon-Thomson C, Chamberlain CG, Hales AM, McAvoy JW. TGF-beta receptor expression in lens: implications for differentiation and cataractogenesis. Exp Eye Res. 2001;72:649–59. doi: 10.1006/exer.2001.1001. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Gordon-Thomson C, de Iongh RU, Hales AM, Chamberlain CG, McAvoy JW. Differential cataractogenic potency of TGF-beta1, -beta2, and -beta3 and their expression in the postnatal rat eye. Invest Ophthalmol Vis Sci. 1998;39:1399–409. [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1995;36:1709–13. [PubMed] [Google Scholar]
- Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994;13:885–90. doi: 10.3109/02713689409015091. [DOI] [PubMed] [Google Scholar]
- Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20:666–74. doi: 10.1101/gad.1388806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Chen P. Planar cell polarity signaling in vertebrates. Bioessays. 2007;29:120–32. doi: 10.1002/bies.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis. 2007;45:157–68. doi: 10.1002/dvg.20277. [DOI] [PubMed] [Google Scholar]
- Labbé E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S, Moses HL, Attisano L. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res. 2007;67:75–84. doi: 10.1158/0008-5472.CAN-06-2559. [DOI] [PubMed] [Google Scholar]
- Liu J, Hales AM, Chamberlain CG, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol Vis Sci. 1994;35:388–401. [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Ang S, Chorazyczewska M, McAvoy JW. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFbeta-induced anterior subcapsular cataract. Dev Neurosci. 2004a;26:446–55. doi: 10.1159/000082286. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signaling. Development. 2001;128:5075–5084. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, McAvoy JW. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. 2002;86:220–6. doi: 10.1136/bjo.86.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest Ophthalmol Vis Sci. 2004b;45:1946–53. doi: 10.1167/iovs.03-1206. [DOI] [PubMed] [Google Scholar]
- Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–9. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006a;25:7461–8. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006b;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-beta signalling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179:11–23. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–23. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–39. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Saika S. Relationship between posterior capsule opacification and intraocular lens biocompatibility. Prog Retin Eye Res. 2004;23:283–305. doi: 10.1016/j.preteyeres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WW, Roberts AB. Smad3 signalling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–63. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Ishida I, Shirai K, Ohnishi Y, Ooshima A, McAvoy JW. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br J Ophthalmol. 2002;86:1428–33. doi: 10.1136/bjo.86.12.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Okada Y, Miyamoto T, Ohnishi Y, Ooshima A, McAvoy JW. Smad translocation and growth suppression in lens epithelial cells by endogenous TGFbeta2 during wound repair. Exp Eye Res. 2001;72:679–86. doi: 10.1006/exer.2001.1002. [DOI] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, McAvoy JW. Inhibition of transforming growth factor-beta-induced cataractous changes in lens explants by ocular media and alpha 2-macroglobulin. Invest Ophthalmol Vis Sci. 1996;37:1509–19. [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–89. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998;101:625–34. doi: 10.1172/JCI1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Lovicu FJ, Ang SL, Pandey SK, McAvoy JW. Lithium stabilizes the polarized lens epithelial phenotype and inhibits proliferation, migration, and epithelial mesenchymal transition. J Pathol. 2006;210:249–57. doi: 10.1002/path.2049. [DOI] [PubMed] [Google Scholar]
- Warner DR, Greene RM, Pisano MM. Cross-talk between the TGFbeta and Wnt signaling pathways in murine embryonic maxillary mesenchymal cells. FEBS Lett. 2005;579:3539–46. doi: 10.1016/j.febslet.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Warner DR, Pisano MM, Roberts EA, Greene RM. Identification of three novel Smad binding proteins involved in cell polarity. FEBS Lett. 2003;539:167–73. doi: 10.1016/s0014-5793(03)00155-8. [DOI] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002;74:337–47. doi: 10.1006/exer.2001.1153. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002;43:2301–8. [PubMed] [Google Scholar]
- Yang YC, Piek E, Zavadil J, Liang D, Xie D, Heyer J, Pavlidis P, Kucherlapati R, Roberts AB, Bottinger EP. Hierarchical model of gene regulation by transforming growth factor beta. Proc Natl Acad Sci U S A. 2003;100:10269–74. doi: 10.1073/pnas.1834070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:10870–6. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]